Magnetic Properties of La0.9A0.1MnO3 (A: Li, Na, K) Nanopowders and Nanoceramics
Abstract
1. Introduction
2. Materials and Methods
3. Results
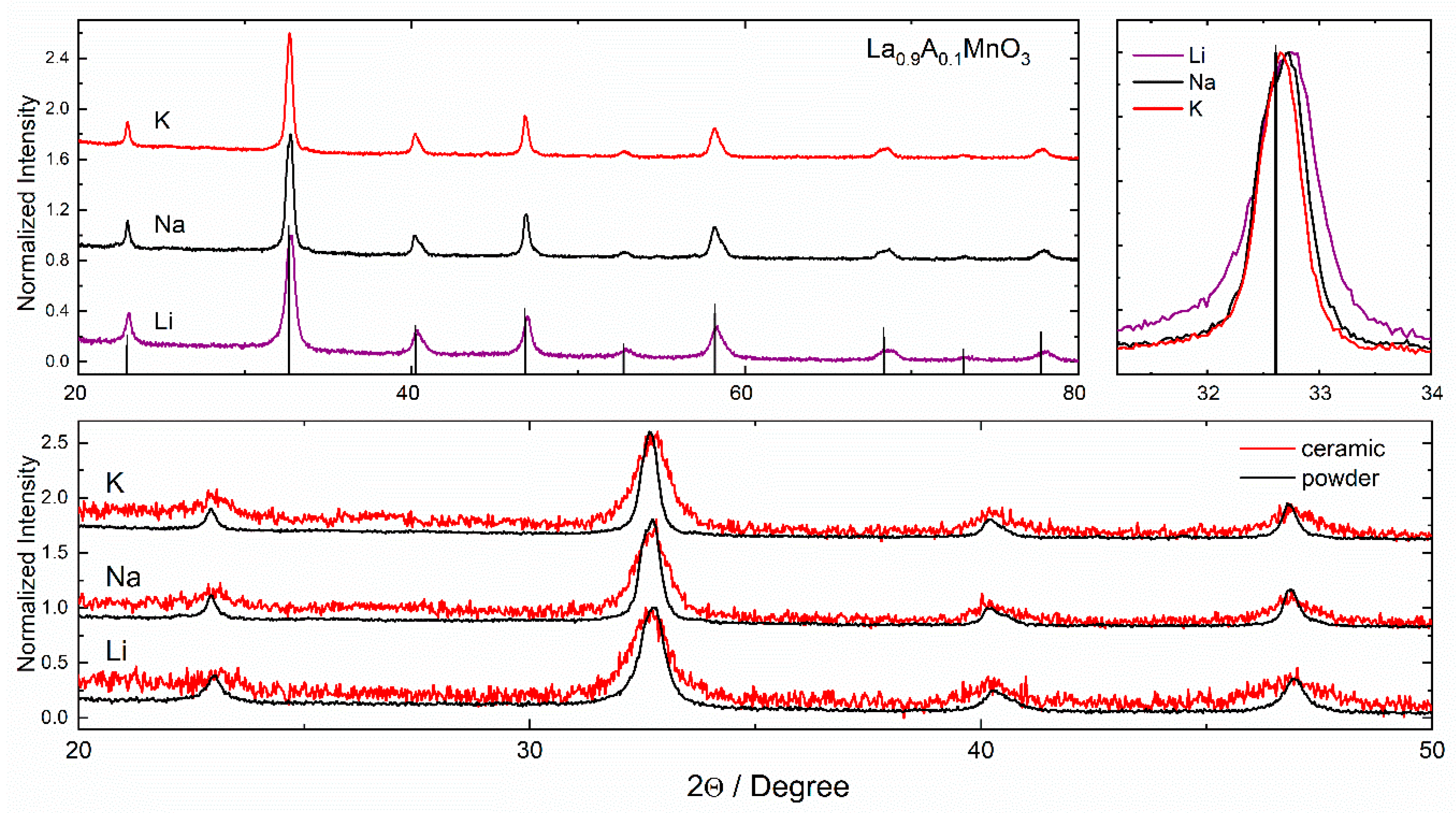
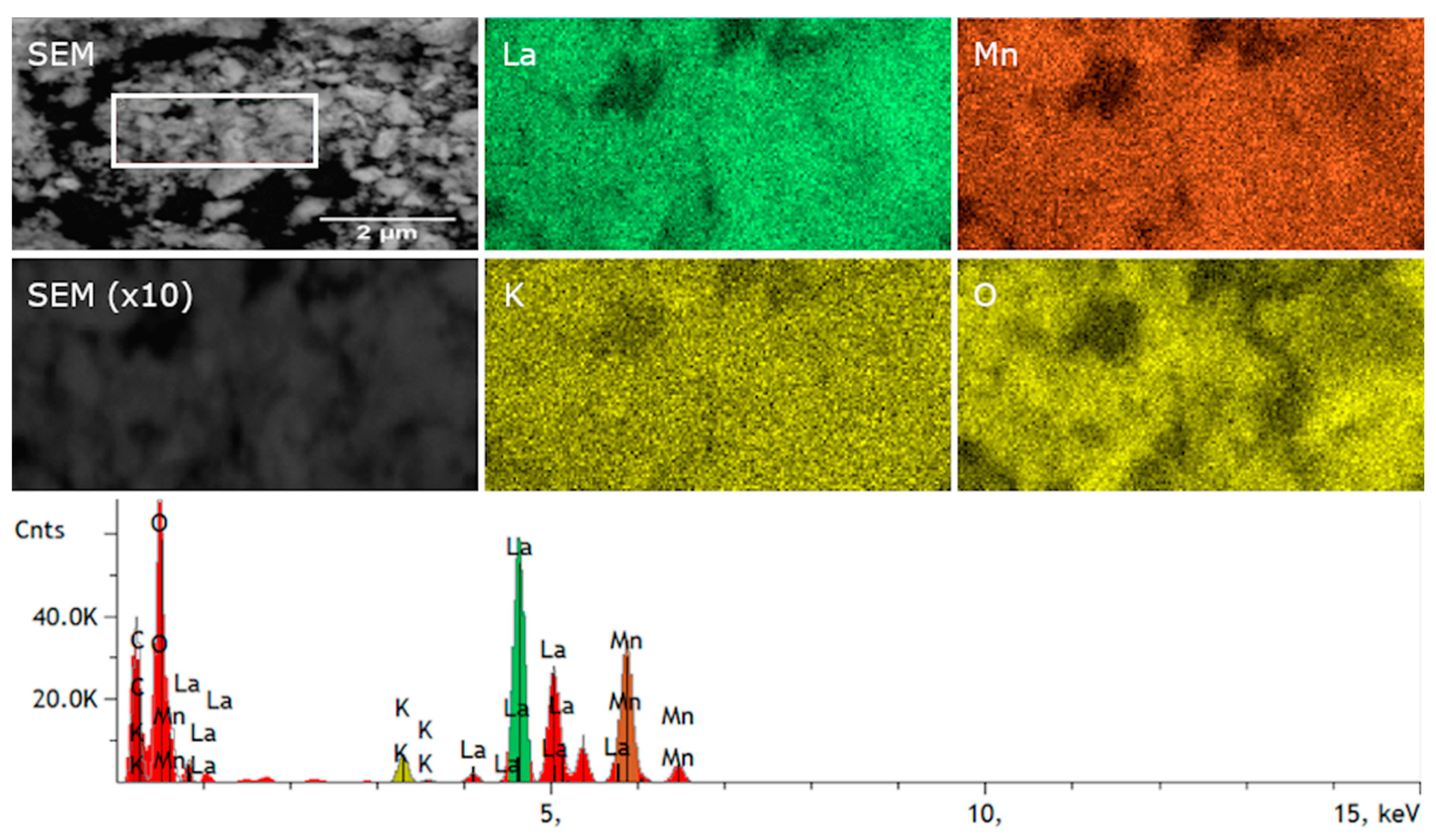
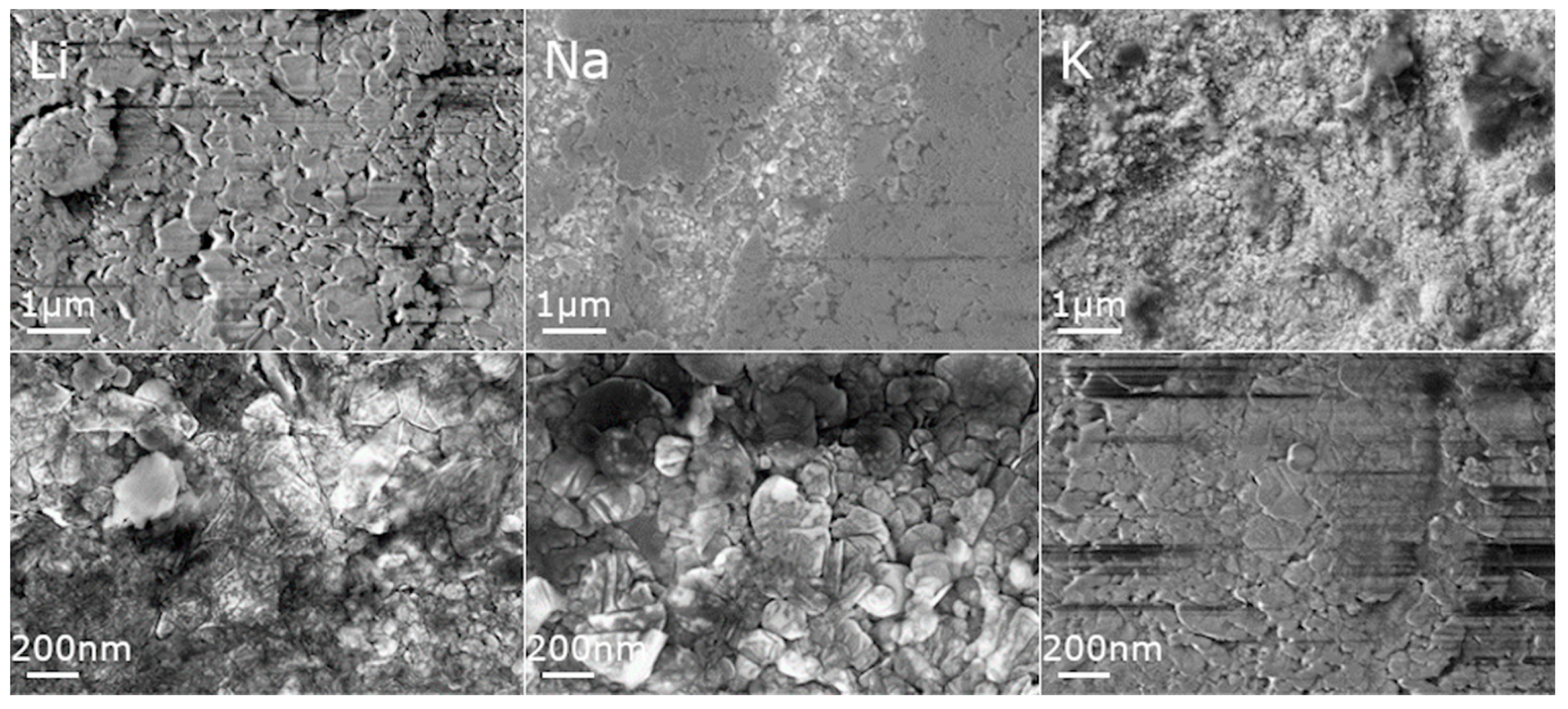
3.1. Structure and Morphology
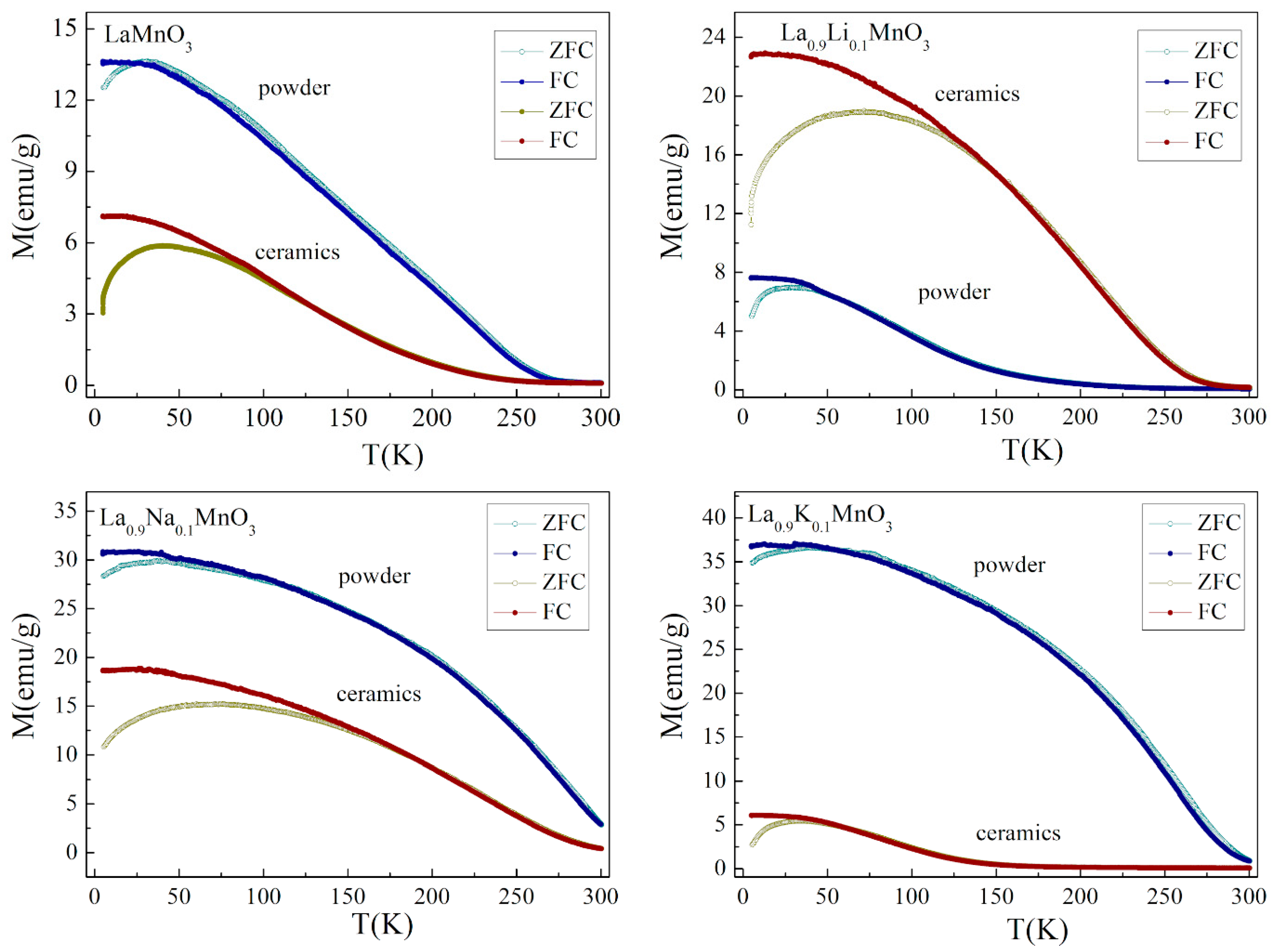
3.2. Magnetic Properties of Powders and Ceramics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dagotto, E.; Hotta, T.; Moreo, A. Colossal magnetoresistant materials: The key role of phase separation. Phys. Rep. 2001, 344, 1–153. [Google Scholar] [CrossRef]
- Baldini, M.; Muramatsu, T.; Sherafati, M.; Mao, H.K.; Malavasi, L.; Postorino, P.; Satpathy, S.; Struzhkin, V.V. Origin of colossal magnetoresistance in LaMnO3 manganite. Proc. Natl. Acad. Sci. USA 2015, 112, 10869–10872. [Google Scholar] [CrossRef] [PubMed]
- Razi, Z.J.; Sebt, S.A.; Khajehnezhad, A. Magnetoresistance temperature dependence of LSMO and LBMO perovskite manganites. J. Theor. Appl. Phys. 2018, 12, 243–248. [Google Scholar] [CrossRef]
- Zener, C. Interaction between the d-shells in the transition metals. II. Ferromagnetic compounds of manganese with Perovskite structure. Phys. Rev. 1951, 82, 403–405. [Google Scholar] [CrossRef]
- Shivakumara, C.; Hegde, M.S.; Subbanna, G.N. Low temperature synthesis of ferromagnetic (LaK)MnO3 from KCl, KBr and KI fluxes. Solid State Sci. 2001, 3, 43–48. [Google Scholar] [CrossRef]
- Satoh, T.; Kikuchi, Y.; Miyano, K.; Pollert, E.; Hejtmánek, J.; Jirák, Z. Irreversible photoinduced insulator-metal transition in the Na-doped manganite Pr0.75Na0.25MnO3. Phys. Rev. B-Condens. Matter Mater. Phys. 2002, 65, 1–4. [Google Scholar] [CrossRef]
- Mahesh, R.; Mahendiran, R.; Raychaudhuri, A.K.; Rao, C.N.R. Effect of the Internal-Pressure Due to the A-Site Cations on the Giant Magnetoresistance and Related Properties of Doped Rare-Earth Manganates, Ln1-xAxMnO3 (Ln=La,Nd,Gd,Y, A=Ca,Sr,Ba,Pb). J. Solid State Chem. 1995, 120, 204–207. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Khomchenko, V.A.; Karpinsky, D.V.; Silibin, M.V.; Trukhanov, A.V.; Lobanovsky, L.S.; Szymczak, H.; Botez, C.E.; Troyanchuk, I.O. A-site ordered state in manganites with perovskite-like structure based on optimally doped compounds Ln0.70Ba0.30MnO3 (Ln = Pr, Nd). J. Rare Earths 2019, 37, 1242–1249. [Google Scholar] [CrossRef]
- Fäth, M.; Freisem, S.; Menovsky, A.A.; Tomioka, Y.; Aarts, J.; Mydosh, J.A. Spatially inhomogeneous metal-insulator transition in doped manganites. Science 1999, 285, 1540–1542. [Google Scholar]
- Tao, J.; Niebieskikwiat, D.; Varela, M.; Luo, W.; Schofield, M.A.; Zhu, Y.; Salamon, M.B.; Zuo, J.M.; Pantelides, S.T.; Pennycook, S.J. Direct imaging of nanoscale phase separation in La0.55Ca0.45MnO3: Relationship to colossal magnetoresistance. Phys. Rev. Lett. 2009, 103, 097202. [Google Scholar] [CrossRef]
- Rout, G.C.; Parhi, N.; Behera, S.N. The influence of band Jahn-Teller effect and magnetic order on the magneto-resistance in manganite systems. Phys. B Condens. Matter 2009, 404, 2315–2323. [Google Scholar] [CrossRef]
- Phan, M.H.; Yu, S.C. Review of the magnetocaloric effect in manganite materials. J. Magn. Magn. Mater. 2007, 308, 325–340. [Google Scholar] [CrossRef]
- Ge, X.S.; Li, Z.Z.; Qi, W.H.; Ji, D.H.; Tang, G.D.; Ding, L.L.; Qian, J.J.; Du, Y.N. Magnetic and electrical transport properties of perovskite manganites Pr0.6Sr0.4 M xMn1-xO3 (M = Fe, Co, Ni). AIP Adv. 2017, 7. [Google Scholar] [CrossRef]
- Kim, M.K.; Moon, J.Y.; Oh, S.H.; Oh, D.G.; Choi, Y.J.; Lee, N. Strong magnetoelectric coupling in mixed ferrimagnetic-multiferroic phases of a double perovskite. Sci. Rep. 2019, 9, 5456. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Parsons, J.; McCloy, J.S. Magnetic properties of double perovskite La2BMnO6 (B = Ni or Co) nanoparticles. Nanoscale 2013, 5, 4720–4728. [Google Scholar] [CrossRef]
- Yamada, S.; Abe, N.; Sagayama, H.; Ogawa, K.; Yamagami, T.; Arima, T. Room-Temperature Low-Field Colossal Magnetoresistance in Double-Perovskite Manganite. Phys. Rev. Lett. 2019, 123, 126602. [Google Scholar] [CrossRef]
- Allodi, G.; De Renzi, R.; Zheng, K.; Sanna, S.; Sidorenko, A.; Baumann, C.; Righi, L.; Orlandi, F.; Calestani, G. Band filling effect on polaron localization in La1-x(Ca ySr1-y)xMnO3 manganites. J. Phys. Condens. Matter 2014, 26, 266004. [Google Scholar] [CrossRef]
- Das, S.; Kavipriya, T.; Nirmala, R. Magnetic and magnetocaloric property studies on nanoparticles of electron-doped manganites R0.15Ca0.85MnO3 (R = Pr and Nd). Mater. Res. Express 2019, 6. [Google Scholar] [CrossRef]
- Bebenin, N.G.; Zainullina, R.I.; Bannikova, N.S.; Elokhina, L.V.; Ustinov, V.V.; Mukovskii, Y.M. Magnetoresistance of the La0.7Ca0.3MnO3 single crystal. Phys. Met. Metallogr. 2009, 108, 232–236. [Google Scholar] [CrossRef]
- Supelano, G.I.; Barón-González, A.J.; Sarmiento Santos, A.; Ortíz, C.; Mejía Gómez, J.A.; Parra Vargas, C.A. Effect of Mg addition on LaMnO3 ceramic system. J. Mater. Res. Technol. 2018, 7, 77–81. [Google Scholar] [CrossRef]
- Markovich, V.; Fita, I.; Mogilyansky, D.; Wisniewski, A.; Puzniak, R.; Titelman, L.; Vradman, L.; Herskowitz, M.; Gorodetsky, G. Magnetic properties of nanocrystalline La1-xMnO 3+δ manganites: Size effects. J. Phys. Condens. Matter 2007, 19, 346210. [Google Scholar] [CrossRef]
- Sacchetti, A.; Postorino, P.; Capone, M. High-pressure phase diagram in the manganites: A two-site model study. New J. Phys. 2006, 8. [Google Scholar] [CrossRef]
- Garbarino, G.; Parón, S.; Monteverde, M.; Acha, C.; Leyva, G.; Vega, D.; Polla, G.; Briático, J.; Alascio, B. High-pressure effects on the resistivity and ferromagnetic transition of ceramic manganite Ca1-xyxMnO3. J. Magn. Magn. Mater. 2001, 226–230, 843–844. [Google Scholar] [CrossRef]
- Głuchowski, P.; Tomala, R.; Kowalski, R.; Ignatenko, O.; Witkowski, M.E.; Drozdowski, W.; Stręk, W.; Ryba-Romanowski, W.; Solarz, P. “Frozen” pressure effect in GGAG:Ce3+ white light emitting nanoceramics. Ceram. Int. 2019, 45, 21870–21877. [Google Scholar] [CrossRef]
- Fedyk, R.; Hreniak, D.; Łojkowski, W.; Stręk, W.; Matysiak, H.; Grzanka, E.; Gierlotka, S.; Mazur, P. Method of preparation and structural properties of transparent YAG nanoceramics. Opt. Mater. 2007, 29, 1252–1257. [Google Scholar] [CrossRef]
- Wang, Q.; He, D.; Peng, F.; Lei, L.; Liu, P.; Yin, S.; Wang, P.; Xu, C.; Liu, J. Unusual compression behavior of nanocrystalline CeO2. Sci. Rep. 2014, 4, 4441. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Gluchowski, P.; Strek, W. Luminescence and excitation spectra of Cr3+:MgAl 2O4 nanoceramics. Mater. Chem. Phys. 2013, 140, 222–227. [Google Scholar] [CrossRef]
- Alonso, J.A. Non-stoichiometry and properties of mixed-valence manganites. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 1998, 356, 1617–1634. [Google Scholar]
- Markovich, V.; Jung, G.; Fita, I.; Mogilyansky, D.; Wu, X.; Wisniewski, A.; Puzniak, R.; Froumin, N.; Titelman, L.; Vradman, L.; et al. Magnetotransport in granular LaMnO3+δ manganite with nano-sized particles. J. Phys. D. Appl. Phys. 2008, 41, 185001. [Google Scholar] [CrossRef]
- Markovich, V.; Fita, I.; Mogilyansky, D.; Wisniewski, A.; Puzniak, R.; Titelman, L.; Vradman, L.; Herskowitz, M.; Gorodetsky, G. Effect of particle size on magnetic properties of LaMnO3 + δ nanoparticles. Superlattices Microstruct. 2008, 44, 476–482. [Google Scholar] [CrossRef]
- Coey, J.M.D.; Viret, M.; Von Molnár, S. Mixed-valence manganites. Adv. Phys. 1999, 48, 167–293. [Google Scholar] [CrossRef]
- Ju, H.L.; Nam, Y.S.; Lee, J.E.; Shin, H.S. Anomalous magnetic properties and magnetic phase diagram of La1-xBaxMnO3. J. Magn. Magn. Mater. 2000, 219, 1–8. [Google Scholar] [CrossRef]
- Von Helmolt, R.; Wecker, J.; Samwer, K.; Haupt, L.; Bärner, K. Intrinsic giant magnetoresistance of mixed valence La-A-Mn oxide (A=Ca,Sr,Ba) (invited). J. Appl. Phys. 1994, 76, 6925–6928. [Google Scholar] [CrossRef]
- Castillo, M.E.; Shvartsman, V.V.; Gobeljic, D.; Gao, Y.; Landers, J.; Wende, H.; Lupascu, D.C. Effect of particle size on ferroelectric and magnetic properties of BiFeO3 nanopowders. Nanotechnology 2013, 24, 355701. [Google Scholar] [CrossRef]
- Huang, F.; Wang, Z.; Lu, X.; Zhang, J.; Min, K.; Lin, W.; Ti, R.; Xu, T.; He, J.; Yue, C.; et al. Peculiar magnetism of BiFeO3 nanoparticles with size approaching the period of the spiral spin structure. Sci. Rep. 2013, 3, 2907. [Google Scholar] [CrossRef]



| a, b | c | V | Strains | Density | |
|---|---|---|---|---|---|
| Å | Å | Å3 | % | g/cm3 | |
| Powders | |||||
| La0.9Li0.1MnO3 | 5.4974 | 13.3149 | 348.49 (58.08) | 0.073 | 6.62 |
| La0.9Na0.1MnO3 | 5.5017 | 13.3382 | 349.65 (58.27) | 0.051 | 6.60 |
| La0.9K0.1MnO3 | 5.5044 | 13.3693 | 350.79 (58.47) | 0.046 | 6.58 |
| Ceramics | |||||
| La0.9Li0.1MnO3 | 5.4979 | 13.3198 | 348.68 (58.11) | 0.136 | 6.91 |
| La0.9Na0.1MnO3 | 5.5080 | 13.3402 | 350.51 (58.42) | 0.094 | 6.86 |
| La0.9K0.1MnO3 | 5.5088 | 13.3798 | 351.64 (58.61) | 0.083 | 6.82 |
| Element | Line | Intensity | Concentration | Concentration | Error |
|---|---|---|---|---|---|
| (c/s) | wt.% | mol% | 2-sig | ||
| C | Ka | 96.28 | 7.79 | - | 0.114 |
| O | Ka | 196.26 | 14.21 | 22.2 | 0.148 |
| K | Ka | 24.95 | 1.69 | 1.08 | 0.068 |
| Mn | Ka | 132 | 23.37 | 10.62 | 0.287 |
| La | La | 228.79 | 52.94 | 9.52 | 0.401 |
| Total | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Głuchowski, P.; Nikonkov, R.; Tomala, R.; Stręk, W.; Shulha, T.; Serdechnova, M.; Zheludkevich, M.; Pakalaniškis, A.; Skaudžius, R.; Kareiva, A.; et al. Magnetic Properties of La0.9A0.1MnO3 (A: Li, Na, K) Nanopowders and Nanoceramics. Materials 2020, 13, 1788. https://doi.org/10.3390/ma13071788
Głuchowski P, Nikonkov R, Tomala R, Stręk W, Shulha T, Serdechnova M, Zheludkevich M, Pakalaniškis A, Skaudžius R, Kareiva A, et al. Magnetic Properties of La0.9A0.1MnO3 (A: Li, Na, K) Nanopowders and Nanoceramics. Materials. 2020; 13(7):1788. https://doi.org/10.3390/ma13071788
Chicago/Turabian StyleGłuchowski, Paweł, Ruslan Nikonkov, Robert Tomala, Wiesław Stręk, Tatsiana Shulha, Maria Serdechnova, Mikhail Zheludkevich, Andrius Pakalaniškis, Ramūnas Skaudžius, Aivaras Kareiva, and et al. 2020. "Magnetic Properties of La0.9A0.1MnO3 (A: Li, Na, K) Nanopowders and Nanoceramics" Materials 13, no. 7: 1788. https://doi.org/10.3390/ma13071788
APA StyleGłuchowski, P., Nikonkov, R., Tomala, R., Stręk, W., Shulha, T., Serdechnova, M., Zheludkevich, M., Pakalaniškis, A., Skaudžius, R., Kareiva, A., Abramov, A., Kholkin, A., Bushinsky, M. V., & Karpinsky, D. (2020). Magnetic Properties of La0.9A0.1MnO3 (A: Li, Na, K) Nanopowders and Nanoceramics. Materials, 13(7), 1788. https://doi.org/10.3390/ma13071788











