Microwave-Assisted Glycerol Etherification Over Sulfonic Acid Catalysts
Abstract
1. Introduction
2. Materials and Methods
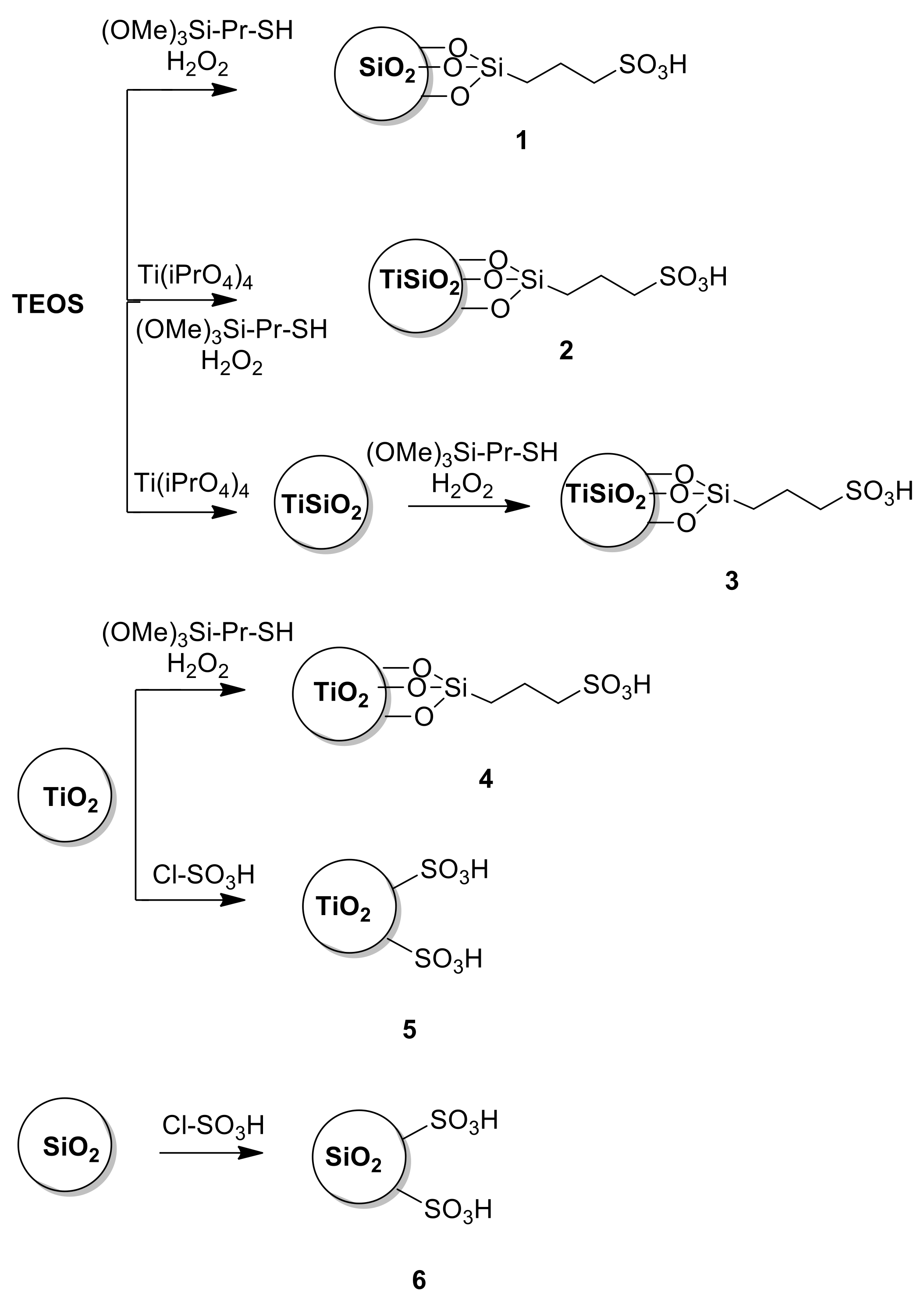
2.1. Preparation of Catalysts
2.1.1. Synthesis of the Propyl SO3H Amorphous SiO2 as It (1) or 10% Ti Doped (2)
2.1.2. Synthesis of 10% Ti Doped Propyl SO3H Amorphous SiO2 (3)
2.1.3. Synthesis of SiO2 Support
2.1.4. Synthesis of TiO2 Support
2.1.5. Synthesis of Propyl Sulfonic Acid Titania (4)
2.1.6. Synthesis of Sulfonic Acid Titania (5) or Silica (6)
2.2. Catalysts Characterization
2.3. Catalytic Reaction
3. Results and Discussion
3.1. Characterization of Catalysts
3.2. Catalytic Activity in the Etherification Reaction
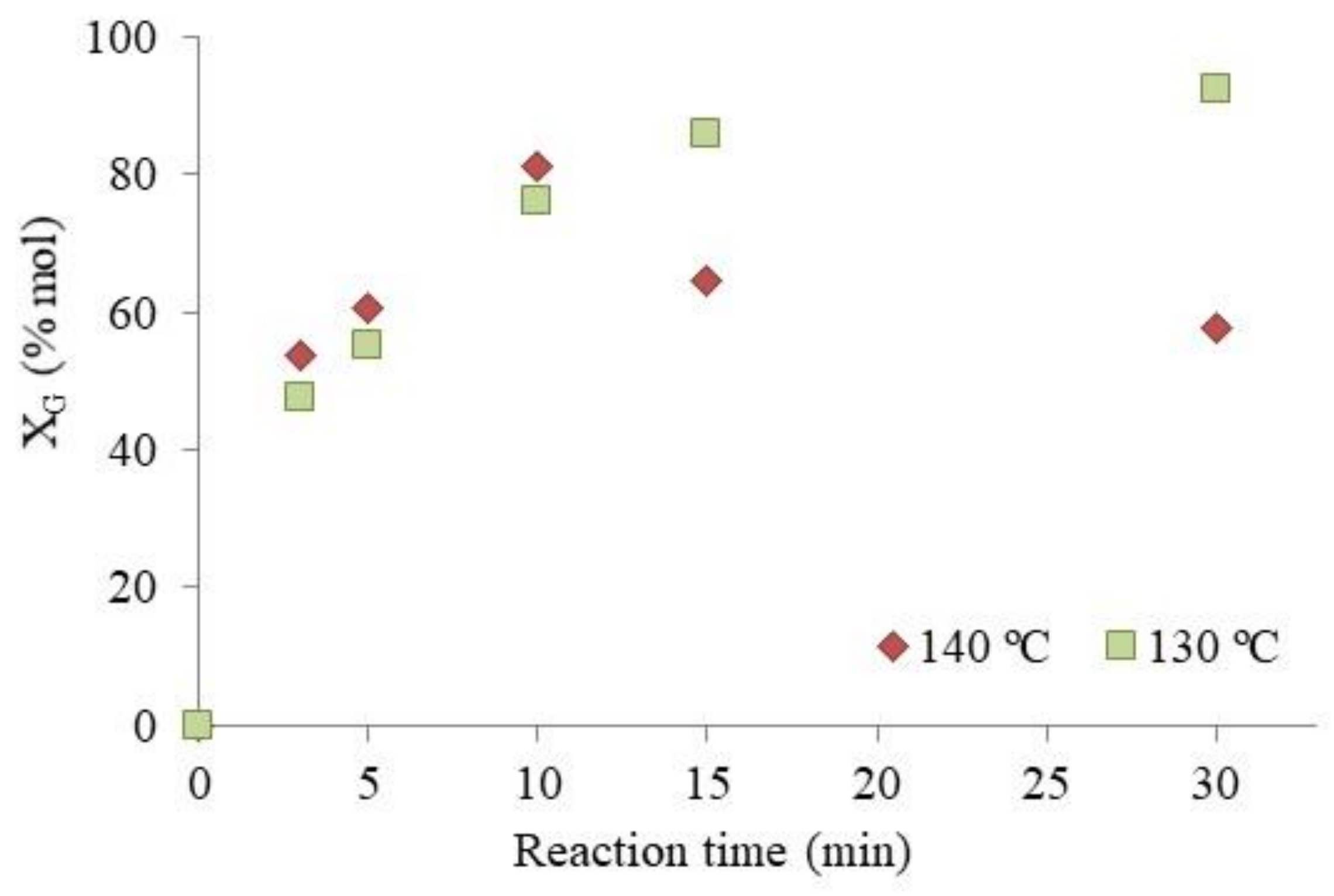
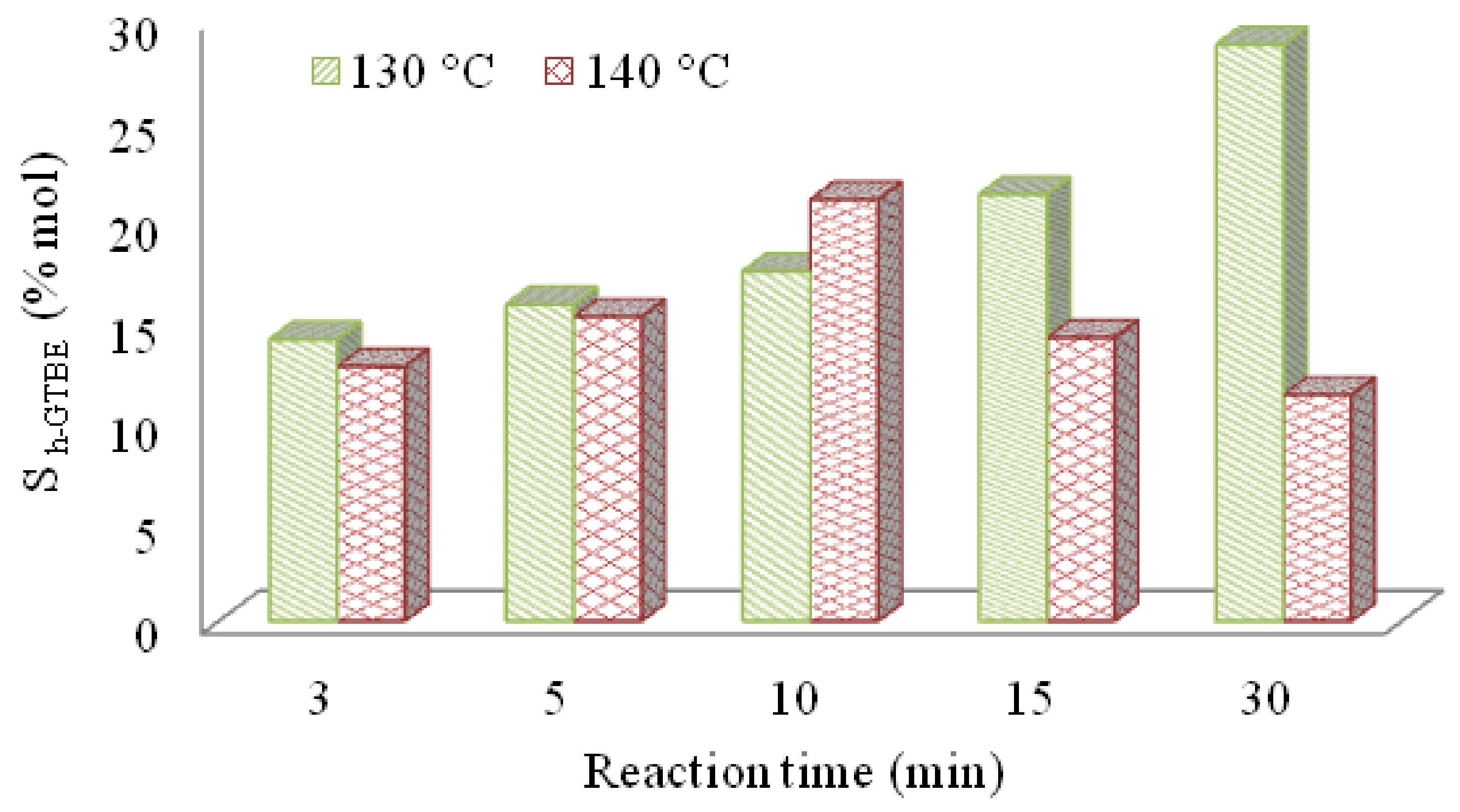
3.2.1. Optimization of Reaction Condition
3.2.2. Screening of the Sulfonic Silica- or Titania-Based Catalysts
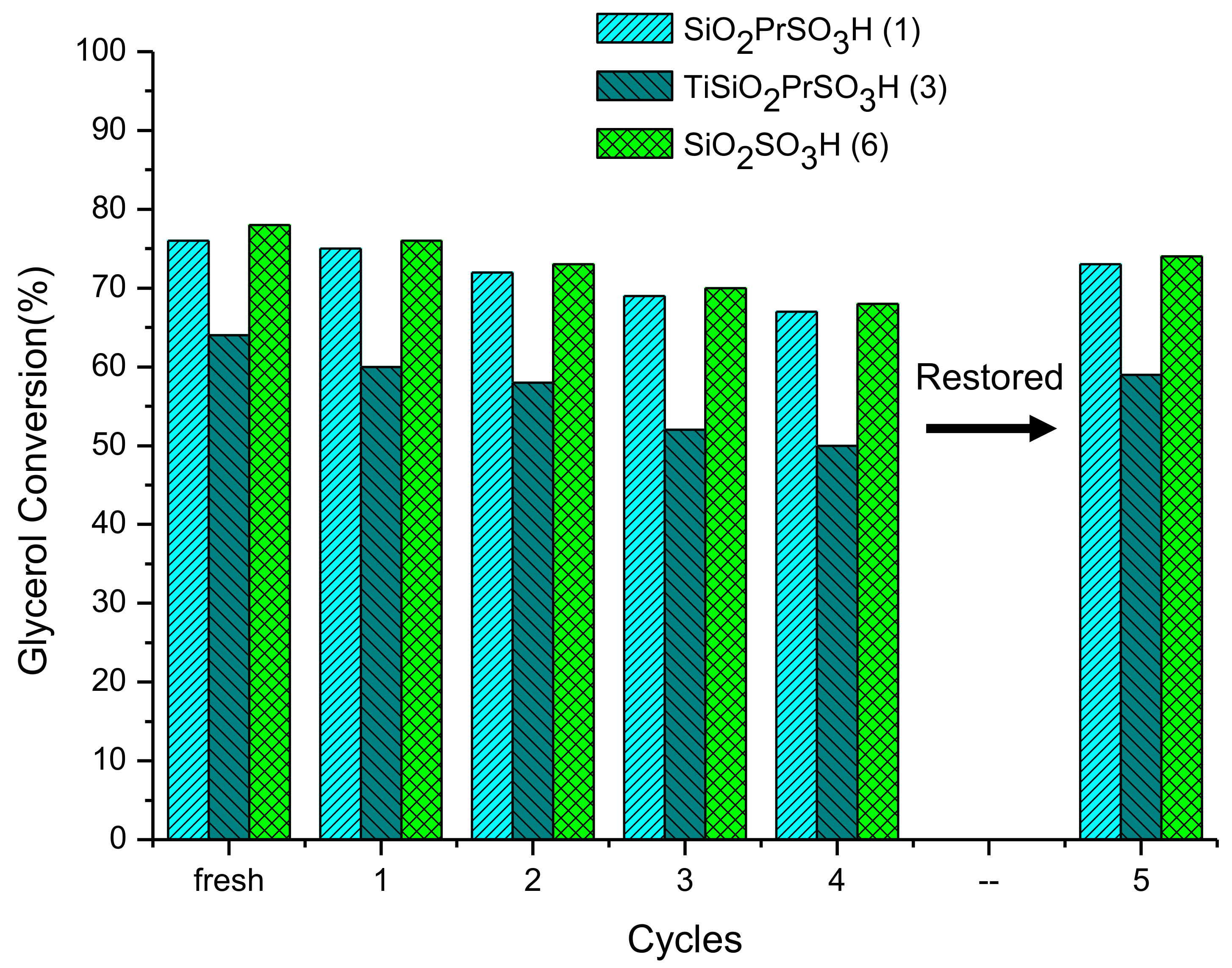
3.2.3. Reusability and Catalyst Stability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Estevez, R.; Aguado-Deblas, L.; Bautista, F.M.; Luna, D.; Luna, C.; Calero, J.; Posadillo, A.; Romero, A.A. Biodiesel at the Crossroads: A Critical Review. Catalysts 2019, 9, 1033. [Google Scholar] [CrossRef]
- Calero, J.; Verdugo, C.; Luna, D.; Sancho, E.D.; Luna, C.; Posadillo, A.; Bautista, F.M.; Romero, A.A. Achievement of a biofuel-like biodiesel by regioselective transesterification of sunflower oil with mucor miehei lipase. New Biotechnol. 2014, 31, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Calero, J.; Luna, D.; Sancho, E.D.; Luna, C.; Bautista, F.M.; Romero, A.A.; Posadillo, A.; Verdugo, C. Development of a new biodiesel that integrates glycerol, by using CaO as heterogeneous catalyst, in the partial methanolysis of sunflower oil. Fuel 2014, 122, 94–102. [Google Scholar] [CrossRef]
- Drago, C.; Liotta, L.F.; la Parola, V.; Testa, M.L.; Nicolosi, G. One-pot microwave assisted catalytic transformation of vegetable oil into glycerol-free biodiesel. Fue 2013, 113, 707–711. [Google Scholar] [CrossRef]
- Rahmat, N.; Abdullah, A.Z.; Mohamed, A.R. Recent progress on innovative and potential technologies for glycerol transformation into fuel additives: A critical review. Renew. Sustain. Energy Rev. 2010, 14, 987–1000. [Google Scholar] [CrossRef]
- Bagheri, S.; Julkapli, N.M.; Yehye, W.A. Catalytic conversion of biodiesel derived raw glycerol to value added products. Renew. Sustain. Energy Rev. 2015, 41, 113–127. [Google Scholar] [CrossRef]
- Nanda, M.R.; Zhang, Y.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Xu, C.C. Catalytic conversion of glycerol for sustainable production of solketal as a fuel additive: A review. Renew. Sustain. Energy Rev. 2016, 56, 1022–1031. [Google Scholar] [CrossRef]
- Cornejo, A.; Barrio, I.; Campoy, M.; Lázaro, J.; Navarrete, B. Oxygenated fuel additives from glycerol valorization. Main production pathways and effects on fuel properties and engine performance: A critical review. Renew. Sustain. Energy Rev. 2017, 79, 1400–1413. [Google Scholar] [CrossRef]
- Estevez, R.; Lopez-Pedrajas, S.; Luna, D.; Bautista, F. Microwave-assisted etherification of glycerol with tert-butyl alcohol over amorphous organosilica-aluminum phosphates. Appl. Catal. B Environ. 2017, 213, 42–52. [Google Scholar] [CrossRef]
- Alptekin, E. Emission, injection and combustion characteristics of biodiesel and oxygenated fuel blends in a common rail diesel engine. Energy 2017, 119, 44–52. [Google Scholar] [CrossRef]
- Beatrice, C.; di Blasio, G.; Lazzaro, M.; Mancaruso, E.; Marialto, R.; Sequino, L.; Vaglieco, B.M. Investigation of the combustion in both metal and optical diesel engines using high-glycerol ethers/diesel blends. Int. J. Engine Res. 2015, 16, 38–51. [Google Scholar] [CrossRef]
- Behr, A.; Obendorf, L. Development of a process for the acid-catalyzed etherification of glycerine and isobutene forming glycerine tertiary butyl ethers. Eng. Life Sci. 2002, 2, 185–189. [Google Scholar] [CrossRef]
- Klepáčová, K.; Mravec, D.; Bajus, M. tert-Butylation of glycerol catalysed by ion-exchange resins. Appl. Catal. A Gen. 2005, 294, 141–147. [Google Scholar] [CrossRef]
- Chang, J.-S.; Zhang, Y.-C.; Chen, C.-C.; Ling, T.-R.; Chiou, Y.-J.; Wang, G.-B.; Chang, K.-T.; Chou, T.-C. One-Step Synthesis of Gasoline Octane Booster and Diesel Fuel from Glycerol and tert-Butyl Alcohol. Ind. Eng. Chem. Res. 2014, 53, 5398–5405. [Google Scholar] [CrossRef]
- González, M.D.; Salagre, P.; Linares, M.; García, R.; Serrano, D.; Cesteros, Y. Effect of hierarchical porosity and fluorination on the catalytic properties of zeolite beta for glycerol etherification. Appl. Catal. A Gen. 2014, 473, 75–82. [Google Scholar] [CrossRef]
- Estevez, R.; Iglesias, I.; Luna, D.; Bautista, F.M. Sulfonic Acid Functionalization of Different Zeolites and Their Use as Catalysts in the Microwave-Assisted Etherification of Glycerol with tert-Butyl Alcohol. Molecules 2017, 22, 2206. [Google Scholar] [CrossRef]
- Melero, J.; Vicente, G.; Morales, G.; Paniagua, M.; Moreno, J.; Roldán, R.; Ezquerro, A.; Pérez, C. Acid-catalyzed etherification of bio-glycerol and isobutylene over sulfonic mesostructured silicas. Appl. Catal. A Gen. 2008, 346, 44–51. [Google Scholar] [CrossRef]
- Gonçalves, M.; Mantovani, M.; Carvalho, W.A.; Rodrigues, R.; Mandelli, D.; Albero, J.S. Biodiesel wastes: An abundant and promising source for the preparation of acidic catalysts for utilization in etherification reaction. Chem. Eng. J. 2014, 256, 468–474. [Google Scholar] [CrossRef]
- Estevez, R.; López, M.; Jiménez-Sanchidrián, C.; Luna, D.; Romero-Salguero, F.; Bautista, F. Etherification of glycerol with tert-butyl alcohol over sulfonated hybrid silicas. Appl. Catal. A Gen. 2016, 526, 155–163. [Google Scholar] [CrossRef]
- Galhardo, T.S.; Simone, N.; Gonçalves, M.; Figueiredo, F.C.; Mandelli, D.; Carvalho, W.A. Preparation of Sulfonated Carbons from Rice Husk and Their Application in Catalytic Conversion of Glycerol. ACS Sustain. Chem. Eng. 2013, 1, 1381–1389. [Google Scholar]
- González, M.D.; Cesteros, Y.; Salagre, P. Establishing the role of Brønsted acidity and porosity for the catalytic etherification of glycerol with tert-butanol by modifying zeolites. Appl. Catal. A Gen. 2013, 450, 178–188. [Google Scholar] [CrossRef]
- Simone, N.; Carvalho, W.A.; Mandelli, D.; Ryoo, R. Nanostructured MFI-type zeolites as catalysts in glycerol etherification with tert-butyl alcohol. J. Mol. Catal. A Chem. 2016, 422, 115–121. [Google Scholar] [CrossRef]
- Gonçalves, M.; Souza, V.C.; Galhardo, T.S.; Mantovani, M.; Figueiredo, F.v.C.; Mandelli, D.; Carvalho, W.A. Glycerol Conversion Catalyzed by Carbons Prepared from Agroindustrial Wastes. Ind. Eng. Chem. Res. 2013, 52, 2832–2839. [Google Scholar] [CrossRef]
- Testa, M.L.; la Parola, V.; Venezia, A.M. Transesterification of short chain esters using sulfonic acid-functionalised hybrid silica. Catal. Today 2014, 223, 115–121. [Google Scholar] [CrossRef]
- Testa, M.L.; la Parola, V.; Liotta, L.F.; Venezia, A.M. Screening of different solid acid catalysts for glycerol acetylation. J. Mol. Catal. A Chem. 2013, 367, 69–76. [Google Scholar] [CrossRef]
- Testa, M.L.; Miroddi, G.; Russo, M.; la Parola, V.; Marci, G. Dehydration of Fructose to 5-HMF over Acidic TiO2 Catalysts. Materials 2020, 13, 1178. [Google Scholar] [CrossRef]
- Serrano, D.P.; Calleja, G.; Sanz, R.; Pizarro, P. Preparation of bimodal micro–mesoporous TiO2 with tailored crystalline properties. Chem. Commun. 2004, 8, 1000–1001. [Google Scholar]
- Shiota, M. Some properties of aqueous titanium isopropoxide-hydrogenperoxide solutions and their decomposition to produce titanium dioxide. J. Mater. Sci. 1988, 23, 1718–1724. [Google Scholar] [CrossRef]
- Nyman, M.; Hobbs, D.T. A Family of Peroxo-titanate Materials Tailored for Optimal Strontium and Actinide Sorption. Chem. Mater. 2006, 18, 6425–6435. [Google Scholar]
- Chang, J.A.; Vithal, M.; Baek, I.C.; Seok, S.I. Evolution of Phase and Morphology of Titanium Dioxide Induced from Peroxo Titanate Complex Aqueous Solution. J. Nanosci. Nanotechnol. 2010, 10, 163–169. [Google Scholar] [CrossRef]
- Frusteri, F.; Arena, F.; Bonura, G.; Cannilla, C.; Spadaro, L.; di Blasi, O. Catalytic etherification of glycerol by tert-butyl alcohol to produce oxygenated additives for diesel fuel. Appl. Catal. A Gen. 2009, 367, 77–83. [Google Scholar] [CrossRef]
- Klepáčová, K.; Mravec, D.; Bajus, M. Etherification of glycerol with tert-butyl alcohol catalysed by ion-exchange resins. Chem. Papers 2006, 60, 224–230. [Google Scholar]
- Beatrice, C.; di Blasio, G.; Lazzaro, M.; Cannilla, C.; Bonura, G.; Frusteri, F.; Asdrubali, F.; Baldinelli, G.; Presciutti, A.; Fantozzi, F. Technologies for energetic exploitation of biodiesel chain derived glycerol: Oxy-fuels production by catalytic conversion. Appl. Energy 2013, 102, 63–71. [Google Scholar] [CrossRef]
- Magar, S.; Kamble, S.; Mohanraj, G.T.; Jana, S.K.; Rode, C. Solid-Acid-Catalyzed Etherification of Glycerol to Potential Fuel Additives Energy. Fuels 2017, 31, 12272–12277. [Google Scholar] [CrossRef]
- Celdeira, P.A.; Goncalves, M.; Figueiredo, F.C.; Bosco, S.M.D.; Mandelli, D.; Carvalho, W.A. Sulfonated niobia and pillared clay as catalysts in etherification reaction of glycerol. Appl. Catal. A Gen. 2014, 478, 98–106. [Google Scholar] [CrossRef]
- Srinivas, M.; Raveendra, G.; Parameswaram, G.; Prasad, P.S.; Lingaiah, N. Cesium exchanged tungstophosphoric acid supported on tin oxide: An efficient solid acid catalyst for etherification of glycerol with tert-butanol to synthesize biofuel additives. J. Mol. Catal. A Chem. 2016, 413, 7–14. [Google Scholar] [CrossRef]

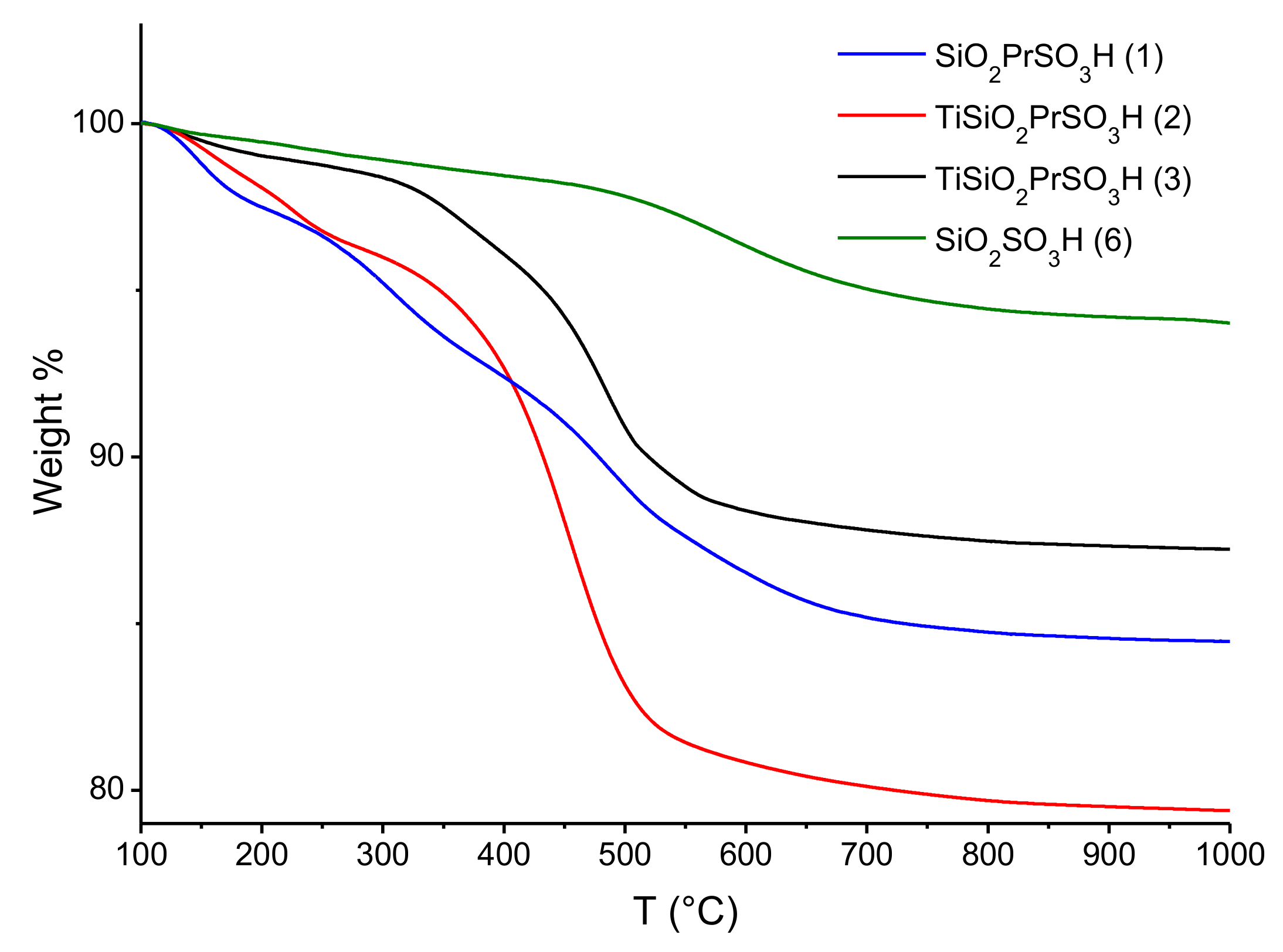
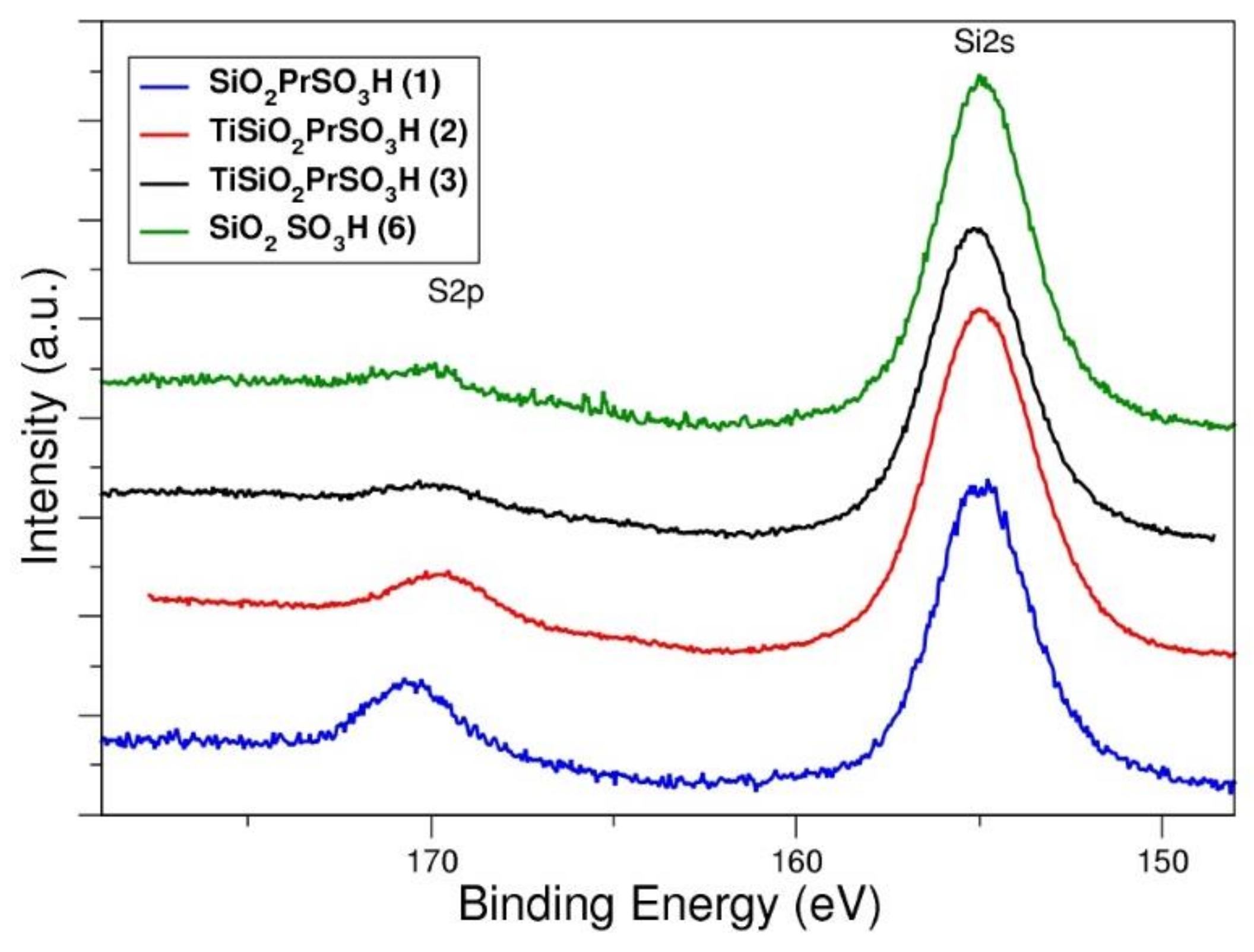

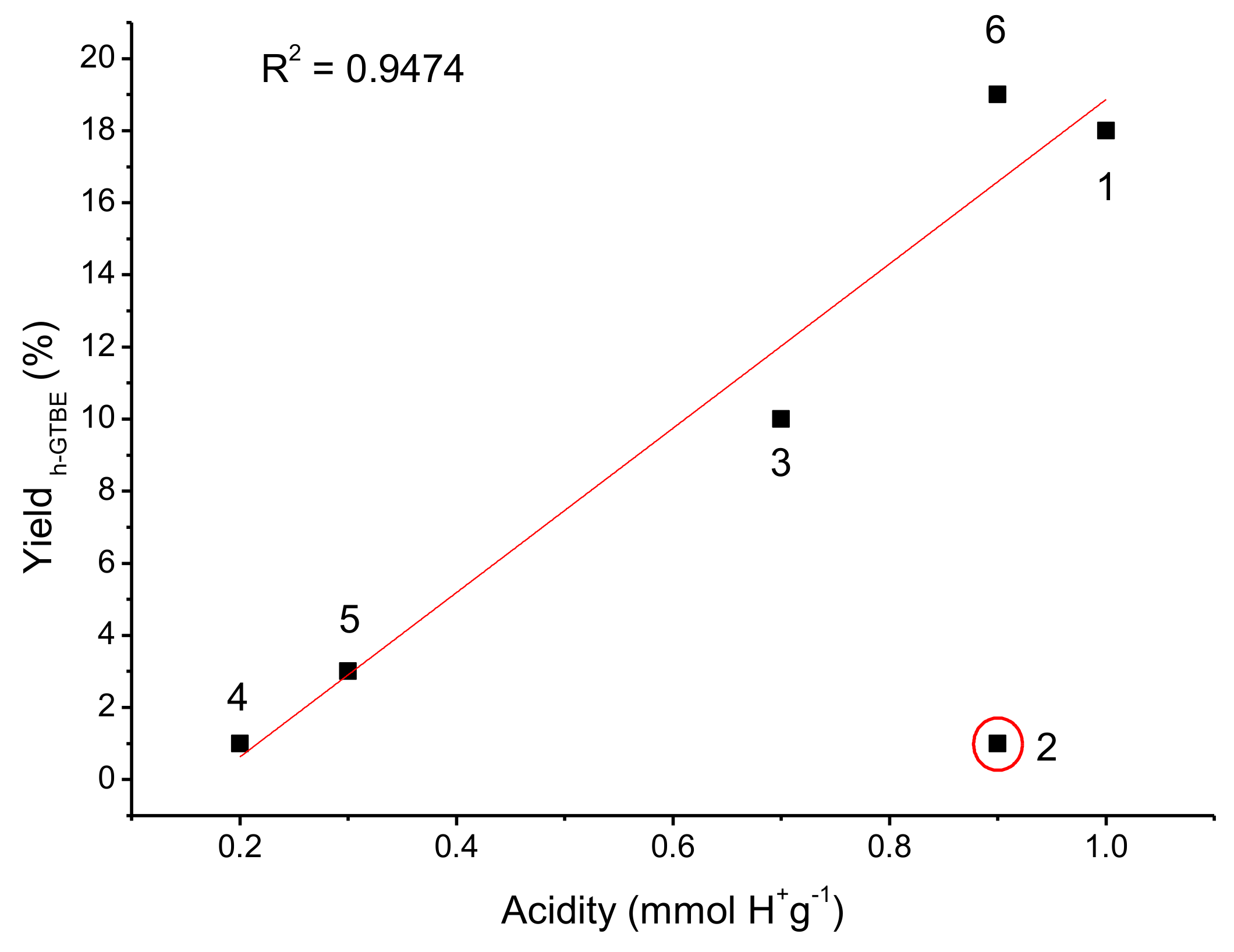
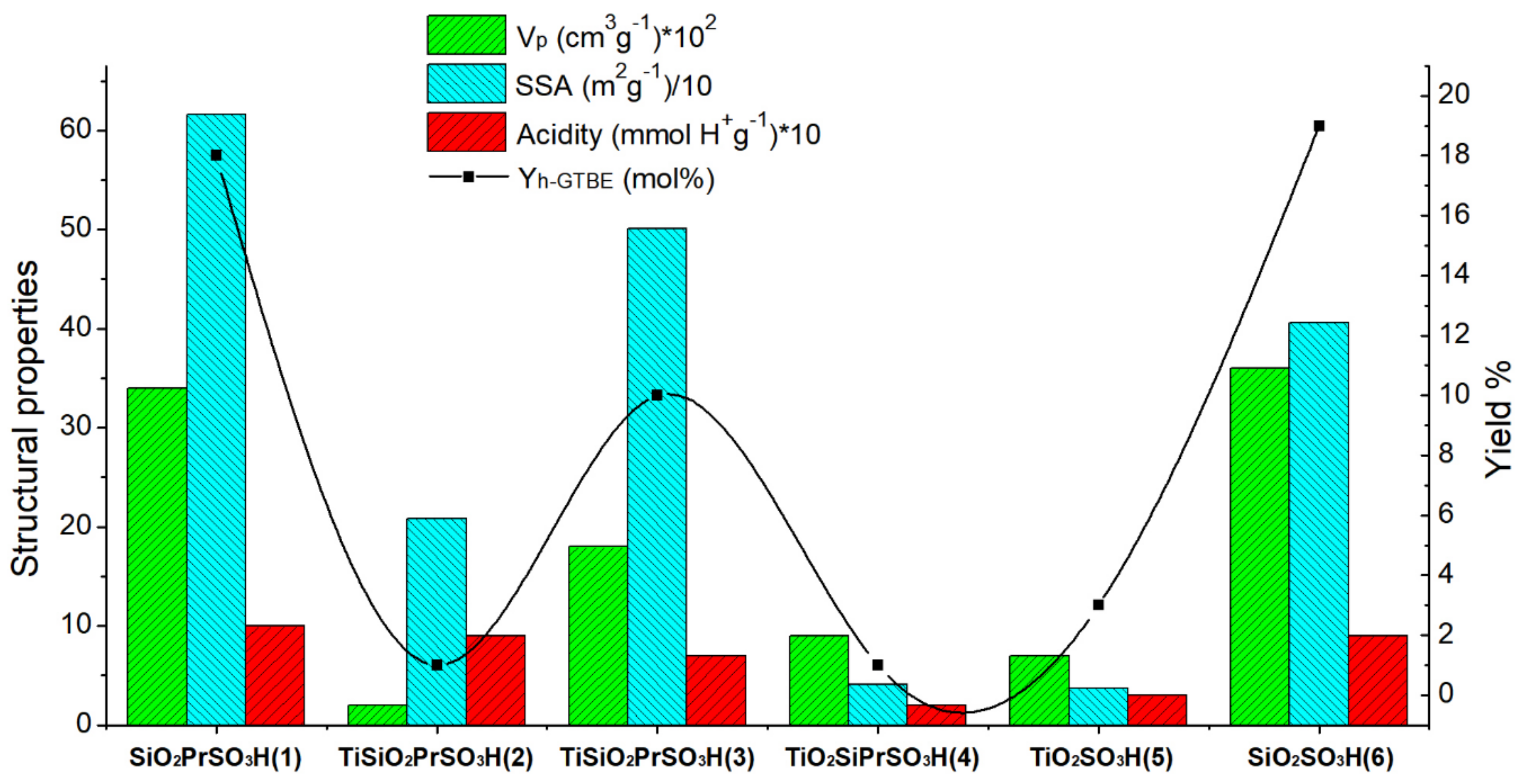

| Catalyst | BET | TGA | XPS | Acidity * (mmol H+g−1) | |||
|---|---|---|---|---|---|---|---|
| SSA (m2g−1) | Vp (cm3g−1) | (% wt) | S/Si | S/Ti | Ti/Si | ||
| SiO2 PrSO3H (1) | 616 | 0.34 | 15.5 | 0.13 | - | - | 1 (0.9) |
| Ti SiO2 PrSO3H (2) | 208 | 0.02 | 20.6 | 0.08 | - | 0.008 | 0.9 |
| Ti SiO2 PrSO3H (3) | 501 | 0.18 | 12.8 | 0.06 | - | 0.010 | 0.7 (0.7) |
| TiO2-SiPrSO3H (4) | 41 | 0.09 | 5.9 | 0.35 | 0.19 | 1.887 | 0.2 |
| TiO2-SO3H (5) | 37 | 0.07 | 2.1 | - | 0.17 | - | 0.3 |
| SiO2 SO3H (6) | 406 | 0.36 | 6.0 | 0.04 | 0.9 (0.8) | ||
| Catalyst | XG* (%) | SMTBG* (%) | Sh-GTBE* (%) | Yh-GTBE* (%) |
|---|---|---|---|---|
| SiO2 PrSO3H (1) | 76 (93) | 76 (73) | 24 (27) | 18 (25.1) |
| Ti SiO2 PrSO3H (2) | 14 (16) | 94 (92) | 6 (8) | 1 (1.3) |
| Ti SiO2 PrSO3H (3) | 64 (78) | 86 (83) | 14 (17) | 10 (13) |
| TiO2-SiPr-SO3H (4) | 16 (19) | 95 (92) | 5 (8) | 1 (1.5) |
| TiO2-SO3H (5) | 22 (24) | 87 (84) | 13 (16) | 3 (3.8) |
| SiO2-SO3H (6) | 78 (93) | 76 (73) | 24 (27) | 19 (26) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguado-Deblas, L.; Estevez, R.; Russo, M.; La Parola, V.; Bautista, F.M.; Testa, M.L. Microwave-Assisted Glycerol Etherification Over Sulfonic Acid Catalysts. Materials 2020, 13, 1584. https://doi.org/10.3390/ma13071584
Aguado-Deblas L, Estevez R, Russo M, La Parola V, Bautista FM, Testa ML. Microwave-Assisted Glycerol Etherification Over Sulfonic Acid Catalysts. Materials. 2020; 13(7):1584. https://doi.org/10.3390/ma13071584
Chicago/Turabian StyleAguado-Deblas, Laura, Rafael Estevez, Marco Russo, Valeria La Parola, Felipa M. Bautista, and Maria Luisa Testa. 2020. "Microwave-Assisted Glycerol Etherification Over Sulfonic Acid Catalysts" Materials 13, no. 7: 1584. https://doi.org/10.3390/ma13071584
APA StyleAguado-Deblas, L., Estevez, R., Russo, M., La Parola, V., Bautista, F. M., & Testa, M. L. (2020). Microwave-Assisted Glycerol Etherification Over Sulfonic Acid Catalysts. Materials, 13(7), 1584. https://doi.org/10.3390/ma13071584









