Fabrication of Hollow and Porous Tin-Doped Indium Oxide Nanofibers and Microtubes via a Gas Jet Fiber Spinning Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Spinning Sol Solution: PVP-InCl3-SnCl4
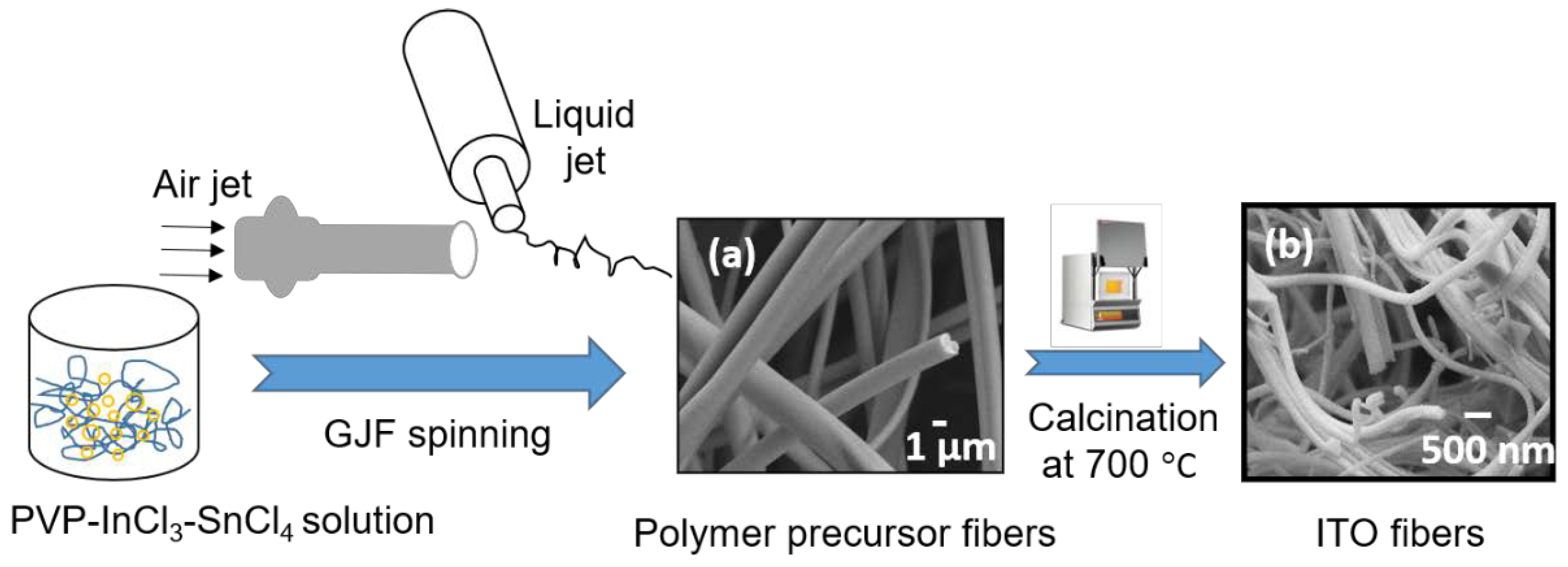
2.2. Fabrication of ITO Fibers
2.3. Materials Characterization
3. Results and Discussion
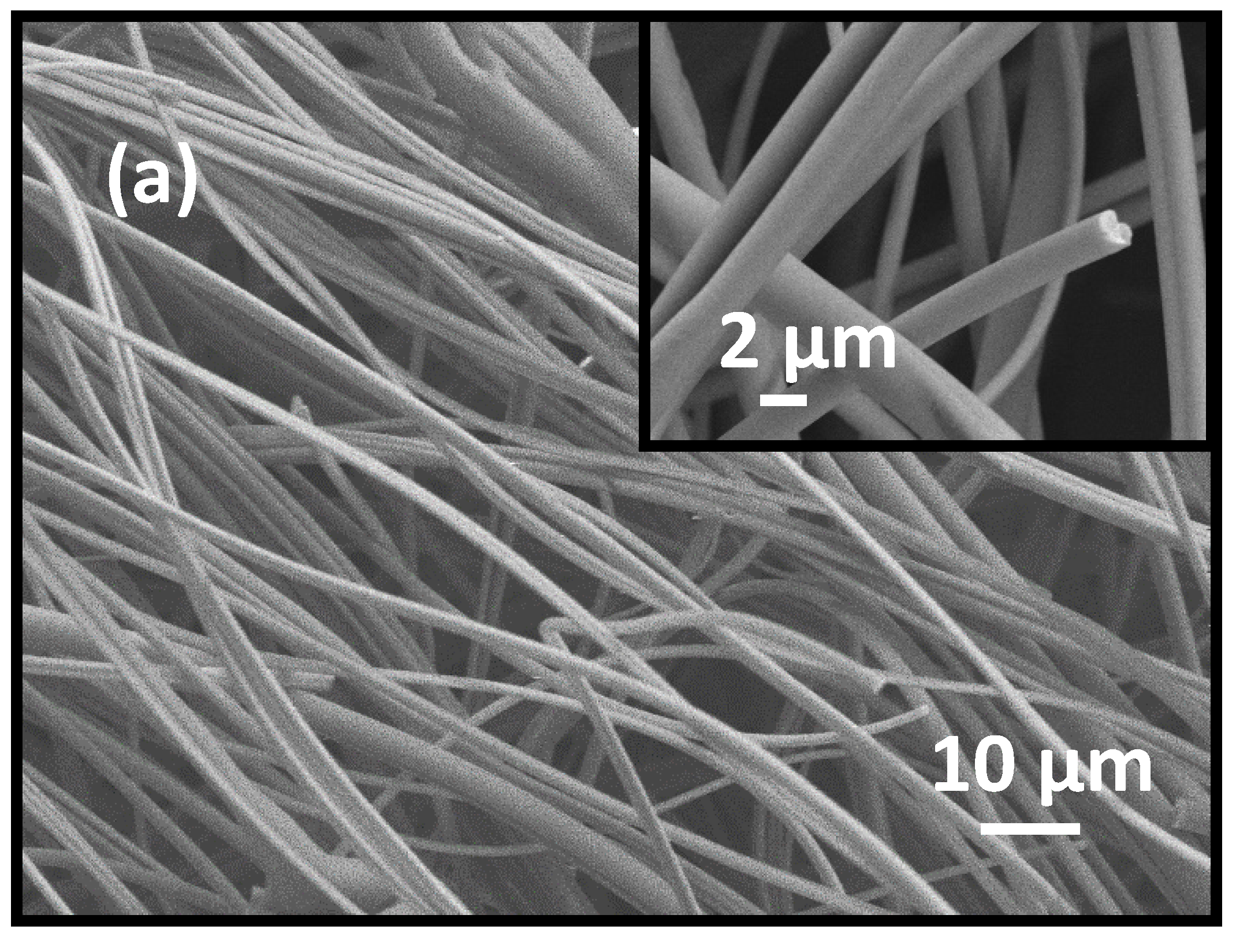
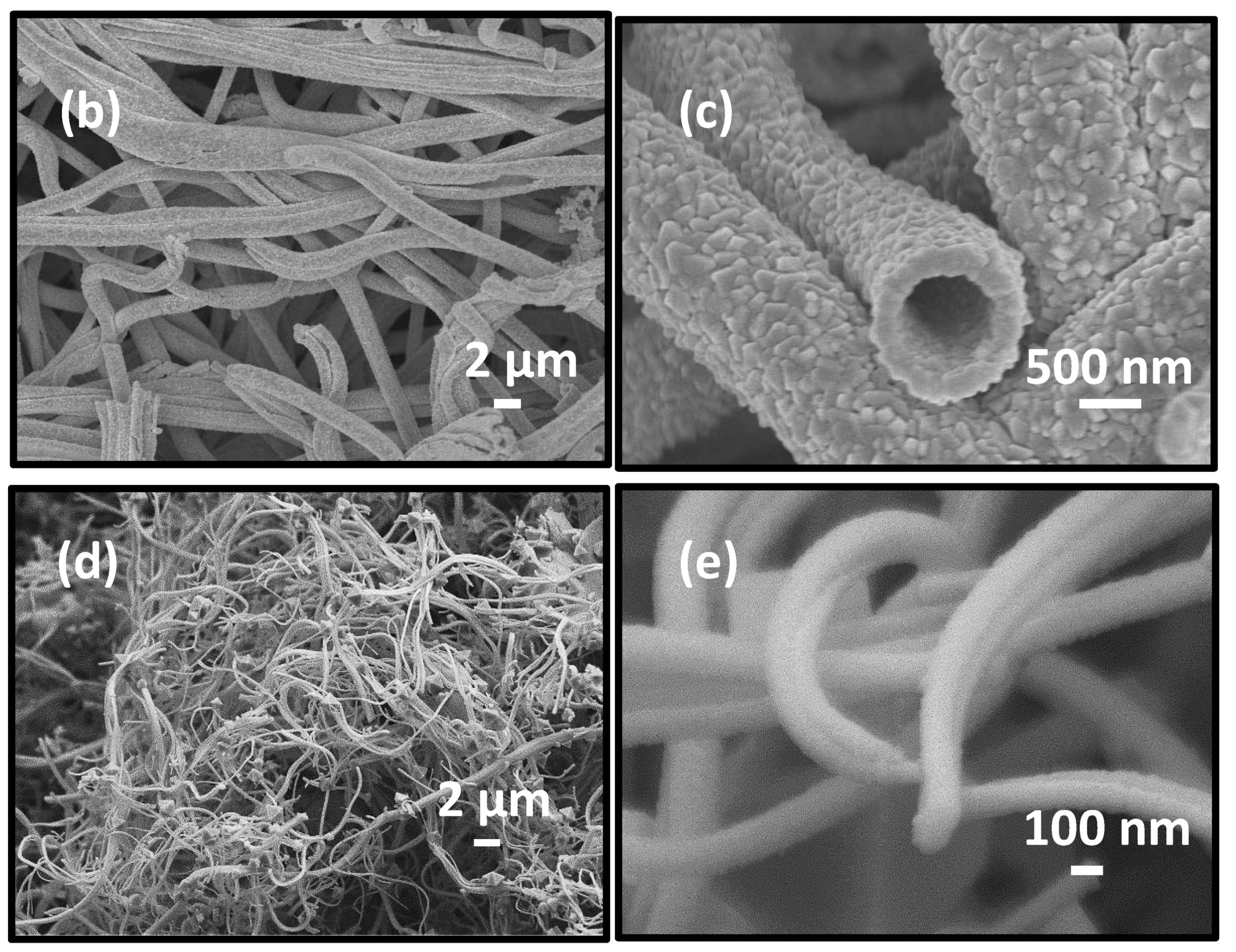
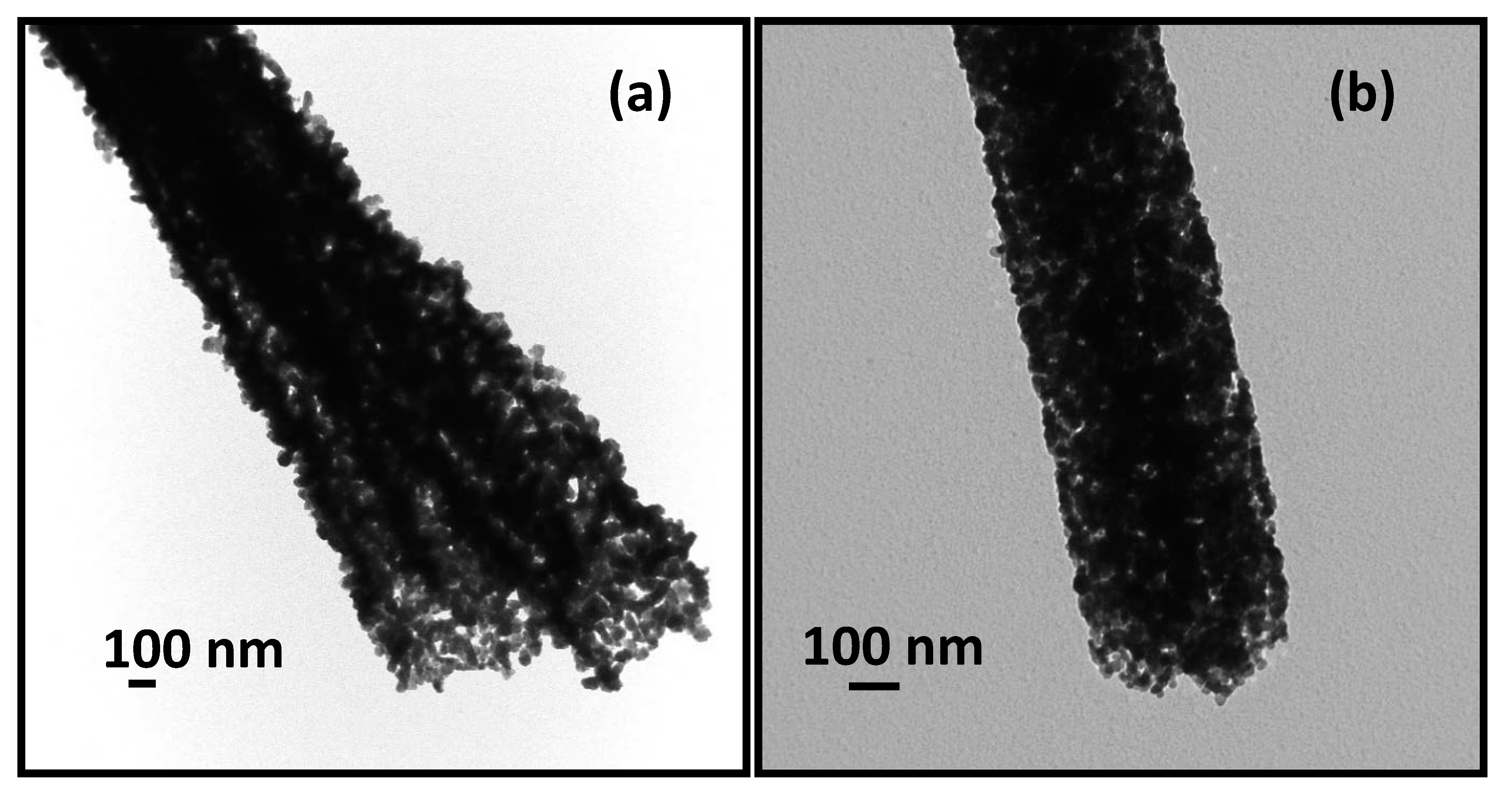
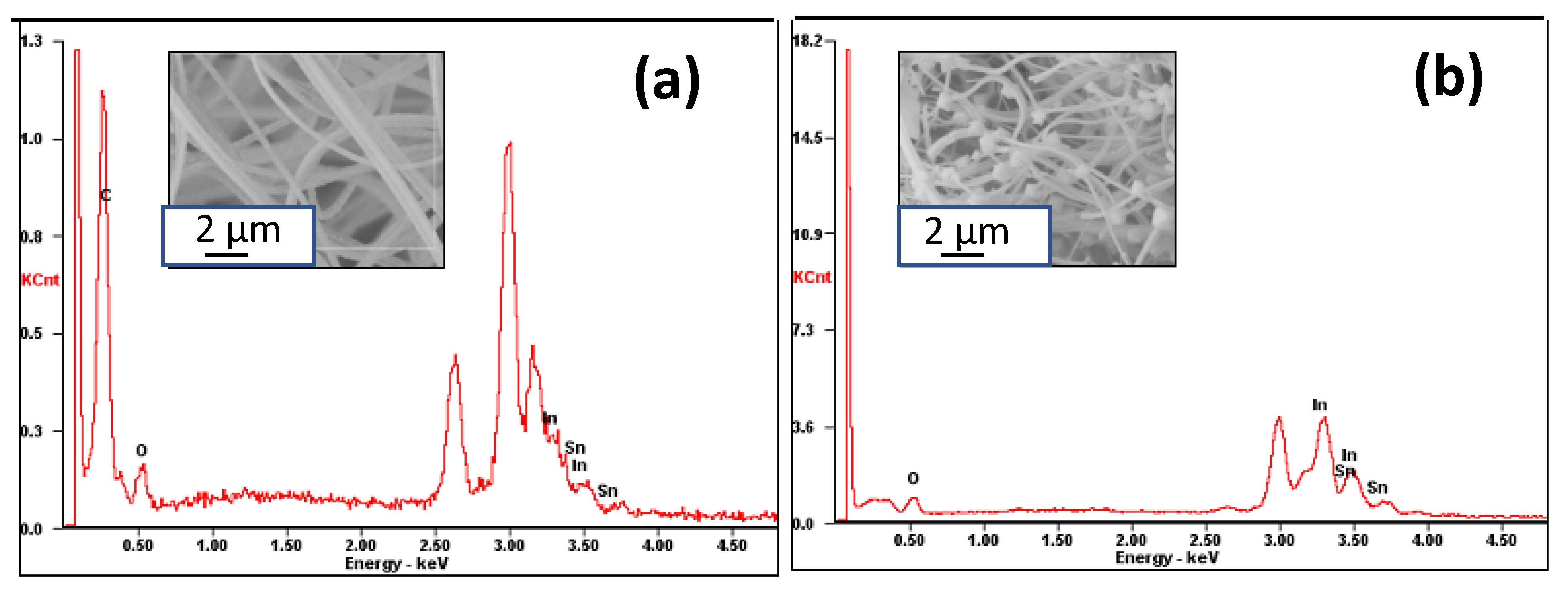
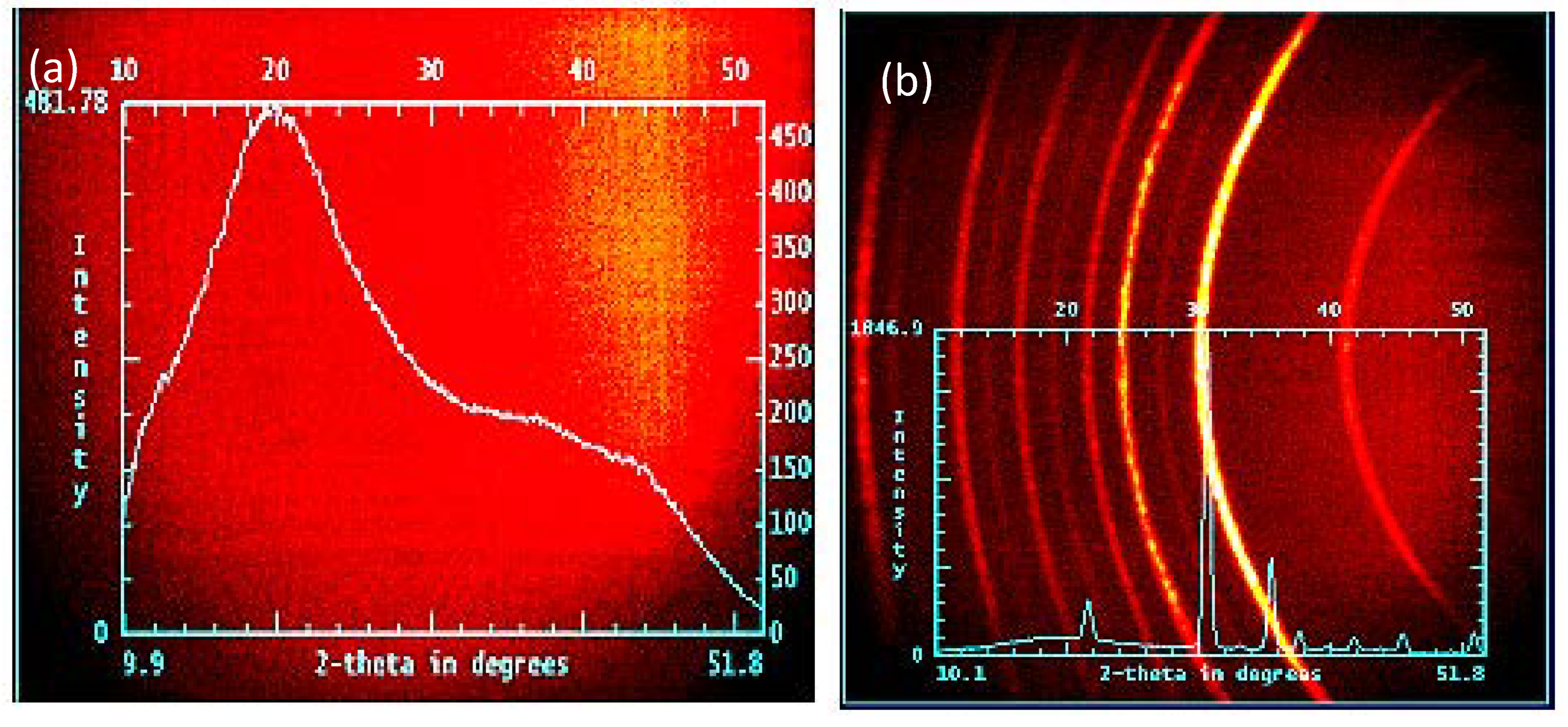
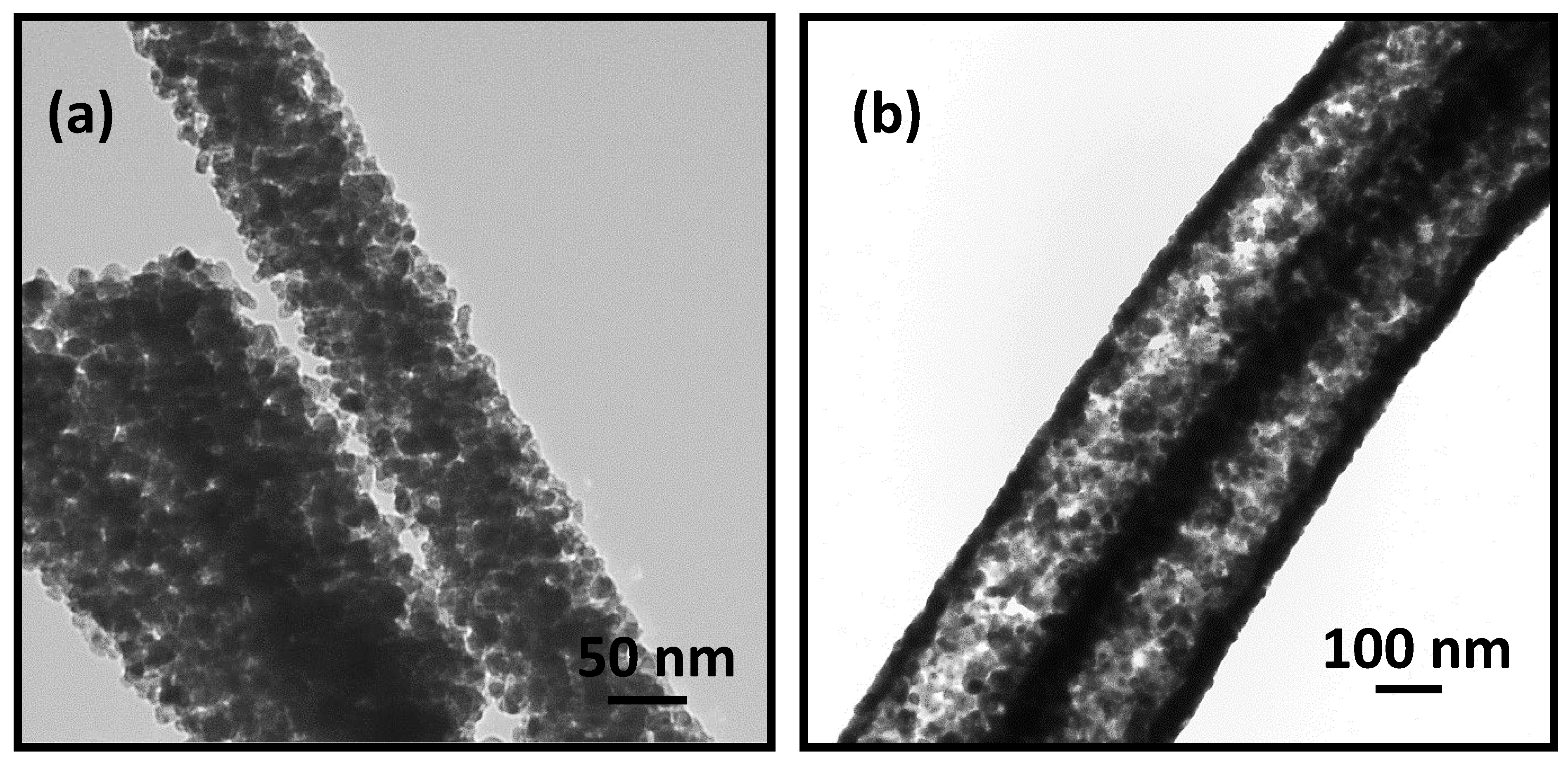
3.1. Morphology and Crystal Structure of ITO Micro/Nano Fiber
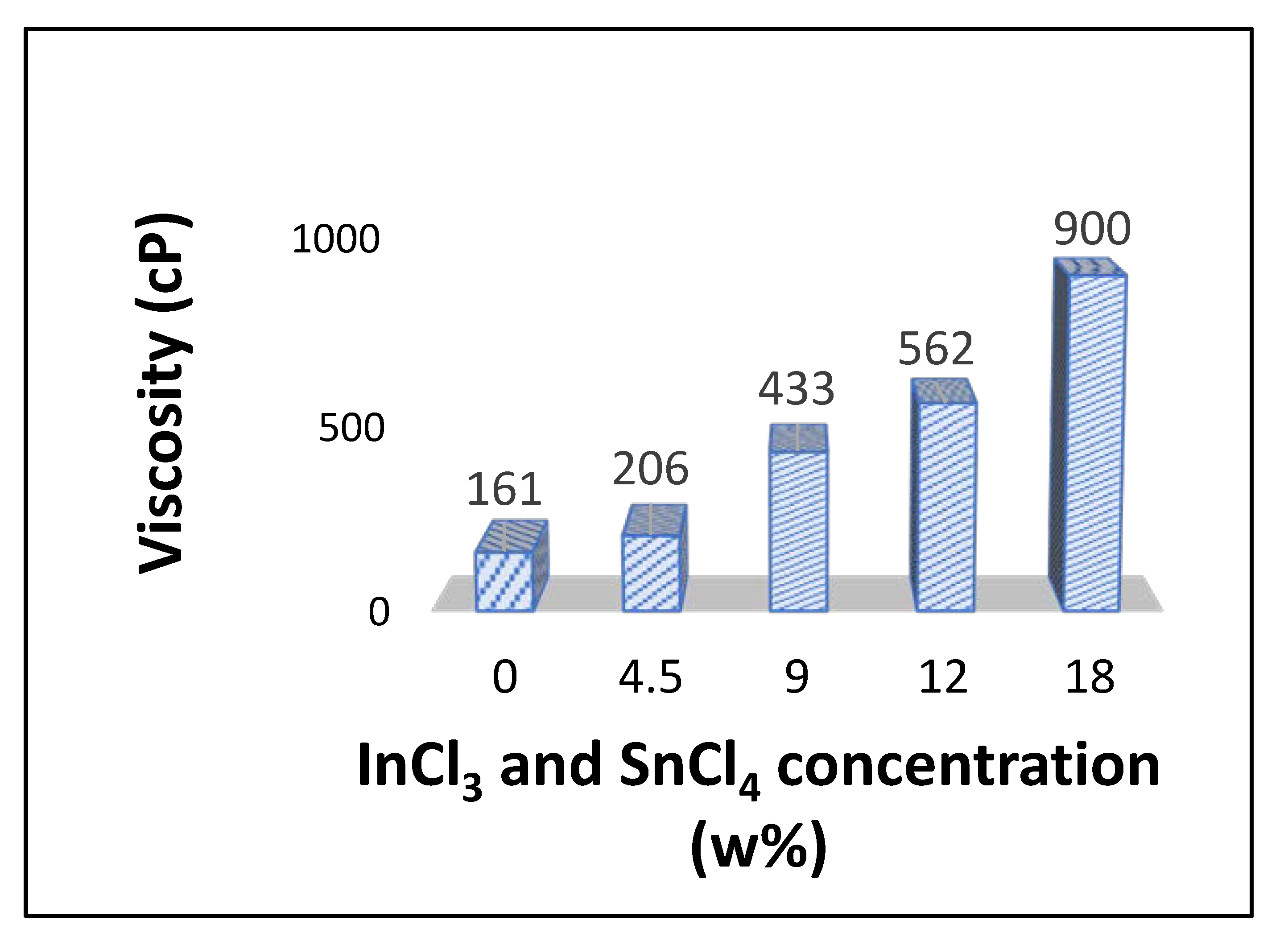
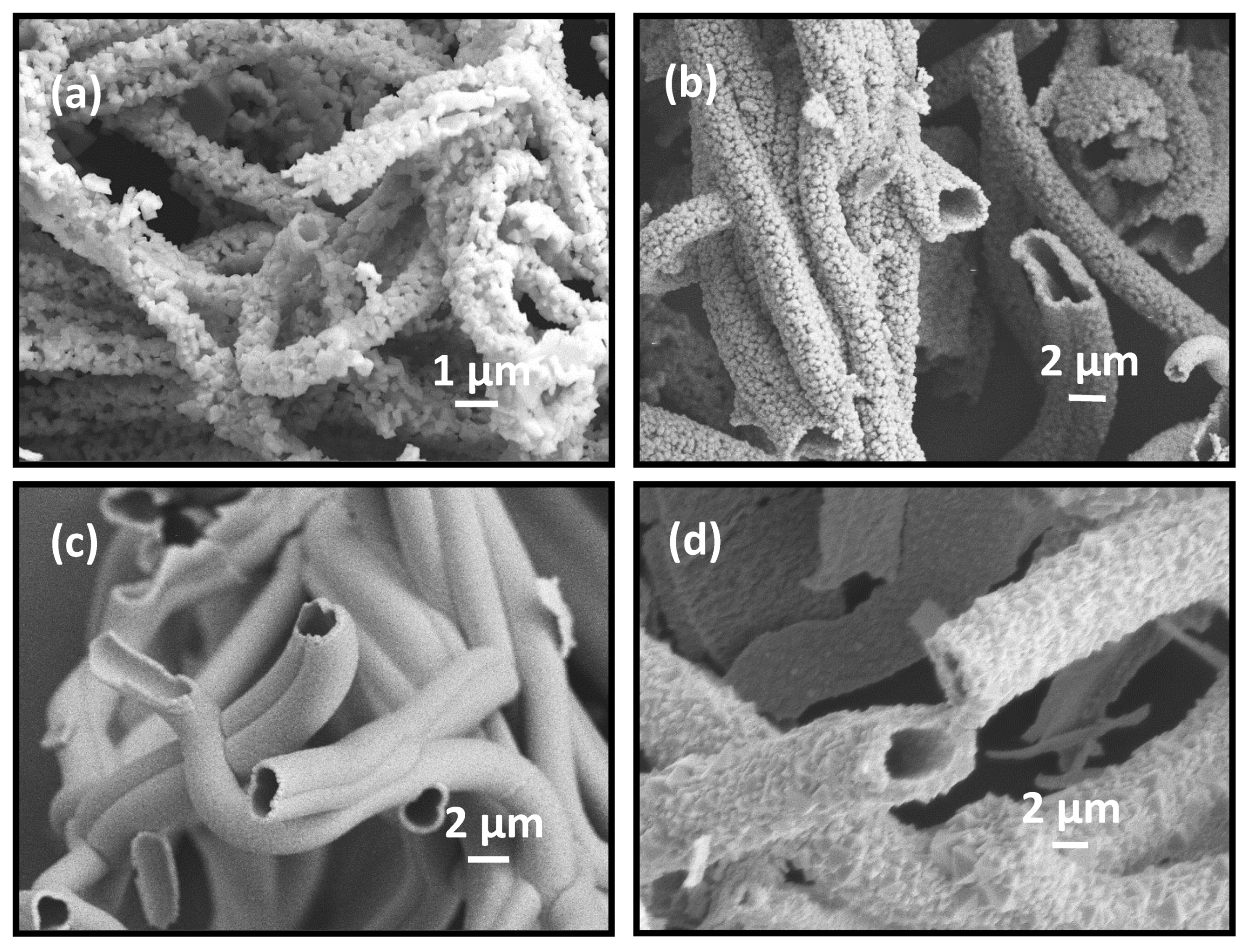
3.2. Morphology of ITO: Effect of ITO Precursor Concentration
3.3. Formation Mechanism of ITO Fibers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yoon, S.; Kim, H.; Shin, E.-S.; Huh, J.N.; Noh, Y.-Y.; Park, B.; Hwang, I. Toward High Conductivity of Electrospun Indium Tin Oxide Nanofibers with Fiber Morphology Dependent Surface Coverage: Postannealing and Polymer Ratio Effects. ACS Appl. Mater. Interfaces 2017, 9, 34305–34313. [Google Scholar] [CrossRef] [PubMed]
- Lang, L.; Wu, D.; Xu, Z. Controllable Fabrication of TiO2 1D-Nano/Micro Structures: Solid, Hollow, and Tube-in-Tube Fibers by Electrospinning and the Photocatalytic Performance. Chem. Eur. J. 2012, 18, 10661–10668. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, Y.; Xia, Y. Electrospinning of Polymeric and Ceramic Nanofibers as Uniaxially Aligned Arrays. Nano Lett. 2003, 3, 1167–1171. [Google Scholar] [CrossRef]
- Mitra, J.; Ghosh, M.; Bordia, R.K.; Sharma, A. Photoluminescent Electrospun Submicron Fibers Of Hybrid Organosiloxane And Derived Silica. RSC Adv. 2013, 3, 7591–7600. [Google Scholar] [CrossRef]
- Xu, S.; Shi, Y. Low Temperature High Sensor Response Nano Gas Sensor Using ITO Nanofibers. Sens. Actuators B Chem. 2009, 143, 71–75. [Google Scholar] [CrossRef]
- Ghosh, M.; Liu, J.; Chuang, S.S.C.; Jana, S.C. Fabrication of Hierarchical V2O5 Nanorods on TiO2 Nanofibers and Their Enhanced Photocatalytic Activity under Visible Light. ChemCatChem 2018, 10, 3305–3318. [Google Scholar] [CrossRef]
- Lang, L.; Xu, Z. Controllable Synthesis of Porous α-Fe2O3 Microtube and Tube-in-tube by Non-coaxial Electrospinning. Chem. Lett. 2013, 42, 750–752. [Google Scholar] [CrossRef]
- Wan, Q.; Wei, M.; Zhi, D.; MacManus-Driscoll, J.L.; Blamire, M.G. Epitaxial Growth of Vertically Aligned and Branched Single-Crystalline Tin-Doped Indium Oxide Nanowire Arrays. Adv. Mater. 2006, 18, 234–238. [Google Scholar] [CrossRef]
- Almasian, A.; Chizari Fard, G.; Maleknia, L. Fabrication Of Hollow And Nonhollow Sio2 Nanofibers For Removal of Cationic Dyes From Aqueous Solutions. Environ. Progress Sustain. Energy 2017, 36, 1390–1404. [Google Scholar] [CrossRef]
- Wang, L.; Luo, X.; Zheng, X.; Wang, R.; Zhang, T. Direct Annealing Of Electrospun Synthesized High-Performance Porous Sno2 Hollow Nanofibers For Gas Sensors. RSC Adv. 2013, 3, 9723–9728. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Wang, C.; Wei, L.; Liu, Y.; Shao, C. ZnO Hollow Nanofibers: Fabrication from Facile Single Capillary Electrospinning and Applications in Gas Sensors. J. Phys. Chem. C 2009, 113, 19397–19403. [Google Scholar] [CrossRef]
- Cheng, J.P.; Wang, B.B.; Zhao, M.G.; Liu, F.; Zhang, X.B. Nickel-Doped Tin Oxide Hollow Nanofibers Prepared By Electrospinning For Acetone Sensing. Sens. Actuators B Chem. 2014, 190, 78–85. [Google Scholar] [CrossRef]
- Einert, M.; Ostermann, R.; Weller, T.; Zellmer, S.; Garnweitner, G.; Smarsly, B.M.; Marschall, R. Hollow A-Fe2O3 Nanofibres for Solar Water Oxidation: Improving The Photoelectrochemical Performance By Formation of A-Fe2O3/ITO-Composite Photoanodes. J. Mater. Chem. A 2016, 4, 18444–18456. [Google Scholar] [CrossRef]
- Zhang, X.; Thavasi, V.; Mhaisalkar, S.G.; Ramakrishna, S. Novel Hollow Mesoporous 1D Tio2 Nanofibers As Photovoltaic And Photocatalytic Materials. Nanoscale 2012, 4, 1707–1716. [Google Scholar] [CrossRef]
- Mao, X.; Si, Y.; CHen, Y.; Yang, L.; Zhao, F.; DIng, B.; Yu, J. Silica Nanofibrous Membranes With Robust Flexibility And Thermal Stability For High-Efficiency Fine Particulate Filtration. RSC Adv. 2012, 2, 12216–12223. [Google Scholar] [CrossRef]
- Lakshmi, B.; Patrissi, C.; Martin, C. Sol−Gel Template Synthesis of Semiconductor Oxide Micro- And Nanostructures. Chem. Mater. 1997, 9, 2544–2550. [Google Scholar] [CrossRef]
- Li, D.; McCann, J.T.; Xia, Y. Use of Electrospinning to Directly Fabricate Hollow Nanofibers with Functionalized Inner and Outer Surfaces. Small 2005, 1, 83–86. [Google Scholar] [CrossRef]
- Srivastava, Y.; Loscertales, I.; Marquez, M.; Thorsen, T. Electrospinning of Hollow And Core/Sheath Nanofibers Using A Microfluidic Manifold. Microfluid. Nanofluidics 2008, 4, 245–250. [Google Scholar] [CrossRef]
- Yasuda, K.; Schmuki, P. Formation of Self-organized Zirconium Titanate Nanotube Layers by Alloy Anodization. Adv. Mater. 2007, 19, 1757–1760. [Google Scholar] [CrossRef]
- Jia, C.J.; Sun, L.D.; Yan, Z.G.; You, L.P.; Luo, F.; Han, X.D.; Pang, Y.C.; Zhang, Z.; Yan, C.H. Single-Crystalline Iron Oxide Nanotubes. Angew. Chem. 2005, 44, 4328–4333. [Google Scholar] [CrossRef]
- Wu, J.; Zeng, D.; Wang, X.; Zeng, L.; Huang, Q.; Tang, G.; Xie, C. mechanistic Insights Into Formation Of Sno2 Nanotubes: Asynchronous Decomposition Of Poly(Vinylpyrrolidone) In Electrospun Fibers During Calcining Process. Langmuir 2014, 30, 11183–11189. [Google Scholar] [CrossRef] [PubMed]
- Benavides, R.E.; Jana, S.C.; Reneker, D.H. Nanofibers from Scalable Gas Jet Process. ACS Macro Lett. 2012, 1, 1032–1036. [Google Scholar] [CrossRef]
- Ghosh, M.; Jana, S.C. Bi-Component Inorganic Oxide Nanofibers from Gas Jet Fiber Spinning Process. RSC Advances 2015, 5, 105313–105318. [Google Scholar] [CrossRef]
- Rajgarhia, S.S.; Jana, S.C. Comparison of Electrospinning and Gas Jet Fiber Processes for Fabrication of Bi-Component Polymer Nanofibers from Single Solutions. Macromol. Symp. 2016, 369, 8–13. [Google Scholar] [CrossRef]
- Ghosh, M.; Jana, S.C. Fabrication, Morphological Evaluation, and Characterization of Semiconducting Oxide Nanofibers from Gas Jet Fiber Spinning Process. In Proceedings of the ANTEC 2016, Indianapolis, IN, USA, 23–25 May 2016; Society of Plastics Engineers: Brookfield, CT, USA, 2016; pp. 640–646, ISBN 123-0-1234567-8-9. [Google Scholar]
- Ghosh, M.; Lohrasbi, M.; Chuang, S.S.C.; Jana, S.C. Mesoporous Titanium Dioxide Nanofibers with a Significantly Enhanced Photocatalytic Activity. ChemCatChem 2016, 8, 2525–2535. [Google Scholar] [CrossRef]
- O’Dwyer, C.; Szachowicz, M.; Visimberga, G.; Lavayen, V.; Newcomb, S.B.; Torres, C.M.S. Bottom-Up Growth Of Fully Transparent Contact Layers Of Indium Tin Oxide Nanowires for Light-Emitting Devices. Nat. Nanotechnol. 2009, 4, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, F.; Suryamas, A.B.; Kawabe, M.; Munir, M.M.; Okuyama, K.; Tarao, T.; Nishitani, T. Indium Tin Oxide Nanofiber Film Electrode for High Performance Dye Sensitized Solar Cells. Jpn. J. Appl. Phys. 2010, 49, 010213. [Google Scholar] [CrossRef]
- Hong-Wen, W.; Chi-Feng, T.; Miao-Ken, H.; Chwei-Huann, C.; Ying-Ling, L.; Zongwen, L.; Kyle, R.R.; Simon, P.R. Three-Dimensional Electrodes For Dye-Sensitized Solar Cells: Synthesis Of Indium–Tin-Oxide Nanowire Arrays And ITO/TiO2 Core–Shell Nanowire Arrays By Electrophoretic Deposition. Nanotechnology 2009, 20, 055601. [Google Scholar]
- Chuangchote, S.; Sagawa, T.; Yoshikawa, S. Indium Tin Oxide Nanofibers and their Applications for Dye-Sensitized Solar Cells. ECS Trans. 2011, 41, 223. [Google Scholar]
- Lee, D.; Kim, B.; Kim, J.; Jeong, S.; Cao, G.; Moon, J. Salami-like Electrospun Si Nanoparticle-ITO Composite Nanofibers with Internal Conductive Pathways for use as Anodes for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 27234–27241. [Google Scholar] [CrossRef]
- Jin, M.-J.; Ma, T.; Ling, T.; Qiao, S.-Z.; Du, X.-W. Three-Dimensional Networks Of ITO/Cds Coaxial Nanofibers For Photovoltaic Applications. J. Mater. Chem. 2012, 22, 13057–13063. [Google Scholar] [CrossRef]
- Luff, B.J.; Wilkinson, J.S.; Perrone, G. Indium Tin Oxide Overlayered Waveguides for Sensor Applications. Appl. Opt. 1997, 36, 7066–7077. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Hu, L.; Carney, T.; Ruan, Z.; Kong, D.; Yu, Z.; Yao, Y.; Cha, J.J.; Zhu, J.; Fan, S.; et al. Low Reflectivity and High Flexibility of Tin-Doped Indium Oxide Nanofiber Transparent Electrodes. J. Am. Chem. Soc. 2011, 133, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Choi, W.; Cho, S.; Goshima, D.; Ham, D.; Kim, K.; Jeong, J.; Lee, J.; Lee, S. Fabrication of Structurally Simple Index-Matched ITO Films Using Roll-to-Roll Sputtering for Touch Screen Panel Devices. Plasma Process. Polym. 2015, 12, 1322–1327. [Google Scholar] [CrossRef]
- Munir, M.M.; Widiyandari, H.; Iskandar, F.; Okuyama, K. Patterned Indium Tin Oxide Nanofiber Films and Their Electrical and Optical Performance. Nanotechnology 2008, 19, 375601. [Google Scholar] [CrossRef]
- Mukhlish, M.Z.B.; Horie, Y.; Higashi, K.; Ichigi, A.; Guo, S.; Nomiyama, T. Self-Standing Conductive ITO-Silica Nanofiber Mats for Use In Flexible Electronics And Their Application In Dye-Sensitized Solar Cells. Ceram. Int. 2017, 43, 8146–8152. [Google Scholar] [CrossRef]
- Muhammad Miftahul, M.; Ferry, I.; Ki Myoung, Y.; Kikuo, O.; Mikrajuddin, A. Optical And Electrical Properties of Indium Tin Oxide Nanofibers Prepared By Electrospinning. Nanotechnology 2008, 19, 145603. [Google Scholar]
- Jiang, H.; Moon, K.-S.; Sun, Y.; Wong, C.P.; Hua, F.; Pal, T.; Pal, A. Tin/Indium Nanobundle Formation from Aggregation Or Growth Of Nanoparticles. J. Nanoparticle Res. 2008, 10, 41–46. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L. Electrospinning Jets and Polymer Nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Cheng, Y.; Zou, B.; Yang, J.; Wang, C.; Liu, Y.; Fan, X.; Zhu, L.; Wang, Y.; Ma, H.; Cao, X. Fabrication Of CoFe2O4 Hollow Fibers By Direct Annealing of The Electrospun Composite Fibers And Their Magnetic Properties. CrystEngComm 2011, 13, 2268–2272. [Google Scholar] [CrossRef]

| Sample | Morphology after Calcination at 700 °C with Heating Rates of | |
|---|---|---|
| Lower (0.5 °C min−1) | Higher (5.0 °C min−1) | |
| 4.5 w% ITO precursor loaded PVP solution | Porous cylindrical nanofiber | Highly porous hollow fiber |
| 9.0 w% ITO precursor loaded PVP solution | Compact cylindrical nanofiber | Porous hollow fiber |
| 12.0 w% ITO precursor loaded PVP solution | Solid fiber with traces of agglomeration | Thin walled hollow microtube |
| 18.0 w% ITO precursor loaded PVP solution | - | Thick walled hollow microtube and agglomerated mass |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, M.; Jana, S.C. Fabrication of Hollow and Porous Tin-Doped Indium Oxide Nanofibers and Microtubes via a Gas Jet Fiber Spinning Process. Materials 2020, 13, 1539. https://doi.org/10.3390/ma13071539
Ghosh M, Jana SC. Fabrication of Hollow and Porous Tin-Doped Indium Oxide Nanofibers and Microtubes via a Gas Jet Fiber Spinning Process. Materials. 2020; 13(7):1539. https://doi.org/10.3390/ma13071539
Chicago/Turabian StyleGhosh, Monoj, and Sadhan C. Jana. 2020. "Fabrication of Hollow and Porous Tin-Doped Indium Oxide Nanofibers and Microtubes via a Gas Jet Fiber Spinning Process" Materials 13, no. 7: 1539. https://doi.org/10.3390/ma13071539
APA StyleGhosh, M., & Jana, S. C. (2020). Fabrication of Hollow and Porous Tin-Doped Indium Oxide Nanofibers and Microtubes via a Gas Jet Fiber Spinning Process. Materials, 13(7), 1539. https://doi.org/10.3390/ma13071539







