Effect of Organics on Heavy Metal-Contaminated River Sediment Treated with Electro-Osmosis and Solidification/Stabilization Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Properties
2.2. Testing Methods
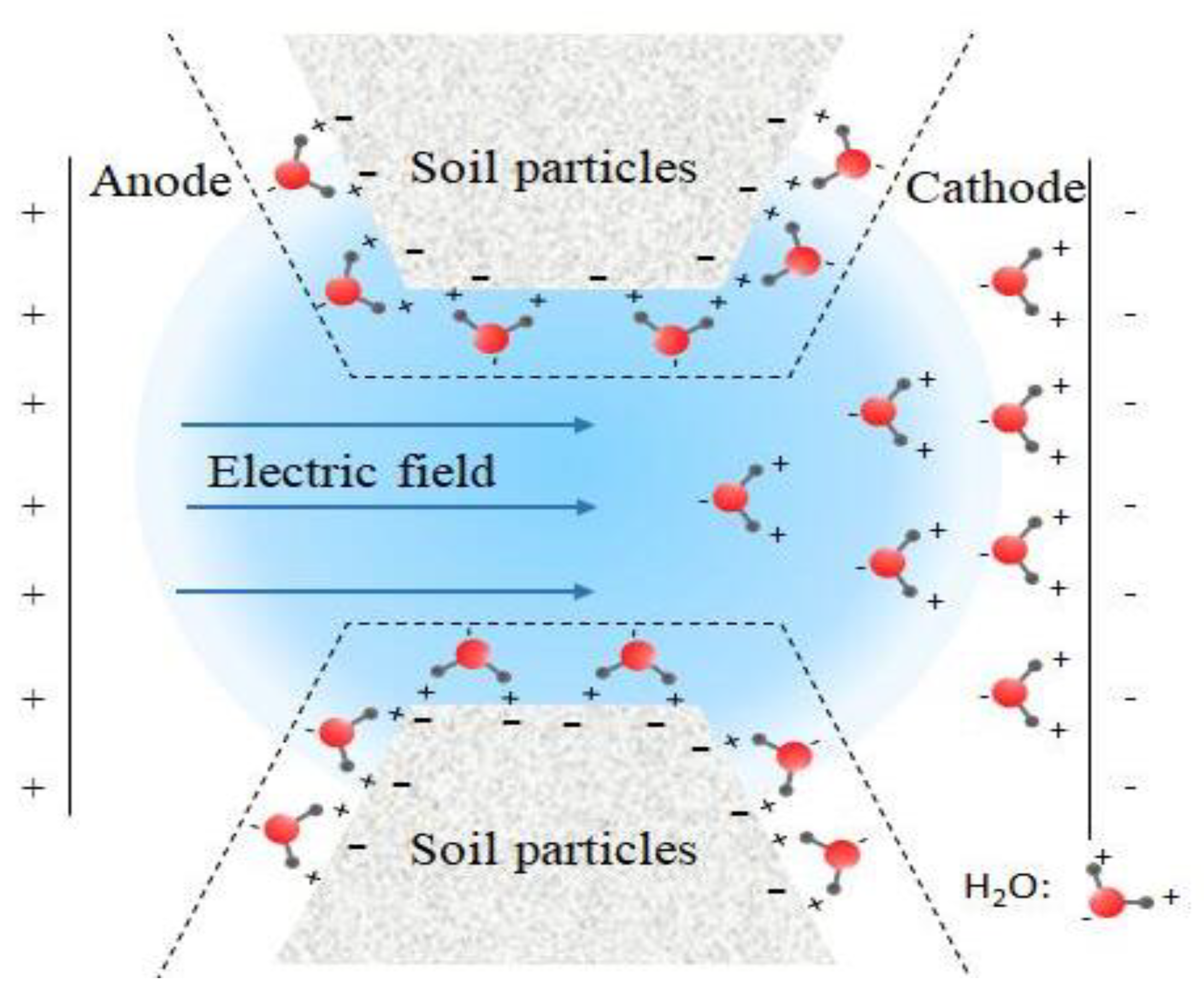
2.2.1. Electro-Osmosis Experiment
2.2.2. Unconfined Compressive Strength (UCS) Experiment
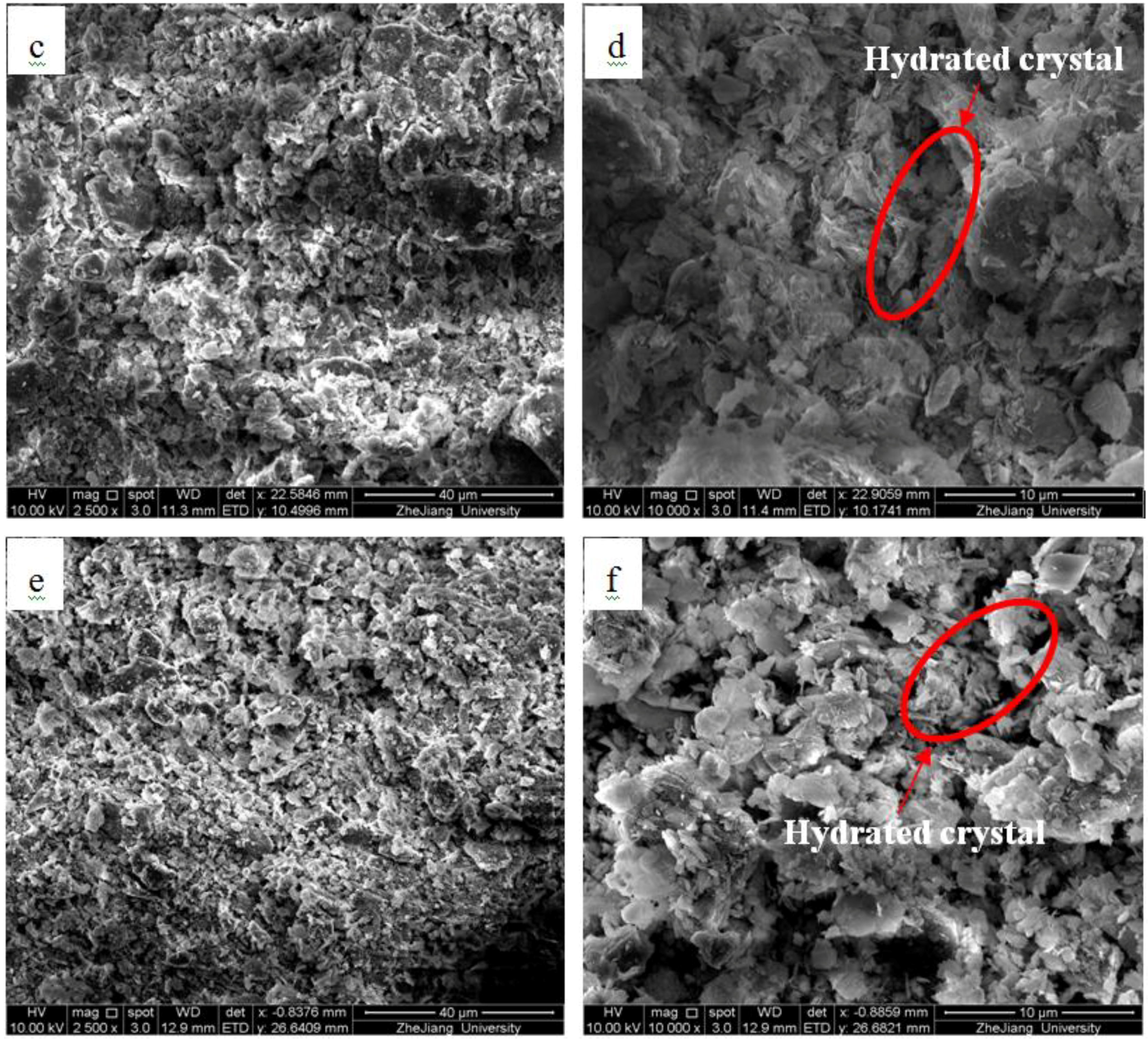
2.2.3. Microscopic Analyzes
3. Results and Discussion
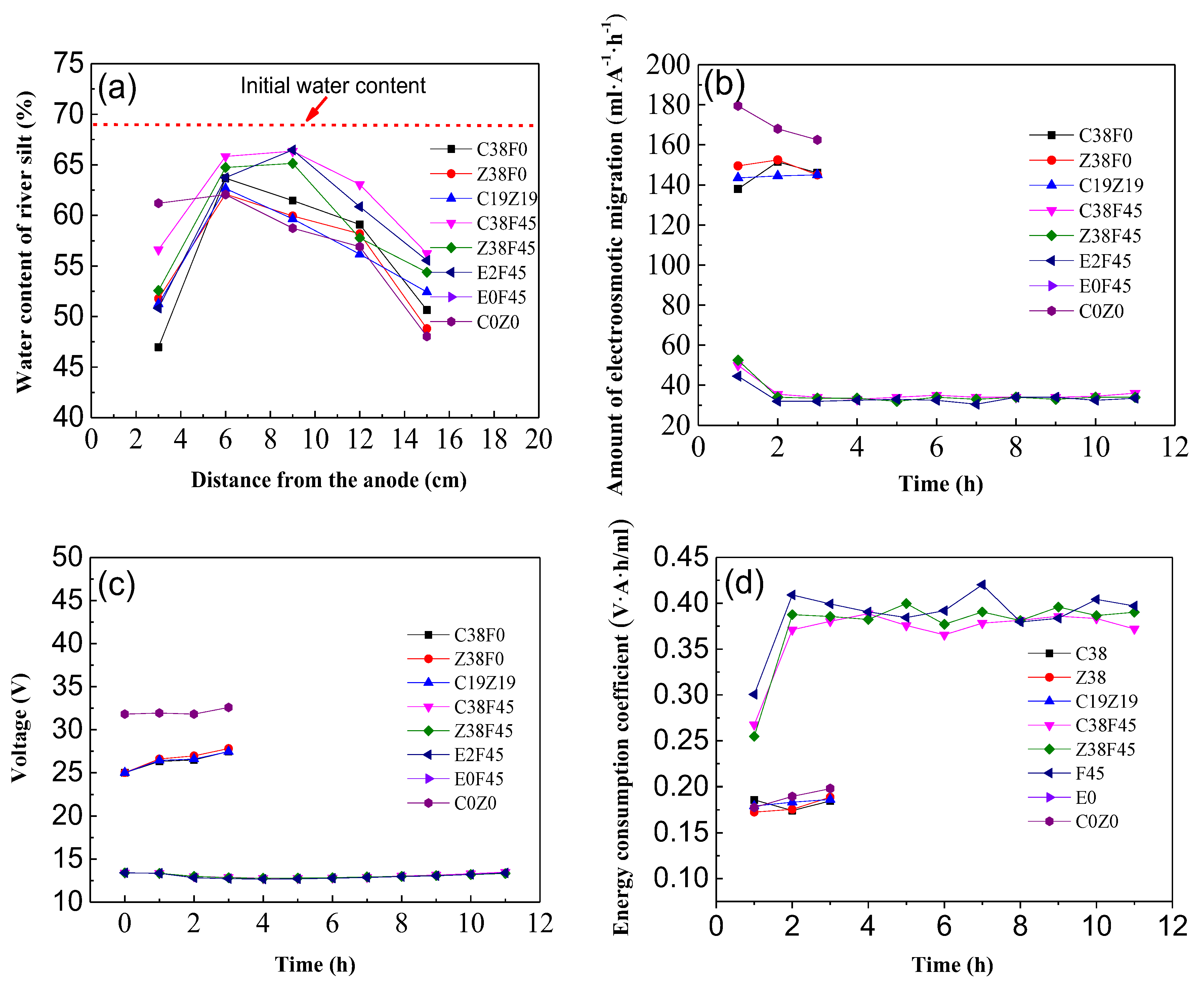
3.1. Effect of Parameters Change of Electro-Osmosis
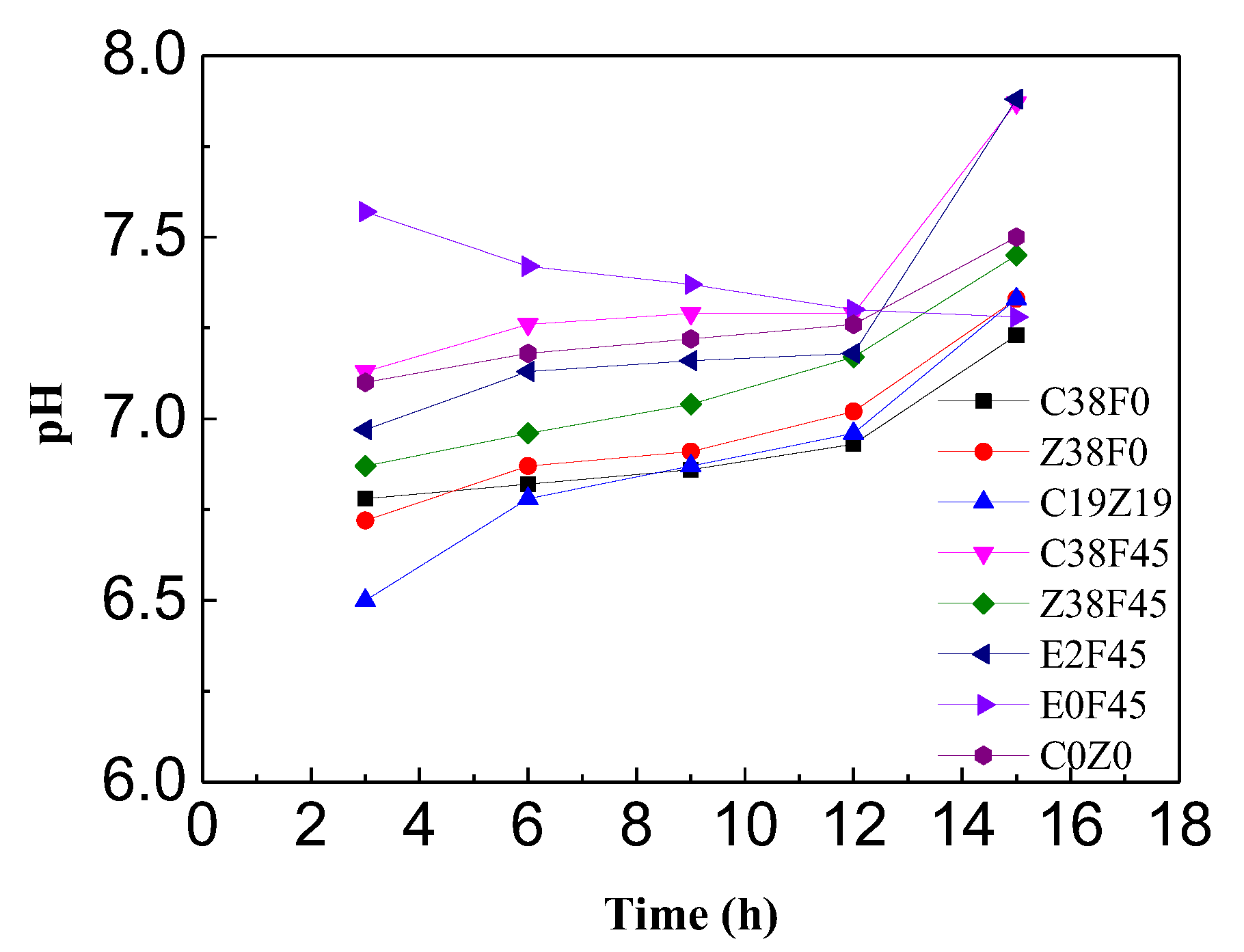
3.2. pH and Water Content of Sediment with Electro-Osmosis Treatment
3.3. Variation of Electro-Osmosis Migration, Voltage and Energy Consumption Coefficient
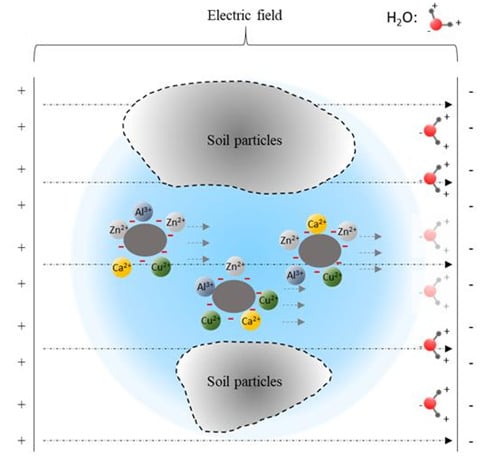
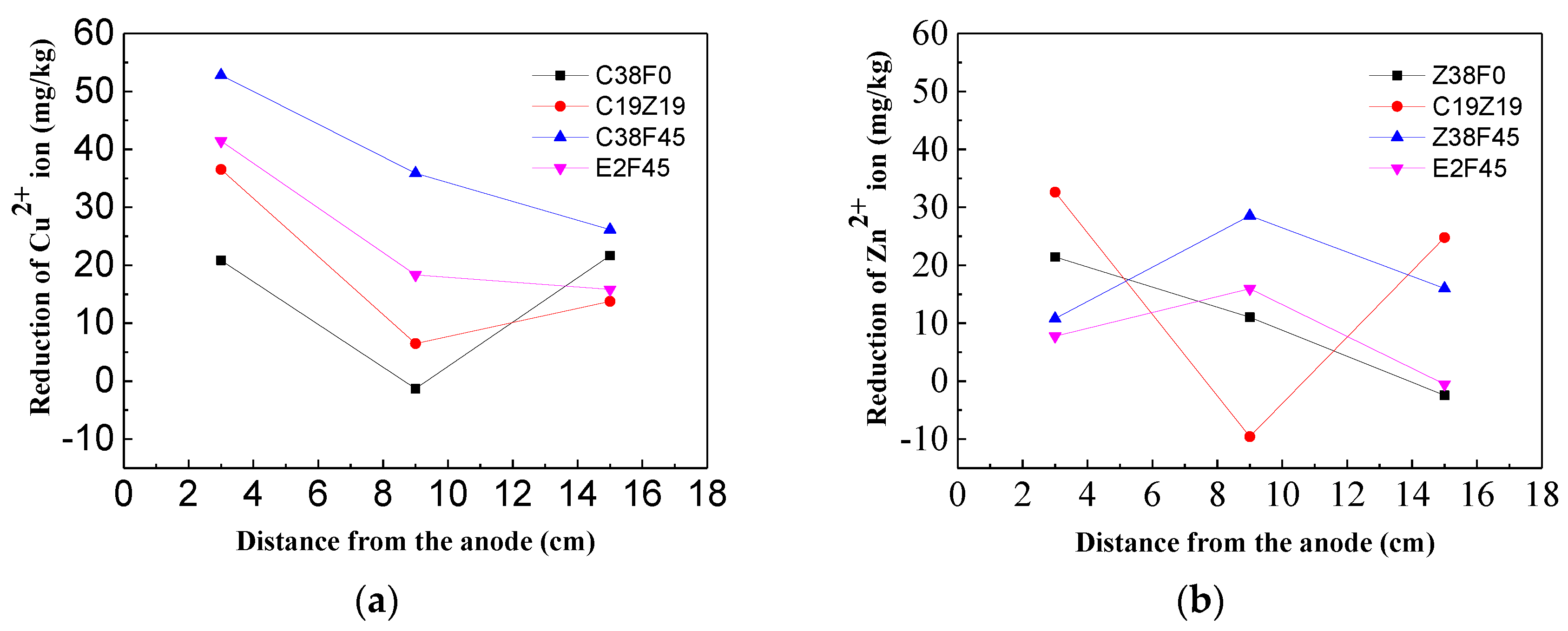
3.4. Effect of Organics on Heavy Metal Ion igration
3.5. Effect of Organics on the Strength of Solidified Sediment
3.6. Effect of Organics and Curing Agents on Solidified Sediment
4. Conclusions
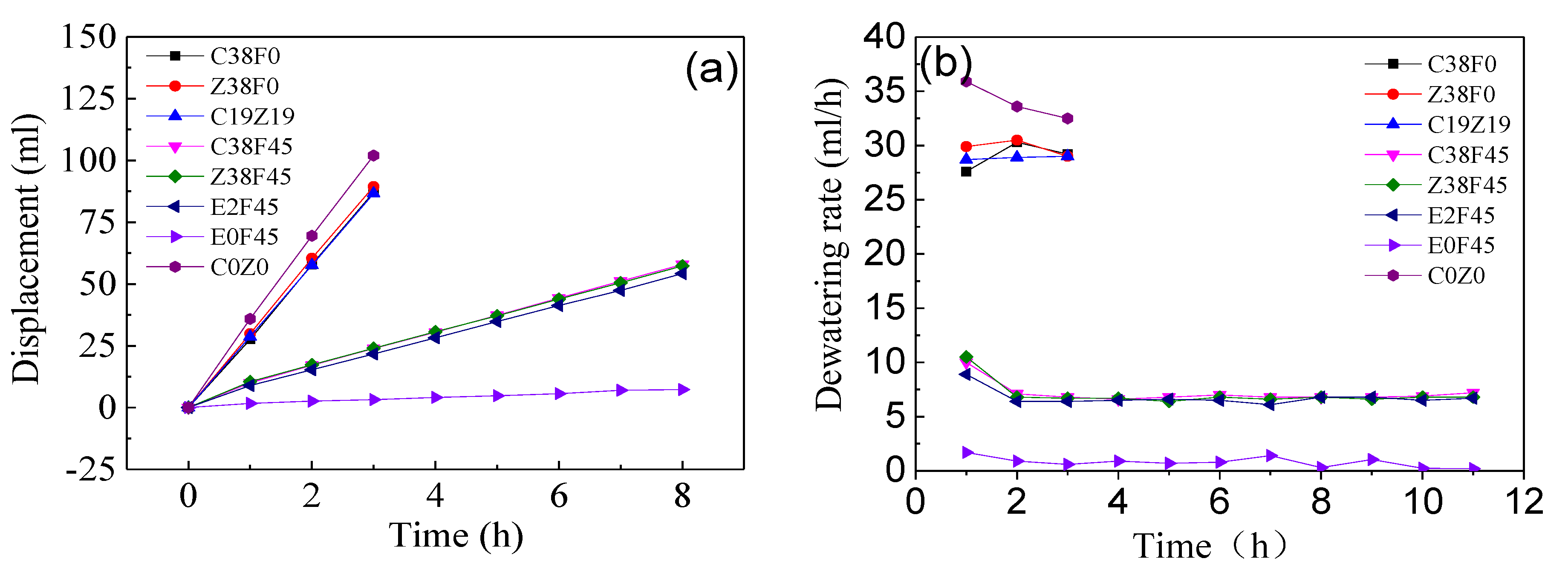
- Fulvic acid can effectively reduce the corrosion of the electrode during electro-osmosis, but the addition of fulvic acid under a constant current results in a decrease in the dewatering rate of electro-osmosis. The energy consumption coefficient of the electroosmotic process in this study is controlled by the equivalent voltage, regardless of the type of electrolyte added. In addition, the experiment revealed that the gravity field still plays an important role in simulating one-dimensional horizontal dewatering electro-osmosis.
- The heavy metals (Zn2+, Cu2+) have less effect on the displacement and dewatering rate of sediment with electro-osmosis treatment, and the fulvic acid in the sediment can enhance the migration ability of Cu2+ and Zn2+ ions through complexation. In addition, the experimental study shows that the average reductions of Cu2+ ions and Zn2+ ions are 28 and 11 mg/kg, respectively, which indicates the migration capacity of Cu2+ ions in sediment is stronger than that of Zn2+ ions.
- The experimental results show that fulvic acid in sediment can reduce the adsorption between Cu2+, Zn2+ ions and soil particles through complexation, enhance the migration ability of Cu2+, Zn2+ ions, and effectively reduce the formation of colloids. Fulvic acid can effectively reduce the corrosion of electrodes during electro-osmosis; meanwhile, the sediment containing a high content of fulvic acid has a stronger plasticity and a weaker electro-osmosis dewatering capacity compared with ordinary sediment. Therefore, electro-osmosis treatment is not recommended for sediment with a high content of fulvic acid.
- The electro-osmosis repair method has a better reinforcement effect on river sediment, but the migration of Cu2+ and Zn2+ ions in the short-time energization process is limited, so it is necessary to combine solidification treatment for heavy metal contained sediment.
- During the solidification process of river sediment, the fulvic acid in organics can be combined with aluminum and calcium produced by cement hydration, delaying the progress of cement hydration, simultaneously decomposing hydration products, hindering the hardening of cement, and thus affecting and destroying the formation of solidified sediment structure and strength.
Author Contributions
Funding
Conflicts of Interest
References
- Dohnalkov, B.; Drochytka, R.; Hodul, J. New possibilities of neutralisation sludge solidification technology. J. Clean. Prod. 2018, 204, 1097–1107. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Cho, D.; Tsang, D.C.W.; Tong, L.; Zhou, Y.; Yang, J.; Hu, Q. Sustainable stabilization/solidification of municipal solid waste incinerator fly ash by incorporation of green materials. J. Clean. Prod. 2019, 222, 335–343. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, D.N. Characterization of sediment for sustainable development: State of theart. Mar. Georesour. Geotechnol. 2015, 33, 447–465. [Google Scholar] [CrossRef]
- Wen, J.; Luo, D.Q.; Luo, X.B.; Fang, Z.F. Ecological risk assessment on heavy metals in the bottom mud of the Qiandao Lake. Res. Soil. Water Conserv. 2006, 13, 11–14. [Google Scholar]
- Gong, X.F.; Chen, C.L.; Zhou, W.B.; Jian, M.F.; Zhang, Z.H. Assessment on heavy metal pollution in the sediment of Poyang Lake. Environ. Sci. 2006, 27, 732–736. [Google Scholar]
- Chen, Q.; Shen, Y.; Fang, Y.; Yan, J.; Li, P.; Zhang, K. Heavy metals pollution risk and characteristics of plant accumulation along Zihu River. TCSAE 2014, 30, 198–205. [Google Scholar]
- Sayadi, M.H.; Sayyed, M.R.; Kumar, S. Short-term accumulative signatures of heavy metals in river bed sediment in the industrial area, Tehran, Iran. Environ. Monit. Assess. 2010, 162, 465–473. [Google Scholar] [CrossRef]
- Paul, D. Research on heavy metal pollution of river Ganga: A review. Ann. Agrar. Sci. 2017, 15, 278–286. [Google Scholar] [CrossRef]
- Ouchir, N.; Aissa, L.B.; Boughdiri, M. Assessment of heavy metal contamination status in sediment and identification of pollution source in Ichkeul Lake and rivers ecosystem, northern Tunisia. Arab. J. Geosci. 2016, 9, 539. [Google Scholar] [CrossRef]
- Son, J.H.; Baek, J.W.; Choi, A.E.S.; Park, H.S. Thiomer solidification of an ASR bottom ash: Optimization based on compressive strength and the characterization of heavy metal leaching. J. Clean. Prod. 2017, 166, 12–20. [Google Scholar] [CrossRef]
- Du, Y.J.; Jin, F.; Liu, S.Y.; Chen, L.; Zhang, F. Review of stabilization/solidification technique for remediation of heavy metals contaminated lands. Rock Soil Mech. 2011, 32, 116–124. [Google Scholar]
- Xu, Y.; Zhang, C.; Zhao, M.; Rong, H.; Zhang, K.; Chen, Q. Comparison of bioleaching and electrokinetic remediation processes for removal of heavy metals from wastewater treatment sludge. Chemosphere 2017, 168, 1152–1157. [Google Scholar] [CrossRef]
- Wang, X.L.; Wang, C.Y.; Wu, X.C. Research progress on the remediation of soils contaminated by heavy metals. Chem. Bioeng. 2019, 36, 1–7. [Google Scholar]
- Myers, T.E. A simple procedure for acceptance testing of freshly prepared solidified waste. In Hazardous and Industrial Solid Waste Testing: Fourth Symposium ASTM STP 886; Peros, J.K., Jr., Lacy, W.J., Conway, R.A., Eds.; American Society of Testing and Materials: Philadelphia, PA, USA, 1986. [Google Scholar]
- Hou, D.Y.; Gu, Q.B.; Ma, F.J.; O’Connell, S. Life cycle assessment comparison of thermal desorption and stabilization/solidification of mercury contaminated soil on agricultural land. J. Clean. Prod. 2016, 139, 949–956. [Google Scholar] [CrossRef]
- Jones, L. Interference mechanisms in waste stabilization/solidification processes. J. Hazard. Mater. 1990, 24, 83–88. [Google Scholar] [CrossRef]
- Pan, Y.Z.; Rossabi, J.; Pan, C.; Xie, X. Stabilization/solidification characteristics of organic clay contaminated by lead when using cement. J. Hazard. Mater. 2019, 362, 132–139. [Google Scholar] [CrossRef]
- Jain, N.; Minocha, A.K.; Verma, C.L. Effect of inorganic materials on the solidification of heavy metal sludge. Cem. Concr. Res. 2003, 33, 1695–1701. [Google Scholar]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal (loid) s contaminated soils–to mobilize or to immobilize. J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef]
- Berman, S.; Drage, D.F.; Tate, M.J. The Effect of Acid Rain on Magnesium Hydroxide Contained in Cement-Lime Mortar. In Masonry: Opportunities for the 21st Century; Throop, D., Klingner, R., Eds.; ASTM International: West Conshohocken, PA, USA, 2002; pp. 51–60. [Google Scholar]
- Park, C.K. Hydration and solidification of hazardous wastes containing heavy metals using modified cementitious materials. Cem. Concr. Res. 2000, 30, 429–435. [Google Scholar] [CrossRef]
- Qiao, Z.X.; Jin, C.J.; Jia, Y.G.; Li, H.J.; Li, Q.S.; Xiang, Y. Technology of electrokinetic remediation of heavy metals-contaminated soils. Technol. Equip. Environ. Pollut. Control 2004, 6, 80–83. [Google Scholar]
- Li, F.Y. Bioremediation in heavy metals contaminated soils. Environ. Sci. Technol. 2011, 34, 148–151. [Google Scholar]
- Conrardy, J.B.; Vaxelaire, J.; Olivier, J. Electro-dewatering of activated sludge: Electrical resistance analysis. Water Res. 2016, 100, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Xiang, W.; Wu, X.T. Influences of alkaline oxidant on strength of cement-stabilized sludge. Chin. J. Geotech. Eng. 2019, 41, 693–699. [Google Scholar]
- Wang, Y.N.; O’Connor, D.; Shen, Z.T.; Lo, I.M.C.; Tsang, D.C.W.; Pehkonen, S.; Pu, S.Y.; Hou, D.Y. Green synthesis of nanoparticles for the remediation of contaminated waters and soils: Constituents, synthesizing methods, and influencing factors. J. Clean. Prod. 2019, 226, 540–549. [Google Scholar] [CrossRef]
- Suzuki, T.; Niinae, M.; Koga, T.; Akita, T.; Ohta, M.; Choso, T. EDDS-enhanced electrokinetic remediation of heavy metal-contaminated clay soils under neutral pH conditions. Colloids Surf. A 2014, 440, 145–150. [Google Scholar] [CrossRef]
- Probstein, R.F. Physicochemical Hydrodynamics: An Introduction; John Wiley & Sons: Hoboken, NJ, USA, 1989; pp. 1–8. [Google Scholar]
- Glendinning, S. Electrochemical remediation technologies for polluted soils, sediment and groundwater. Eur. J. Soil Sci. 2010, 61, 623–624. [Google Scholar] [CrossRef]
- Hu, K.W.; Jia, D.Y.; Yan, L.; Guan, L.Z. Effect of bentonite on competitive adsorption of heavy metals ions. Chin. J. Soil Sci. 2011, 42, 467–470. [Google Scholar]
- Ye, W.M.; Yong, H.; Chen, Y.G.; Chen, B.; Cui, Y.J. Adsorption, Desorption and Competitive Adsorption of Heavy Metal Ions from Aqueous Solution onto GMZ01 Bentonite. Eng. Geol. Soc. Terr. 2015, 6, 533–536. [Google Scholar]
- Sun, H.L. Study of adsorption capability and mechanism of organic pollutant and heavy metal onto organobentonites. Chem. Res. Appl. 2007, 19, 745–751. [Google Scholar]
- Kim, K.J.; Kim, D.H.; Yoo, J.C.; Baek, K. Electrokinetic extraction of heavy metals from dredged marine sediment. Sep. Purif. Technol. 2011, 79, 164–169. [Google Scholar] [CrossRef]
- Amrate, S.; Akretche, D.E. Modeling EDTA enhanced electrokinetic remediation of lead contaminated soils. Chemosphere 2005, 60, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Tang, X.W.; Wang, Y. Competitive adsorption behavior and mechanism of loess towards Pb(II), Cu(II) and Cd(II). Chin. J. Geotech. Eng. 2014, 36, 327–333. [Google Scholar]
- Zheng, L.W. Electro-Osmosis Reinforcement Technical Research of Coastal Soft Soil Foundation; Zhejiang University: Hangzhou, China, 2018. [Google Scholar]
- Shen, C.N.; Fang, X.W.; Wang, H.W.; Sun, S.G.; Guo, J.F. Research on effects of suction, water content and dry density on shear strength of remolded unsaturated soils. Rock Soil Mech. 2009, 30, 1347–1351. [Google Scholar]
- Chien, S.C.; Ou, C.Y.; Lee, Y.C. A novel electroosmotic chemical treatment technique for soil improvement. Appl. Clay Sci. 2010, 50, 481–492. [Google Scholar] [CrossRef]
- Shang, J.Q.; Lo, K.Y.; Inculet, I.I. Polarization and Conduction of Clay-Water-Electrolyte Systems. J. Geotech. Eng. 1995, 121, 243–248. [Google Scholar] [CrossRef]
- Jiao, D.; Gong, X.N.; Li, W. Experimental study of consolidation of soft clay USING electro-osmosis method. Chin. J. Rock Mech. Eng. 2011, S1, 3208–3216. [Google Scholar]
- Li, Y.W. Experimental Research on The Effect of Electrodes Array to Electro-Osmotic Dewatering; Zhejiang University: Hangzhou, China, 2013. [Google Scholar]
- Tao, Y.L.; Zhou, J.; Gong, X.N.; Chen, Z. Experimental research of the influence of current intermittence on electro-osmotic effect. J. Harbin. Inst. Technol. 2014, 46, 78–83. [Google Scholar]
- Wang, J.; Wang, Y.; Feiyu, L. Test of reinforcement by intermittent vacuum preloading-electroosmosis in dredger soft clay. Chin. J. Highw. Transp. 2016, 29, 37–45. [Google Scholar]
- Li, Y.; Gong, X.N.; Guo, B.; Zhou, Z. Research on conductivity characteristics of soft clay during electro-osmosis and its conductive mechanism. Chin. J. Rock Mech. Eng. 2010, 29, 4027–4032. [Google Scholar]
- Xie, X.Y.; Zheng, L.W.; Xie, K.H.; Zhou, Z. Laboratory tests of electroosmosis-solidification on river sludge. Chin. Civ. Eng. J. 2019, 52, 108–114. [Google Scholar]
- Li, Z.Z.; Sheng, J.W.; Gan, C. Research on model and mechanism of solute transport under multi-field coupling. Rock Soil Mech. 2011, s2, 279–283. [Google Scholar]
- Gabrieli, L.; Alshawabkeh, A.N. Influence of boundary conditions on transient excess pore pressure during electrokinetic applications in soils. J. Appl. Electrochem. 2010, 40, 1113–1121. [Google Scholar] [CrossRef]
- Jeyakanthan, V.; Gnanendran, C.T.; Lo, S.C.R. Laboratory assessment of electro-osmotic stabilization of soft clay. Can. Geotech. J. 2011, 48, 1788–1802. [Google Scholar] [CrossRef]
- Li, Y.; Gong, X.N. Experimental study on effect of soil salinity on electro-osmotic dewatering in soft clay. Chin. J. Geotech. Eng. 2011, 8, 1254–1259. [Google Scholar]
- Tao, Y.L.; Zhou, J.; Gong, X.N. Experimental study on transport process ofelectro-osmosis based on Hangzhou soft soil. J. Cent. South Univ. 2018, 49, 1672. [Google Scholar]
- Glendinning, S.; Lamont-Black, J.; Jones, C.J.F.P. Treatment of sewage sludge using electrokinetic geosynthetics. J. Hazard. Mater. 2007, 139, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.F. Theory and design method for electro-osmotic consolidation. Chin. J. Geotech. Eng. 2016, 38, 152–155. [Google Scholar]
- Hu, X.H. Research on the Impact of Electrolyte and Nano-Materials for Electroosmosis for Consolidating Weak Soils; Wuhan Polytechnic University: Wuhan, China, 2016. [Google Scholar]
- Gong, X.N.; Jiao, D. Experimental study on electro-osmotic consolidation of soft clay under intermittent current condition. J. Cent. South. Univ. 2011, 42, 1725–1730. [Google Scholar]
- Liu, F.; Wei, M.; Wang, J.; Hong-tao, F. Influence of applying stepped voltage in electroosmotic reinforcement of dredger fill. Chin. J. Rock Mech. Eng. 2014, 33, 2582–2591. [Google Scholar]
- Qian, W. Experimental Study on the Effects of Fulvic on Electrokinetic Remediation and Electro-Osmotic Consolidation of Plumbum Contaminated Soil; Zhejiang University: Hangzhou, China, 2017. [Google Scholar]
- An, H.; Zhan, M.H.; Cheng, H.F.; Li, Y. Effect of electrokinetic remediation on heavy metal contaminated soil by adding acetic acid. Chin. J. Environ. Eng. 2017, 11, 5283–5290. [Google Scholar]
- Zhao, S.N.; Fan, L.; Hou, J.; Zhou, M.Y.; Zhu, X.F.; Li, X.L. Electrokinetic remediation of kaolin contaminated with multiple heavy metals. Environ. Prot. Chem. Ind. 2017, 37, 481–486. [Google Scholar]
- Chien, S.C.; Ou, C.Y.; Wang, M.K. Injection of saline solutions to improve the electro-osmotic pressure and consolidation of foundation soil. Appl. Clay Sci. 2009, 44, 218–224. [Google Scholar] [CrossRef]
- Wang, J.; Ma, J.J.; Liu, F.Y.; Mi, W.; Cai, Y.Q.; Fu, H.T.; Wang, P. Experimental study on the improvement of marine clay slurry by electroosmosis-vacuum preloading. Geotext. Geomembr. 2016, 44, 615–622. [Google Scholar] [CrossRef]
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Giannopoulou, I.; Dimas, D.; Maragkos, I.; Panias, D. Utilization of metallurgical solid by-products for the development of inorganic polymeric construction materials. Glob. Nest. J. 2009, 11, 127–136. [Google Scholar]
- Pan, Z.; Li, D.; Yu, J.; Yang, N. Properties and microstructure of the hardened alkali-activated red mud-slag cementitious material. Cem. Concr. Res. 2003, 33, 1437–1441. [Google Scholar] [CrossRef]
- Huang, X.; Hu, T.A. Soft soil consolidation by the mixture of industrial waste gypsum and cement. Arch. Technol. 2001, 3, 161–163. [Google Scholar]
- Zhou, S.G.; Chen, S.; Yuan, Y.; Lu, Q. Influence of Humic Acid Complexation with Metal Ions on Extracellular Electron Transfer Activity. Sci. Rep. 2015, 23, 17067. [Google Scholar] [CrossRef]
- Lai, Y.X.; Zhan, D.M. Application of the cement mixing pile in reinforcing organic soil. Geotech. Found. 2004, 2, 9–11. [Google Scholar]
- Lin, C.; Zhu, W.; Han, J. Strength and Leachability of Solidified Sewage Sludge with Different Additives. J. Mater. Civil Eng. 2013, 25, 1594–1601. [Google Scholar] [CrossRef]
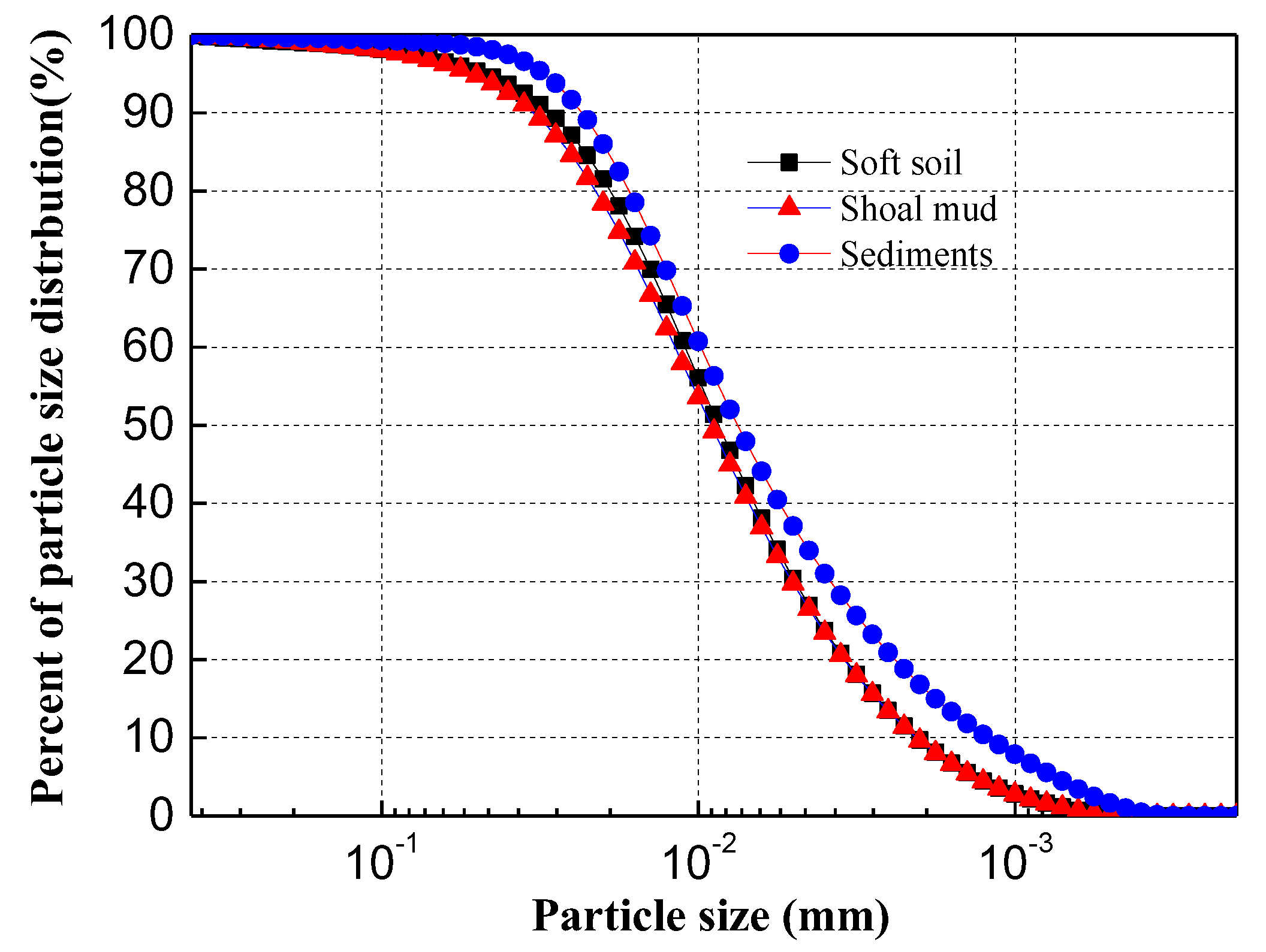




| Relative Density Gs | Density ρ (g·cm−3) | Initial Water Content w0 (%) | Plastic Limit wp (%) | Liquid Limit wL (%) | pH | Cu2+ (mg/kg) | Zn2+ (mg/kg) |
|---|---|---|---|---|---|---|---|
| 2.64 | 1.36 | 68 | 30 | 42 | 7.26 | 10 | 20 |
| Sample | Electricity (A) | Cu2+ (mg/kg) | Zn2+ (mg/kg) | Fulvic Acid Dosage (%) |
|---|---|---|---|---|
| C38F0 | 0.2 | 380 | 0 | 0 |
| Z38F0 | 0.2 | 0 | 380 | 0 |
| C19Z19 | 0.2 | 190 | 190 | 0 |
| C38F45 | 0.2 | 380 | 0 | 4.5 |
| Z38F45 | 0.2 | 0 | 380 | 4.5 |
| E2F45 | 0.2 | 190 | 190 | 4.5 |
| E0F45 | 0 | 190 | 190 | 4.5 |
| C0Z0 | 0.2 | 0 | 0 | 4.5 |
| Sample | Fulvic Acid/% | 7 d Intensity/MPa | 28 d Intensity/MPa |
|---|---|---|---|
| F0 | 0 | 0.35 | 0.58 |
| F15 | 1.5 | 0.28 | 0.45 |
| F3 | 3 | 0.13 | 0.39 |
| F45 | 4.5 | 0.04 | 0.25 |
| Sample | Curing Agent Dosage/% | Organics Content/% | 7d/MPa | 28d/MPa |
|---|---|---|---|---|
| C3 | 3 | 0 | 0.11 | 0.18 |
| C5 | 5 | 0 | 0.18 | 0.34 |
| C5O3 | 5 | 3 | 0.10 | 0.26 |
| C8 | 8 | 0 | 0.35 | 0.58 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, C.; Chen, K.; Chen, D. Effect of Organics on Heavy Metal-Contaminated River Sediment Treated with Electro-Osmosis and Solidification/Stabilization Methods. Materials 2020, 13, 1466. https://doi.org/10.3390/ma13061466
Pan C, Chen K, Chen D. Effect of Organics on Heavy Metal-Contaminated River Sediment Treated with Electro-Osmosis and Solidification/Stabilization Methods. Materials. 2020; 13(6):1466. https://doi.org/10.3390/ma13061466
Chicago/Turabian StylePan, Chonggen, Keyu Chen, and Danting Chen. 2020. "Effect of Organics on Heavy Metal-Contaminated River Sediment Treated with Electro-Osmosis and Solidification/Stabilization Methods" Materials 13, no. 6: 1466. https://doi.org/10.3390/ma13061466
APA StylePan, C., Chen, K., & Chen, D. (2020). Effect of Organics on Heavy Metal-Contaminated River Sediment Treated with Electro-Osmosis and Solidification/Stabilization Methods. Materials, 13(6), 1466. https://doi.org/10.3390/ma13061466