Bone Regeneration and Soft Tissue Enhancement Around Zygomatic Implants: Retrospective Case Series
Abstract
1. Introduction
2. Material and Methods
2.1. Patient Selection and Operating Procedure
2.1.1. Indication for Regenerative or Enhancement Method
2.1.2. Bone Regeneration
2.1.3. Buccal Soft Tissue Enhancement
2.1.4. Immediate Loading
2.1.5. Follow-up
2.2. Data Gathering
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bedrossian, E.; Stumpel, L.; Beckeley, M.; Inderson, T. The zygomatic implant: Preliminary data on treatment of severely resorbed maxillae. A clinical report. Int. J. Oral. Maxillofac. Implant. 2002, 17, 861–865. [Google Scholar]
- Ahlgren, F.; Størksen, K.; Tornes, K. A study of 25 zygomatic dental implants with 11 to 49 months’ follow-up after loading. Int. J. Oral. Maxillofac. Implant. 2006, 21, 421–425. [Google Scholar]
- Malevez, C.; Abarca, W.; Durdu, F.; Daelemans, P. Clinical outcome of 103 consecutive zygomatic implants. Clin. Oral Implant. Res. 2004, 15, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Zwahlen, R.A.; Graetz, K.W.; Oeschlin, C.K.; Studer, S.P. Survival rate of zygomatic implants in atrophic or partially resected maxillae prior to functional loading: A retrospective clinical report. Int. J. Oral. Maxillofac. Implant. 2006, 21, 413–420. [Google Scholar]
- Stella, J.P.; Warner, M.R. Sinus slot technique for simplification and improved orientation of zygomaticus dental implants: A technical note. Int. J. Oral. Maxillofac. Implant. 2000, 15, 889–893. [Google Scholar]
- Cawood, J.I.; Howell, R.A. A classification of the edentulous jaws. Int. J. Oral Maxillofac. Surg. 1988, 17, 232–236. [Google Scholar] [CrossRef]
- Peñarrocha, M.; Carrillo, C.; Boronat, A.; Martí, E. Level of satisfaction in patients with maxillary full-arch fixed prostheses: Zygomatic versus conventional implants. Int. J. Oral Maxillofac. Implant. 2007, 22, 769–773. [Google Scholar]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Survival and complications of zygomatic implants: An updated systematic review. J. Oral Maxillofac. Surg. 2016, 74, 1949–1964. [Google Scholar] [CrossRef]
- Aparicio, C.; Ouazzani, W.; Hatano, N. The use of zygomatic implants for prosthetic rehabilitation of the severely resorbed maxilla. Periodontol. 2000 2008, 47, 162–171. [Google Scholar] [CrossRef]
- Peñarrocha, M.; Uribe, R.; García, B.; Martí, E. Zygomatic implants using the sinus slot technique: Clinical report of a patient series. Int. J. Oral Maxillofac. Implant. 2005, 20, 788–792. [Google Scholar]
- Peñarrocha, M.; García, B.; Martí, E.; Boronat, A. Rehabilitation of severely atrophic maxillae with fixed implant-supported prostheses using zygomatic implants placed using the sinus slot technique: Clinical report on a series of 21 patients. Int. J. Oral Maxillofac. Implant. 2007, 22, 645–650. [Google Scholar]
- Migliorança, R.; Ilg, J.P.; Serrano, A.S.; Souza, R.P.; Zamperlini, M.S. Sinus exteriorization of the zygoma fixtures: A new surgical protocol. Implant. News 2006, 3, 30–35. [Google Scholar]
- Ouazzani, W.; Arevalo, X.; Sennerby, L.; Lundgren, S.; Aparicio, C. Zygomatic implants: A new surgical approach. J. Clin. Periodontol. 2006, 33, 126. [Google Scholar]
- Maloó, P.; de Araujo Nobre, M.; Lopes, I. A new approach to rehabilitate the severely atrophic maxilla using extramaxillary anchored implants in immediate function: A pilot study. J. Prosthet. Dent. 2008, 100, 354–366. [Google Scholar] [CrossRef]
- Aparicio, C.; Ouazzani, W.; Aparicio, A.; Fortes, V.; Muela, R.; Pascual, A.; Codesal, M.; Barluenga, N.; Manresa, C.; Franch, M. Extrasinus zygomatic implants: A new surgical approach for patients with pronounced buccal concavities in the edentulous maxilla. Clin. Implant. Dent. Relat. Res. 2010, 12, 55–61. [Google Scholar] [CrossRef]
- Aparicio, C. A proposed classification for zygomatic implant patient based on the zygoma anatomy guided approach (ZAGA): A cross-sectional survey. Eur. J. Oral Implant. 2011, 4, 269–275. [Google Scholar]
- Aparicio, C.; Manresa, C.; Francisco, K.; Aparicio, A.; Nunes, J.; Claros, P.; Potau, J.M. Zygomatic implants placed using the zygomatic anatomy-guided approach versus the classical technique: A proposed system to report rhinosinusitis diagnosis. Clin. Implant. Dent. Relat. Res. 2014, 16, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, C.; Manresa, C.; Francisco, C.; Claros, P.; Alandez, J.; Gonzalez, O.; Albrektsson, T. Zygomatic implants: Indications, techniques and outcomes, and the Zygomatic Success Code. Periodontol. 2000 2014, 66, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, C.; López-Piriz, R.; Albrektsson, T. ORIS criteria of success for the Zygoma related rehabilitation. The (revisited) Zygoma Success Code. Int. J. Oral. Maxillofac. Implant. 2020, 35, 366–378. [Google Scholar] [CrossRef]
- Aizcorbe-Vicente, J.; Peñarrocha-Oltra, D.; Canullo, L.; Soto-Peñaloza, D.; Peñarrocha-Diago, M. Influence of facial bone thickness after implant placement into the healed ridges on the remodeled facial bone and considering soft tissue recession: A systematic review. Int. J. Oral Maxillofac. Implant. 2020, 35, 107–119. [Google Scholar] [CrossRef]
- Linkevicius, T.; Apse, P.; Grybauskas, S.; Puisys, A. The influence of soft tissue thickness on crestal bone changes around implants: A 1-year prospective controlled clinical trial. Int. J. Oral Maxillofac. Implant. 2009, 24, 712–719. [Google Scholar]
- Lin, G.H.; Chan, H.L.; Wang, H.L. The significance of keratinized mucosa on implant health: A systematic review. J. Periodontol. 2013, 84, 1755–1767. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.; Wat, P.; Hui, E.; Lee, P.; Li, W. A new method to eliminate the risk of maxillary sinusitis with zygoma implants. Int. J. Oral Maxillofac. Implant. 2010, 25, 1–7. [Google Scholar]
- Hinze, M.; Vrielinck, L.; Thalmair, T.; Wachtel, H.; Wolfgang, B. Zygomatic implant placement in conjunction with sinus bone grafting: The “extended sinus elevation technique”. a case-cohort study. Int. J. Oral Maxillofac. Implant. 2013, 28, 376–385. [Google Scholar] [CrossRef][Green Version]
- Guennal, P.; Guiol, J. Use of buccal fat pads to prevent vestibular gingival recession of zygomatic implants. J. Stomatol. Oral Maxillofac. Surg. 2018, 119, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, C.; Sanz, A. The ZAGA “Scarf Graft” to prevent soft tissue dehiscence around zygomatic implants. A technical note. J. Oral Maxillofac. Implant. 2020, 35, 21–26. [Google Scholar] [CrossRef]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D. The CARE guidelines: Consensus-based clinical case reporting guideline development. J. Med. Case Rep. 2013, 7, 223. [Google Scholar] [CrossRef]
- Lanza, D.C.; Kennedy, D.W. Adult rhinosinusitis defined. Otolaryngol. Head Neck Surg. 1997, 117, s1–s7. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Abreu, M.H. Survival and complications of zygomatic implants: A systematic review. Oral Maxillofac. Surg. 2013, 17, 81–93. [Google Scholar] [CrossRef]
- Kahnberg, K.E.; Henry, P.J.; Hirsch, J.M.; Ohnell, L.O.; Andreasson, L.; Brånemark, P.I.; Chiapasco, M.; Gynther, G.; Finne, K.; Higuchi, K.W.; et al. Clinical evaluation of the zygoma implant: 3-year follow-up at 16 clinics. J. Oral Maxillofac. Surg. 2007, 65, 2033–2038. [Google Scholar] [CrossRef]
- Aparicio, C.; Ouazzani, W.; Garcia, R.; Arevalo, X.; Muela, R.; Fortes, V. A prospective clinical study on titanium implants in the zygomatic arch for prosthetic rehabilitation of the atrophic edentulous maxilla with a follow-up of 6 months to 5 years. Clin. Implant. Dent. Relat. Res. 2006, 8, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Farzad, P.; Andersson, L.; Gunnarsson, S.; Johansson, B. Rehabilitation of severely resorbed maxillae with zygomatic implants: An evaluation of implant stability, tissue conditions, and patients’ opinion before and after treatment. Int. J. Oral Maxillofac. Implant. 2006, 21, 399–404. [Google Scholar]
- Hirsch, J.M.; Ohrnell, L.O.; Henry, P.J.; Andreasson, L.; Brånemark, P.I.; Chiapasco, M.; Gynther, G.; Finne, K.; Higuchi, K.W.; Isaksson, S.; et al. A clinical evaluation of the zygoma fixture: One year of follow-up at 16 clinics. J. Oral Maxillofac. Surg. 2004, 62, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Peñarrocha, M.; Carrillo, C.; Boronat, A.; Balaguer, J.; Peñarrocha, M. Palatal positioning of implants in severely resorbed edentulous maxillae. Int. J. Oral Maxillofac. Implant. 2009, 24, 527–533. [Google Scholar]
- Candel-Martí, E.; Peñarrocha-Oltra, D.; BagáN, L.; Peñarrocha-Diago, M.; Peñarrocha-Diago, M. Palatal positioned implants in severely atrophic maxillae versus conventional implants to support fixed full-arch prostheses: Controlled retrospective study with 5 years of follow-up. Med. Oral Patol. Oral Y Cir. Bucal 2015, 20, e357–e364. [Google Scholar]
- Wessing, B.; Lettner, S.; Zechner, W. Guided bone regeneration with collagen membranes and particulate graft materials: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 87–100. [Google Scholar] [CrossRef]
- Jung, R.E.; Benic, G.I.; Scherrer, D.; Hämmerle, C.H. Cone beam computed tomography evaluation of regenerated buccal bone 5 years after simultaneous implant placement and guided bone regeneration procedures--a randomized, controlled clinical trial. Clin. Oral Implant. Res. 2015, 26, 28–34. [Google Scholar] [CrossRef]
- Aparicio, C.; Ouazzani, W.; Aparicio, A.; Fortes, V.; Muela, R.; Pascual, A.; Codesal, M.; Barluenga, N.; Franch, M. Immediate/early loading of zygomatic implants: Clinical experiences after 2 to 5 years of follow-up. Clin. Implant. Dent. Relat. Res. 2010, 12, 77–82. [Google Scholar] [CrossRef]
- Thoma, D.S.; Naenni, N.; Figuero, E.; Hämmerle, C.H.; Schwarz, F.; Jung, R.E.; Sanz-Sánchez, I. Effects of soft tissue augmentation procedures on peri-implant health or disease: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29, 32–49. [Google Scholar] [CrossRef]
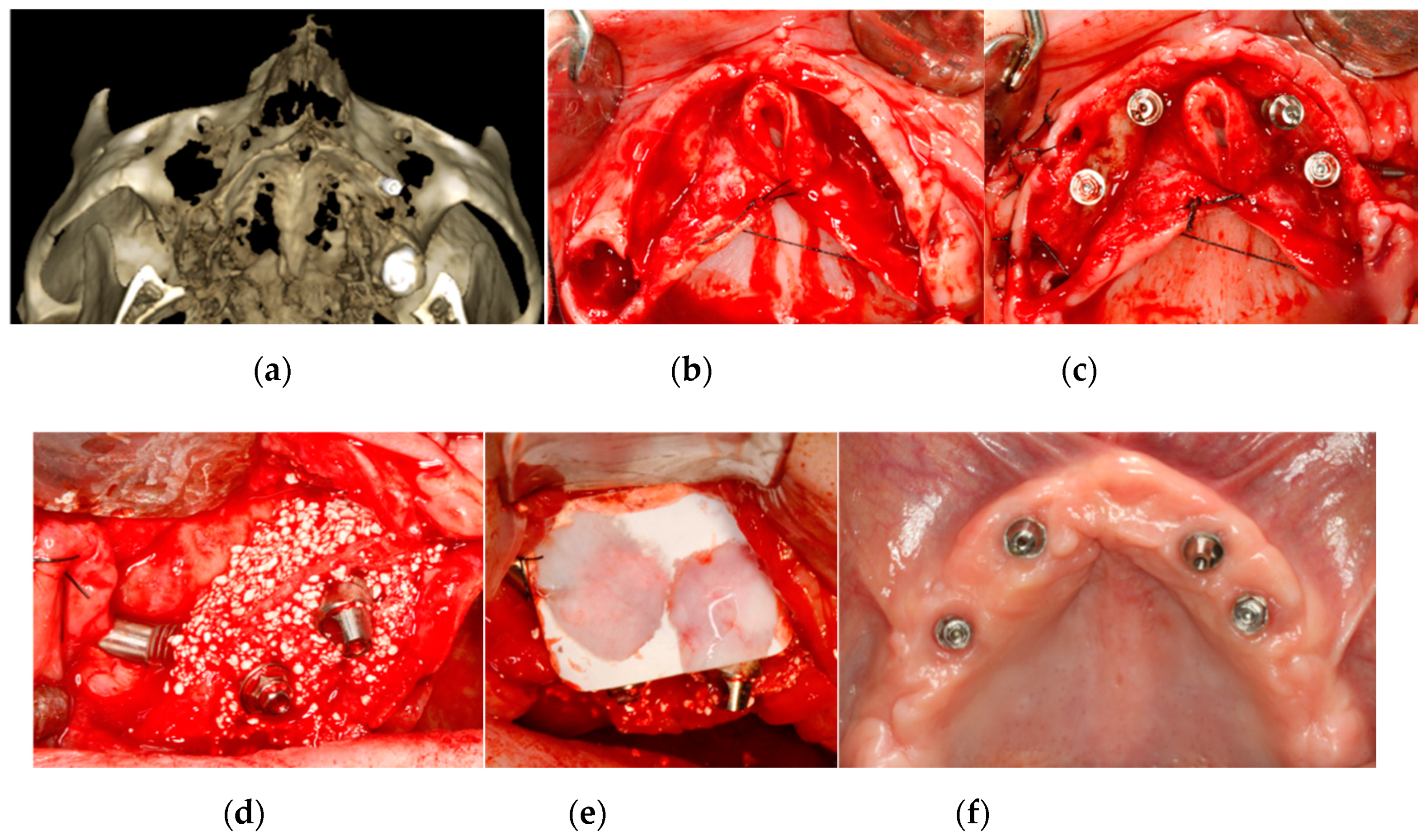
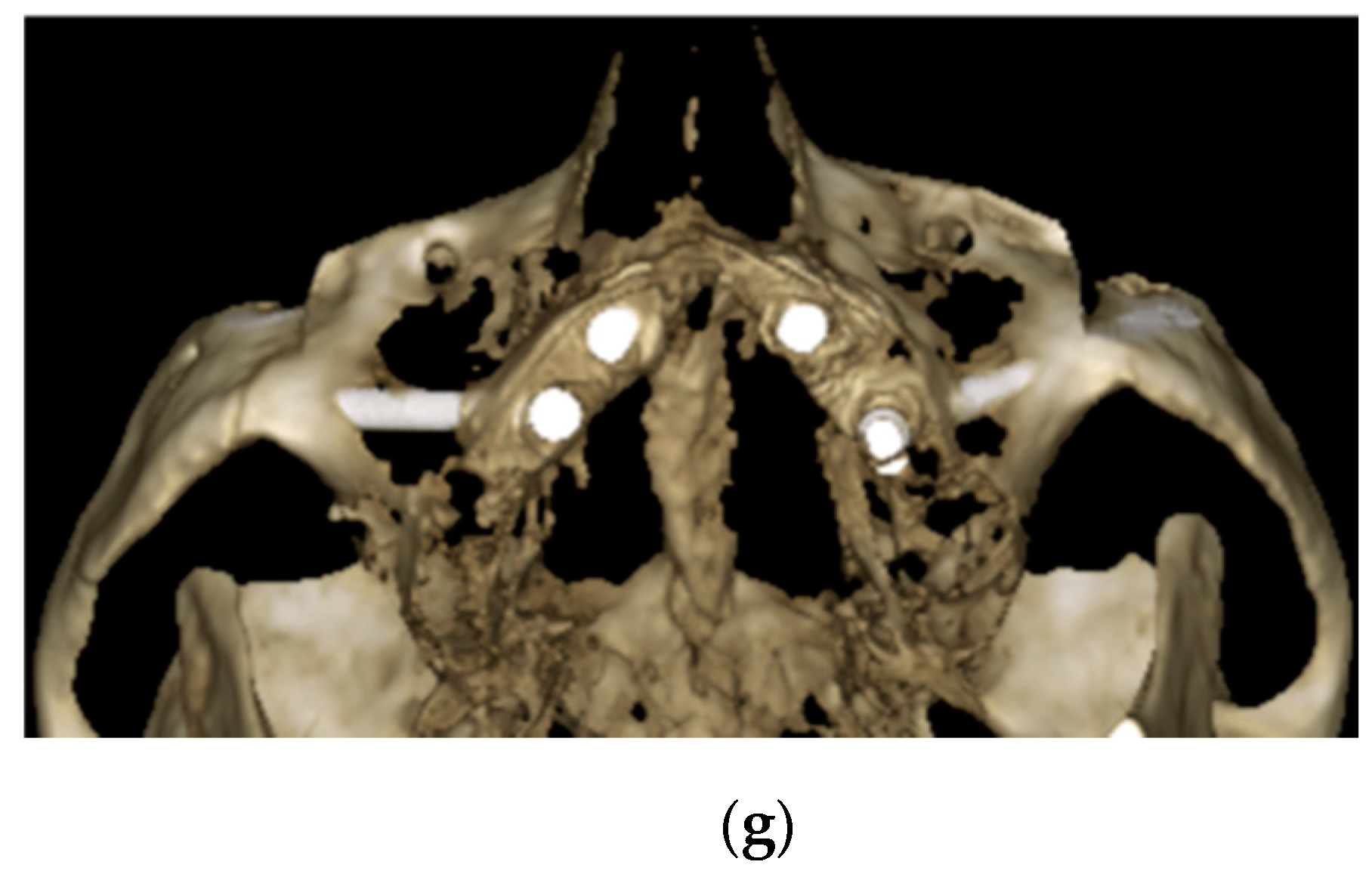
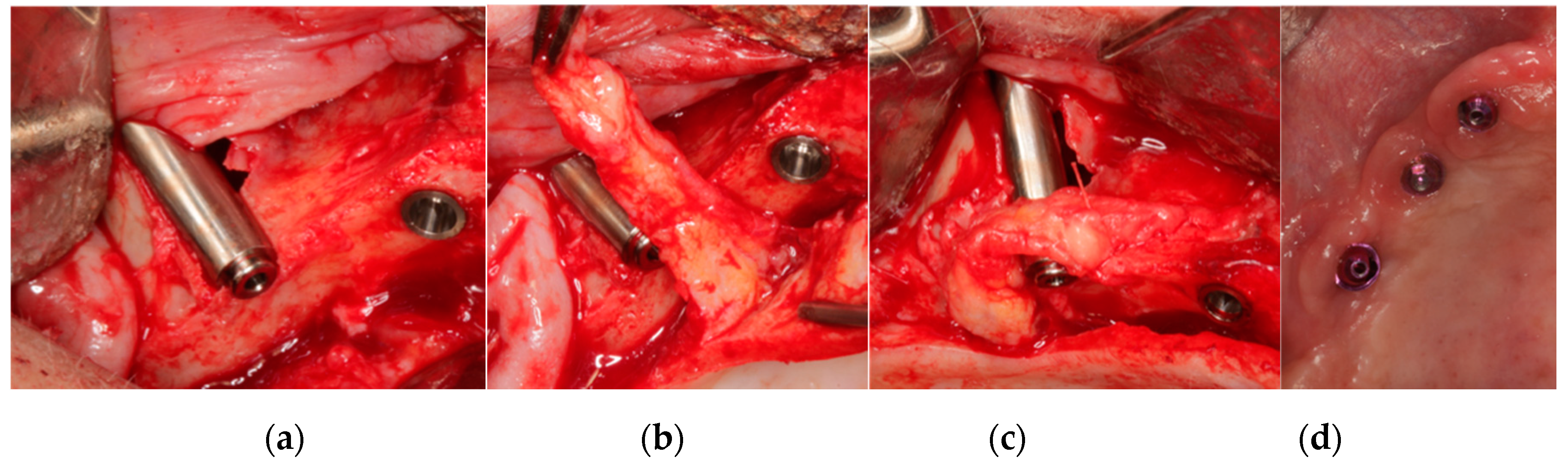
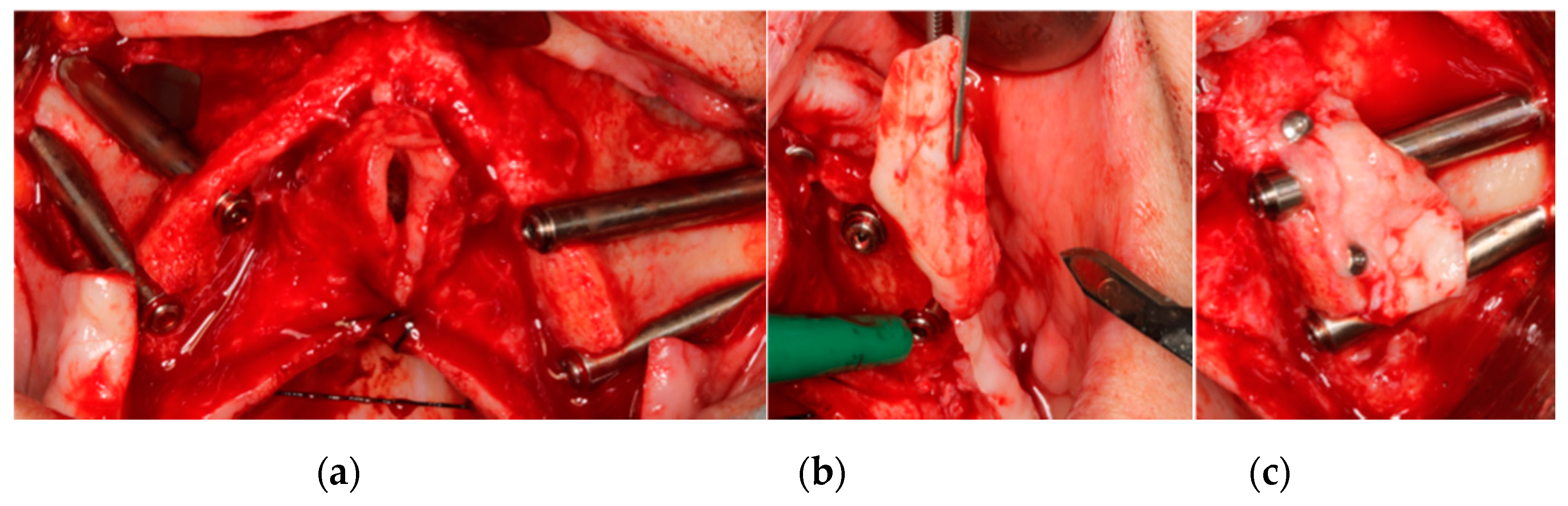


| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Patient with indication for zygomatic implant treatment for atrophic maxillae (Cawood–Howell grade V and VI). | Cases with less than 12 months of follow-up. |
| Zygomatic implants with simultaneous bone regeneration or enhancement of peri-implant soft tissue. | Incomplete medical history and incomplete radiographic examination. |
| Indicated Technique | Implant Emergence | Alveolar Process State | Illustrated Scheme * | |
|---|---|---|---|---|
| Width and Length of Palatal Bone Crest | Width and Length of Buccal Bone Crest | |||
| Palatal bone regeneration | Palatal emergence. | Non-existent | Preserved. |  |
| Palatal and buccal bone regeneration | Crestal emergence. | Non-existent or < 2 mm in both directions | Non-existent or < 2 mm in both directions. |  |
| Buccal bone regeneration | Moderate buccal emergence with at least more than half of the implant diameter inside the alveolar bone crest. | Preserved. | Non-existent or < 2 mm in both directions. |  |
| Soft tissue enhancement | Total buccal emergence or more than half of the implant diameter outside the alveolar bone crest. | Preserved. | Non-existent. |  |
| Type of Bone Regeneration Technique | No. P | No. ZI | ZAGA Classification (%) | Immediate Loading. (%) | Successful Regeneration Technique (%) | Bone Gain at the 6 Months (mm) | Biological Complications (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | Width | Length | ||||||||
| P | B | P | B | |||||||||||
| Buccal | 4 | 5 | 20 | 80 | 0 | 0 | 0 | 20 | 80 | - | 3 | - | 9.2 | 0 |
| Palatal | 4 | 7 | 14.3 | 71.4 | 14.3 | 0 | 0 | 28.6 | 100 | 3.7 | - | 5 | - | 0 |
| Buccal and Palatal | 2 | 4 | 0 | 100 | 0 | 0 | 0 | 50 | 100 | 2.3 | 2.3 | 8 | 7.4 | 0 |
| Total | 10 | 16 | 12.5 | 81.2 | 6.2 | 0 | 0 | 31.25 | 93.75 | 3 | 2.65 | 6.5 | 8.3 | 0 |
| Type of Soft Tissue Enhancement Technique | No. P | No. ZI | ZAGA Classification (%) | Immediate Loading (%) | Successful Enhancement Technique (%) | Buccal Keratinized Mucosa Gain > 2 mm (mm) | Biological Complications (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |||||||
| Pediculate Connective Tissue Graft | 8 | 11 | 0 | 90.9 | 9.1 | 0 | 0 | 72.7 | 100 | 100 | 0 |
| Free Connective Tissue Graft | 1 | 4 | 50 | 50 | 0 | 0 | 0 | 0 | 100 | 100 | 0 |
| Total | 9 | 15 | 13.1 | 80 | 6.7 | 0 | 0 | 53.3 | 100 | 100 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peñarrocha-Diago, M.; Bernabeu-Mira, J.C.; Fernández-Ruíz, A.; Aparicio, C.; Peñarrocha-Oltra, D. Bone Regeneration and Soft Tissue Enhancement Around Zygomatic Implants: Retrospective Case Series. Materials 2020, 13, 1577. https://doi.org/10.3390/ma13071577
Peñarrocha-Diago M, Bernabeu-Mira JC, Fernández-Ruíz A, Aparicio C, Peñarrocha-Oltra D. Bone Regeneration and Soft Tissue Enhancement Around Zygomatic Implants: Retrospective Case Series. Materials. 2020; 13(7):1577. https://doi.org/10.3390/ma13071577
Chicago/Turabian StylePeñarrocha-Diago, Miguel, Juan Carlos Bernabeu-Mira, Alberto Fernández-Ruíz, Carlos Aparicio, and David Peñarrocha-Oltra. 2020. "Bone Regeneration and Soft Tissue Enhancement Around Zygomatic Implants: Retrospective Case Series" Materials 13, no. 7: 1577. https://doi.org/10.3390/ma13071577
APA StylePeñarrocha-Diago, M., Bernabeu-Mira, J. C., Fernández-Ruíz, A., Aparicio, C., & Peñarrocha-Oltra, D. (2020). Bone Regeneration and Soft Tissue Enhancement Around Zygomatic Implants: Retrospective Case Series. Materials, 13(7), 1577. https://doi.org/10.3390/ma13071577





