Effect of the Compositions on the Biocompatibility of New Alumina–Zirconia–Titania Dental Ceramic Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Ceramic Composites Manufacturing
2.2. Wettability Measurements
2.3. Cell Culture and Treatment
2.4. Cell Viability Assay
2.5. Analysis of Cell Morphology
2.6. Single-Cell Gel Electrophoresis (SCGE, Comet Assay)
3. Results and Discussion
3.1. Wettability
3.2. Cytotoxicity
3.2.1. Cell Viability
3.2.2. Relationship between Cell Viability and Surface Wettability
3.2.3. Cell Morphology
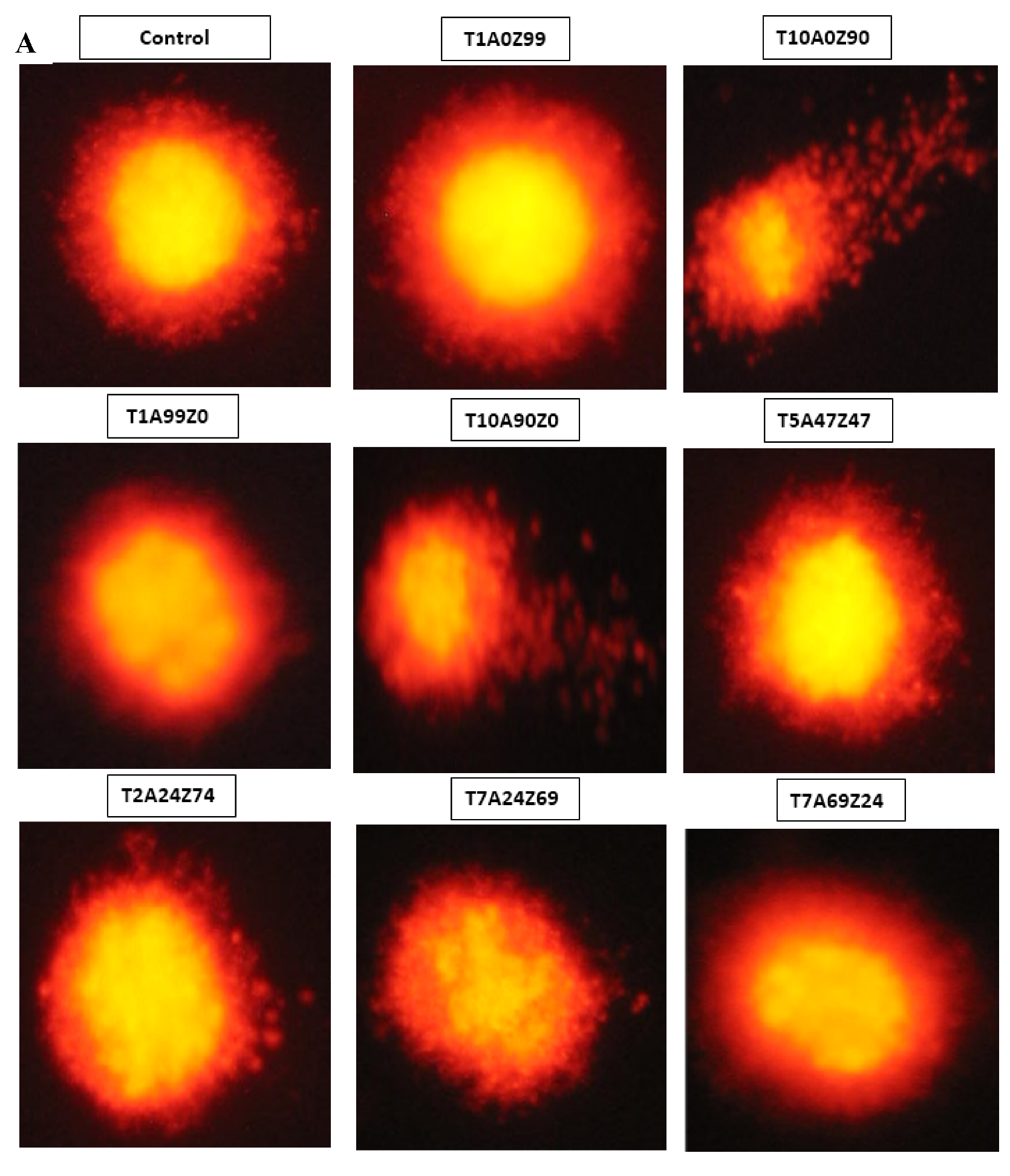
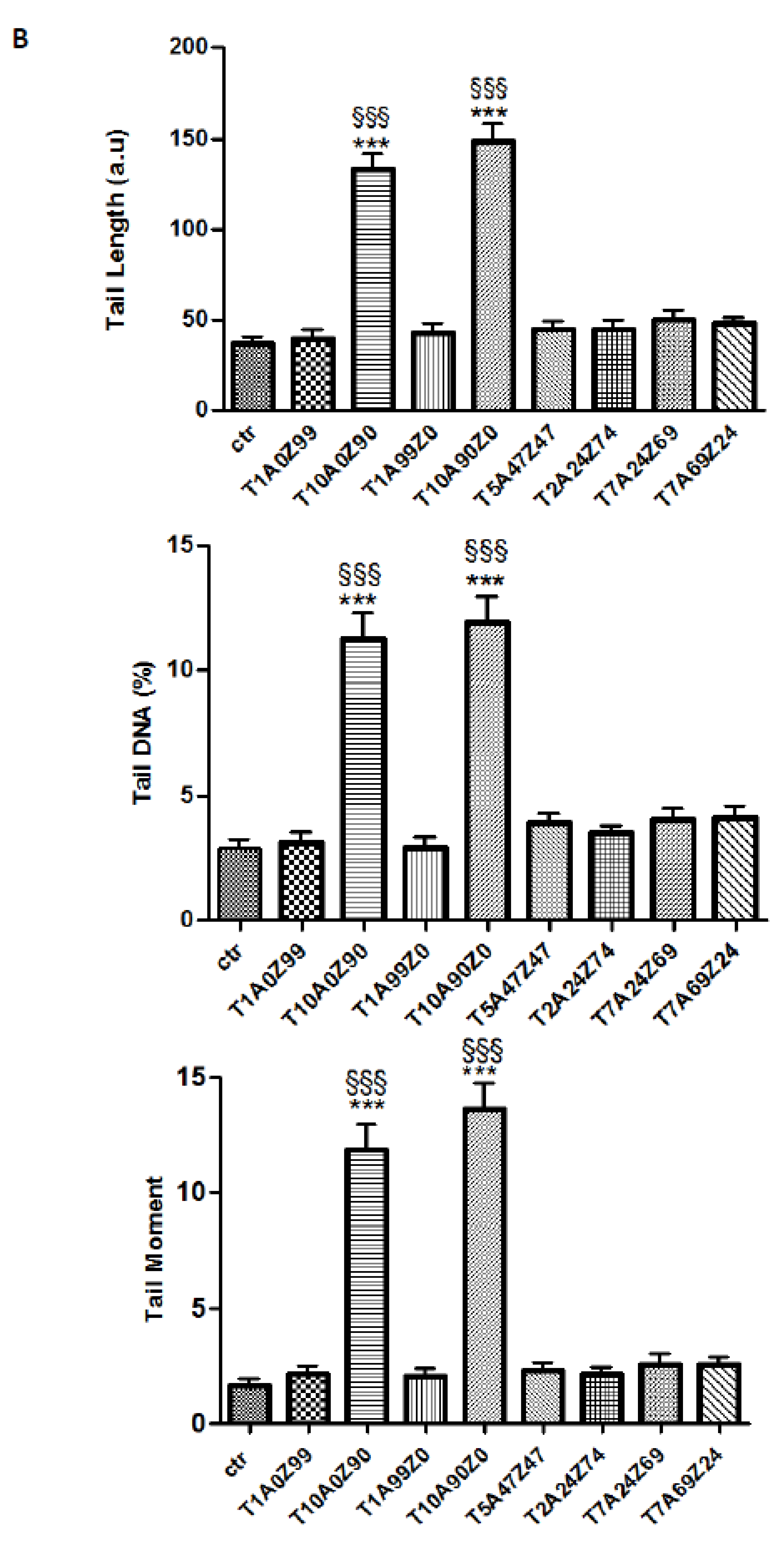
3.3. Genotoxicity
4. Conclusions
- The studied ceramic composites with high wettability could be used to manufacture dental implants with a high capacity of osseointegration.
- The results of the biological tests highlighted the absence of cytotoxicity when compared to the control.
- The DNA damage is closely related to TiO2 content. Genotoxicity towards human gingival fibroblasts was mainly attributed to composites containing 10 wt.% TiO2.
Author Contributions
Funding
Conflicts of Interest
References
- Hagiwara, Y.; Nakajima, K.; Dds, Y.H.; Nakajima, K. Application of Ce-TZP/Al2O3 nanocomposite to the framework of an implant-fixed complete dental prosthesis and a complete denture. J. Prosthodont. Res. 2016, 60, 337–343. [Google Scholar] [CrossRef]
- Palmero, P.; Fornabaio, M.; Montanaro, L.; Reveron, H. Towards long lasting zirconia-based composites for dental implants. Part I: Innovative synthesis, microstructural characterization and in vitro stability. Biomaterials 2015, 50, 38–46. [Google Scholar] [CrossRef]
- Christopher, B.; Sauter, C.; Wolkewitz, M.; Kohal, R. Alumina reinforced zirconia implants: Effects of cyclic loading and abutment modification on fracture resistance. Dent. Mater. 2014, 31, 262–272. [Google Scholar]
- Osman, R.; Swain, M. A Critical Review of Dental Implant Materials with an Emphasis on Titanium versus Zirconia. Materials 2015, 8, 932–958. [Google Scholar] [CrossRef] [PubMed]
- Sansone, V.; Pagani, D.; Melato, M. The effects on bone cells of metal ions released from orthopaedic implants. A review. Clin. Cases Miner. Bone Metab. 2013, 10, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Huet, R.; Sakona, A.; Kurtz, S.M. Strength and reliability of alumina ceramic femoral heads: Review of design, testing, and retrieval analysis. J. Mech. Behav. Biomed. Mater. 2011, 4, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Lutton, P.; Ben-Nissan, B. The Status of Biomaterials for Orthopedic and Dental Applications: Part I—Materials. Mater. Technol. 1997, 12, 59–64. [Google Scholar] [CrossRef]
- Wang, J.; Stevens, R. Surface toughening of TZP ceramics by low temperature ageing. Ceram. Int. 1989, 15, 15–21. [Google Scholar] [CrossRef]
- Pandey, A.K.; Jena, U.R.; Biswas, K. In vitro ageing and wear behaviour of ceria stabilized zirconia toughened alumina (CSZ-TA) bio-ceramic. Mater. Chem. Phys. 2014, 146, 456–463. [Google Scholar] [CrossRef]
- Jayaseelan, D.; Nishikawa, T.; Awaji, H.; Gnanam, F.D. Pressureless sintering of sol-gel derived alumina—Zirconia composites. Mater. Sci. Eng. A 1998, 256, 265–270. [Google Scholar] [CrossRef]
- Manshor, H.; Md Aris, S.; Azhar, A.Z.A.; Abdullah, E.C.; Ahmad, Z.A. Effects of TiO2 addition on the phase, mechanical properties, and microstructure of zirconia-toughened alumina ceramic composite. Ceram. Int. 2015, 41, 3961–3967. [Google Scholar] [CrossRef]
- Agac, O.; Gozutok, M.; Turkoglu, H.; Ozturk, A.; Park, J. Mechanical and biological properties of Al2O3 and TiO2 co-doped zirconia ceramics. Ceram. Int. 2017, 43, 10434–10441. [Google Scholar] [CrossRef]
- Khaskhoussi, A.; Calabrese, L.; Bouaziz, J.; Proverbio, E. Effect of TiO2 addition on microstructure of zirconia/alumina sintered ceramics. Ceram. Int. 2017, 43, 10392–10402. [Google Scholar] [CrossRef]
- Khaskhoussi, A.; Calabrese, L.; Bouhamed, H.; Kamoun, A. Mixture design approach to optimize the performance of TiO2 modified zirconia/alumina sintered ceramics. Mater. Des. 2018, 137, 1–8. [Google Scholar] [CrossRef]
- Khaskhoussi, A.; Calabrese, L.; Bouaziz, J.; Proverbio, E. Performances and aging stability of new Al2O3-ZrO2-TiO2 ternary ceramic composites. Mater. Chem. Phys. 2020, 243, 122586. [Google Scholar] [CrossRef]
- Milleding, P.; Wennerberg, A.; Alaeddin, S.; Karlsson, S.; Simon, E. Surface corrosion of dental ceramics in vitro. Biomaterials 1999, 20, 733–746. [Google Scholar] [CrossRef]
- Khaskhoussi, A.; Bouhamed, H.; Calabrese, L.; Proverbio, E.; Bouaziz, J. Properties and microstructural aspects of TiO2-doped sintered Alumina-Zirconia composite ceramics. Int. J. Appl. Ceram. Technol. 2018, 15, 1532–1541. [Google Scholar] [CrossRef]
- Camposilvan, E.; Leone, R.; Gremillard, L.; Sorrentino, R.; Zarone, F.; Ferrari, M.; Chevalier, J. Aging resistance, mechanical properties and translucency of different yttria-stabilized zirconia ceramics for monolithic dental crown applications. Dent. Mater. 2018, 34, 879–890. [Google Scholar] [CrossRef]
- Hao, L.; Lawrence, J. Effects of Nd:YAG laser treatment on the wettability characteristics of a zirconia-based bioceramic. Opt. Lasers Eng. 2006, 44, 803–814. [Google Scholar] [CrossRef]
- Puleo, D.A.; Nanci, A. Understanding and controlling the bone implant-interface. Biomaterials 1999, 20, 2311–2321. [Google Scholar] [CrossRef]
- Wei, J.; Igarashi, T.; Okumori, N.; Igarashi, T.; Maetani, T.; Liu, B.; Yoshinari, M. Influence of surface wettability on competitive protein adsorption and initial attachment of osteoblasts. Biomed. Mater. 2009, 4, 05002. [Google Scholar] [CrossRef] [PubMed]
- Picerno, I.; Chirico, C.; Condello, S.; Visalli, G.; Ferlazzo, N.; Gorgone, G.; Caccamo, D.; Ientile, R. Homocysteine induces DNA damage and alterations in proliferative capacity of T-lymphocytes: A model for immunosenescence? Biogerontology 2007, 8, 111–119. [Google Scholar] [CrossRef]
- Rupp, F.; Gittens, R.A.; Scheideler, L.; Marmur, A.; Boyan, B.D.; Schwartz, Z.; Geis-Gerstorfer, J. A review on the wettability of dental implant surfaces I: Theoretical and experimental aspects. Acta Biomater. 2014, 10, 2894–2906. [Google Scholar] [CrossRef] [PubMed]
- Yilbas, B.S. Laser treatment of zirconia surface for improved surface hydrophobicity. J. Alloys Compd. 2015, 625, 208–215. [Google Scholar] [CrossRef]
- González-Martín, M.L.; Labajos-Broncan, L.; Jańczuk, B.; Bruque, J.M. Wettability and Surface Free Energy of Graphene Films. J. Mater. Sci. 1999, 34, 5923–5926. [Google Scholar] [CrossRef]
- Huang, H.L.; Chang, Y.Y.; Weng, J.C.; Chen, Y.C.; Lai, C.H.; Shieh, T.M. Anti-bacterial performance of Zirconia coatings on Titanium implants. Thin Solid Films 2013, 528, 151–156. [Google Scholar] [CrossRef]
- Nowak, E.; Combes, G.; Stitt, E.H.; Pacek, A.W. A comparison of contact angle measurement techniques applied to highly porous catalyst supports. Powder Technol. 2013, 233, 52–64. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, Z.; Liu, Z.; Zhai, J.; Jiang, L. Light-gating titania/alumina heterogeneous nanochannels with regulatable ion rectification characteristic. Adv. Funct. Mater. 2014, 24, 424–431. [Google Scholar] [CrossRef]
- Samandi, M.; Gudze, M.; Evans, P. Application of ion implantation to ceramic/metal joining. Nucl. Instrum. Methods Phys. Res. B 1997, 128, 669–672. [Google Scholar] [CrossRef]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Lopes, M.A.; Monteiro, F.J.; Santos, J.D.; Serro, A.P.; Saramago, B. Hydrophobicity, surface tension, and zeta potential measurements of glass-reinforced hydroxyapatite composites. J. Biomed. Mater. Res. 1999, 45, 370–375. [Google Scholar]
- Hao, L.; Lawrence, J. Albumin and fibronectin protein adsorption on CO2-laser-modified biograde stainless steel. J. Eng. Med. 2005, 220, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Piconi, C.; Maccauro, G.; Muratori, F.; Prever, E.B.D.E.L. Alumina and zirconia ceramics in joint replacements. J. Appl. Biomater. Biomech. 2003, 1, 19–32. [Google Scholar] [PubMed]
- Hao, L.; Lawrence, J. The adsorption of human serum albumin (HSA) on CO2 laser modified magnesia partially stabilised zirconia (MgO-PSZ). Colloids Surf. B Biointerfaces 2004, 34, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Lawrence, J. Effects of CO2 laser irradiation on the wettability and human skin fibroblast cell response of magnesia partially stabilised zirconia. Mater. Sci. Eng. C 2003, 23, 627–639. [Google Scholar] [CrossRef]
- Hao, L.; Lawrence, J.; Chian, K.S. Osteoblast cell adhesion on a laser modified zirconia based bioceramic. J. Mater. Sci. Mater. Med. 2005, 16, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, S.; Fernandes, M.H.; Neves, N.; Almeida, M.M. Development and characterization of zirconia–alumina composites for orthopedic implants. Ceram. Int. 2017, 43, 693–703. [Google Scholar] [CrossRef]
- Marchi, J.; Ussui, V.; Delfino, C.S.; Bressiani, A.H.A.; Marques, M.M. Analysis in vitro of the cytotoxicity of potential implant materials. I: Zirconia-titania sintered ceramics. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 94, 305–311. [Google Scholar] [CrossRef]
- Kaluđerović, M.R.; Schreckenbach, J.P.; Graf, H.L. Zirconia coated titanium for implants and their interactions with osteoblast cells. Mater. Sci. Eng. C 2014, 44, 254–261. [Google Scholar] [CrossRef]
- Biological Evaluation of Medical Devices Part 5: Tests for in Vitro Cytotoxicity, International Organization for Standardization (ISO) 10993-5, 3rd ed.; ISO: Geneva, Switzerland, 2009.
- Chiriaco, F.; Conversano, F.; Sbenaglia, E.A.; Casciaro, S.; Leporatti, S.; Lay-Ekuakille, A. Cytotoxicity measurements of Halloysite Nanotubes for nanomedicine applications. In Proceedings of the 2014 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Lisboa, Portugal, 11–12 June 2014. [Google Scholar]
- Wu, J.M.; Hayakawa, S.; Tsuru, K.; Osaka, A. Low-temperature preparation of anatase and rutile layers on titanium substrates and their ability to induce in vitro apatite deposition. J. Am. Ceram. Soc. 2004, 87, 1635–1642. [Google Scholar] [CrossRef]
- Capel, F.; Moure, C.; Durán, P. Structural characterization and mixed conductivity of TiO2-doped ceria stabilized tetragonal zirconia. Ceram. Int. 2002, 28, 627–636. [Google Scholar] [CrossRef]
- Spagnuolo, G.; D’Antò, V.; Cosentino, C.; Schmalz, G.; Schweikl, H.; Rengo, S. Effect of N-acetyl-L-cysteine on ROS production and cell death caused by HEMA in human primary gingival fibroblasts. Biomaterials 2006, 27, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Josset, Y.; Oum’hamed, Z.; Zarrinpour, A.; Lorenzato, M.; Adnet, J.J.; Laurent-Maquin, D. In vitro reactions of human osteoblasts in culture with zirconia and alumina ceramics. J. Biomed. Mater. Res. 1999, 47, 481–493. [Google Scholar] [CrossRef]
- Douglas, G. IPCS guidelines for the monitoring of genotoxic effects of carcinogens in humans IPCS guidelines for the monitoring of genotoxic effects of carcinogens in humans. Mutat. Res. 2000, 463, 111–172. [Google Scholar]
- Takami, Y.; Nakazawa, T.; Makinouchi, K.; Glueck, J.; Nose, Y. Biocompatibility of alumina ceramic and polyethylene as materials for pivot bearings of a centrifugal blood pump. J. Biomed. Mater. Res. 1997, 36, 381–386. [Google Scholar] [CrossRef]
- Covacci, V.; Bruzzese, N.; Maccauro, G.; Andreassi, C.; Ricci, G.A.; Piconi, C.; Marmo, E.; Burger, W.; Cittadini, A. In vitro evaluation of the mutagenic and carcinogenic power of high purity zirconia ceramic. Biomaterials 1999, 20, 371–376. [Google Scholar] [CrossRef]
- Maccauro, G.; Bianchino, G.; Sangiorgi, S.; Magnani, G.; Marotta, D.; Manicone, P.F.; Raffaelli, L.; Rossi Iommetti, P.; Stewart, A.; Cittadini, A.; et al. Development of a new zirconia-toughened alumina: Promising mechanical properties and absence of in vitro carcinogenicity. Int. J. Immunopathol. Pharmacol. 2009, 22, 773–779. [Google Scholar] [CrossRef]
- Noushad, M.; Kannan, T.P.; Husein, A.; Abdullah, H.; Ismail, A.R. Toxicology in Vitro Genotoxicity evaluation of locally produced dental porcelain—An in vitro study using the Ames and Comet assays. Toxicol. Vitr. 2009, 23, 1145–1150. [Google Scholar] [CrossRef]
- Di Bucchianico, S.; Cappellini, F.; Le Bihanic, F.; Zhang, Y.; Dreij, K.; Karlsson, H.L. Genotoxicity of TiO2 nanoparticles assessed by mini-gel comet assay and micronucleus scoring with flow cytometry. Mutagenesis 2017, 32, 127–137. [Google Scholar] [CrossRef]
- Simon, A.; Gouget, B.; Mayne, M.; Herlin, N.; Reynaud, C.; Degrouard, J.; Carriere, M. In vitro investigation of TiO2,Al2O3, Au nanoparticles and mutli-walled carbon nanotubes cyto- and genotoxicity on lung, kidney cells and hepatocytes. Toxicol. Lett. 2007, 172, S36. [Google Scholar] [CrossRef]
- Lu, P.J.; Ho, I.C.; Lee, T.C. Induction of sister chromatid exchanges and micronuclei by titanium dioxide in Chinese hamster ovary-K1 cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1998, 414, 15–20. [Google Scholar] [CrossRef]
- Ghosh, M.; Bandyopadhyay, M.; Mukherjee, A. Genotoxicity of titanium dioxide (TiO2) nanoparticles at two trophic levels: Plant and human lymphocytes. Chemosphere 2010, 81, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Chakraborty, A.; Mukherjee, A. Cytotoxic, genotoxic and the hemolytic effect of titanium dioxide (TiO2) nanoparticles on human erythrocyte and lymphocyte cells in vitro. J. Appl. Toxicol. 2013, 33, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F. Single Cell Gel/Comet Assay: Guidelines for In Vitro and In Vivo Genetic Toxicology Testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Matsumoto, M.; Filho, H.N.; Ferrari, R.; Fernandes, K.; Renno, A.C.; Ribeiro, D. Genotoxicity of Endosseous Implants Using Two Cellular Lineages In Vitro. J. Oral Implantol. 2014, 40, 25–29. [Google Scholar] [CrossRef]
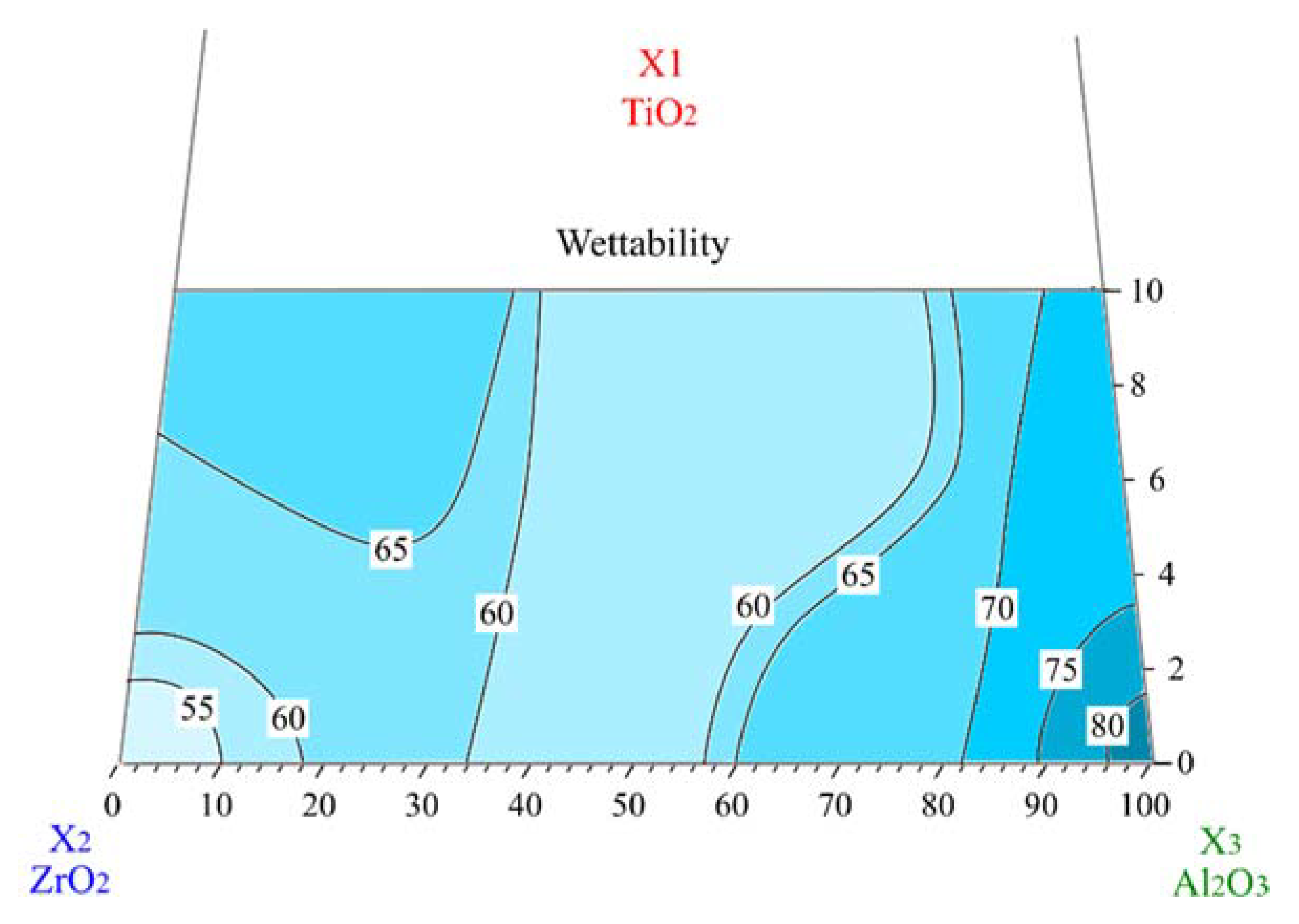
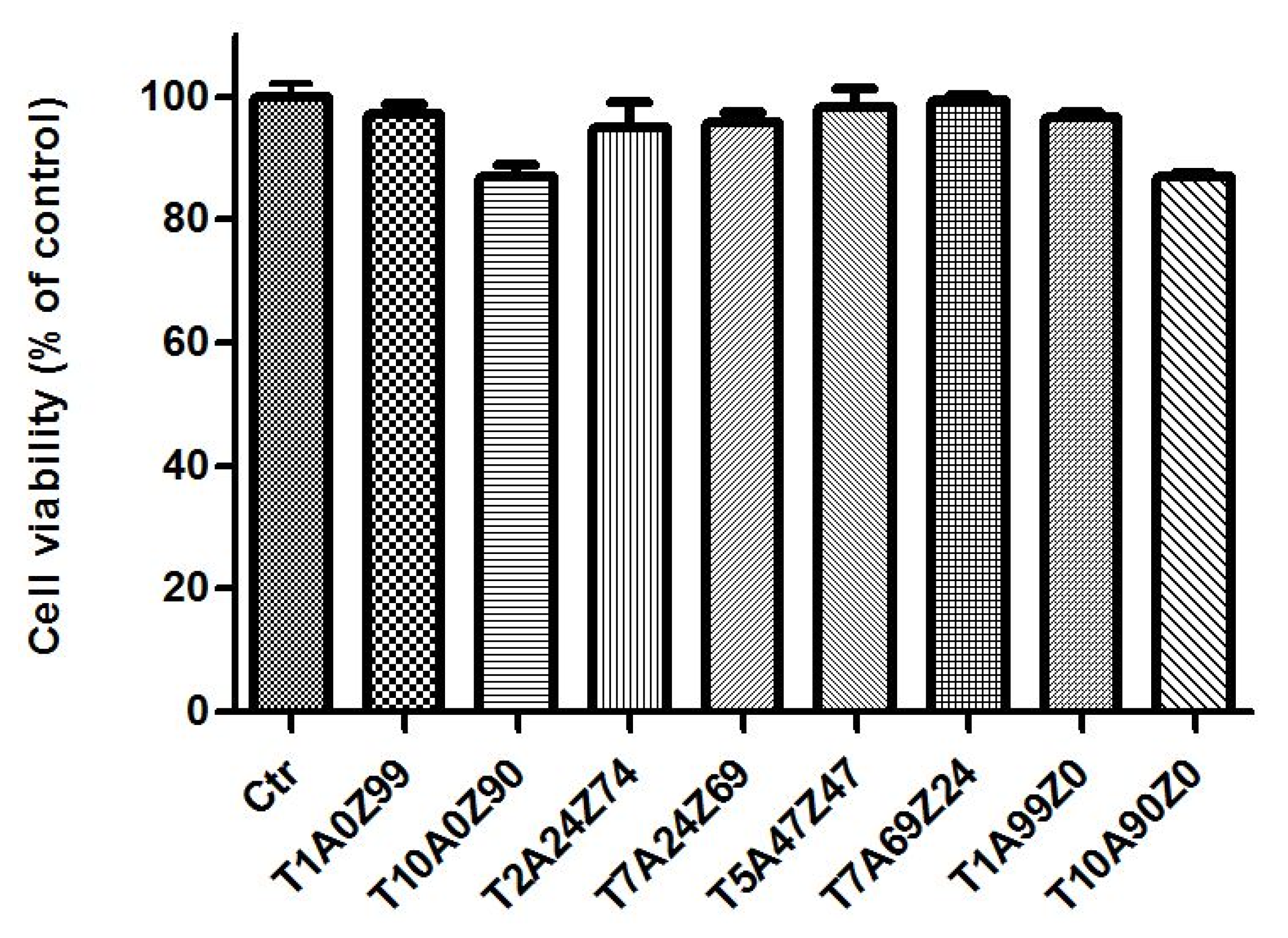
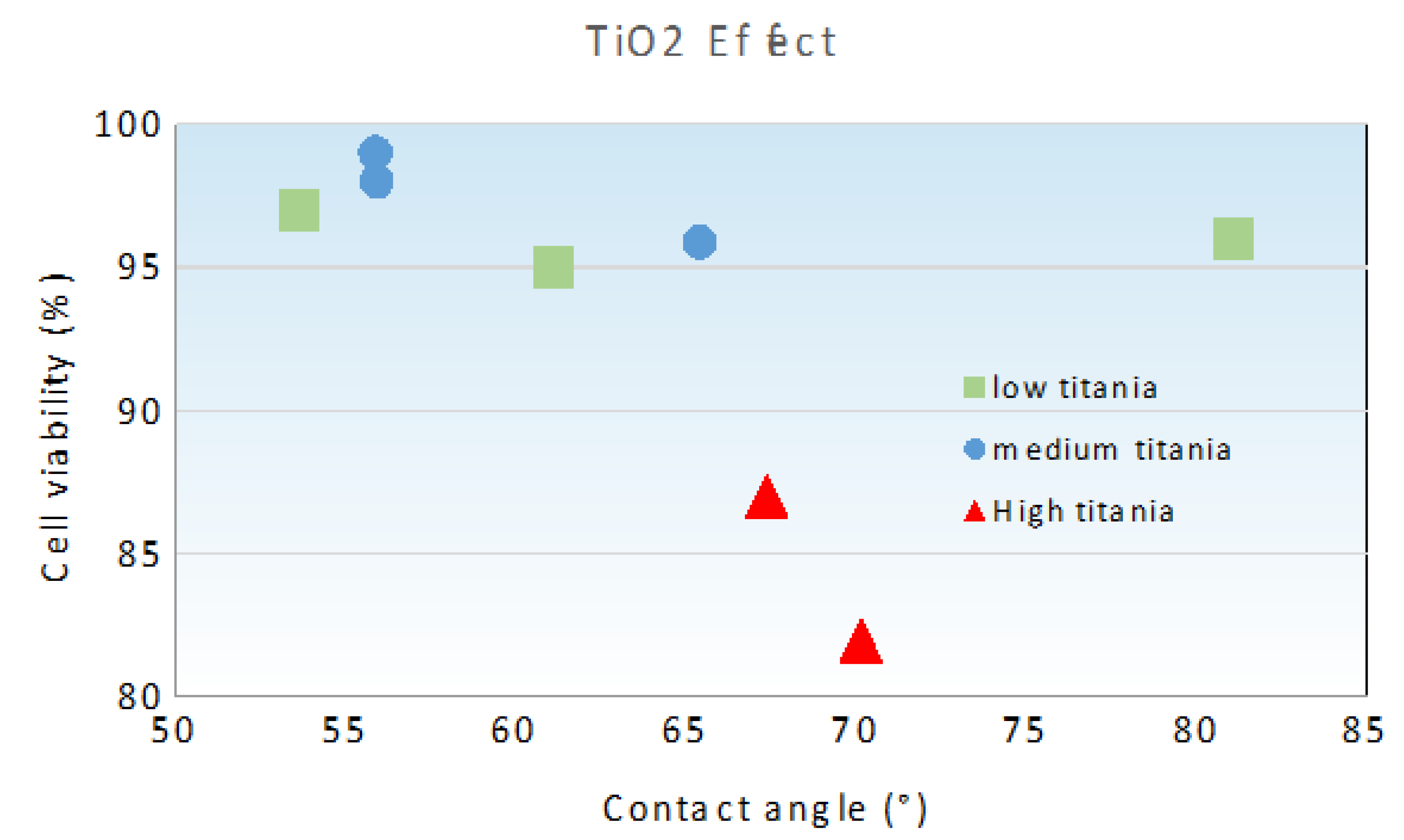
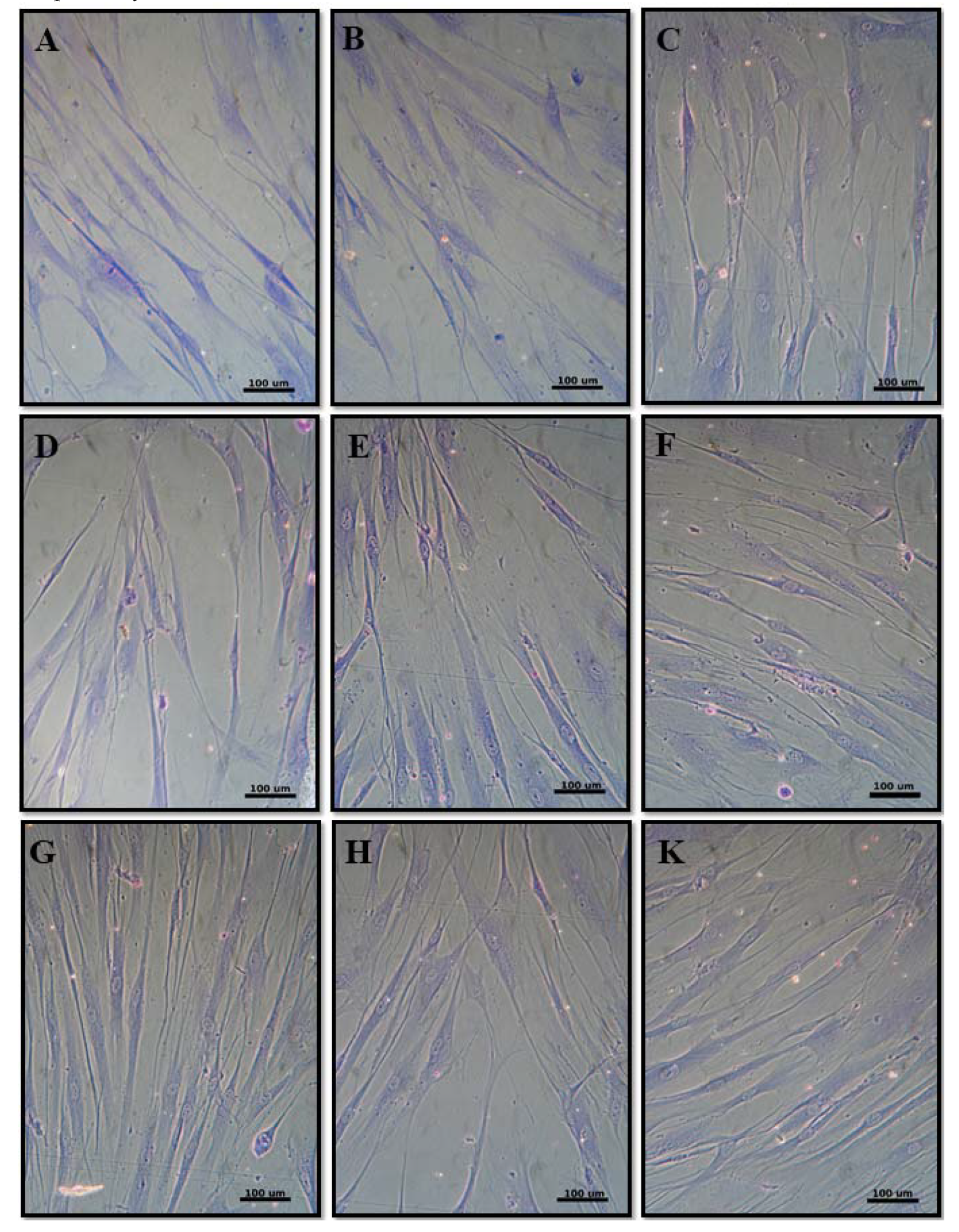
| Code | TiO2 | Al2O3 | ZrO2 (12 mol.% CeO2) |
|---|---|---|---|
| T1A0Z99 | 0.27 | 0 | 99.73 |
| T10A0Z90 | 10 | 0 | 90 |
| T1A99Z0 | 0.27 | 99.73 | 0 |
| T10A90Z0 | 10 | 90 | 0 |
| T5A0Z95 | 5 | 0 | 95 |
| T1A50Z50 | 0.27 | 49.87 | 49.87 |
| T10A45Z45 | 10 | 45 | 45 |
| T5A95Z0 | 5 | 95 | 0 |
| T5A47Z47 | 5 | 47.50 | 47.50 |
| T2A24Z74 | 2.5 | 23.75 | 73.75 |
| T7A24Z69 | 7.5 | 23.75 | 68.75 |
| T2A74Z24 | 2.5 | 73.75 | 23.75 |
| T7A69Z24 | 7.5 | 68.75 | 23.75 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaskhoussi, A.; Calabrese, L.; Currò, M.; Ientile, R.; Bouaziz, J.; Proverbio, E. Effect of the Compositions on the Biocompatibility of New Alumina–Zirconia–Titania Dental Ceramic Composites. Materials 2020, 13, 1374. https://doi.org/10.3390/ma13061374
Khaskhoussi A, Calabrese L, Currò M, Ientile R, Bouaziz J, Proverbio E. Effect of the Compositions on the Biocompatibility of New Alumina–Zirconia–Titania Dental Ceramic Composites. Materials. 2020; 13(6):1374. https://doi.org/10.3390/ma13061374
Chicago/Turabian StyleKhaskhoussi, Amani, Luigi Calabrese, Monica Currò, Riccardo Ientile, Jamel Bouaziz, and Edoardo Proverbio. 2020. "Effect of the Compositions on the Biocompatibility of New Alumina–Zirconia–Titania Dental Ceramic Composites" Materials 13, no. 6: 1374. https://doi.org/10.3390/ma13061374
APA StyleKhaskhoussi, A., Calabrese, L., Currò, M., Ientile, R., Bouaziz, J., & Proverbio, E. (2020). Effect of the Compositions on the Biocompatibility of New Alumina–Zirconia–Titania Dental Ceramic Composites. Materials, 13(6), 1374. https://doi.org/10.3390/ma13061374








