Effect of the Formation of Amorphous Networks on the Structure and Hydration Characteristics of Granulated Blast Furnace Slag
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation Process of Air-Quenched Slag
2.1.1. Raw Materials
2.1.2. Chemical Composition Design
2.1.3. Quenching Granulation and Powder Grinding Process
2.2. Methods
2.2.1. Mineral Composition Analysis
2.2.2. Hydration Activity Analysis
2.2.3. Early Ion Dissolution Characteristics Analysis
2.2.4. Microstructure of Hydration Products Analysis
2.2.5. Structure Analysis of the Amorphous Phase
2.2.6. Exothermic Characteristic of Hydration Process
3. Results
3.1. Analysis of Slag with Different S/A
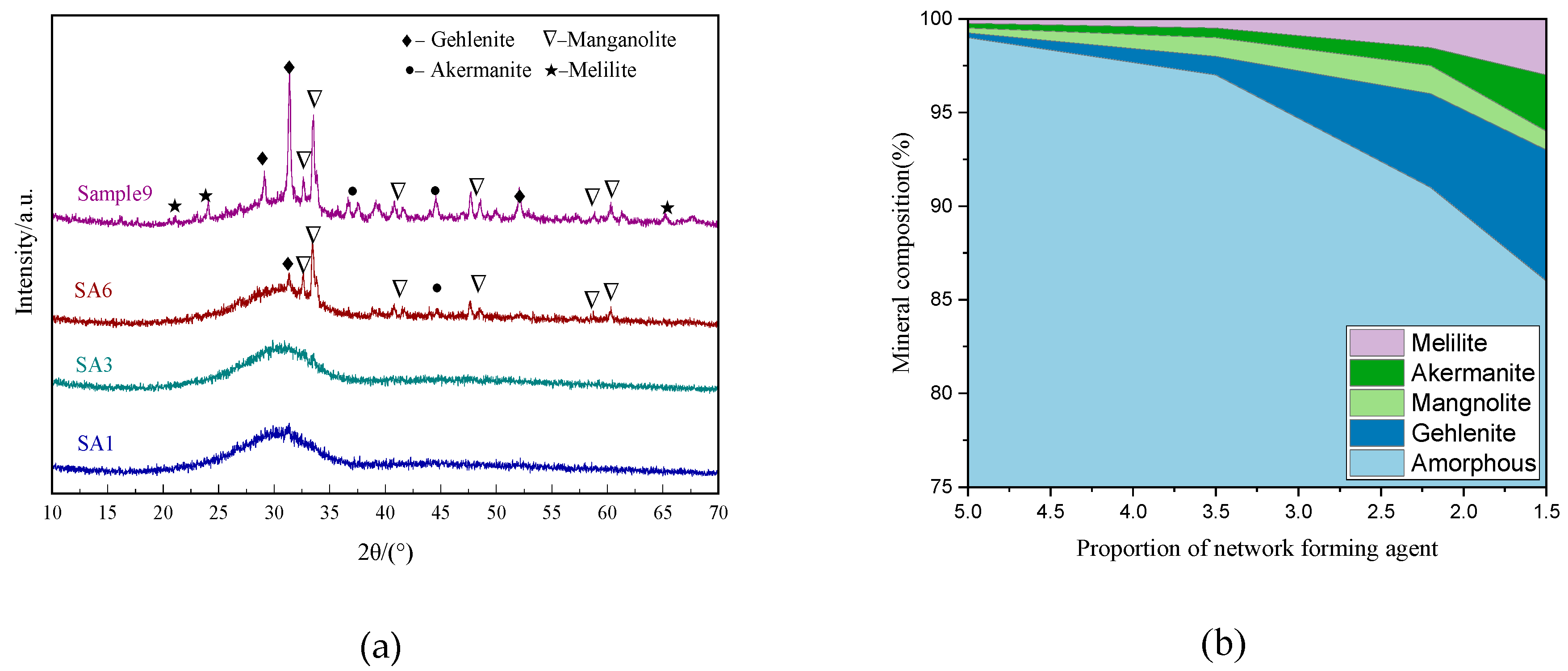
3.1.1. Mineral Composition
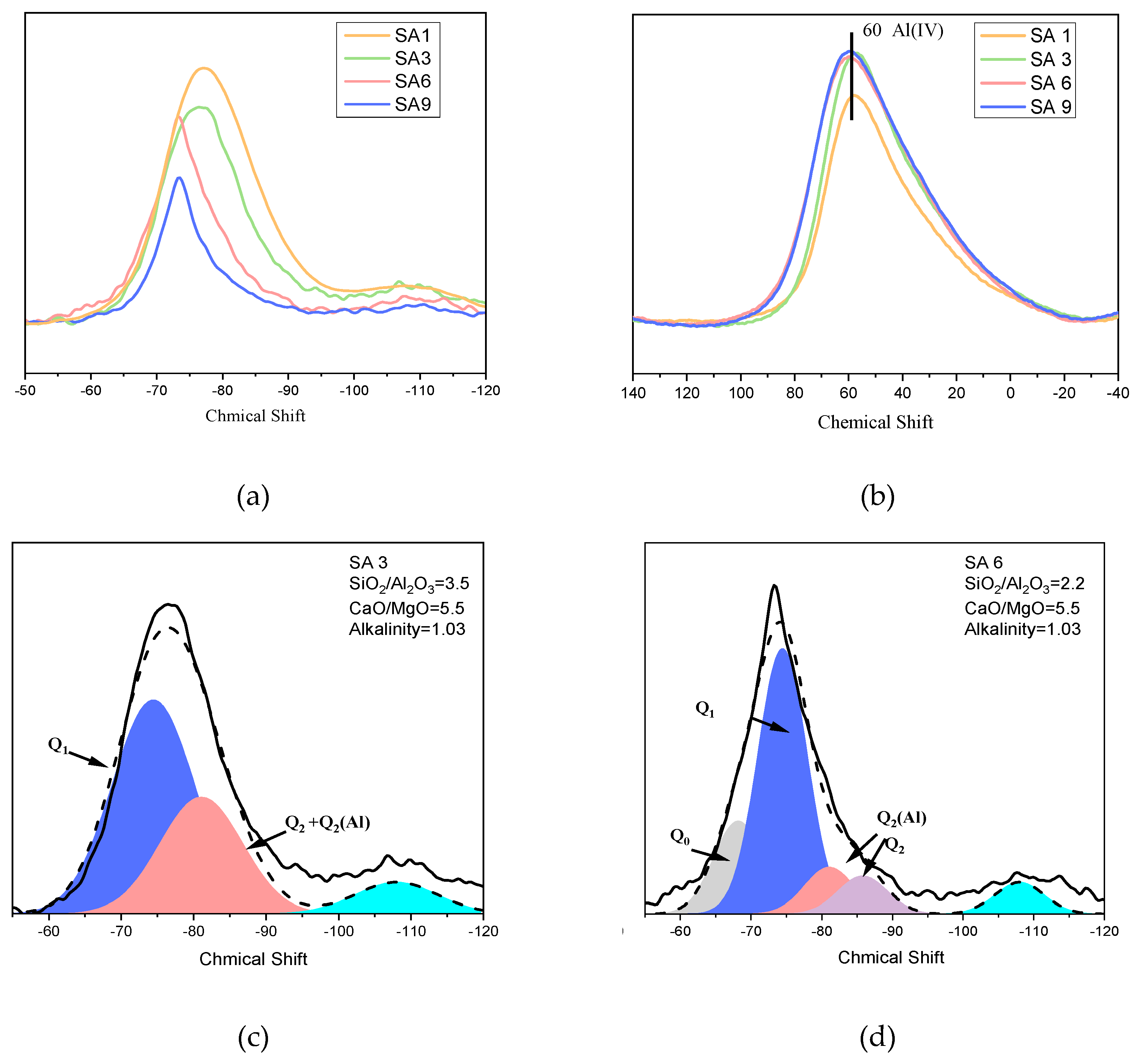
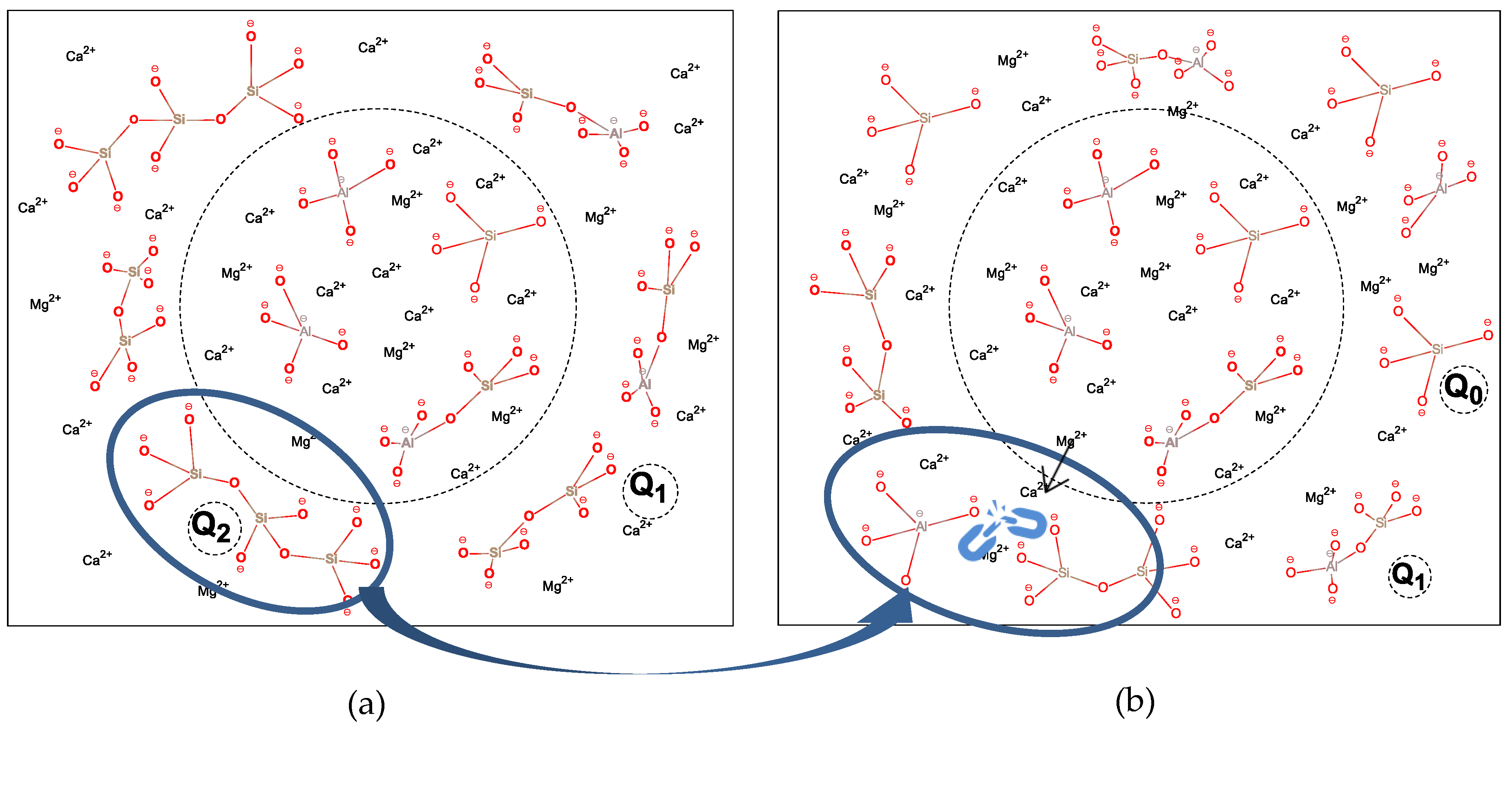
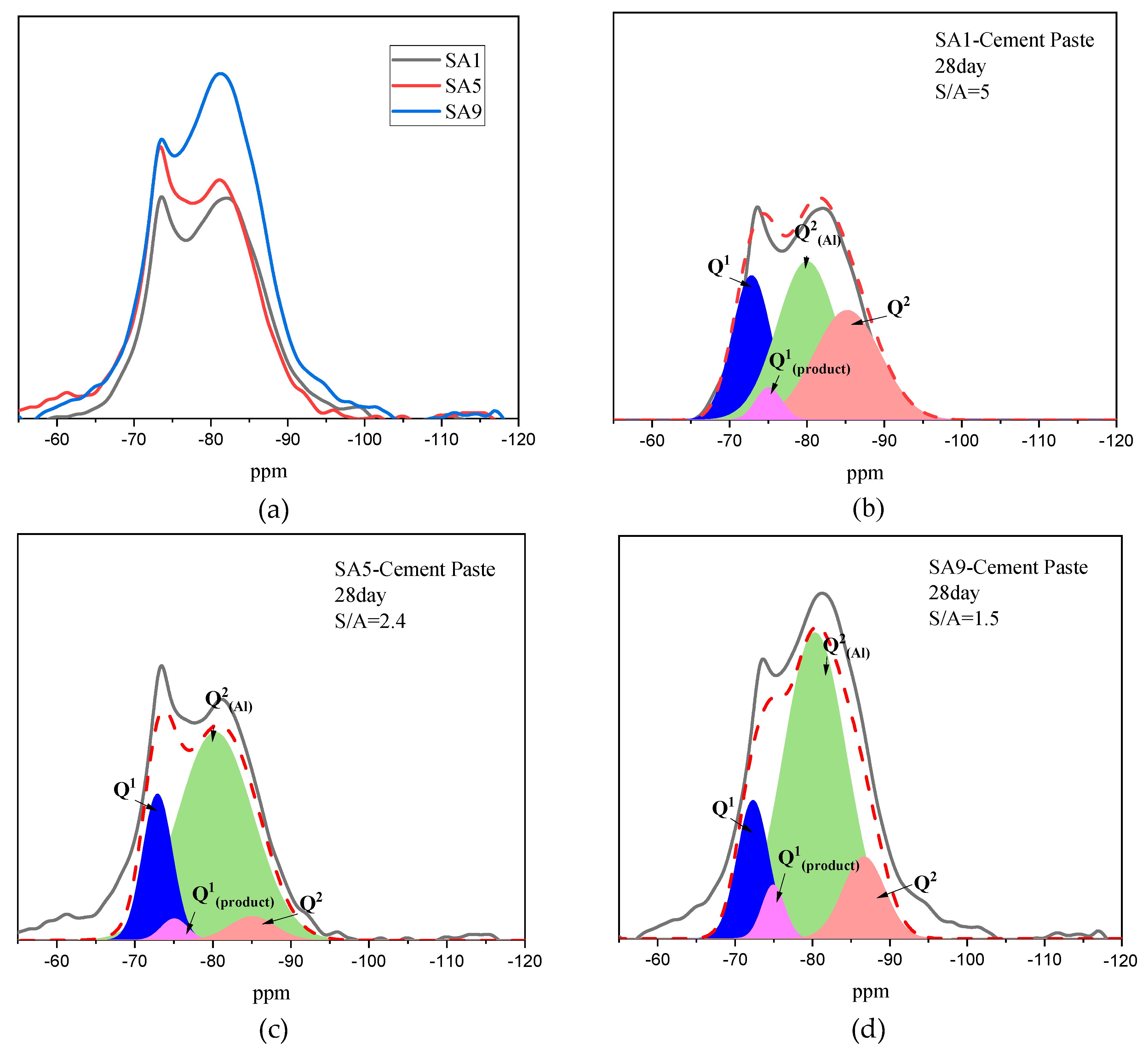
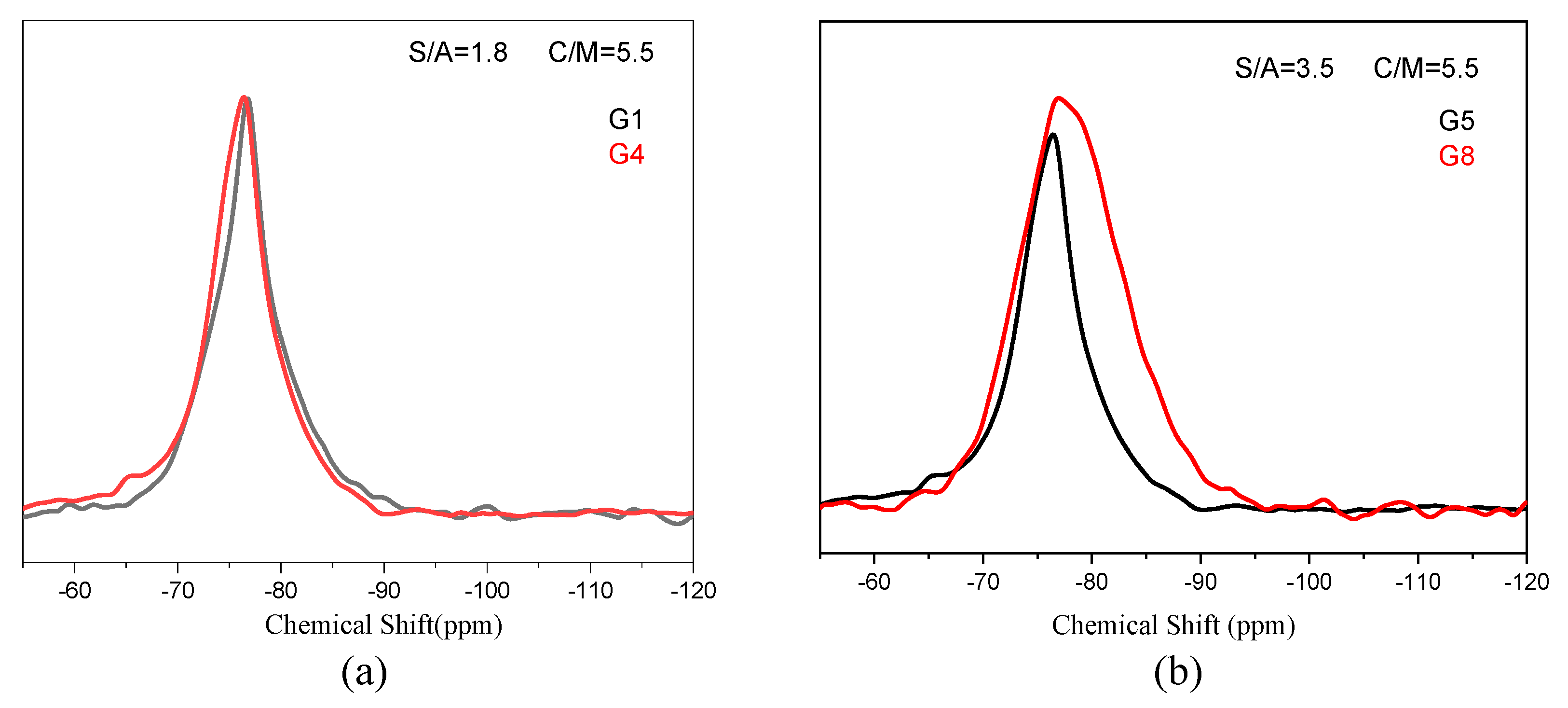
3.1.2. Amorphous Phase Structure
- Influence the mineral composition;
- Change the polymerization degree of network formers;
- Rearrange the distribution of metal cations located in the network.
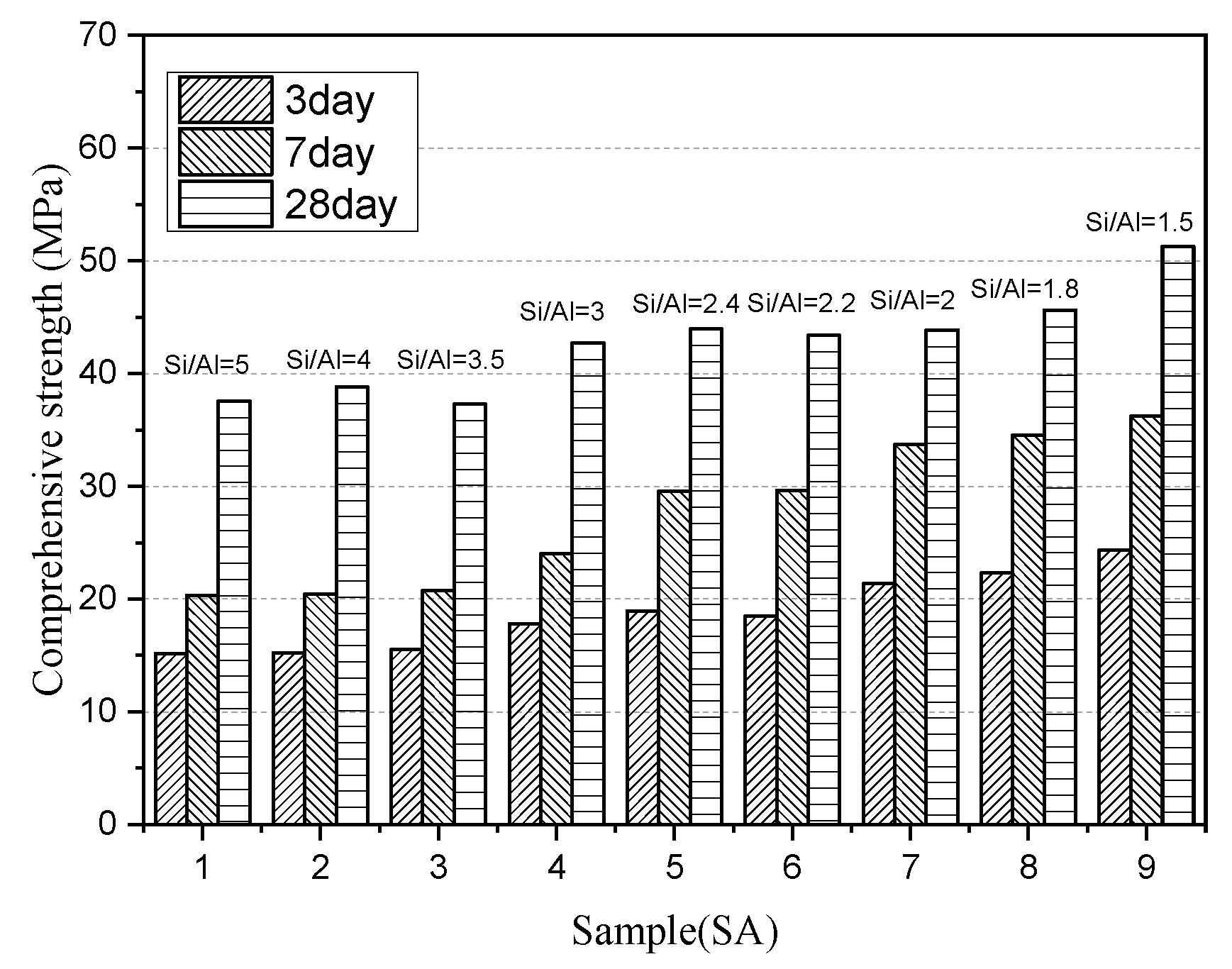
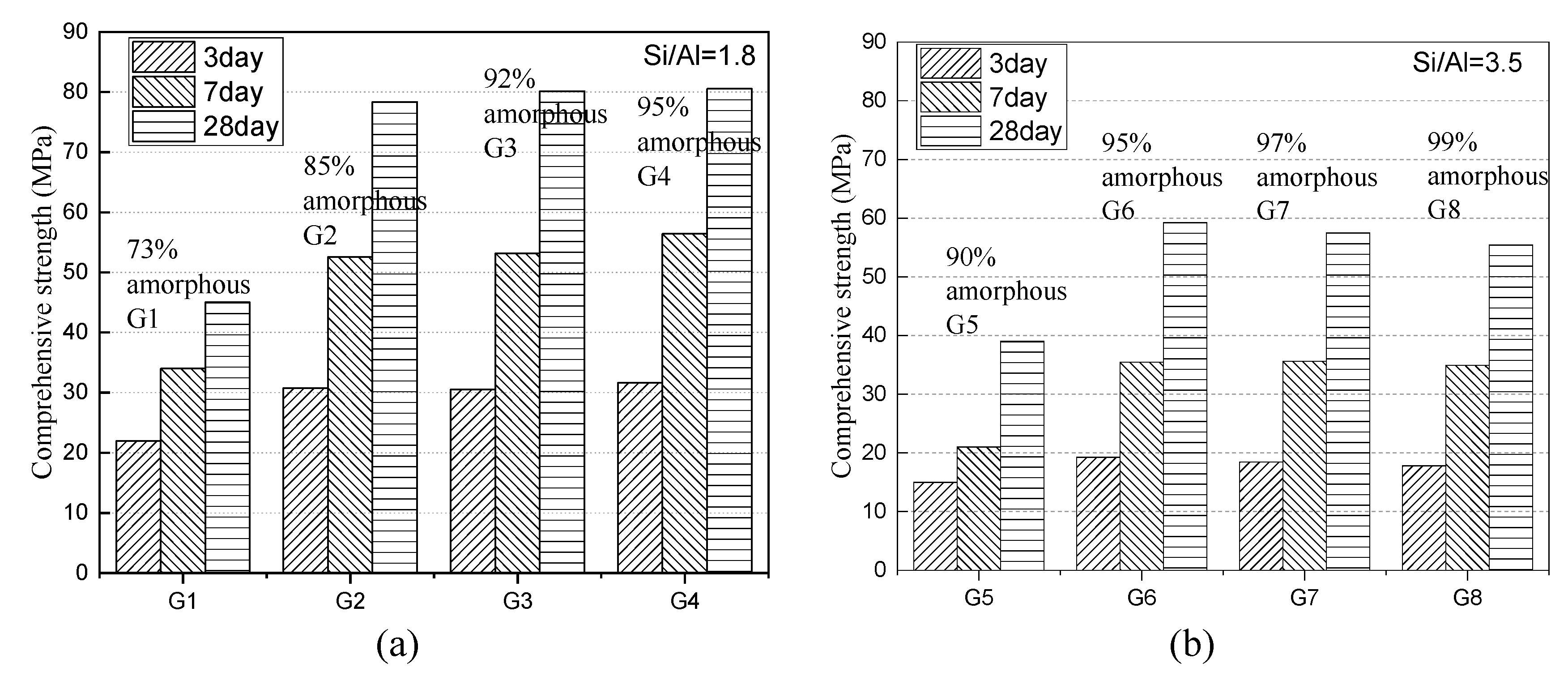
3.1.3. Compressive Strength Development of Paste
- Higher S/A slag contains a high concentration of Q1 and Q2 species, which need to consume a large amount of OH- during the hydration process to destroy the structure and lead to reduced activity effectively.
- Although the lower S/A slag forms a more crystalline phase, the formed amorphous phase has a relatively lower degree of polymerization and is more activated to hydrolysis, so it exhibits higher compressive strength. However, such slag can provide less active substance, so its activity may fall behind the slag of higher S/A after prolonged hydration age.
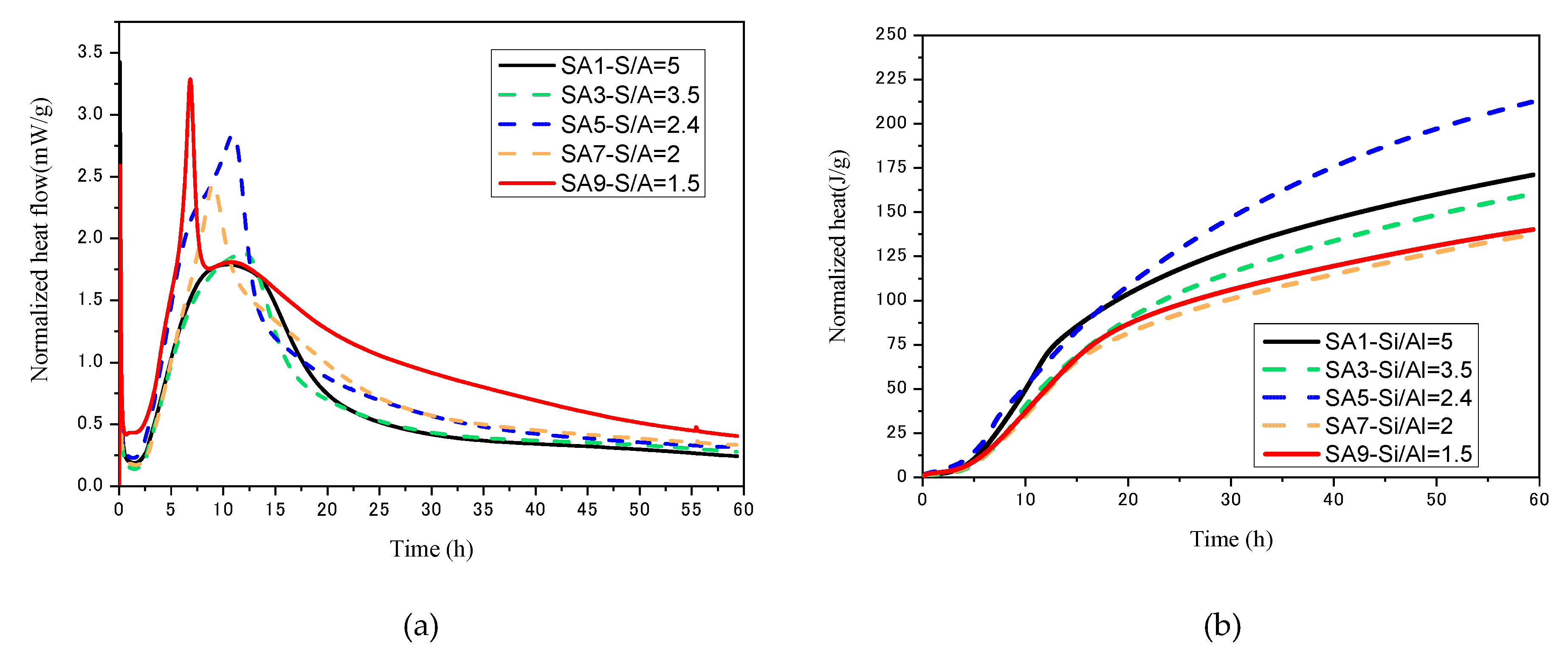
3.1.4. Hydration Kinetics
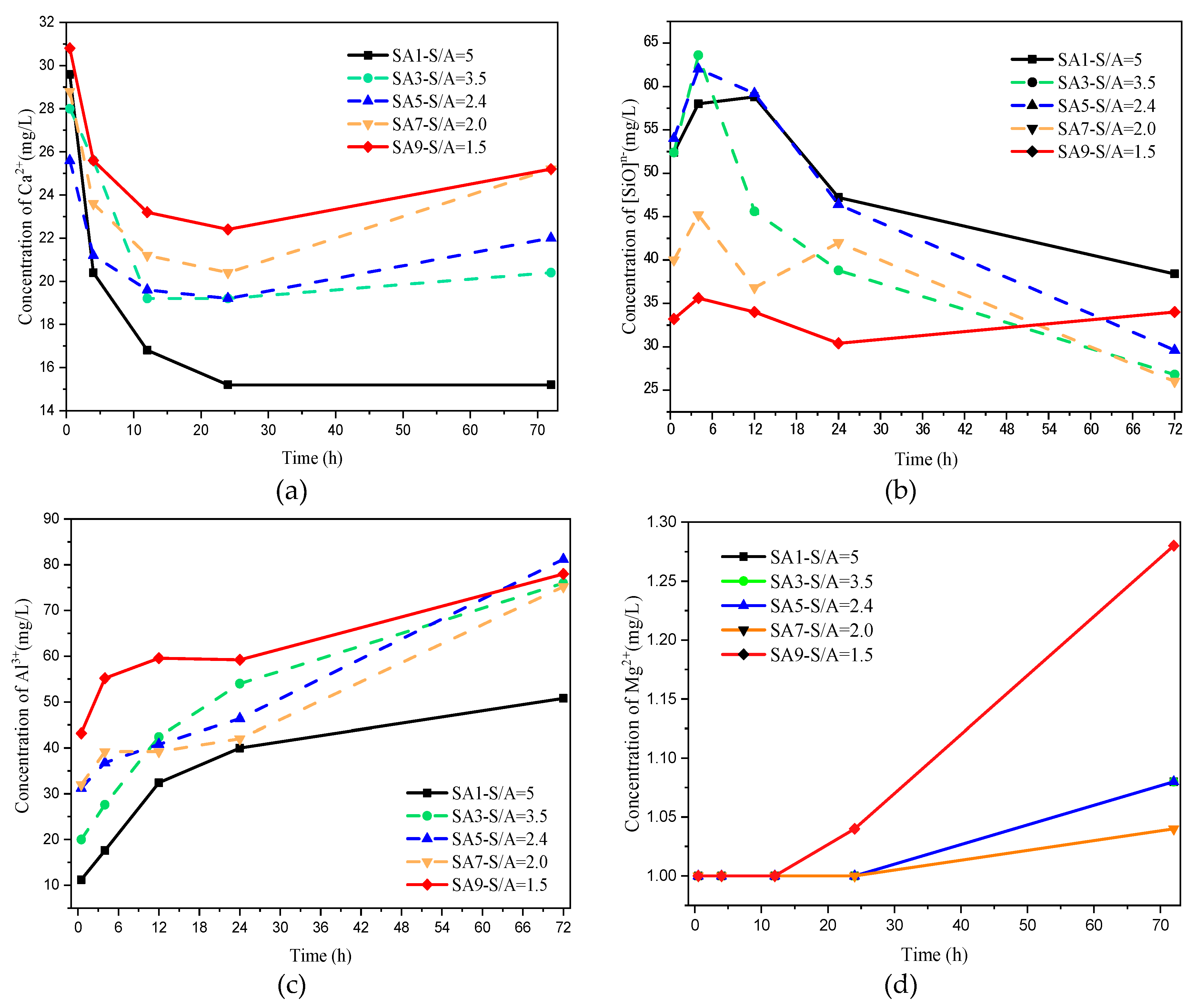
3.1.5. Early Ion Dissolution Characteristics
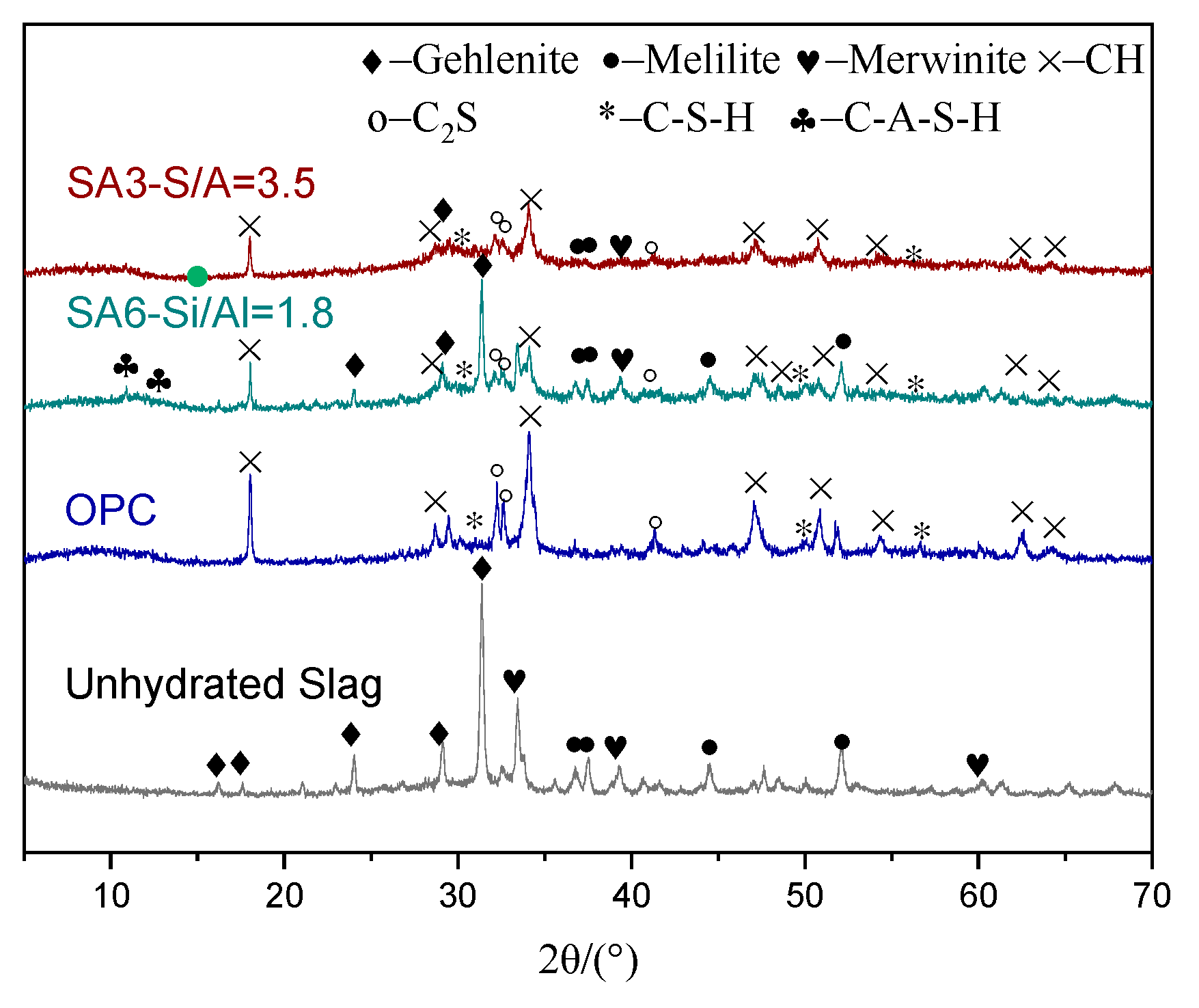
3.1.6. Structural Evolution of Slag During Hydration
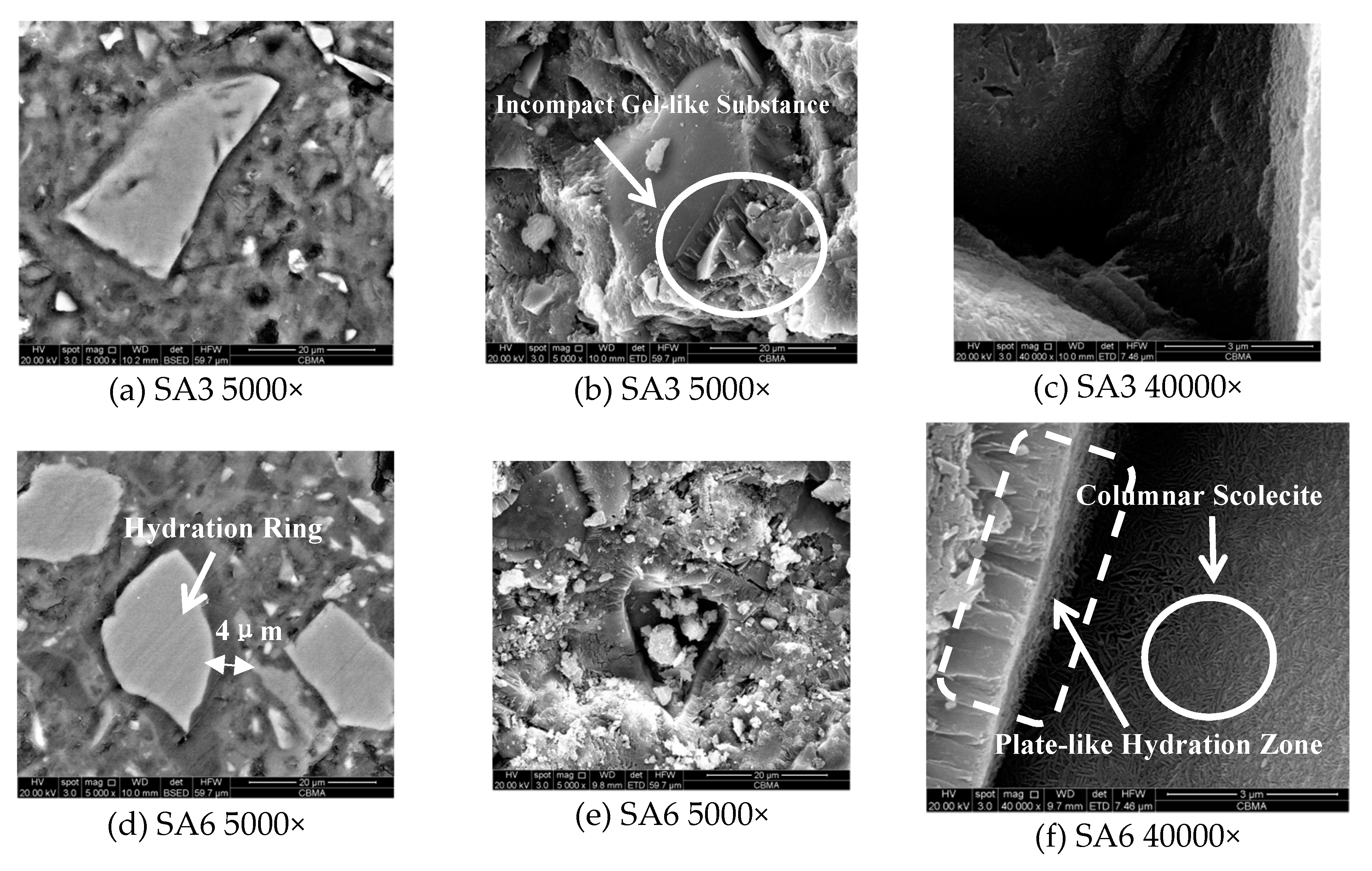
3.1.7. Hydration Products and Micro-Morphology
3.2. Analysis of Slag with Different Contents
3.2.1. Mineral Composition
3.2.2. Amorphous Phase Structure
3.2.3. Compressive Strength Development of Paste
4. Discussion
5. Conclusion
- The decrease of S/A value in chemical composition can effectively reduce the degree of polymerization in slag, as evidenced by the change of Q2 to Q0 and Q1, which is accompanied by the homogenization of metal cations distribution among the network. However, it increases the content of the crystalline phase in the mineral composition.
- The degree of polymerization of the amorphous phase network in the slag decreases with the S/A value. This phenomenon causes the advance of slag’s hydration starting, and the cumulative heat release increases, the Ca/Si ratio and the Al/Si ratio in the liquid phase increase, the hydration product converts from C−S−H gel to C−A−S−H gel, and finally causes the increase in compressive strength through the macroscopic perspective.
- The regularity that the compressive strength rises with the increase of S/A is more evident in the early stage of hydration. Increasing the S/A value of 3.5 can increase the three-day and seven-day strengths of the slag-cement paste by 67% and 85%, respectively. However, for the longer age, the content of the active amorphous phase is reduced due to the high crystal phase content in high S/A value slag, so the 28-day hydration activity increase is only 36%, and the differences in the activity is gradually reduced.
- Slag with high S/A tends to form a highly polymerized amorphous network structure. When the content of the amorphous phase is higher than 95%, the degree of polymerization of amorphous increases as the content of it sequentially increases, resulting in a small decrease in the hydration activity. In contrast, the low S/A value slag rarely forms an amorphous phase with a high degree of polymerization, and the compressive strength of paste is completely proportional to the content of amorphous.
Author Contributions
Funding
Conflicts of Interest
References
- Provis, J.L.; Bernal, S.A. Geopolymers and Related Alkali-Activated Materials. Annu. Rev. Mater. Sci. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Huang, H.; Tang, M.; Shen, X. The Resourcing of Industrial Waste and Its Sustainable Development (I)-The Characteristics of Typical Industrial Waste and the Present Status of Utilization. Mater. Rev. 2006, 20, 450–454. [Google Scholar]
- Gu, H.; Zhu, C. China’s steel imports and exports in 2017. Chin. Steel Foc. 2018, 351, 25–28. [Google Scholar]
- Chen, E.; Li, T. Development status and analysis of slag powder industry in China. Chin. Concr. 2015, 07, 56–59. [Google Scholar]
- Han, C.; Yang, X.; Zhou, H. Steel Slag and Its Application in Cement Industries. Mater. Rev. 2010, 24, 440–443. [Google Scholar]
- Puertas, F.; MartíNez-RamíRez, S.; Alonso, S. Alkali-activated fly ash slag cements: Strength behaviour and hydration products. Cem. Concr. Res. 2000, 30, 1625–1632. [Google Scholar] [CrossRef]
- Maria, S.K.; Surendra, P.S. Hydration and properties of novel blended cements based on cement kiln dust and blast furnace slag. Cem. Concr. Res. 2003, 33, 1269–1276. [Google Scholar]
- Ricarda, T.; Anja, B.; Dietmar, S. Effect of slag chemistry on the hydration of alkali-activated blast-furnace slag. Mater. Struct. 2015, 48, 629–641. [Google Scholar]
- Itoh, T. Rapid discrimination of the character of the water-cooled blast furnace slag used for Portland slag cement. J. Mater. Sci. 2004, 39, 2191–2193. [Google Scholar] [CrossRef]
- Yan, Z.; Lv, X.; Zhang, J. Influence of MgO, Al2O3 and CaO/SiO2 on the viscosity of blast furnace type slag with high Al2O3 and 5 wt-% TiO2. Can. Metall. Q. 2016, 55, 186–194. [Google Scholar] [CrossRef]
- Wang, H.; Cui, S.; Shang, X. Optimization Chemical Composition of the Blast Furnace Slag with Uniform Design. Mater. Sci. Forum 2013, 744, 210–215. [Google Scholar] [CrossRef]
- Haha, M.B.; Lothenbach, B.; Saout, G.L. Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag-Part I: Effect of MgO. Cem. Concr. Res. 2011, 41, 955–963. [Google Scholar] [CrossRef]
- Wang, D.; Ma, K.; Yang, X. Influences of CaO/SiO2/MgO/Al2O3 on the Formation Behavior of FeO-Bearing Primary-Slags in Blast Furnace. In Applications of Process Engineering Principles in Materials Processing, Energy and Environmental Technologies; Wang, S., Free, M., Alam, S., Zhang, M., Taylor, P.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 251–258. [Google Scholar]
- Yan, Z.; Lv, X.; Dong, L. Transition of Blast Furnace Slag from Silicates-Based to Aluminates-Based: Viscosity. Metall. Mater. Trans. 2017, 48, 1092–1099. [Google Scholar] [CrossRef]
- Liu, S.; Han, W.; Li, Q. Hydration Properties of Ground Granulated Blast-Furnace Slag (GGBS) Under Different Hydration Environments. Mater. Sci. 2017, 23, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Yip, C.; Lukey, G.; Deventer, J. Effect of Blast Furnace Slag Addition on Microstructure and Properties of Metakaolinite Geopolymeric Materials. Ceram. Trans. 2004, 153, 187–209. [Google Scholar]
- Zhang, W.; Zhang, L.; Zhang, J. Crystallization and Coarsening Kinetics of Rutile Phase in Modified Ti-Bearing Blast Furnace Slag. Ind. Eng. Chem. Res. 2012, 51, 12294–12298. [Google Scholar]
- Yu, L.; Sun, H.; Liu, X. Effect of phase separation structure on cementitious reactivity of blast furnace slag. Sci. China 2009, 52, 2695–2699. [Google Scholar]
- Li, C.; Sun, H.; Li, L. Glass Phase Structure of Blast Furnace Slag. Adv. Mater. Res. 2010, 168–170, 3–7. [Google Scholar] [CrossRef]
- Zhou, S. Advances in the study of slag microstructure. Shandong Metall. 2008, 30, 12–16. [Google Scholar]
- Schilling, P.; Butler, L.; Roy, A. 29Si and 27Al MAS-NMR of NaOH Activated Blast Furnace Slag. J. Am. Ceram. Soc. 2010, 77, 2363–2368. [Google Scholar] [CrossRef]
- Dembovska, L.; Pundiene, I.; Bajare, D. Effect of the Ratio SiO2/Al2O3 on the Structure, Properties, and Thermal Stability of Geopolymer Refractory Materials. Glass Ceram. 2018, 75, 112–117. [Google Scholar] [CrossRef]
- Ruiz-Santaquiteria, C.; Fernandez-Jimenez, A.; Skibsted, J. Clay reactivity: Production of alkali activated cements. Appl. Clay Sci. 2013, 73, 11–16. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.; Liu, J. Phase-Structure Transformation in Low-Si/Al-Ratio Geopolymers. Key Eng. Mater. 2014, 602–603, 76–79. [Google Scholar] [CrossRef]
- Cong, X.; Kirkpatrick, R. 29Si MAS NMR study of the structure of calcium silicate hydrate. Adv. Cem. Based Mater. 1996, 3, 144–156. [Google Scholar] [CrossRef]
- Faucon, P.; Charpentier, T.; Bertrandie, D. Characterization of Calcium Aluminate Hydrates and Related Hydrates of Cement Pastes by 27Al MQ-MAS NMR. Inorg. Chem. 1998, 37, 3726–3733. [Google Scholar] [CrossRef]
- Wen, X.; Yue, B. NMR Research on Silicon-Oxide Polyhedron Structure in Activated Fly Ash. J. Mater. Sci. Eng. 2004, 22, 561–563. [Google Scholar]
- Porteneuve, C.; Hélène, Z.; Petit, D. Structure-texture correlation in ultra-high-performance concrete: A nuclear magnetic resonance study. Cem. Concr. Res. 2002, 32, 97–101. [Google Scholar] [CrossRef]
- Cannas, C.; Casu, M.; Lai, A. XRD, TEM and 29Si MAS NMR study of sol-gel ZnO-SiO2 nanocomposites. J. Mater. Chem. 1999, 9, 1765–1769. [Google Scholar] [CrossRef]
- Nie, Y.; Xia, M.; Niu, F. 29Si and 27Al MAS-NMR Studies of Fly Ash Changes during the Polymerization of Mineral Polymer. Adv. Mater. Res. 2011, 156–157, 794–798. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Sun, H. Mechanism of phase separation in BFS (blast furnace slag) glass phase. Sci. China Ser. E Technol. Sci. 2011, 54, 105–109. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Hou, L. Preparation of CaO-Al2O3-SiO2 system glass from molten blast furnace slag. Int. J. Miner. Metall. Mater. 2014, 21, 169–174. [Google Scholar] [CrossRef]
- Kolani, B.; Buffo-Lacarrière, L.; Sellier, A. Hydration of slag-blended cements. Cem. Concr. Compos. 2012, 34, 1009–1018. [Google Scholar] [CrossRef]
- Stamboulis, A.; Matsuya, S.; Hill, R.G. MAS-NMR spectroscopy studies in the setting reaction of glass ionomer cements. J. Dent. 2006, 34, 581. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Y.; Cui, S. Reactivity and Hydration Property of Synthetic Air Quenched Slag with Different Chemical Compositions. Materials 2019, 12, 932. [Google Scholar] [CrossRef] [Green Version]
- Sadrnezhaad, K.; Simchi, A. Simulation of the Iron Smelting-Reduction Process. J. Mater. Sci. Technol. 1999, 15, 121–127. [Google Scholar]
- GB/T 203. Granulated Blast Furnace Slag Used for Cement Production; China Standards Press: Beijing, China, 2008. [Google Scholar]
- GB/T 18046. Ground Granulated Blast Furnace Slag Used for Cement and Concrete; China Standards Press: Beijing, China, 2017. [Google Scholar]
- GB/T 17671. Method of Testing Cements-Determination of Strength; China Standards Press: Beijing, China, 1999. [Google Scholar]
- Sherriff, B.L.; Grundy, H.D. Calculations of 29Si MAS NMR chemical shift from silicate mineral structure. Nature 1988, 332, 819–822. [Google Scholar] [CrossRef]
- Silva, A.; Queiroz, C.M.; Agathopoulos, S. Structure of SiO2–MgO–Na2O glasses by FTIR, Raman and 29Si MAS NMR. J. Mol. Struct. 2011, 986, 16–21. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C. 29Si NMR study of structural ordering in alumino-silicate geopolymer gels. Langmuir 2005, 21, 3028–3036. [Google Scholar] [CrossRef]
- Singh, P.S.; Trigg, M.; Burgar, I. Geopolymer formation processes at room temperature studied by 29Si and 27Al MAS-NMR. Mater. Sci. Eng. A 2005, 396, 392–402. [Google Scholar] [CrossRef]
- Rocha, J. Single and Triple-Quantum 27Al MAS NMR Study of the Thermal Transformation of Kaolinite. Met. Sci. J. 2013, 7, 24–27. [Google Scholar]
- Urbanova, M.; Kobera, L.; Brus, J. Factor analysis of 27Al MAS NMR spectra for identifying nanocry- stalline phases in amorphous geopolymers. Magn. Reson. Chem. 2013, 51, 734–742. [Google Scholar] [PubMed]
- Pérez, G.; Guerrero, A.; Gaitero, J.J. Structural characterization of C-S-H gel through an improved deconvolution analysis of NMR spectra. J. Mater. Sci. 2014, 49, 142–152. [Google Scholar] [CrossRef]
- ASTM C1679. Standard Practice for Measuring Hydration Kinetics of Hydraulic Cementitious Mixtures Using Isothermal Calorimetry; ASTM International: Washington, DC, USA, 2017. [Google Scholar]
- Liu, R.; Yan, P. Hydration Characteristics of Slag in Cement–Slag Complex Binder. J. Chin. Ceram. Soc. 2012, 40, 1112–1118. [Google Scholar]
- Biernacki, J.J.; Richardson, J.M.; Stutzman, P.E. Kinetics of Slag Hydration in the Presence of Calcium Hydroxide. J. Am. Ceram. Soc. 2002, 85, 2261–2267. [Google Scholar] [CrossRef]
- García, I. C-S-H gels: Interpretation of 29Si MAS-NMR spectra. J. Am. Ceram. Soc. 2012, 95, 1–7. [Google Scholar]
- Zhang, J.; Ding, H.; Dai, K. Microstructure and properties of hydration products of slag-fly ash mixed cementitious materials. J. Chin. Ceram. Soc. 2007, 35, 633–637. [Google Scholar]
- Puertas, F.; Palacios, M.; Manzano, H. A model for the C–A–S–H gel formed in alkali-activated slag cements. J. Eur. Ceram. Soc. 2011, 31, 2043–2056. [Google Scholar] [CrossRef]

| No. | CaO (wt.%) | SiO2 (wt.%) | Al2O3 (wt.%) | MgO (wt.%) | S/A | C/M | (C + M)/(S + A) |
|---|---|---|---|---|---|---|---|
| SA1 | 39.96 | 36.98 | 9.24 | 7.43 | 5.0 | 5.5 | 1.03 |
| SA2 | 39.96 | 35.95 | 10.27 | 7.43 | 4.0 | 5.5 | 1.03 |
| SA3(G5) | 39.96 | 34.67 | 11.56 | 7.43 | 3.5 | 5.5 | 1.03 |
| SA4 | 39.96 | 32.60 | 13.62 | 7.43 | 3.0 | 5.5 | 1.03 |
| SA5 | 39.96 | 27.73 | 18.49 | 7.43 | 2.4 | 5.5 | 1.03 |
| SA6 | 39.96 | 32.60 | 13.62 | 7.43 | 2.2 | 5.5 | 1.03 |
| SA7 | 39.96 | 31.78 | 14.44 | 7.43 | 2.0 | 5.5 | 1.03 |
| SA8(G1) | 39.96 | 30.81 | 15.41 | 7.43 | 1.8 | 5.5 | 1.03 |
| SA9 | 39.96 | 29.71 | 16.51 | 7.43 | 1.5 | 5.5 | 1.03 |
| No. | BaO (wt.%) | No. | BaO (wt.%) |
|---|---|---|---|
| SA8(G1) | 0 | SA3(G5) | 0 |
| G2 | 0.25 | G6 | 0.25 |
| G3 | 0.5 | G7 | 0.5 |
| G4 | 0.75 | G8 | 0.75 |
| Parameter | Pulse Program (PULPROG) | Time Domain Data Size (TD) | Number of Scans (NS) | Receiver Gain (RG) |
| value | hpdec | 409,600 | 912 | 1030 |
| Parameter | Acquisition Time (AQ) | Sweep Width in Hz (SWH) | Dwell Time (DW) | Relaxing Delay (D1) |
| value | 0.0425 (s) | 48067 (Hz) | 10.4 (μs) | 60 (s) |
| No. | Q1 | Q1(product) | Q2(Al) | Q2 |
|---|---|---|---|---|
| SA1 | 24% | 4% | 41% | 32% |
| SA5 | 20% | 3% | 72% | 5% |
| SA9 | 15% | 4% | 68% | 12% |
| No. | Q0 | Q1 | Q2 |
|---|---|---|---|
| G1 | 42% | 37% | 20% |
| G4 | 41% | 36% | 22% |
| G5 | 15% | 50% | 35% |
| G8 | 0% | 50% | 50% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Wang, Y.; Wei, Q.; Cui, S.; Hao, L. Effect of the Formation of Amorphous Networks on the Structure and Hydration Characteristics of Granulated Blast Furnace Slag. Materials 2020, 13, 1462. https://doi.org/10.3390/ma13061462
Yao Y, Wang Y, Wei Q, Cui S, Hao L. Effect of the Formation of Amorphous Networks on the Structure and Hydration Characteristics of Granulated Blast Furnace Slag. Materials. 2020; 13(6):1462. https://doi.org/10.3390/ma13061462
Chicago/Turabian StyleYao, Yuhan, Yali Wang, Qi Wei, Suping Cui, and Liwei Hao. 2020. "Effect of the Formation of Amorphous Networks on the Structure and Hydration Characteristics of Granulated Blast Furnace Slag" Materials 13, no. 6: 1462. https://doi.org/10.3390/ma13061462
APA StyleYao, Y., Wang, Y., Wei, Q., Cui, S., & Hao, L. (2020). Effect of the Formation of Amorphous Networks on the Structure and Hydration Characteristics of Granulated Blast Furnace Slag. Materials, 13(6), 1462. https://doi.org/10.3390/ma13061462




