Evaluation of Implants with Different Macrostructures in Type I Bone—Pre-Clinical Study in Rabbits
Abstract
1. Introduction
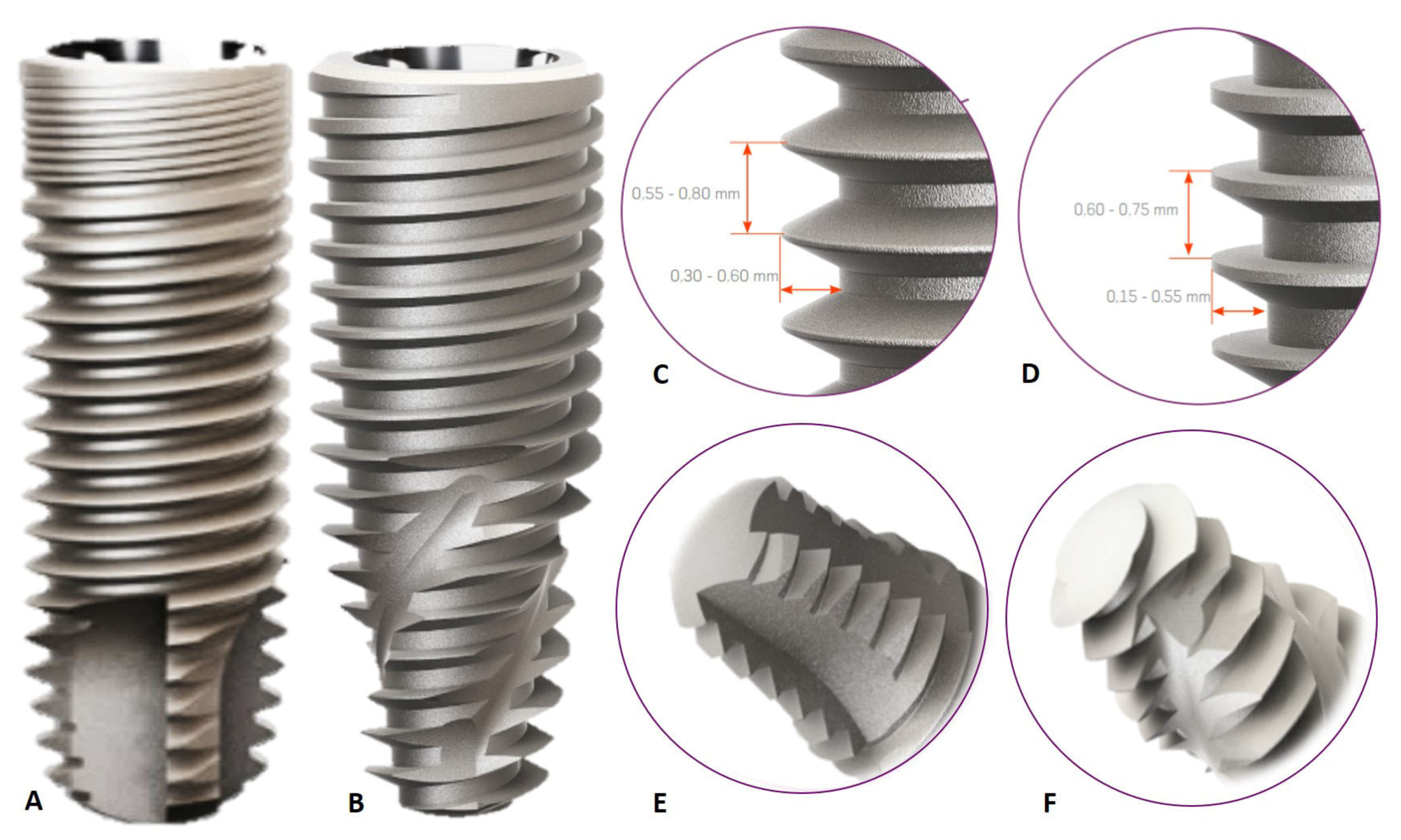
2. Materials and Methods
2.1. Experimental Outline
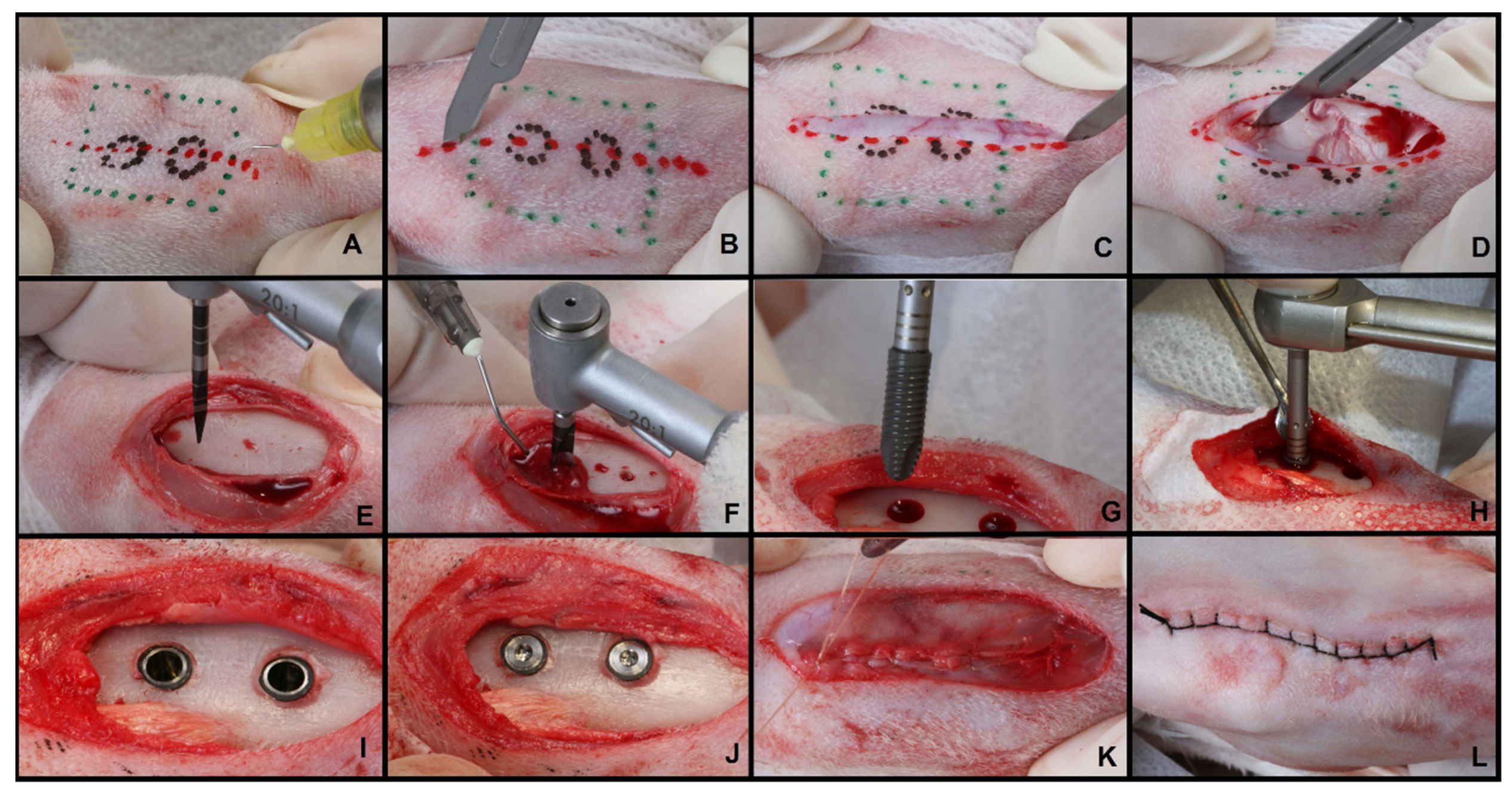
2.2. Surgical Procedure
2.3. Biomechanical Assessment (Insertion Torque and Removal Torque)
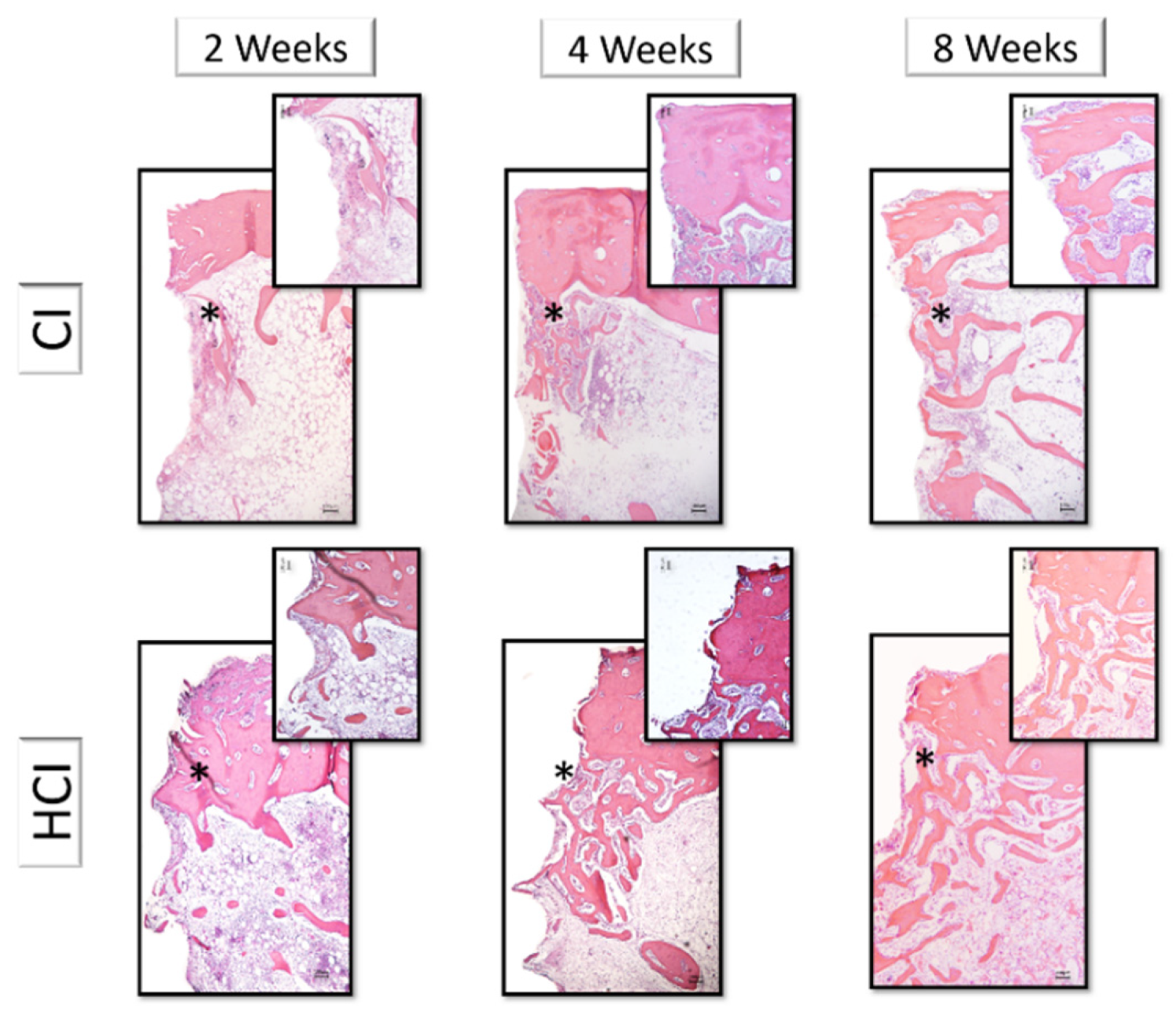
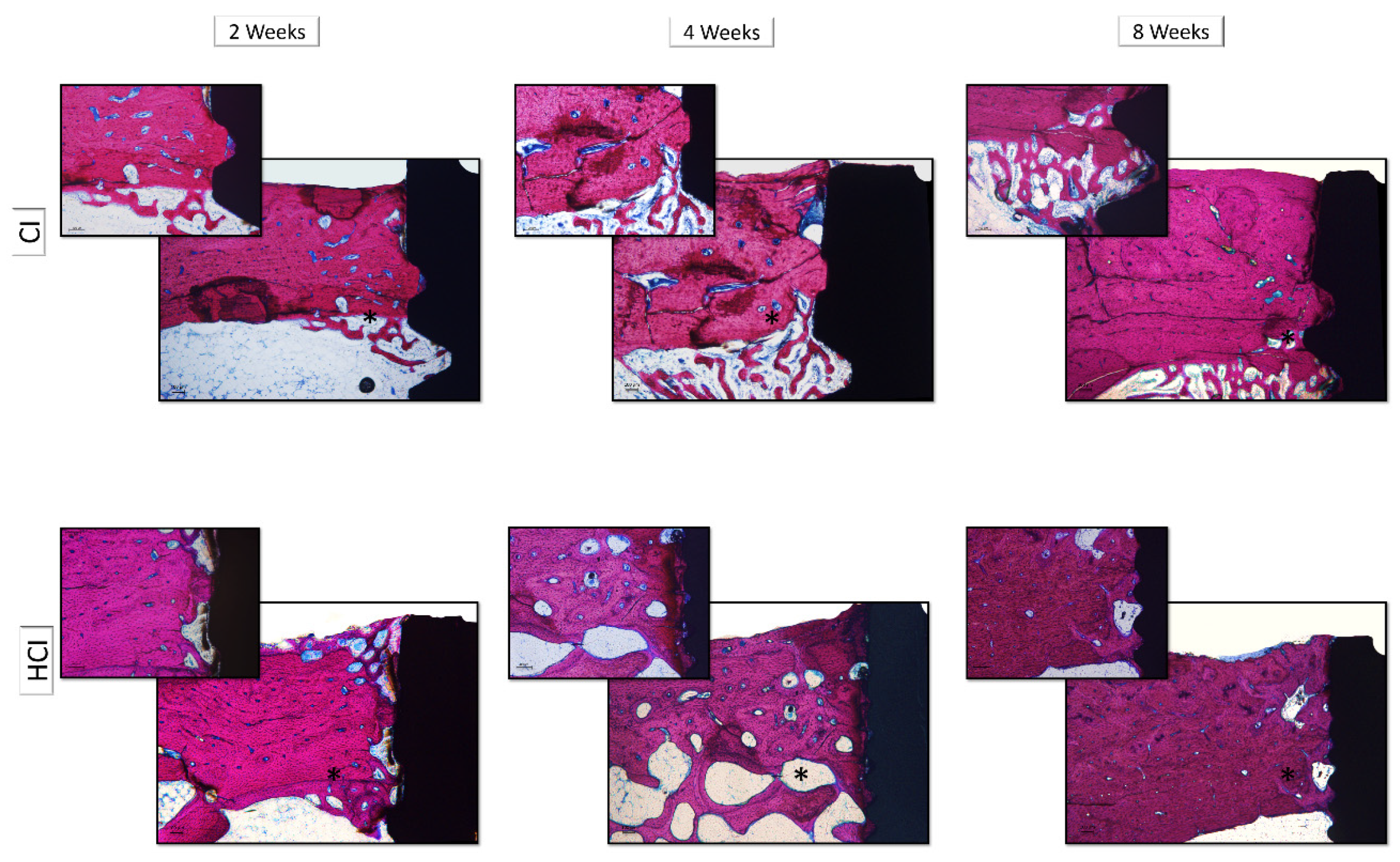
2.4. Descriptive Histology of Decalcified Sections
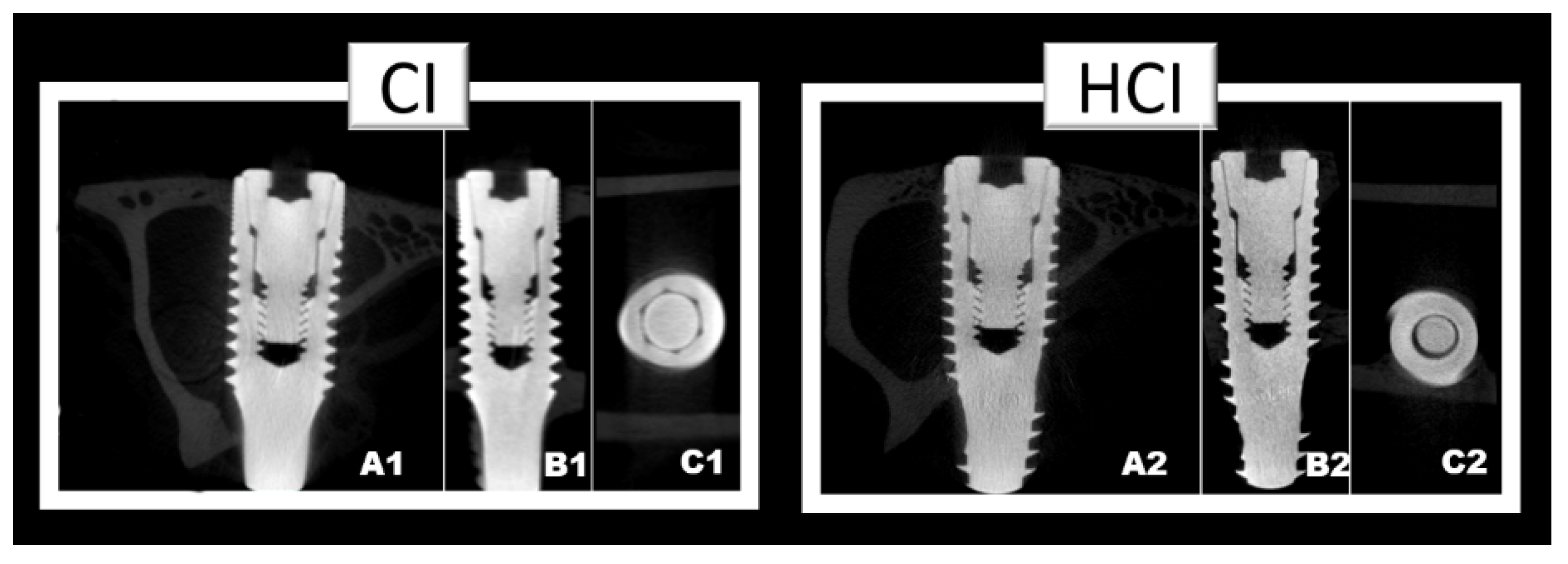
2.5. Microtomographic Analysis (µCT)
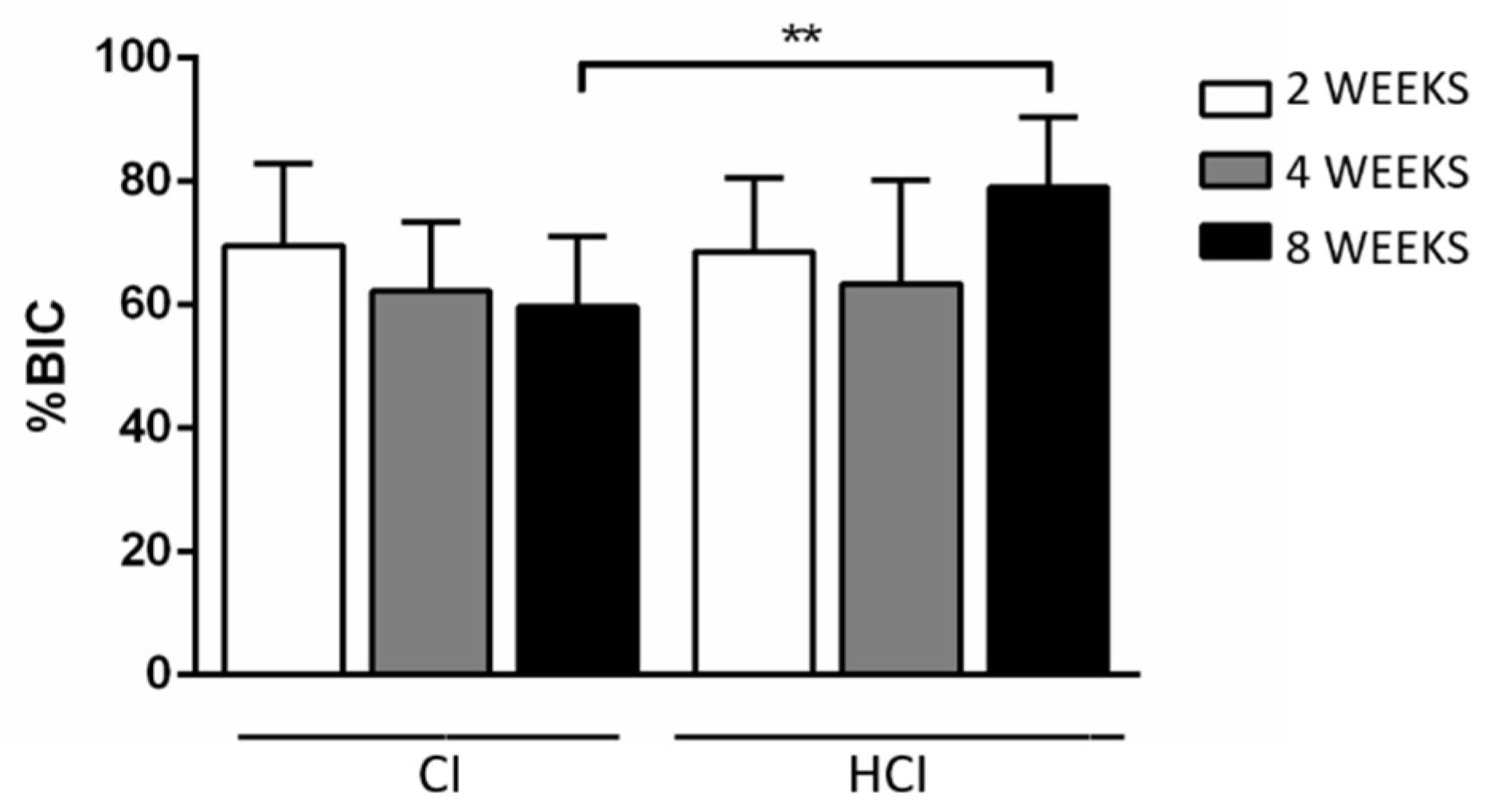
2.6. Histometric Analysis (BIC)
2.7. Statistical Analysis
3. Results
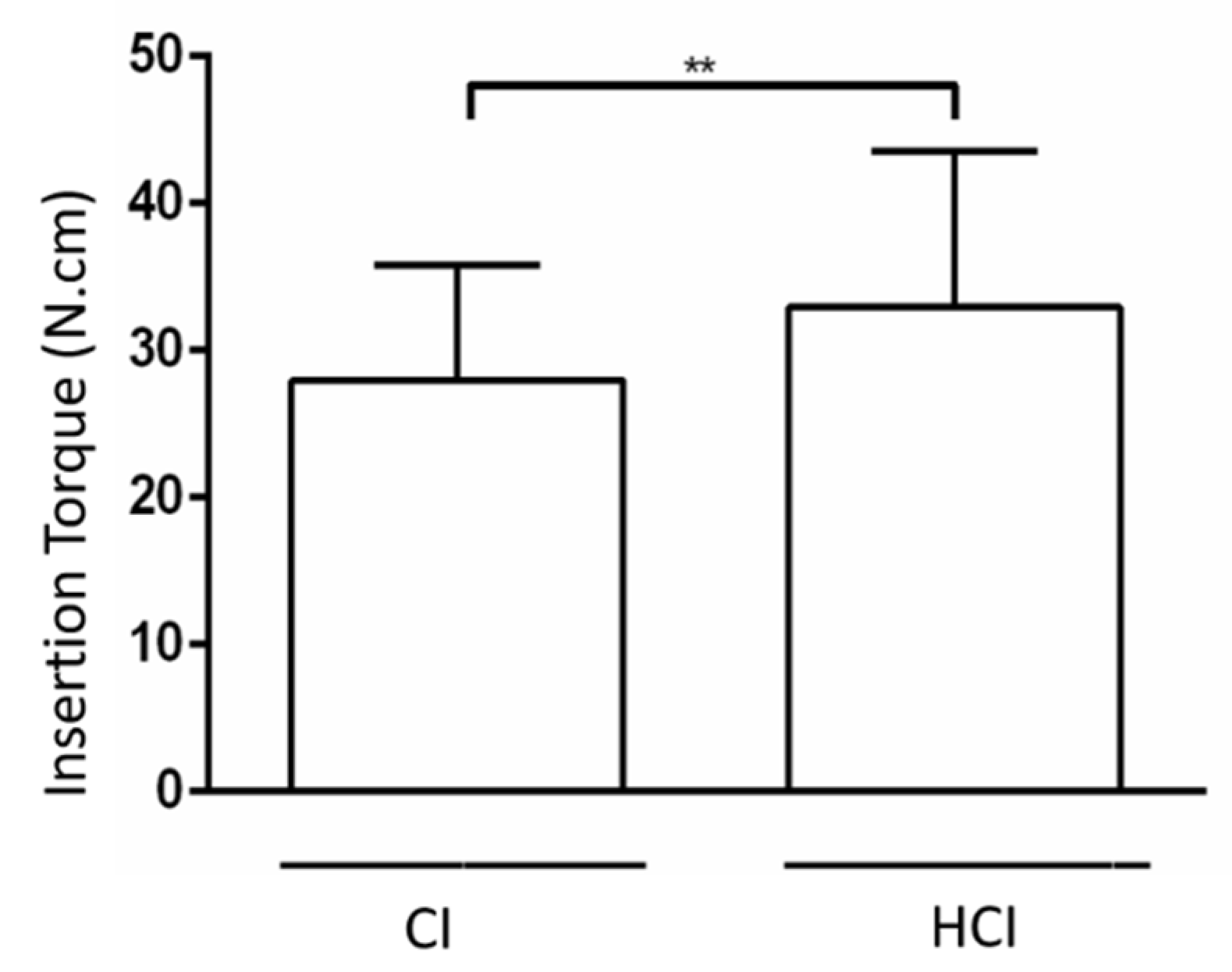
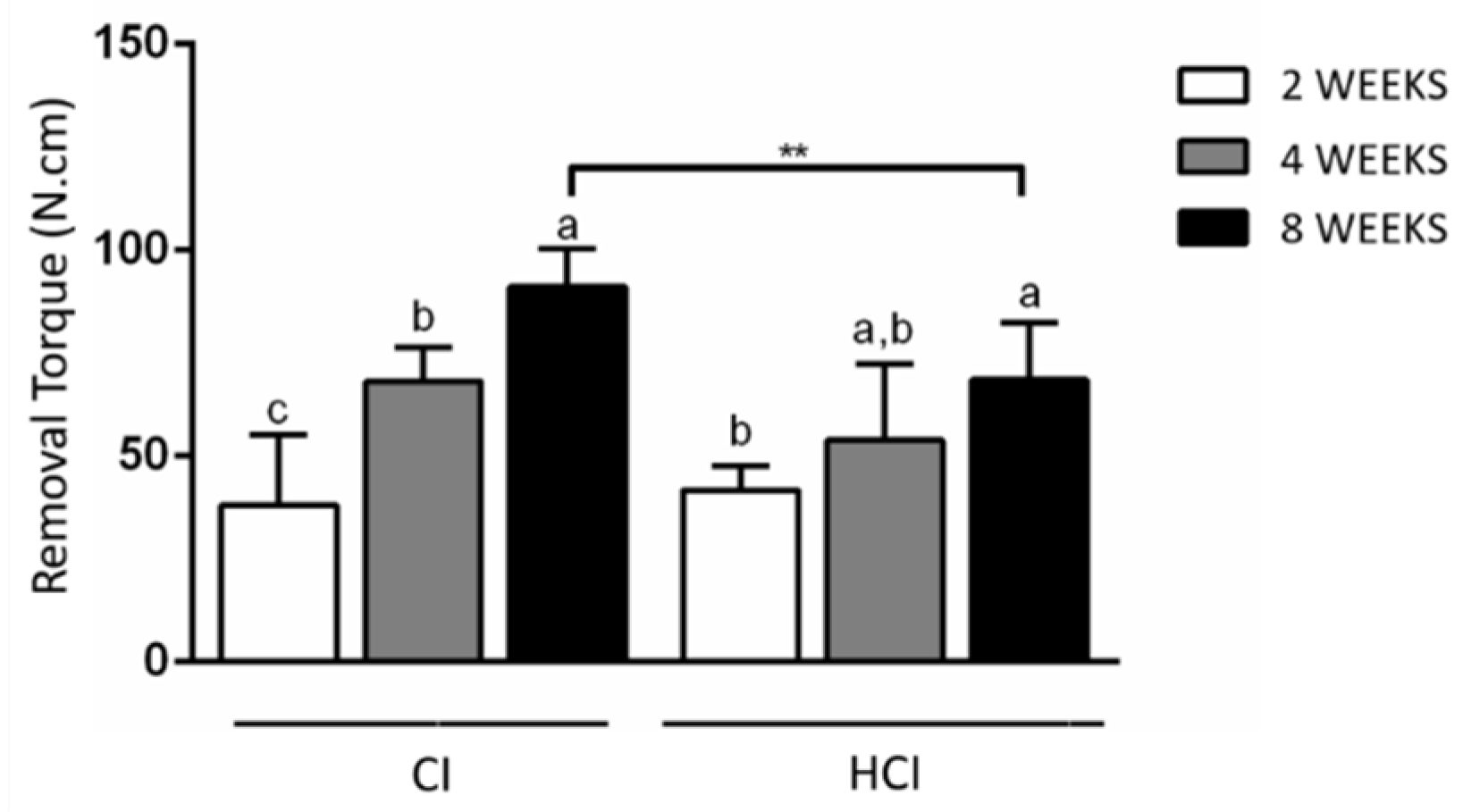
3.1. Biomechanical Analysis
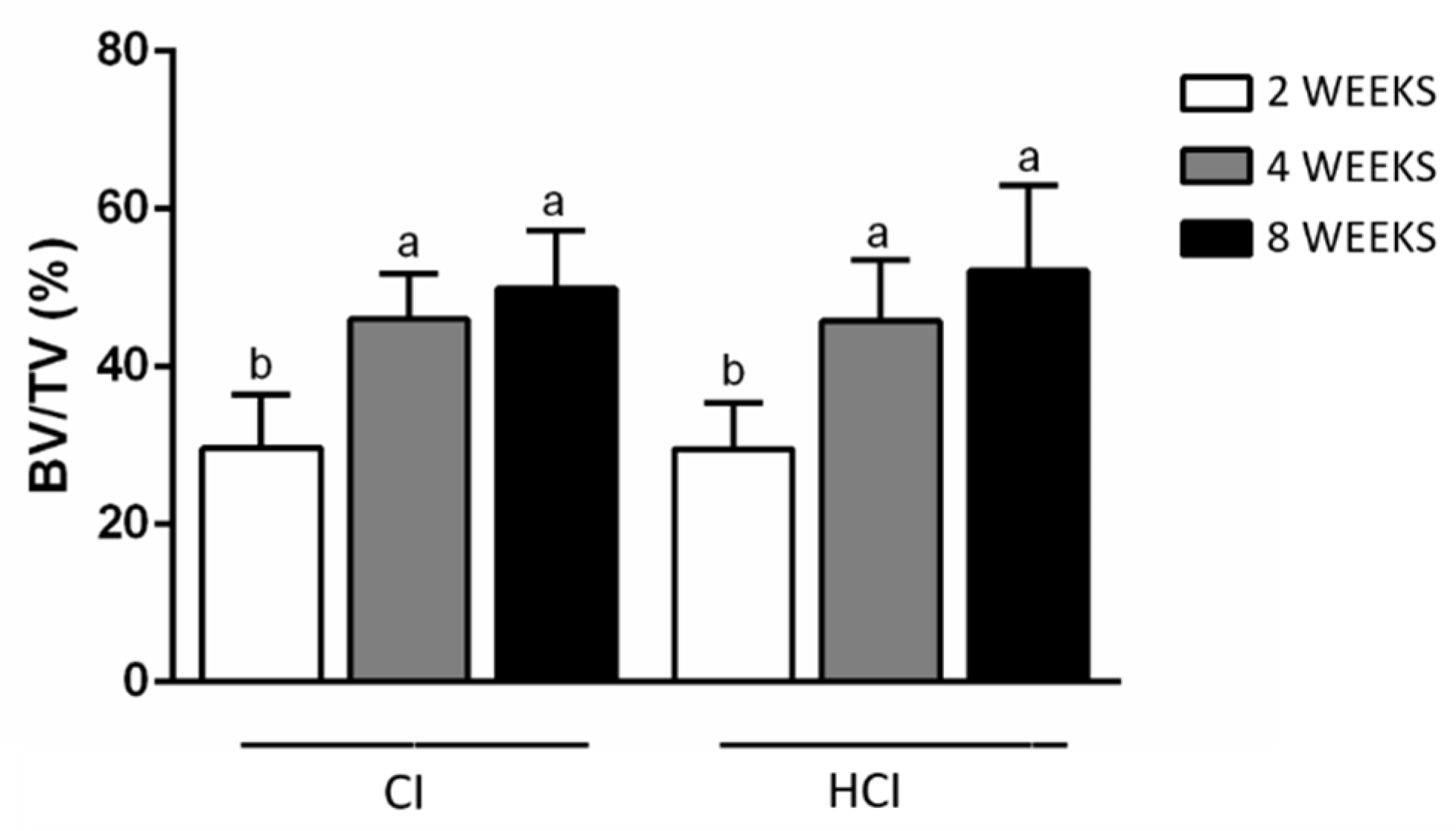
3.2. Microtomographic Analysis
3.3. Descriptive and Histometric Histological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Papaspyridakos, P.; Mokti, M.; Chen, C.-J.; Benic, G.I.; Gallucci, G.O.; Chronopoulos, V. Implant and Prosthodontic Survival Rates with Implant Fixed Complete Dental Prostheses in the Edentulous Mandible after at Least 5 Years: A Systematic Review. Clin. Implant. Dent. Relat. Res. 2013, 16, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Åstrand, P.; Ahlqvist, J.; Gunne, J.; Nilson, H. Implant Treatment of Patients with Edentulous Jaws: A 20-Year Follow-Up. Clin. Implant. Dent. Relat. Res. 2008, 10, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-T.; Cho, S.A. Biomechanical evaluation of laser-etched Ti implant surfaces vs. chemically modified SLA Ti implant surfaces: Removal torque and resonance frequency analysis in rabbit tibias. J. Mech. Behav. Biomed. Mater. 2016, 61, 299–307. [Google Scholar] [CrossRef] [PubMed]
- El-Askary, A.S.; Meffert, R.M.; Griffin, T. Why do dental implants fail? Part I. Implant. Dent. 1999, 8, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Battula, S.; Lee, J.; Wen, H.; Papanicolaou, S.; Collins, M.; Romanos, G. Evaluation of Different Implant Designs in a Ligature-Induced Peri-implantitis Model: A Canine Study. Int. J. Oral Maxillofac. Implant. 2015, 30, 534–545. [Google Scholar] [CrossRef]
- Cameron, H.U.; Pilliar, R.M.; Macnab, I. The effect of movement on the bonding of porous metal to bone. J. Biomed. Mater. Res. 1973, 7, 301–311. [Google Scholar] [CrossRef]
- Steigenga, J.T.; Al-Shammari, K.F.; Nociti, F.H.; Misch, C.E.; Wang, H.-L. Dental implant design and its relationship to long-term implant success. Implant. Dent. 2003, 12, 306–317. [Google Scholar] [CrossRef]
- Junker, R.; Dimakis, A.; Thoneick, M.; Jansen, J.A. Effects of implant surfasse coatings and composition on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 185–206. [Google Scholar] [CrossRef]
- Pilliar, R.M. Overview of surface variability of metallic endosseous dental implants: Textured and porous surface-structured designs. Implant. Dent. 1998, 7, 305–314. [Google Scholar] [CrossRef]
- Castilho, G.A.A.; Martins, M.; Macedo, W.A.A. Surface characterization of titanium Based dental implants. Braz. J. Phys. 2006, 36, 1004–1008. [Google Scholar] [CrossRef]
- Javed, F.; Romanos, G.E. The role of primary stability for successful immediate loading of dental implants. A literature review. J. Dent. 2010, 38, 612–620. [Google Scholar] [CrossRef]
- Salerno, M.; Itri, A.; Frezzato, M.; Rebaudi, A. Surface Microstructure of Dental Implants Before and After Insertion. Implant. Dent. 2015, 24, 1–255. [Google Scholar] [CrossRef]
- Chiapasco, M.; Gatti, C.; Rossi, E.; Haefliger, W.; Markwalder, T.H. Implant-retained mandibular overdentures with immediate loading. A retrospective multicenter study on 226 consecutive cases. Clin. Oral Implant. Res. 1997, 8, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Chong, L.; Khocht, A.; Suzuki, J.B.; Gaughan, J. Effect of Implant Design on Initial Stability of Tapered Implants. J. Oral Implant. 2009, 35, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Albrektsson, T.; Andersson, B.; Krol, J.J. A histomorghometric study of screw-shaped and removal torque titanium implants with three different surface topographies. Clin. Oral Implant. Res. 1995, 6, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Negri, B.; Calvo-Guirado, J.L.; Maté Sánchez de Val, J.E.; Delgado Ruiz, R.A.; Ramirez Fernandez, M.P.; Gomez Moreno, G.; Aguilar Salvatierra, A.; Guardia, J.; Munoz Guzon, F. Biomechanical and Bone Histomorphological Evaluation of Two Surfaces on Tapered and Cylindrical Root Form Implants: An Experimental Study in Dogs. Clin. Implant. Dent. Relat. Res. 2012, 15, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Guirado, J.L.; Gomez Moreno, G.; Aguilar-Salvatierra, A.; Mate Sanchez de Val, J.E.; Abboud, M.; Nemcovsky, C.E. Bone remodeling at implants with different configurations and placed immediately at different depth into extraction sockets. Experimental study in dogs. Clin. Oral Implant. Res. 2014, 26, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Martínez, C.P.-A.; Piattelli, A.; Shibli, J.A.; Markovic, A.; Guirado, J.L.C. The influence of three different apical implant designs at stability and osseointegration process: Experimental study in rabbits. Clin. Oral Implant. Res. 2016, 28, 355–361. [Google Scholar] [CrossRef]
- Calvo-Guirado, J.L.; Torres, J.A.L.; Dard, M.; Javed, F.; Martínez, C.P.-A.; De Val, J.E.M.S. Evaluation of extrashort 4-mm implants in mandibular edentulous patients with reduced bone height in comparison with standard implants: A 12-month results. Clin. Oral Implant. Res. 2015, 27, 867–874. [Google Scholar] [CrossRef]
- Pinotti, F.E.; De Oliveira, G.J.P.L.; Aroni, M.A.T.; Marcantonio, R.A.C.; Marcantonio, E. Analysis of osseointegration of implants with hydrophilic surfaces in grafted areas: A Preclinical study. Clin. Oral Implant. Res. 2018, 29, 963–972. [Google Scholar] [CrossRef]
- Faeda, R.S.; Spin-Neto, R.; Marcantonio, E.; Guastaldi, A.C. Laser ablation in titanium implants followed by biomimetic hydroxyapatite coating: Histomorphometric study in rabbits. Microsc. Res. Tech. 2012, 75, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Akkocaoglu, M.; Uysal, S.; Tekdemir, I.; Akca, K.; Cehreli, M.C. Implant design and intraosseous stability of immediately placed implants: A human cadaver study. Clin. Oral Implant. Res. 2005, 16, 202–209. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, G.J.P.L.; Barros-Filho, L.A.B.; Queiroz, T.; Marcantonio, É.; Barros, L.A.B. In Vitro Evaluation of the Primary Stability of Short and Conventional Implants. J. Oral Implant. 2016, 42, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Sakoh, J.; Wahlmann, U.; Stender, E.; Nat, R.; Al-Nawas, B.; Wagner, W. Primary stability of a conical implant and a hybrid, cylindric screw-type implant in vitro. Int. J. Oral Maxillofac. Implant. 2006, 21, 560–566. [Google Scholar]
- Turkyilmaz, I.; Sennerby, L.; McGlumphy, E.A.; Tozum, T. Biomechanical Aspects of Primary Implant Stability: A Human Cadaver Study. Clin. Implant. Dent. Relat. Res. 2009, 11, 113–119. [Google Scholar] [CrossRef]
- Marquezan, M.; Osório, A.; Sant’Anna, E.; Souza, M.M.; Maia, L.C. Does bone mineral density influence the primary stability of dental implants? A systematic review. Clin. Oral Implant. Res. 2011, 23, 767–774. [Google Scholar] [CrossRef]
- Markovic, A.; Mišić, T.; Milicic, B.; Calvo-Guirado, J.L.; Aleksić, Z.; Đinić, A. Heat generation during implant placement in low-density bone:effect of surgical technique, insertion torque and implant macro design. Clin. Oral Implant. Res. 2012, 24, 798–805. [Google Scholar] [CrossRef]
- Duyck, J.; Roesems, R.; Cardoso, M.V.; Ogawa, T.; Camargos, G.D.V.; Vandamme, K. Effect of insertion torque on titanium implant osseointegration: An animal experimental study. Clin. Oral Implant. Res. 2013, 26, 191–196. [Google Scholar] [CrossRef]
- Lee, J.; Frias, V.; Lee, K.; Wright, R.F. Effect of implant size and shape on implant success rates: A literature review. J. Prosthet. Dent. 2005, 94, 377–381. [Google Scholar] [CrossRef]
- Montes, C.C.; Pereira, F.A.; Thomé, G.; Alves, E.D.M.; Acedo, R.V.; De Souza, J.R.; Melo, A.C.M.; Trevilatto, P. Failing Factors Associated with Osseointegrated Dental Implant Loss. Implant. Dent. 2007, 16, 404–412. [Google Scholar] [CrossRef]
- Gill, A.; Rao, P. Primary stability: The password of implant integration. J. Dent. Implant. 2012, 2, 103. [Google Scholar] [CrossRef]
- Bataineh, A.; Al-Dakes, A.M. The influence of length of implant on primary stability: An in vitro study using resonance frequency analysis. J. Clin. Exp. Dent. 2017, 9, e1–e6. [Google Scholar] [CrossRef] [PubMed]
| - | Insertion Torque | Removal Torque | ||
|---|---|---|---|---|
| Implant type/Period | - | 2 weeks | 4 weeks | 8 weeks |
| CI | 27.99 ± 7.80 * | 38.01 ± 17.09 c | 68.01 ± 8.46 b | 91.05 ± 9.32 **,a |
| HCI | 32.93 ± 10.61 * | 41.69 ± 5.98 b | 53.88 ± 18.50 a,b | 68.62 ± 13.70 **,a |
| Implant Type/Period | 2 Weeks | 4 Weeks | 8 Weeks |
|---|---|---|---|
| CI | 29.68 ± 6.77 b | 46.06 ± 5.68 a | 49.95 ± 7.36 a |
| HCI | 29.58 ± 5.78 b | 45.80 ± 7.78 a | 52.19 ± 10.77 a |
| Implant Type/Period | 2 Weeks | 4 Weeks | 8 Weeks |
|---|---|---|---|
| CI | 69.64 ± 13.22 | 62.21 ± 11.19 | 59.72 ± 11.29 * |
| HCI | 68.62 ± 11.97 | 63.49 ± 16.77 | 79.08 ± 11.31 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Carvalho Silva Leocádio, A.; Silva Júnior, M.; José Pimentel Lopes de Oliveira, G.; da Col Santos Pinto, G.; Silveira Faeda, R.; Marques Padovan, L.E.; Marcantonio Júnior, É. Evaluation of Implants with Different Macrostructures in Type I Bone—Pre-Clinical Study in Rabbits. Materials 2020, 13, 1521. https://doi.org/10.3390/ma13071521
de Carvalho Silva Leocádio A, Silva Júnior M, José Pimentel Lopes de Oliveira G, da Col Santos Pinto G, Silveira Faeda R, Marques Padovan LE, Marcantonio Júnior É. Evaluation of Implants with Different Macrostructures in Type I Bone—Pre-Clinical Study in Rabbits. Materials. 2020; 13(7):1521. https://doi.org/10.3390/ma13071521
Chicago/Turabian Stylede Carvalho Silva Leocádio, Amanda, Matusalém Silva Júnior, Guilherme José Pimentel Lopes de Oliveira, Gustavo da Col Santos Pinto, Rafael Silveira Faeda, Luis Eduardo Marques Padovan, and Élcio Marcantonio Júnior. 2020. "Evaluation of Implants with Different Macrostructures in Type I Bone—Pre-Clinical Study in Rabbits" Materials 13, no. 7: 1521. https://doi.org/10.3390/ma13071521
APA Stylede Carvalho Silva Leocádio, A., Silva Júnior, M., José Pimentel Lopes de Oliveira, G., da Col Santos Pinto, G., Silveira Faeda, R., Marques Padovan, L. E., & Marcantonio Júnior, É. (2020). Evaluation of Implants with Different Macrostructures in Type I Bone—Pre-Clinical Study in Rabbits. Materials, 13(7), 1521. https://doi.org/10.3390/ma13071521





