Active Transiency: A Novel Approach to Expedite Degradation in Transient Electronics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
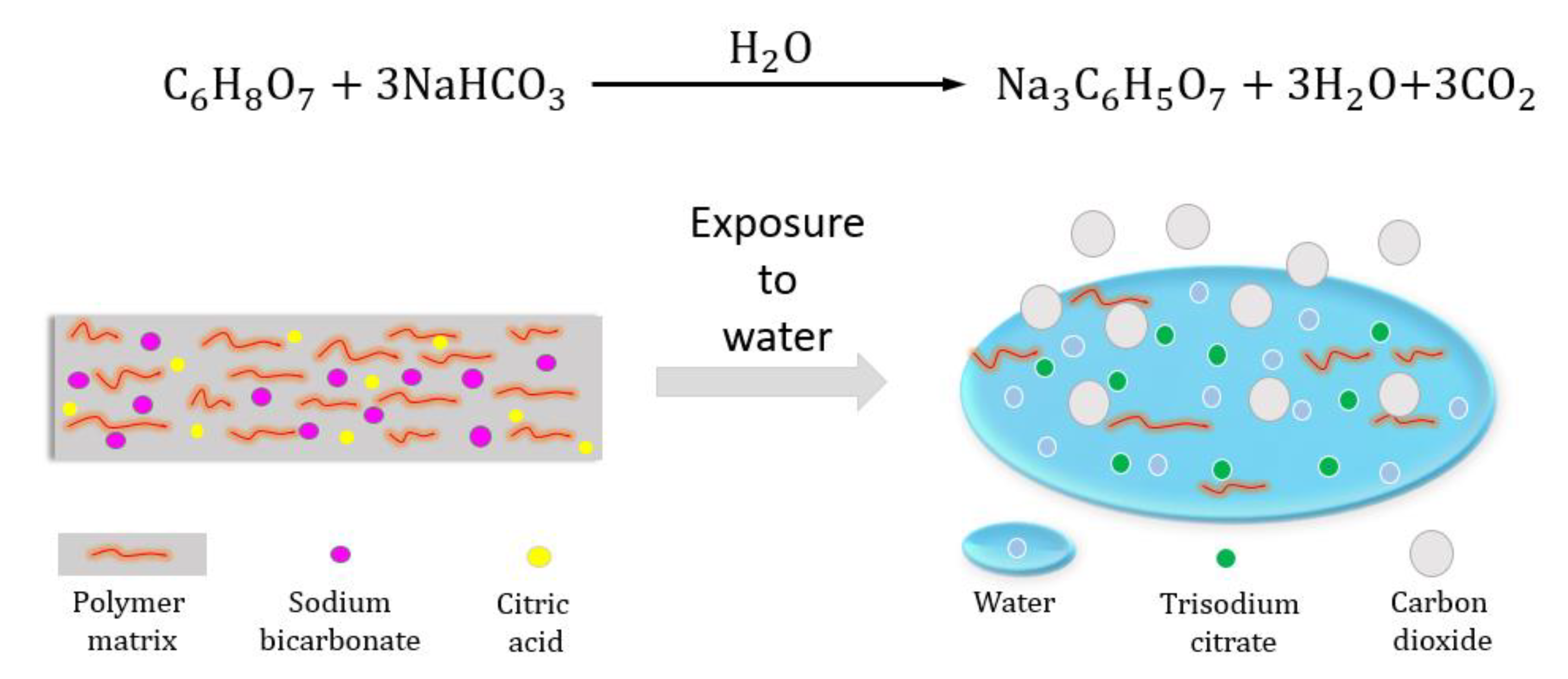
2.2. Preparation of Polymer Films
2.3. Electrically Conductive Patterns
2.4. Transiency
2.4.1. Substrates
2.4.2. Devices
2.5. Mechanical Characterizations
2.6. Infrared Spectroscopy
2.7. Electrical Characterization
3. Results and Discussion
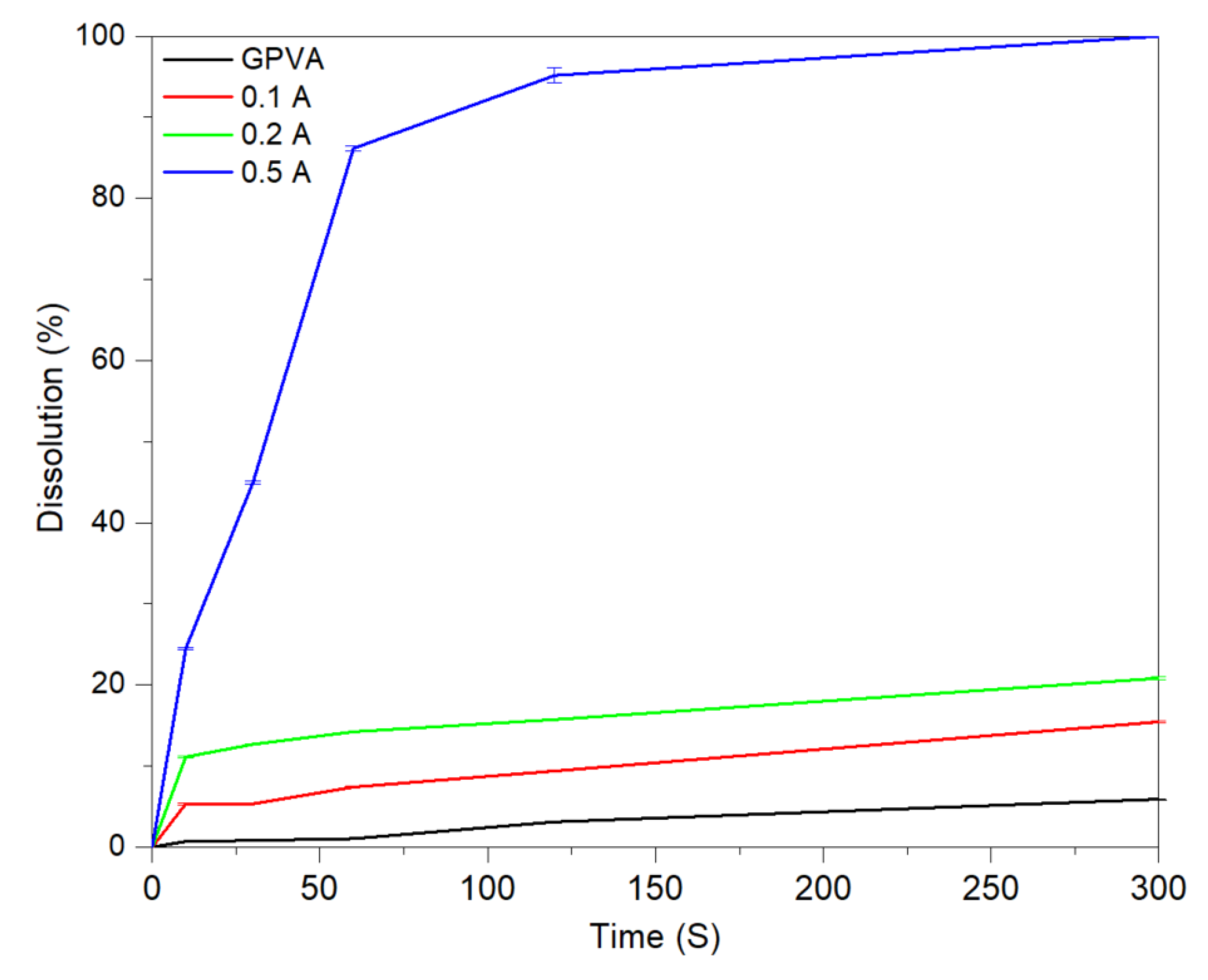
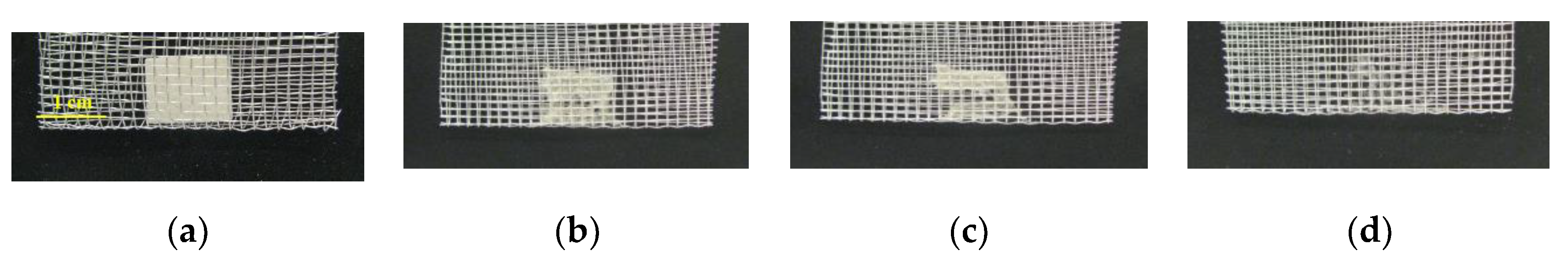
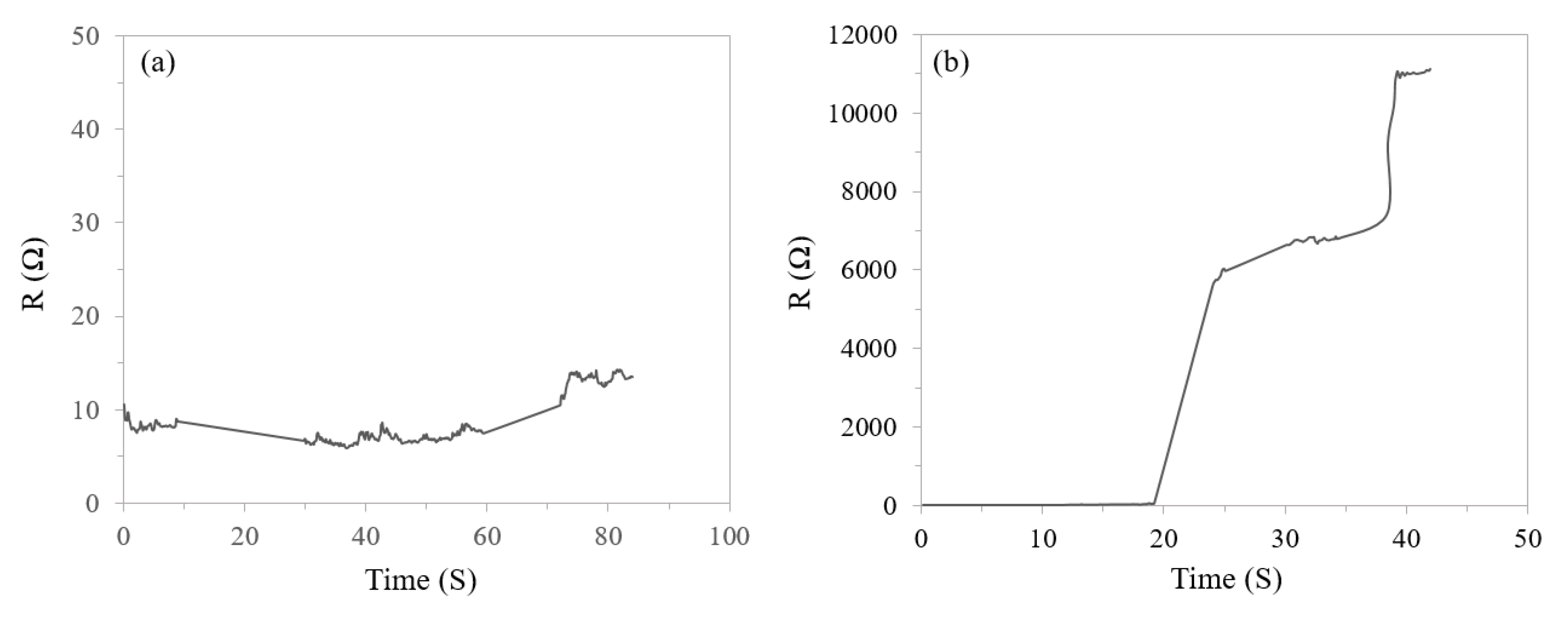
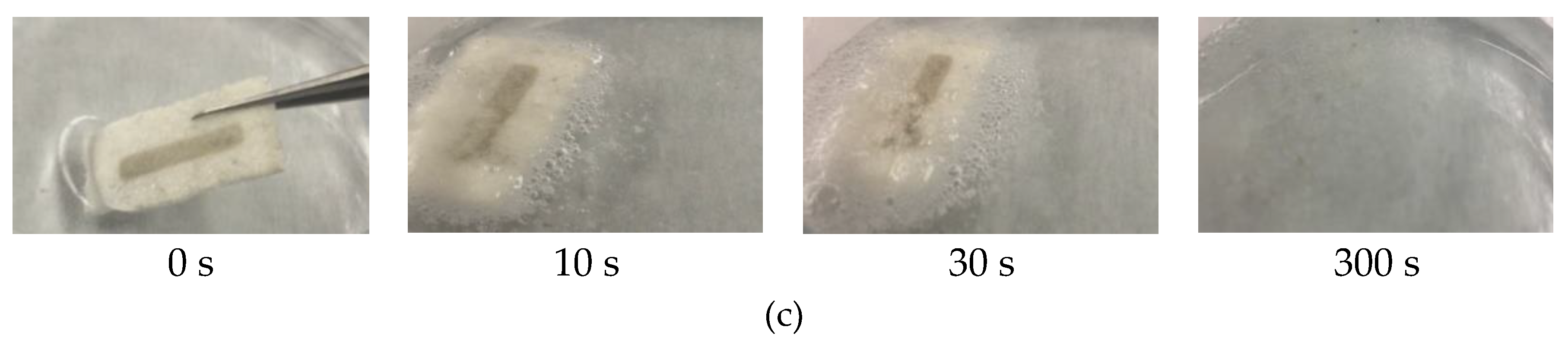
3.1. Transiency
3.1.1. Substrates
3.1.2. Devices
3.2. Mechanical Characterizations
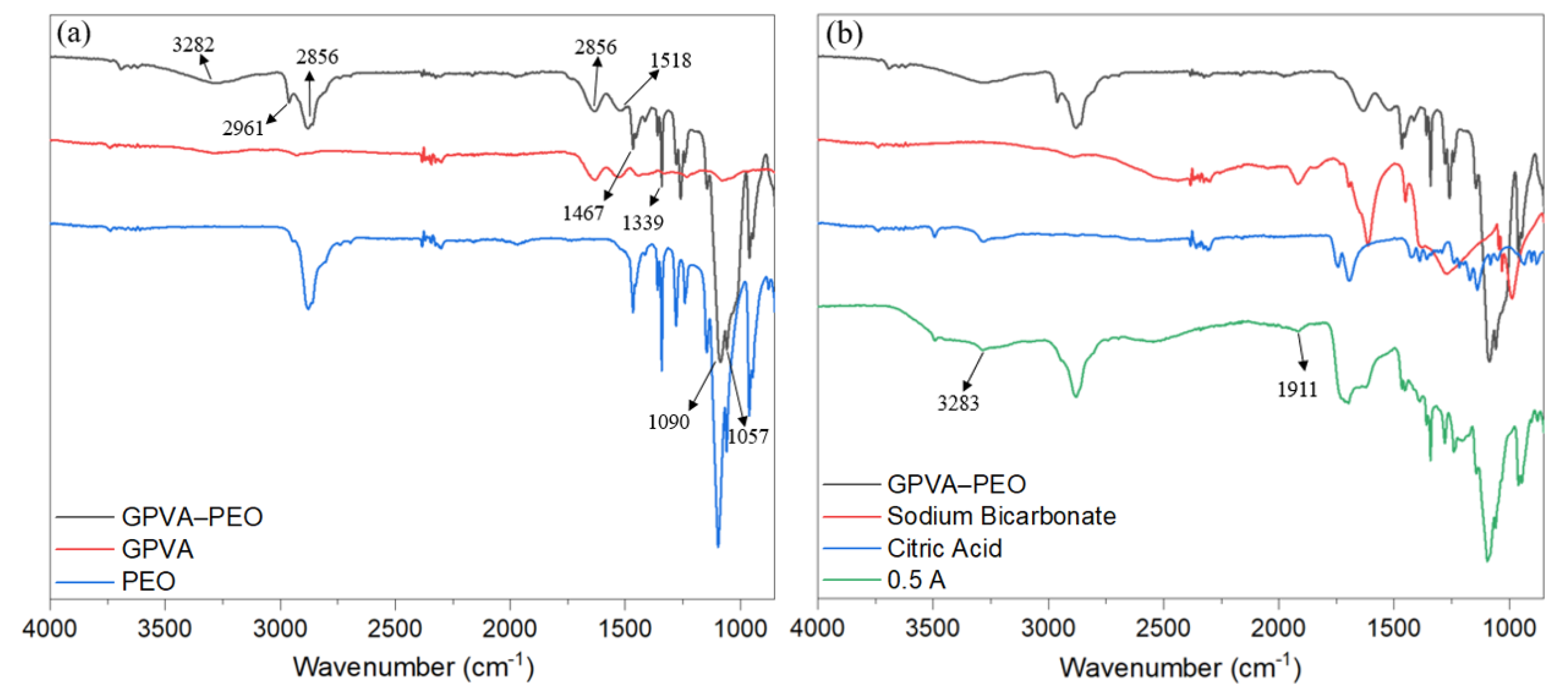
3.3. Chemical Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hwang, S.W.; Kim, D.H.; Tao, H.; Kim, T.i.; Kim, S.; Yu, K.J.; Panilaitis, B.; Jeong, J.W.; Song, J.K.; Omenetto, F.G. Materials and Fabrication Processes for Transient and Bioresorbable High-Performance Electronics. Adv. Funct. Mater. 2013, 23, 4087–4093. [Google Scholar] [CrossRef]
- Bettinger, C.J.; Bao, Z. Organic thin-film transistors fabricated on resorbable biomaterial substrates. Adv. Mater. 2010, 22, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Irimia-Vladu, M.; Troshin, P.A.; Reisinger, M.; Shmygleva, L.; Kanbur, Y.; Schwabegger, G.; Bodea, M.; Schwödiauer, R.; Mumyatov, A.; Fergus, J.W. Biocompatible and Biodegradable Materials for Organic Field-Effect Transistors. Adv. Funct. Mater. 2010, 20, 4069–4076. [Google Scholar] [CrossRef]
- Hwang, S.-W.; Lee, C.H.; Cheng, H.; Jeong, J.-W.; Kang, S.-K.; Kim, J.-H.; Shin, J.; Yang, J.; Liu, Z.; Ameer, G.A. Biodegradable elastomers and silicon nanomembranes/nanoribbons for stretchable, transient electronics, and biosensors. Nano Lett. 2015, 15, 2801–2808. [Google Scholar] [CrossRef]
- Fu, K.K.; Wang, Z.; Dai, J.; Carter, M.; Hu, L. Transient electronics: Materials and devices. Chem. Mater. 2016, 28, 3527–3539. [Google Scholar] [CrossRef]
- Dagdeviren, C.; Hwang, S.W.; Su, Y.; Kim, S.; Cheng, H.; Gur, O.; Haney, R.; Omenetto, F.G.; Huang, Y.; Rogers, J.A. Transient, biocompatible electronics and energy harvesters based on ZnO. Small 2013, 9, 3398–3404. [Google Scholar] [CrossRef]
- Yin, L.; Huang, X.; Xu, H.; Zhang, Y.; Lam, J.; Cheng, J.; Rogers, J.A. Materials, designs, and operational characteristics for fully biodegradable primary batteries. Adv. Mater. 2014, 26, 3879–3884. [Google Scholar] [CrossRef]
- Chen, Y.; Jamshidi, R.; White, K.; Çınar, S.; Gallegos, E.; Hashemi, N.; Montazami, R. Physical–chemical hybrid transiency: A fully transient li-ion battery based on insoluble active materials. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 2021–2027. [Google Scholar] [CrossRef]
- Hwang, S.W.; Huang, X.; Seo, J.H.; Song, J.K.; Kim, S.; Hage-Ali, S.; Chung, H.J.; Tao, H.; Omenetto, F.G.; Ma, Z. Materials for bioresorbable radio frequency electronics. Adv. Mater. 2013, 25, 3526–3531. [Google Scholar] [CrossRef]
- Jamshidi, R.; Cinar, S.; Chen, Y.; Hashemi, N.; Montazami, R. Transient bioelectronics: Electronic properties of silver microparticle-based circuits on polymeric substrates subjected to mechanical load. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 1603–1610. [Google Scholar] [CrossRef]
- Kim, D.-H.; Viventi, J.; Amsden, J.J.; Xiao, J.; Vigeland, L.; Kim, Y.-S.; Blanco, J.A.; Panilaitis, B.; Frechette, E.S.; Contreras, D. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 2010, 9, 511. [Google Scholar] [CrossRef]
- Hwang, S.-W.; Tao, H.; Kim, D.-H.; Cheng, H.; Song, J.-K.; Rill, E.; Brenckle, M.A.; Panilaitis, B.; Won, S.M.; Kim, Y.-S. A physically transient form of silicon electronics. Science 2012, 337, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Acar, H.; Banerjee, S.; Shi, H.; Jamshidi, R.; Hashemi, N.; Cho, M.W.; Montazami, R. Transient Biocompatible Polymeric Platforms for Long-Term Controlled Release of Therapeutic Proteins and Vaccines. Materials 2016, 9, 321. [Google Scholar] [CrossRef] [PubMed]
- Acar, H.; Çınar, S.; Thunga, M.; Kessler, M.R.; Hashemi, N.; Montazami, R. Study of Physically Transient Insulating Materials as a Potential Platform for Transient Electronics and Bioelectronics. Adv. Funct. Mater. 2014, 24, 4135–4143. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Hwang, S.W.; Kang, S.K.; Patnaik, D.; Cortes, J.F.; Rogers, J.A. Biodegradable Materials for Multilayer Transient Printed Circuit Boards. Adv. Mater. 2014, 26, 7371–7377. [Google Scholar] [CrossRef] [PubMed]
- Irimia-Vladu, M. “Green” electronics: Biodegradable and biocompatible materials and devices for sustainable future. Chem. Soc. Rev. 2014, 43, 588–610. [Google Scholar] [CrossRef]
- Irimia-Vladu, M.; Głowacki, E.D.; Voss, G.; Bauer, S.; Sariciftci, N.S. Green and biodegradable electronics. Mater. Today 2012, 15, 340–346. [Google Scholar] [CrossRef]
- Brenckle, M.A.; Cheng, H.; Hwang, S.; Tao, H.; Paquette, M.; Kaplan, D.L.; Rogers, J.A.; Huang, Y.; Omenetto, F.G. Modulated degradation of transient electronic devices through multilayer silk fibroin pockets. ACS Appl. Mater. Interfaces 2015, 7, 19870–19875. [Google Scholar] [CrossRef]
- Park, C.W.; Kang, S.K.; Hernandez, H.L.; Kaitz, J.A.; Wie, D.S.; Shin, J.; Lee, O.P.; Sottos, N.R.; Moore, J.S.; Rogers, J.A. Thermally Triggered Degradation of Transient Electronic Devices. Adv. Mater. 2015, 27, 3783–3788. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, S.; Cheng, H.; Peng, R.; Luo, Z. Thermally Triggered Vanishing Bulk Polyoxymethylene for Transient Electronics. Sci. Rep. 2019, 9, 18107. [Google Scholar] [CrossRef]
- Hernandez, H.L.; Kang, S.K.; Lee, O.P.; Hwang, S.W.; Kaitz, J.A.; Inci, B.; Park, C.W.; Chung, S.; Sottos, N.R.; Moore, J.S. Triggered transience of metastable poly (phthalaldehyde) for transient electronics. Adv. Mater. 2014, 26, 7637–7642. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.K.; Farghaly, A.A.; Wang, C.; Collinson, M.M.; Kundu, S.C.; Yadavalli, V.K. Conducting polymer-silk biocomposites for flexible and biodegradable electrochemical sensors. Biosens. Bioelectron. 2016, 81, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Cheng, H.; Mao, S.; Haasch, R.; Liu, Y.; Xie, X.; Hwang, S.W.; Jain, H.; Kang, S.K.; Su, Y. Dissolvable Metals for Transient Electronics. Adv. Funct. Mater. 2014, 24, 645–658. [Google Scholar] [CrossRef]
- Hwang, S.W.; Song, J.K.; Huang, X.; Cheng, H.; Kang, S.K.; Kim, B.H.; Kim, J.H.; Yu, S.; Huang, Y.; Rogers, J.A. High-Performance Biodegradable/Transient Electronics on Biodegradable Polymers. Adv. Mater. 2014, 26, 3905–3911. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.K.; Hwang, S.W.; Yu, S.; Seo, J.H.; Corbin, E.A.; Shin, J.; Wie, D.S.; Bashir, R.; Ma, Z.; Rogers, J.A. Biodegradable thin metal foils and spin-on glass materials for transient electronics. Adv. Funct. Mater. 2015, 25, 1789–1797. [Google Scholar] [CrossRef]
- Jung, Y.H.; Chang, T.-H.; Zhang, H.; Yao, C.; Zheng, Q.; Yang, V.W.; Mi, H.; Kim, M.; Cho, S.J.; Park, D.-W. High-performance green flexible electronics based on biodegradable cellulose nanofibril paper. Nat. Commun. 2015, 6, 7170. [Google Scholar] [CrossRef]
- Kang, S.-K.; Murphy, R.K.; Hwang, S.-W.; Lee, S.M.; Harburg, D.V.; Krueger, N.A.; Shin, J.; Gamble, P.; Cheng, H.; Yu, S. Bioresorbable silicon electronic sensors for the brain. Nature 2016, 530, 71–76. [Google Scholar] [CrossRef]
- Jin, S.H.; Kang, S.-K.; Cho, I.-T.; Han, S.Y.; Chung, H.U.; Lee, D.J.; Shin, J.; Baek, G.W.; Kim, T.-i.; Lee, J.-H. Water-soluble thin film transistors and circuits based on amorphous indium–gallium–zinc oxide. ACS Appl. Mater. Interfaces 2015, 7, 8268–8274. [Google Scholar] [CrossRef]
- Kim, Y.J.; Chun, S.-E.; Whitacre, J.; Bettinger, C.J. Self-deployable current sources fabricated from edible materials. J. Mater. Chem. B 2013, 1, 3781–3788. [Google Scholar] [CrossRef]
- Hosseini, N.R.; Lee, J.S. Biocompatible and Flexible Chitosan-Based Resistive Switching Memory with Magnesium Electrodes. Adv. Funct. Mater. 2015, 25, 5586–5592. [Google Scholar] [CrossRef]
- Hwang, S.W.; Park, G.; Cheng, H.; Song, J.K.; Kang, S.K.; Yin, L.; Kim, J.H.; Omenetto, F.G.; Huang, Y.; Lee, K.M. 25th anniversary article: Materials for high-performance biodegradable semiconductor devices. Adv. Mater. 2014, 26, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.K.; Hwang, S.W.; Cheng, H.; Yu, S.; Kim, B.H.; Kim, J.H.; Huang, Y.; Rogers, J.A. Dissolution behaviors and applications of silicon oxides and nitrides in transient electronics. Adv. Funct. Mater. 2014, 24, 4427–4434. [Google Scholar] [CrossRef]
- Andrade, G.I.; Barbosa-Stancioli, E.F.; Mansur, A.A.P.; Vasconcelos, W.L.; Mansur, H.S. Small-angle X-ray scattering and FTIR characterization of nanostructured poly (vinyl alcohol)/silicate hybrids for immunoassay applications. J. Mater. Sci. 2008, 43, 450–463. [Google Scholar] [CrossRef]
- Acar, H.; Garifullin, R.; Guler, M.O. Self-assembled template-directed synthesis of one-dimensional silica and titania nanostructures. Langmuir 2011, 27, 1079–1084. [Google Scholar] [CrossRef]
- Pawde, S.M.; Kalim, D. Characterization of polyvinyl alcohol/gelatin blend hydrogel films for biomedical applications. J. Appl. Polym. Sci. 2008, 109, 3431–3437. [Google Scholar] [CrossRef]


| Notation | GPVA (wt%) | PEO (wt%) | Sodium Bicarbonate (wt%) | Citric Acid (wt%) |
|---|---|---|---|---|
| GPVA-PEO | 25 | 25 | 0 | 0 |
| 0.1 A | 25 | 25 | 2.5 | 2.5 |
| 0.2 A | 25 | 25 | 5 | 5 |
| 0.5 A | 25 | 25 | 12.5 | 12.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamshidi, R.; Chen, Y.; Montazami, R. Active Transiency: A Novel Approach to Expedite Degradation in Transient Electronics. Materials 2020, 13, 1514. https://doi.org/10.3390/ma13071514
Jamshidi R, Chen Y, Montazami R. Active Transiency: A Novel Approach to Expedite Degradation in Transient Electronics. Materials. 2020; 13(7):1514. https://doi.org/10.3390/ma13071514
Chicago/Turabian StyleJamshidi, Reihaneh, Yuanfen Chen, and Reza Montazami. 2020. "Active Transiency: A Novel Approach to Expedite Degradation in Transient Electronics" Materials 13, no. 7: 1514. https://doi.org/10.3390/ma13071514
APA StyleJamshidi, R., Chen, Y., & Montazami, R. (2020). Active Transiency: A Novel Approach to Expedite Degradation in Transient Electronics. Materials, 13(7), 1514. https://doi.org/10.3390/ma13071514






