In Vitro Studies on Nanoporous, Nanotubular and Nanosponge-Like Titania Coatings, with the Use of Adipose-Derived Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Titania Nanoporous, Nanotubular, and Nanosponge-Like Coatings
2.2. Characterization of Titania Coatings
2.2.1. Morphological and Structural Evaluation
2.2.2. The Wettability and Surface Free Energy
2.2.3. Nanomechanical Properties and Surface Roughness
2.3. Biological Activity Studies of TNT Coatings
2.3.1. Cell Culture
2.3.2. Cell Proliferation
2.3.3. Cell Morphology
2.3.4. Alkaline Phosphatase Activity
2.3.5. Statistical Analysis
3. Results
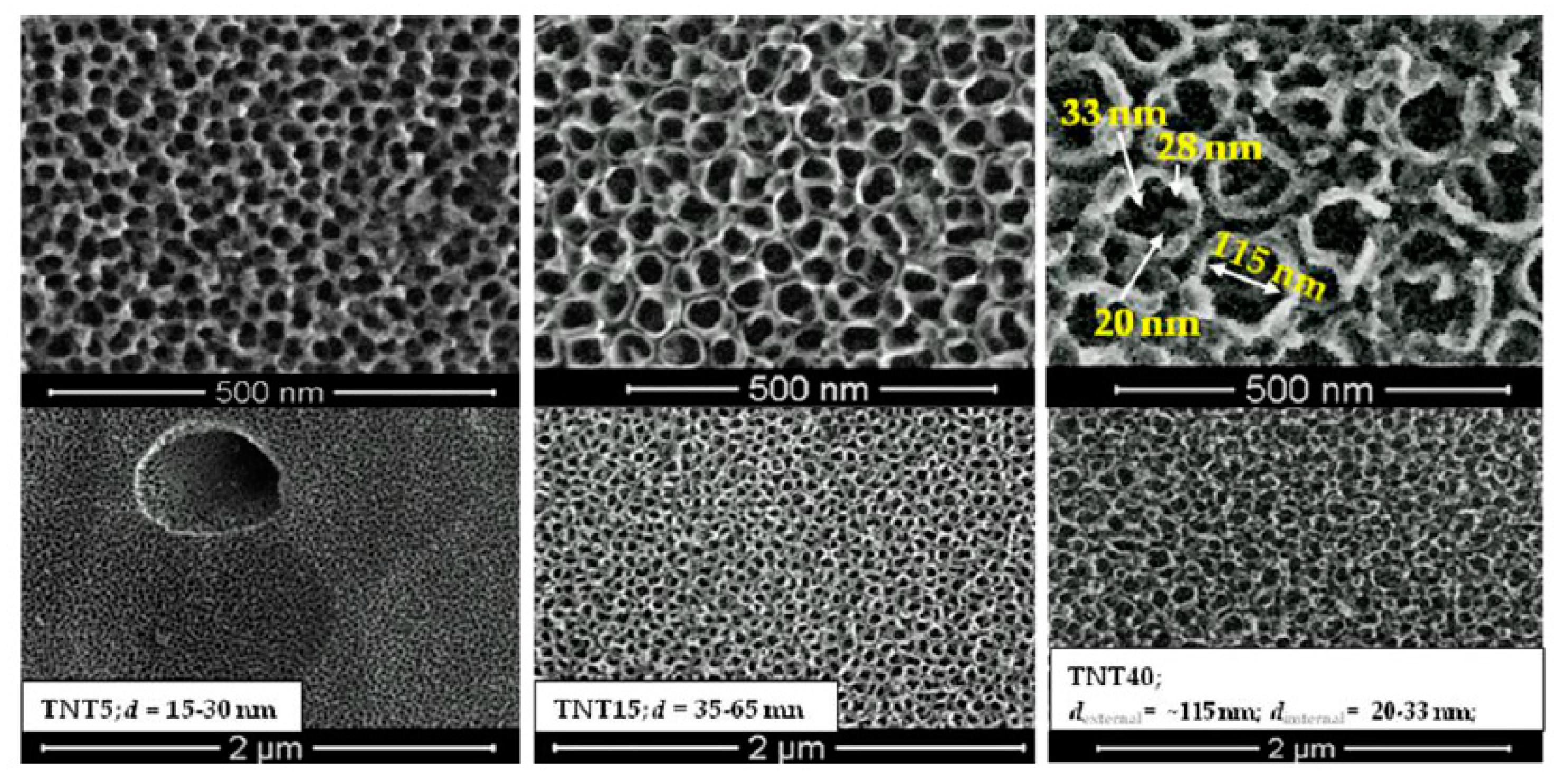
3.1. TNT Coatings Selected to Biological Experiments; Synthesis, Morphology, and Structure Characterization.
3.2. The Wettability and Surface Free Energy
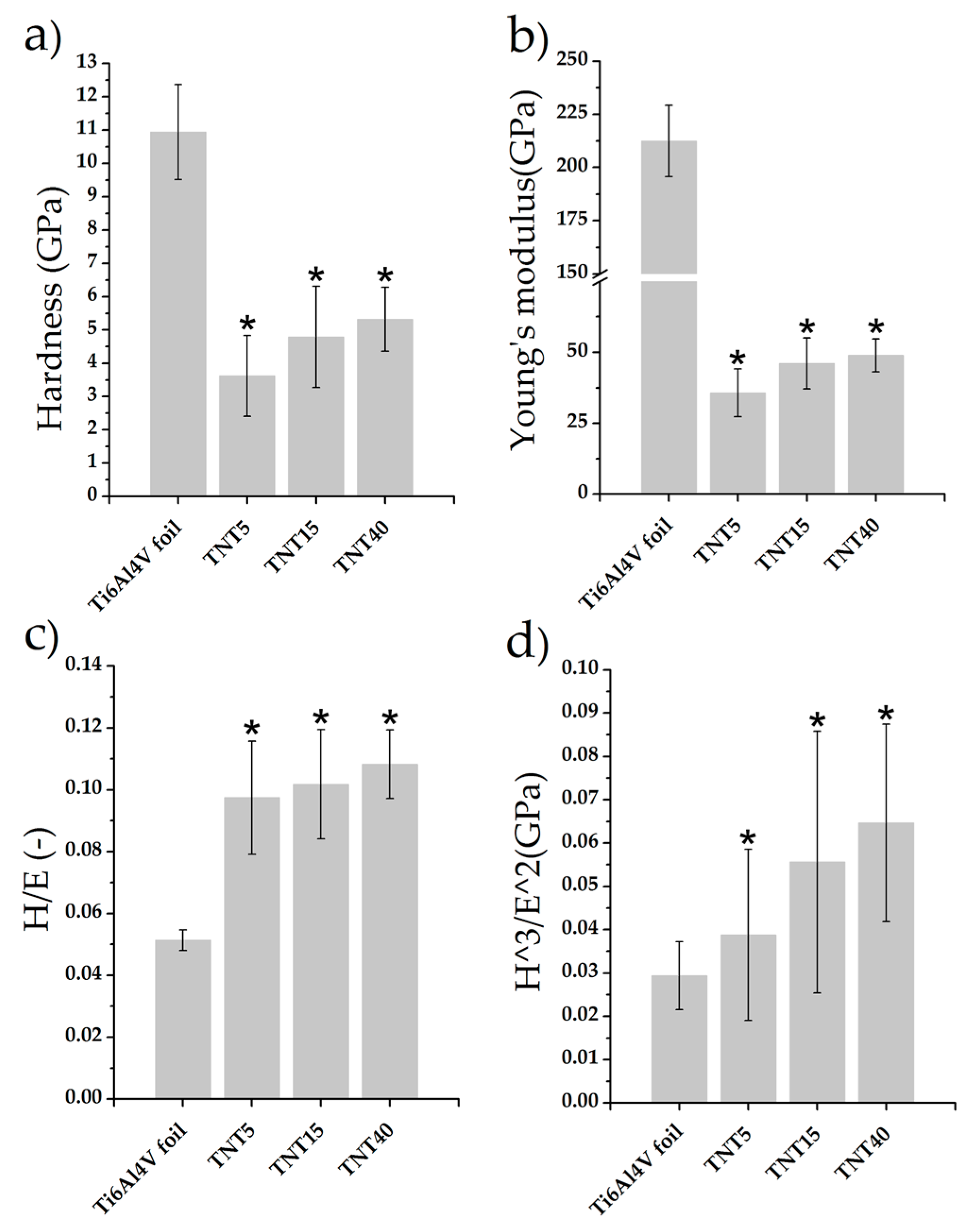
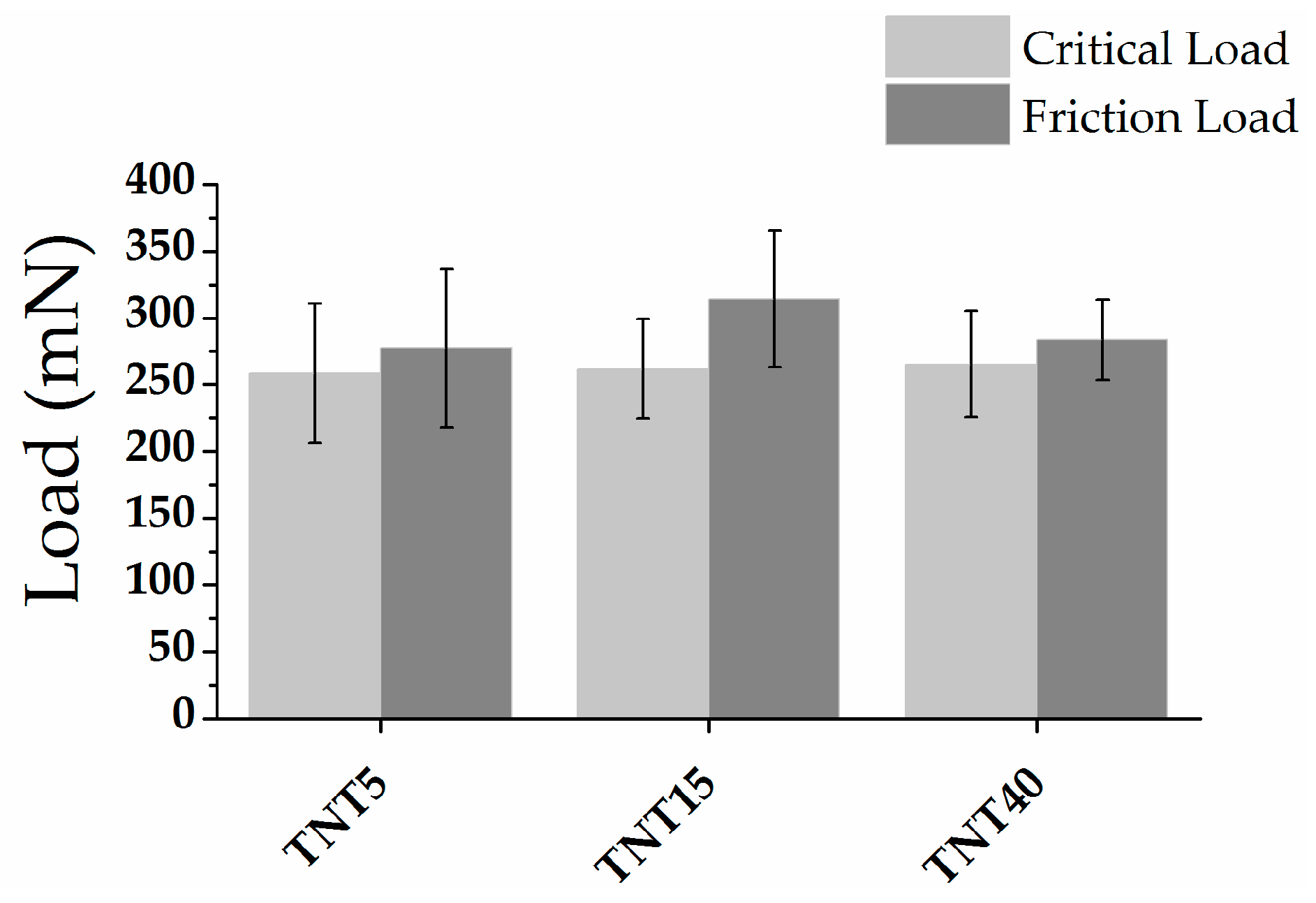
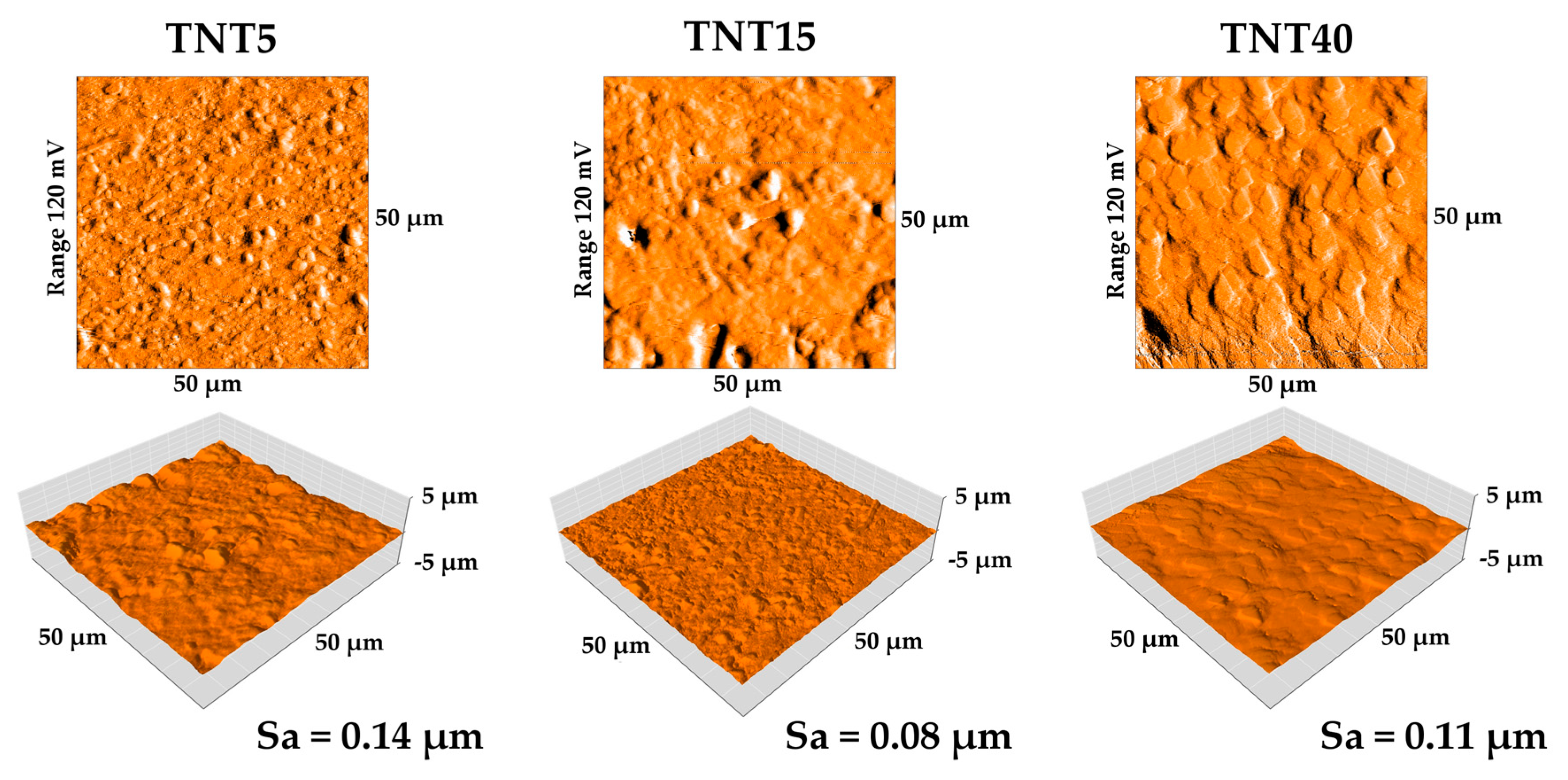
3.3. Nanomechanical Properties and Surface Topography
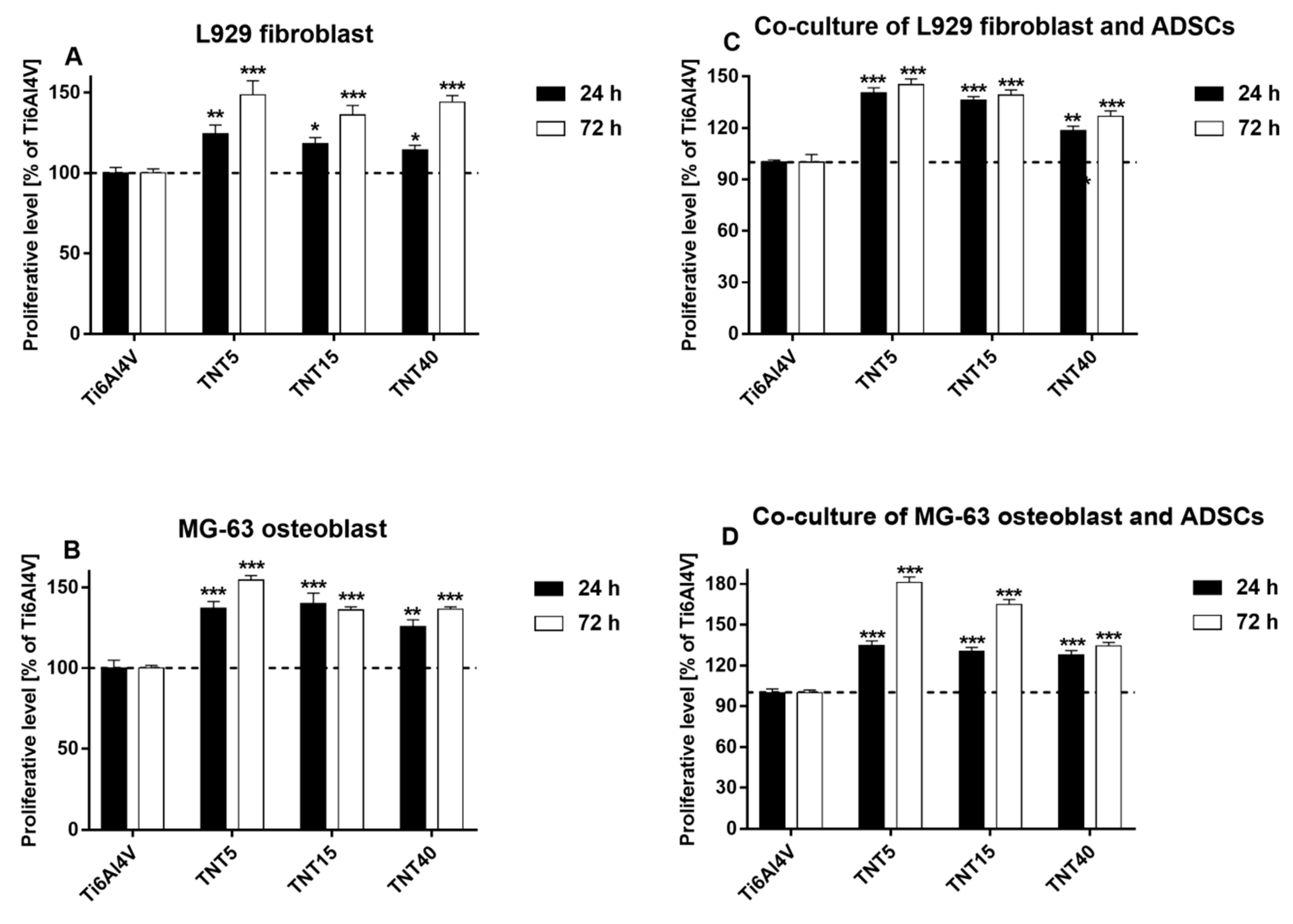
3.4. Proliferation Level of Cells Growing on TNT Coatings
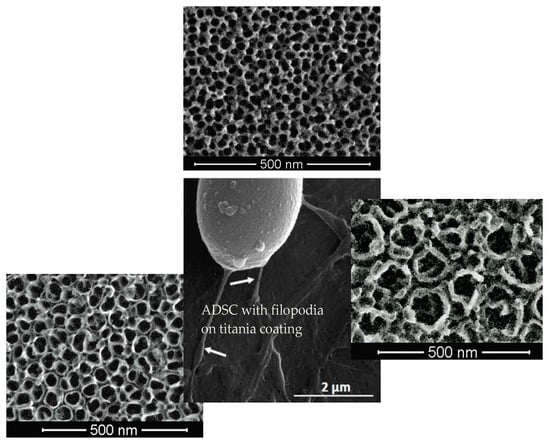
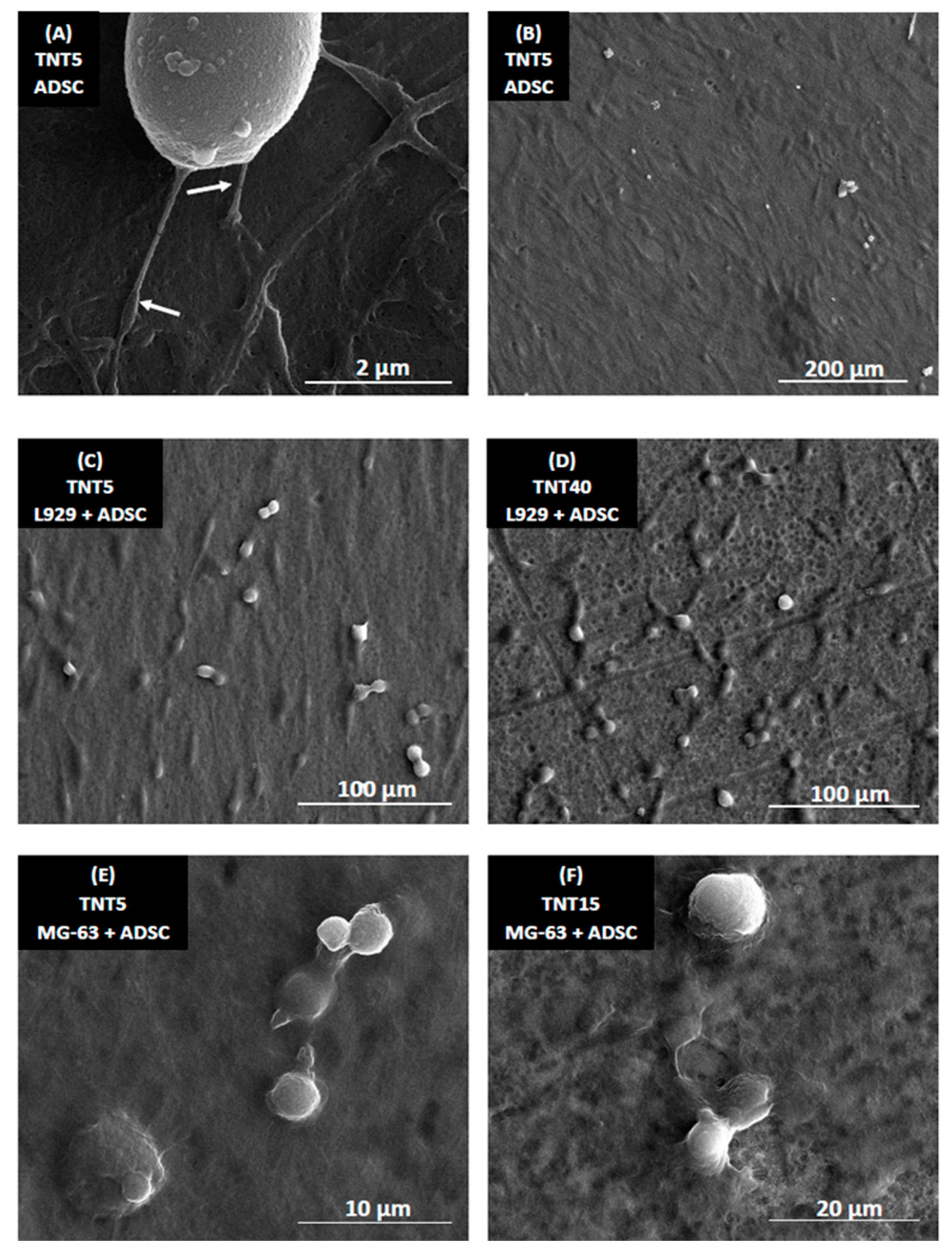
3.5. Morphology of Cells Culture on TNT Coatings
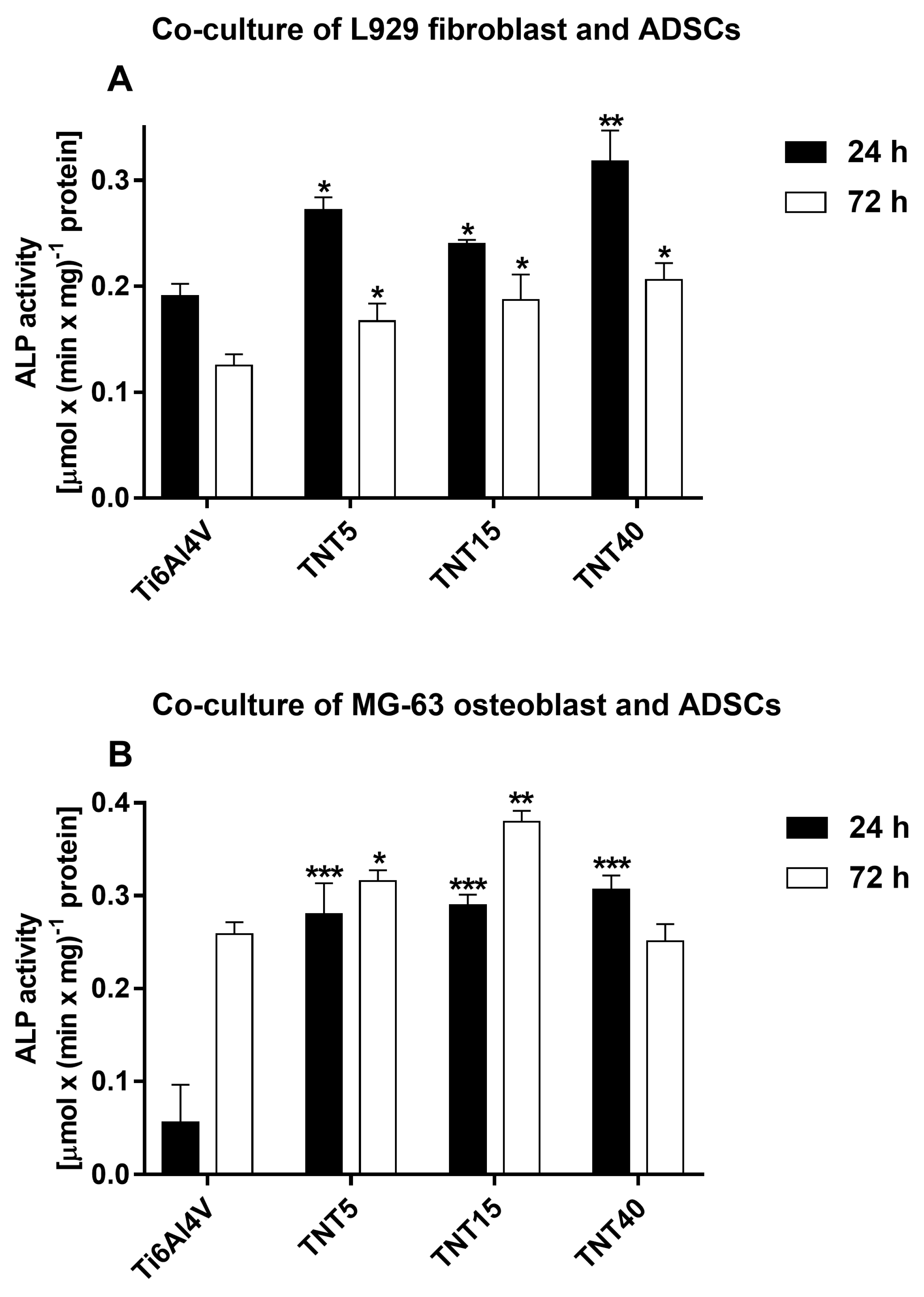
3.6. Alkaline Phosphatase Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Roach, P.; Eglin, D.; Rohde, K.; Perry, C.C. Modern biomaterials: A review—Bulk properties and implications of surface modifications. J. Mater. Sci. Mater. Med. 2007, 18, 1263–1277. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Michiardi, A.; Castano, O.; Planell, J. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Madni, A.; Webster, T.J. The era of biofunctional biomaterials in orthopedics: What does the future hold? Expert Rev. Med. Devices 2018, 15, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Swami, N.; Cui, Z.; Nair, L.S. Titania nanotubes: Novel nanostructures for improved osseointegration. J. Heat Transf. 2011, 133. [Google Scholar] [CrossRef]
- Minagar, S.; Berndt, C.C.; Wang, J.; Ivanova, E.; Wen, C. A review of the application of anodization for the fabrication of nanotubes on metal implant surfaces. Acta Biomater. 2012, 8, 2875–2888. [Google Scholar] [CrossRef]
- Kodama, A.; Bauer, S.; Komatsu, A.; Asoh, H.; Ono, S.; Schmuki, P. Bioactivation of titanium surfaces using coatings of TiO2 nanotubes rapidly pre-loaded with synthetic hydroxyapatite. Acta Biomater. 2009, 5, 2322–2330. [Google Scholar] [CrossRef]
- Veronovski, N. TiO2 Applications as a Function of Controlled Surface Treatment. In Titanium Dioxide—Material for a Sustainable Environment; Yang, D., Ed.; IntechOpen Ltd.: London, UK, 2018; Volume 21, pp. 421–443. [Google Scholar]
- Mydin, R.B.S.M.N.; Hazan, R.; FaridWajidi, M.F.; Sreekantan, S. Titanium Dioxide Nanotube Arrays for Biomedical Implant Materials and Nanomedicine Applications. In Titanium Dioxide—Material for a Sustainable Environment; Yang, D., Ed.; IntechOpen Ltd.: London, UK, 2018; Volume 23, pp. 469–483. [Google Scholar]
- Sulka, G.D.; Kapusta-Kołodziej, J.; Brzózka, A.; Jaskuła, M. Fabrication of nanoporous TiO2 by electrochemical anodization. Electrochim. Acta 2010, 55, 4359–4367. [Google Scholar] [CrossRef]
- Saharudin, K.A.; Sreekantan, S.; Aziz, S.N.Q.A.A.; Hazan, R.; Lai, C.W.; Mydin, R.B.S.M.N.; Mat, I. Surface modification and bioactivity of anodic Ti6Al4V alloy. J. Nanosci. Nanotechnol. 2013, 13, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Saharudin, K.A.; Sreekantan, S.; Mydin, R.B.S.N.M.; Basiron, N.; Krengvirat, W. Factor Affecting Geometry of TiO2 Nanotube Arrays (TNAs) in Aqueous and Organic Electrolyte. In Titanium Dioxide—Material for a Sustainable Environment; Yang, D., Ed.; IntechOpen Ltd.: London, UK, 2018; Volume 6, pp. 117–130. [Google Scholar]
- Macak, J.M.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 nanotubes: Self-organized electrochemical formation, properties and applications. Curr. Opin. Solid State Mater. Sci. 2007, 11, 3–18. [Google Scholar] [CrossRef]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Shankar, K.; Grimes, C.A. A review on highly ordered, vertically oriented TiO2 nanotube arrays: Fabrication, material properties, and solar energy applications. Sol. Energy Mater. Sol. Cells 2006, 90, 2011–2075. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, J.Y.; Li, H.Q.; Chen, Z.; Zhao, A.Z.J.; Wang, Y.; Zhang, K.Q.; Sun, H.T.; Al-Deyab, S.S.; Lai, Y.K. TiO2 nanotube platforms for smart drug delivery: A review. Int. J. Nanomed. 2016, 11, 4819–4834. [Google Scholar]
- Jonášová, L.; Müller, F.A.; Helebrant, A.; Strnad, J.; Greil, P. Biomimetic apatite formation on chemically treated titanium. Biomaterials 2005, 25, 1187–1194. [Google Scholar] [CrossRef]
- Hayakawa, S.; Okamoto, K.; Yoshioka, T. Accelerated induction of in vitro apatite formation by parallel alignment of hydrothermally oxidized titanium substrates separated by sub-millimeter gaps. J. Asian Ceram. Soc. 2019, 7, 90–100. [Google Scholar] [CrossRef]
- Radtke, A.; Piszczek, P.; Topolski, A.; Lewandowska, Ż.; Talik, E.; Hald Andersen, I.; Nielsen, L.P.; Heikkilä, M.; Leskelä, M. The structure and the photocatalytic activity of titania nanotube and nanofiber coatings. Appl. Surf. Sci. 2016, 368, 165–172. [Google Scholar] [CrossRef]
- Radtke, A.; Topolski, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B.; Wieckowska-Szakiel, M.; Piszczek, P. Bioactivity Studies on Titania Coatings and the Estimation of Their Usefulness in the Modification of Implant Surfaces. Nanomaterials 2017, 7, 90. [Google Scholar] [CrossRef]
- Radtke, A.; Topolski, A.; Jędrzejewski, T.; Sadowska, B.; Więckowska-Szakiel, M.; Szubka, M.; Talik, E.; Nielsen, L.P.; Piszczek, P. Studies on the bioactivity and photocatalytic properties of titania nanotube coatings produced with the use of the low potential anodization of Ti6Al4V alloy surface. Nanomaterials 2017, 7, 197. [Google Scholar] [CrossRef]
- Radtke, A.; Bal, M.; Jędrzejewski, T. Novel titania nanocoatings produced by the anodic anodization with the use of the cyclically changing potential; their photocatalytic activity and biocompability. Nanomaterials 2018, 8, 712. [Google Scholar] [CrossRef]
- Radtke, A.; Ehlert, M.; Bartmański, M.; Jędrzejewski, T. The morphology, structure, mechanical properties and biocompatibility of nanotubular titania coatings before and after autoclaving process. J. Clin. Med. 2019, 8, 272. [Google Scholar] [CrossRef]
- Radtke, A. Photocatalytic activity of nanostructured titania films obtained by electrochemical, chemical, and thermal oxidation of Ti6Al4V alloy—Comparative analysis. Catalysts 2019, 9, 279. [Google Scholar] [CrossRef]
- Radtke, A.; Ehlert, M.; Jędrzejewski, T.; Sadowska, B.; Więckowska-Szakiel, M.; Holopainen, J.; Ritala, M.; Leskela, M.; Bartmański, M.; Szkodo, M.; et al. Titania Nanotubes/Hydroxyapatite Nanocomposites Produced with the Use of the Atomic Layer Deposition Technique: Estimation of Bioactivity and Nanomechanical Properties. Nanomaterials 2019, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B.; Więckowska-Szakiel, M.; Talik, E.; Mäkelä, M.; Leskelä, M.; Piszczek, P. Optimization of the silver clusters PEALD process on the surface of 1-D titania coatings. Nanomaterials 2017, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Piszczek, P.; Lewandowska, Ż.; Radtke, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B.; Szubka, M.; Talik, E.; Fiori, F. Biocompatibility of Titania Nanotube Coatings Enriched with Silver Nanograins by Chemical Vapor Deposition. Nanomaterials 2017, 7, 274. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Grodzicka, M.; Ehlert, M.; Muzioł, T.; Szkodo, M.; Bartmański, M.; Piszczek, P. Studies on silver ions releasing processes and mechanical properties of surface-modified titanium alloy implants. Int. J. Mol. Sci. 2018, 19, 3962. [Google Scholar] [CrossRef]
- Radtke, A.; Grodzicka, M.; Ehlert, M.; Jędrzejewski, T.; Wypij, M.; Golińska, P. “To be microbiocidal and not to be cytotoxic at the same time …”—Silver nanoparticles in their main role on the surface of titanium alloy implants. J. Clin. Med. 2019, 8, 334. [Google Scholar] [CrossRef]
- Marini, F.; Luzi, E.; Fabbri, S.; Ciuffi, S.; Sorace, S.; Tognarini, I.; Galli, G.; Zonefrati, R.; Sbaiz, F.; Brandi, M.L. Osteogenic differentiation of adipose tissue-derived mesenchymal stem cells on nanostructured Ti6Al4V and Ti13Nb13Zr. Clin. Cases Miner. Bone Metab. 2015, 12, 224–237. [Google Scholar] [CrossRef]
- Dias-Netipanyj, M.F.; Cowden, K.; Sopchenski, L.; Cogo, S.C.; Elifio-Esposito, S.; Popat, K.C.; Soares, P. Effect of crystalline phases of titania nanotube arrays on adipose derived stem cell adhesion and proliferation. Mater. Sci. Eng. C 2019, 103, 109850. [Google Scholar] [CrossRef]
- Bressan, E.; Sbricoli, L.; Guazzo, R.; Tocco, I.; Roman, M.; Vindigni, V.; Stellini, E.; Gardin, C.; Ferroni, L.; Sivolella, S.; et al. Nanostructured Surfaces of Dental Implants. Int. J. Mol. Sci. 2013, 14, 1918–1931. [Google Scholar] [CrossRef]
- Martino, S.; D’Angelo, F.; Armentano, I.; Kenny, J.M.; Orlacchio, A. Stem cell-biomaterial interactions for regenerative medicine. Biotechnol. Adv. 2012, 30, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Cowden, K.; Dias-Netipanyj, M.F.; Popat, K.C. Adhesion and Proliferation of Human Adipose-Derived Stem Cells on Titania Nanotube Surfaces. Regen. Eng. Transl. Med. 2019, 5, 435–445. [Google Scholar] [CrossRef]
- Ciuffi, S.; Zonefrati, R.; Brandi, M.L. Adipose stem cells for bone tissue repair. Clin. Cases Miner. Bone Metab. 2017, 14, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Lavenus, S.; Berreur, M.; Trichet, V.; Pilet, P.; Louarn, G.; Layrolle, P. Adhesion and osteogenic differentiation of human mesenchymal stem cells on titanium nanopores. Eur. Cells Mater. 2011, 22, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Vercellino, M.; Ceccarelli, G.; Cristofaro, F.; Balli, M.; Bertoglio, F.; Bruni, G.; Benedetti, L.; Avanzini, M.A.; Imbriani, M.; Visai, L. Nanostructured TiO2 Surfaces Promote Human Bone Marrow Mesenchymal Stem Cells Differentiation to Osteoblasts. Nanomaterials 2016, 6, 124. [Google Scholar] [CrossRef]
- James, A.W.; Zara, J.N.; Zhang, X.; Askarinam, A.; Goyal, R.; Chiang, M.; Yuan, W.; Chang, L.; Corselli, M.; Shen, J.; et al. Perivascular stem cells: A prospectively purified mesenchymal stem cell population for bone tissue engineering. Stem Cells Transl. Med. 2012, 1, 510–519. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef]
- Lindroos, B.; Suuronen, R.; Miettinen, S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev. Rep. 2011, 7, 269–291. [Google Scholar] [CrossRef]
- Mazini, L.; Rochette, L.; Amine, M.; Malka, G. Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). Int. J. Mol. Sci. 2019, 20, 2523. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adiposed-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Hattori, H.; Sato, M.; Masuoka, K.; Ishihara, M.; Kikuchi, T.; Matsui, T.; Takase, B.; Ishizuka, T.; Kikuchi, M.; Fujikawa, K.; et al. Osteogenic potential of human adipose tissue derived stromal cells as an alternative stem cell source. Cells Tissues Organs 2004, 178, 2–12. [Google Scholar] [CrossRef] [PubMed]
- De Ugarte, D.A.; Morizono, K.; Elbarbary, A.; Alfonso, Z.; Zuk, P.A.; Zhu, M.; Dragoo, J.L.; Ashjian, P.; Thomas, B.; Benhaim, P.; et al. Comparison of multi-lineage cell from human adipose tissue and bone marrow. Cells Tissues Organs 2003, 174, 101–109. [Google Scholar] [CrossRef]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef]
- Lewallen, E.A.; Jones, D.L.; Dudakovic, A.; Thaler, R.; Paradise, C.R.; Kremers, H.M.; Abdel, M.P.; Kakar, S.; Dietz, A.B.; Cohene, R.C.; et al. Osteogenic potential of human adipose-tissue-derived mesenchymal stromal cells cultured on 3D-printed porous structured titanium. Gene 2016, 581, 95–106. [Google Scholar] [CrossRef]
- Cowden, K.; Dias-Netipanyj, M.F.; Popat, K.C. Effects of titania nanotube surfaces on osteogenic differentiation of human adipose-derived stem cells. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 380–390. [Google Scholar] [CrossRef]
- Ehlert, M.; Roszek, K.; Jędrzejewski, T.; Bartmański, M.; Radtke, A. Titania Nanofiber Scaffolds with Enhanced Biointegration Activity-Preliminary in vitro Studies. Int. J. Mol. Sci. 2019, 20, 5642. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, T.R. Chapter 1 Contact Angle and Wetting Properties. In Surface Science Techniques, Springer Series in Surface Sciences; Bracco, G., Holst, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3–34. [Google Scholar]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Zhang, W.F.; He, Y.L.; Zhang, M.S.; Yin, Z.; Chen, Q. Raman scattering study on anatase TiO2 nanocrystals. J. Phys. D Appl. Phys. 2000, 33, 912. [Google Scholar] [CrossRef]
- Mazza, T.; Barborini, E.; Piseri, P.; Milani, P.; Cattaneo, D.; Li Bassi, A.; Bottani, C.E.; Ducati, C. Raman spectroscopy characterization of TiO2 rutile nanocrystals. Phys. Rev. B 2007, 75, 045416. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Hardcastle, F. Raman Spectroscopy of Titania (TiO2) Nanotubular Water-Splitting Catalysts. J. Ark. Acad. Sci. 2011, 65, 43–48. [Google Scholar]
- Busani, T.; Devine, R.A.B. Dielectric and infrared properties of TiO2 films containing anatase and rutile. Semicond. Sci. Technol. 2005, 20, 870. [Google Scholar] [CrossRef]
- Coy, E.; Yate, L.; Kabacińska, Z.; Jancelewicz, M.; Jurga, S.; Iatsunskyi, I. Topographic reconstruction and mechanical analysis of atomic layer deposited Al2O3/TiO2 nanolaminates by nanoindentation. Mater. Des. 2016, 111, 584–591. [Google Scholar] [CrossRef]
- Bartmanski, M.; Zielinski, A.; Jazdzewska, M.; Głodowska, J.; Kalka, P. Effects of electrophoretic deposition times and nanotubular oxide surfaces on properties of the nanohydroxyapatite/nanocopper coating on the Ti13Zr13Nb alloy. Ceram. Int. 2019, 45, 20002–20010. [Google Scholar] [CrossRef]
- Roach, P.; Eglin, D.; Ronde, K.; Perry, C.C.J. Surface Tailoring for Controlled Protein Adsorption: Effect of Topography at the Nanometer Scale and Chemistry. Mater. Sci. Mater. Med. 2007, 18, 1263–1277. [Google Scholar] [CrossRef]
- Comelles, J.; Estévez, M.; Martínez, E.; Samitier, J. The role of surface energy of technical polymers in serum protein adsorption and MG-63 cells adhesion. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 44–51. [Google Scholar] [CrossRef]
- Tanaka, M. Design of novel 2D and 3D biointerfaces using self-organization to control cell behaviour. Biochim. Biophys. Acta 2011, 1810, 251–258. [Google Scholar] [CrossRef]
- Yang, Y.; Leong, K.W. Nanoscale surfacing for regenerative medicine. WIRES Nanomed. Nanobiotechnol. 2010, 2, 478–495. [Google Scholar] [CrossRef]
- Ricci, J.L.; Grew, J.C.; Alexander, H. Connective-tissue responses to defined biomaterial surfaces. I. Growth of rat fibroblast and bone marrow cell colonies on microgrooved substrates. J. Biomed. Mater. Res. A 2007, 85, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Kunzler, T.P.; Drobek, T.; Schuler, M. Spencer, Systematic study of osteoblast and fibroblast response to roughness by means of surface-morphology gradients. Biomaterials 2007, 28, 2175–2182. [Google Scholar] [CrossRef] [PubMed]
- Łopacińska, J.M.; Gradinaru, C.; Wierzbicki, R.; Købler, C.; Schmidt, M.S.; Madsen, M.T.; Skolimowski, M.; Dufva, M.; Flyvbjerg, H.; Mølhave, K. Cell motility, morphology, viability and proliferation in response to nanotopography on silicon black. Nanoscale 2012, 4, 3739–3745. [Google Scholar] [CrossRef] [PubMed]
- Takamori, E.R.; Cruz, R.; Gonçalvez, F.; Zanetti, R.V.; Zanetti, A.; Granjeiro, J.M. Effect of roughness of zirconia and titanium on fibroblast adhesion. Artif. Organs 2008, 32, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hao, M.; Nian, X.; Qiao, H.; Zhang, X.; Zhang, X.; Song, G.; Guo, J.; Pang, X.; Zhang, H. Strontium and copper co-substituted hydroxyapatite-based coatings with improved antibacterial activity and cytocompatibility fabricated by electrodeposition. Ceram. Int. 2016, 42, 11876–11888. [Google Scholar] [CrossRef]
- Mohan, L.; Durgalakshmi, D.; Geetha, M.; Sankara Narayanan, T.S.N.; Asokamani, R. Electrophoretic deposition of nanocomposite (HAp+TiO2) on titanium alloy for biomedical applications. Ceram. Int. 2012, 38, 3435–3443. [Google Scholar] [CrossRef]
- Rautray, T.R.; Narayanan, R.; Kim, K.H. Ion implantation of titanium based biomaterials. Prog. Mater. Sci. 2011, 56, 1137–1177. [Google Scholar] [CrossRef]
- Gross, K.A.; Babovic, M. Influence of abrasion on the surface characteristics of thermally sprayed hydroxyapatitae coatings. Biomaterials 2002, 23, 4731–4737. [Google Scholar] [CrossRef]
- Alam, F.; Balani, K. Adhesion force of staphylococcus aureus on various biomaterial surfaces. J. Mech. Behav. Biomed. Mater. 2017, 65, 872–880. [Google Scholar] [CrossRef]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. BioMatter 2012, 2, 176–194. [Google Scholar] [CrossRef]
- Kubacka, A.; Diez, M.S.; Rojo, D.; Bargiela, R.; Ciordia, S.; Zapico, I.; Albar, J.P.; Barbas, C.; Martins Dos Santos, V.A.P.; Fernández-García, M.; et al. Understanding the antimicrobial mechanism of TiO2-based nanocomposite films in a pathogenic bacterium. Sci. Rep. 2014, 4, 4134. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, H.; Bär, H.; Kreplak, L.; Strelkov, S.V.; Aebi, U. Intermediate filaments: From cell architecture to nanomechanics. Nat. Rev. Mol. Cell Biol. 2007, 8, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Corni, I.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic deposition: From traditional ceramics to nanotechnology. J. Eur. Ceram. Soc. 2008, 28, 1353–1367. [Google Scholar] [CrossRef]
- Von Wilmowsky, C.; Bauer, S.; Lutz, R.; Meisel, M.; Neukam, F.W.; Toyoshima, T.; Schmuki, P.; Nkenke, E.; Schlegel, K.A. In Vivo Evaluation of Anodic TiO2 Nanotubes; An Experimental Study in the Pig. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 89, 165–171. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.; Roy, P.; Birajdar, B.I.; Spiecker, E.; Schmuki, P. Formation of a non-thickness-limited titanium dioxide mesosponge and its use in dye-sensitized solar cells. Angew. Chem. Int. Ed. 2009, 48, 9326–9329. [Google Scholar] [CrossRef]
- Noyama, Y.; Miura, T.; Ishimoto, T.; Itaya, T.; Niinomi, M.; Nakano, T. Bone loss and reduced bone quality of the human femur after total hip arthroplasty under stress-shielding effects by titanium-based implant. Mater. Trans. 2012, 53, 565–570. [Google Scholar] [CrossRef]
- Asgharzadeh Shirazi, H.; Ayatollahi, M.R.; Asnafi, A. To reduce the maximum stress and the stress shielding effect around a dental implant–bone interface using radial functionally graded biomaterials. Comput. Methods Biomech. Biomed. Eng. 2017, 20, 750–759. [Google Scholar] [CrossRef]
- Huiskes, R.; Weinans, H.; Van Rietbergen, B. The relationship between stress shielding and bone resorption around total hip stems and the effects of flexible materials. Clin. Orthop. Relat. Res. 1992, 274, 124–134. [Google Scholar] [CrossRef]
- Piszczek, P.; Radtke, A.; Ehlert, M.; Jędrzejewski, T.; Sznarkowska, A.; Sadowska, B.; Bartmański, M.; Erdoğan, Y.K.; Ercan, B.; Jędrzejczyk, W. Comprehensive Evaluation of the Biological Properties of Surface-Modified Titanium Alloy Implants. J. Clin. Med. 2020, 9, 342. [Google Scholar] [CrossRef]

| Biomaterial Sample | Average Contact Angle (Θ) [°] ± Standard Deviation | Surface Free Energy ± Standard Deviation [mJ/m2] | |
|---|---|---|---|
| Measuring Liquid | |||
| Water | Diiodomethane | ||
| Ti6Al4V | 81.3 ± 0.2 | 49.2 ± 0.9 | 38.5 ± 0.3 |
| TNT5 | 94.4 ± 0.4 | 22.4 ± 1.0 | 47.8 ± 0.3 |
| TNT15 | 123.3 ± 0.1 | 31.5 ± 0.7 | 61.4 ± 0.3 |
| TNT40 | 85.3 ± 0.9 | 18.5 ± 0.8 | 47.6 ± 0.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehlert, M.; Radtke, A.; Jędrzejewski, T.; Roszek, K.; Bartmański, M.; Piszczek, P. In Vitro Studies on Nanoporous, Nanotubular and Nanosponge-Like Titania Coatings, with the Use of Adipose-Derived Stem Cells. Materials 2020, 13, 1574. https://doi.org/10.3390/ma13071574
Ehlert M, Radtke A, Jędrzejewski T, Roszek K, Bartmański M, Piszczek P. In Vitro Studies on Nanoporous, Nanotubular and Nanosponge-Like Titania Coatings, with the Use of Adipose-Derived Stem Cells. Materials. 2020; 13(7):1574. https://doi.org/10.3390/ma13071574
Chicago/Turabian StyleEhlert, Michalina, Aleksandra Radtke, Tomasz Jędrzejewski, Katarzyna Roszek, Michał Bartmański, and Piotr Piszczek. 2020. "In Vitro Studies on Nanoporous, Nanotubular and Nanosponge-Like Titania Coatings, with the Use of Adipose-Derived Stem Cells" Materials 13, no. 7: 1574. https://doi.org/10.3390/ma13071574
APA StyleEhlert, M., Radtke, A., Jędrzejewski, T., Roszek, K., Bartmański, M., & Piszczek, P. (2020). In Vitro Studies on Nanoporous, Nanotubular and Nanosponge-Like Titania Coatings, with the Use of Adipose-Derived Stem Cells. Materials, 13(7), 1574. https://doi.org/10.3390/ma13071574