Improvement of AZ91 Alloy Corrosion Properties by Duplex NI-P Coating Deposition
Abstract
1. Introduction
2. Experimental Material and Procedures
3. Results and Discussion
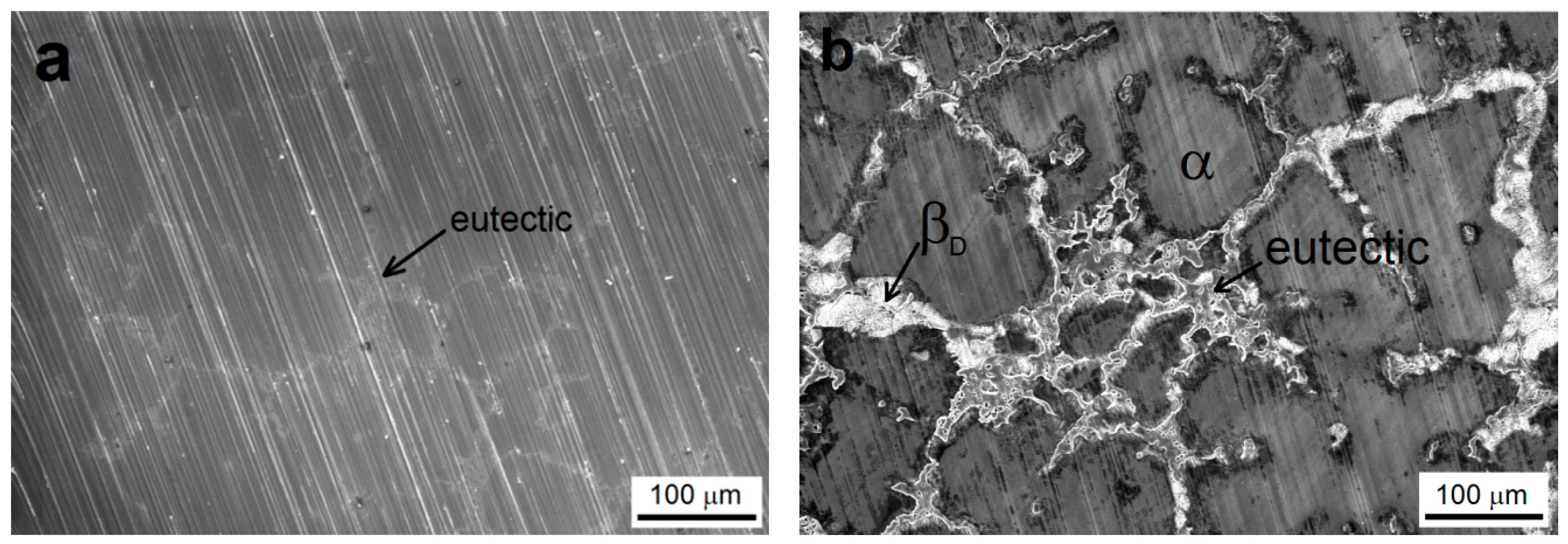
3.1. Pre-Treated AZ91 Magnesium Alloy Samples’ Surface Morphology
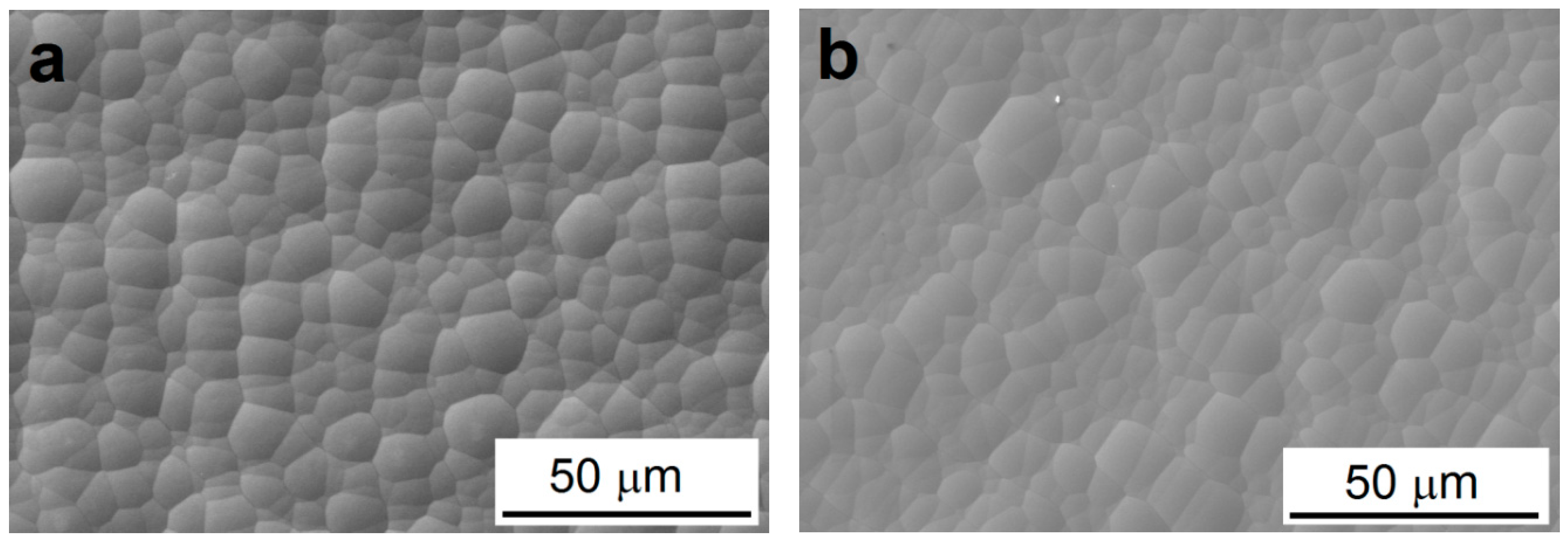
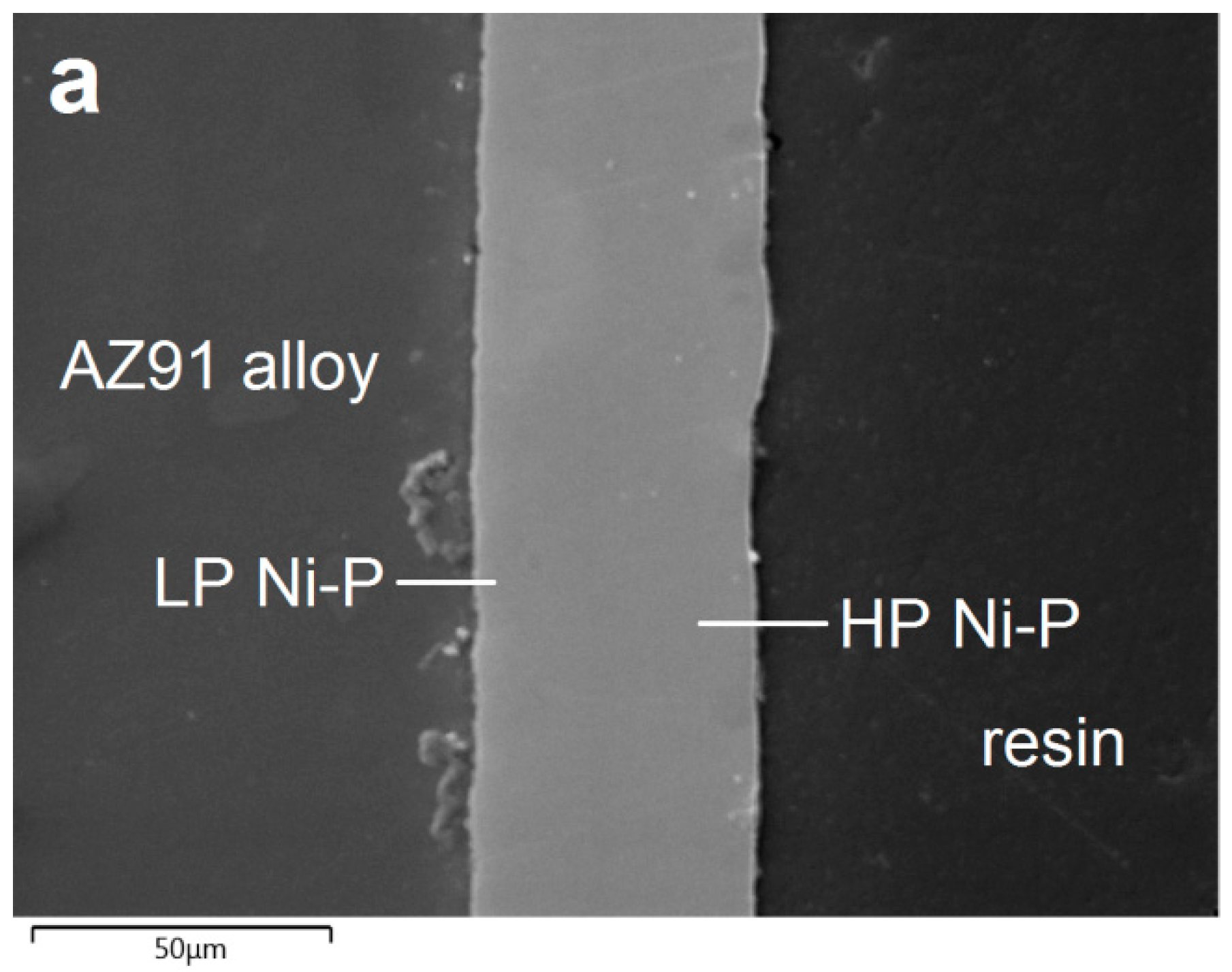
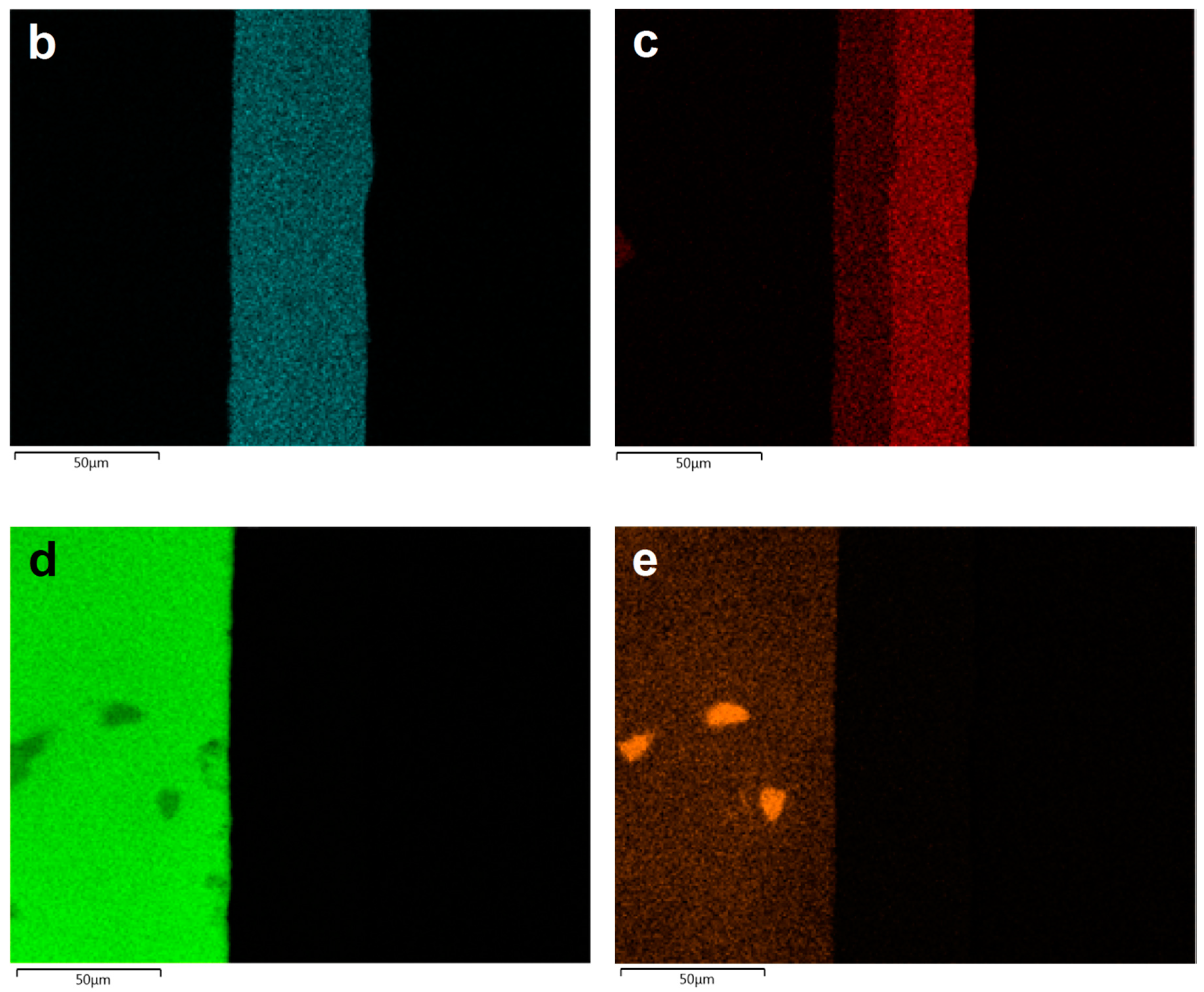
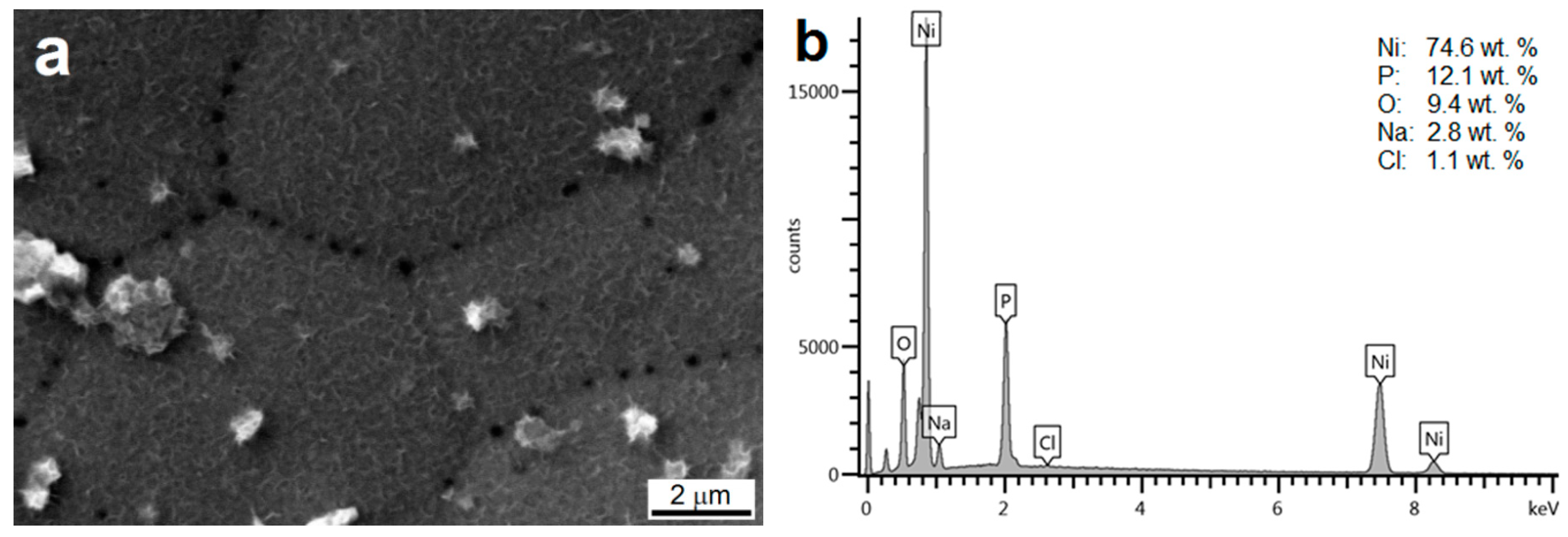
3.2. Morphology and Chemical Composition of Deposited NI-P Coatings
3.3. Microhardness of Deposited Coatings
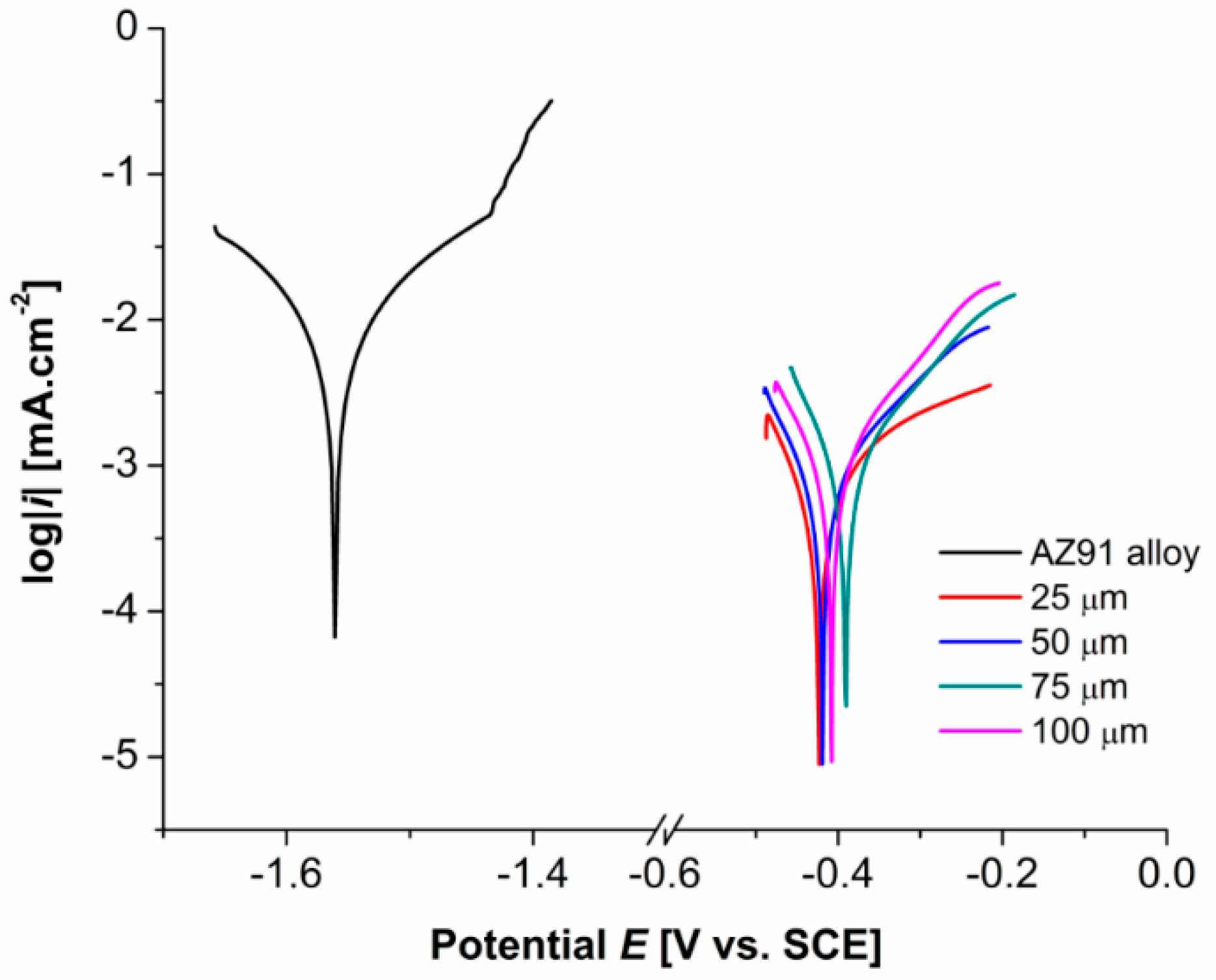
3.4. Electrochemical Polarization Measurements
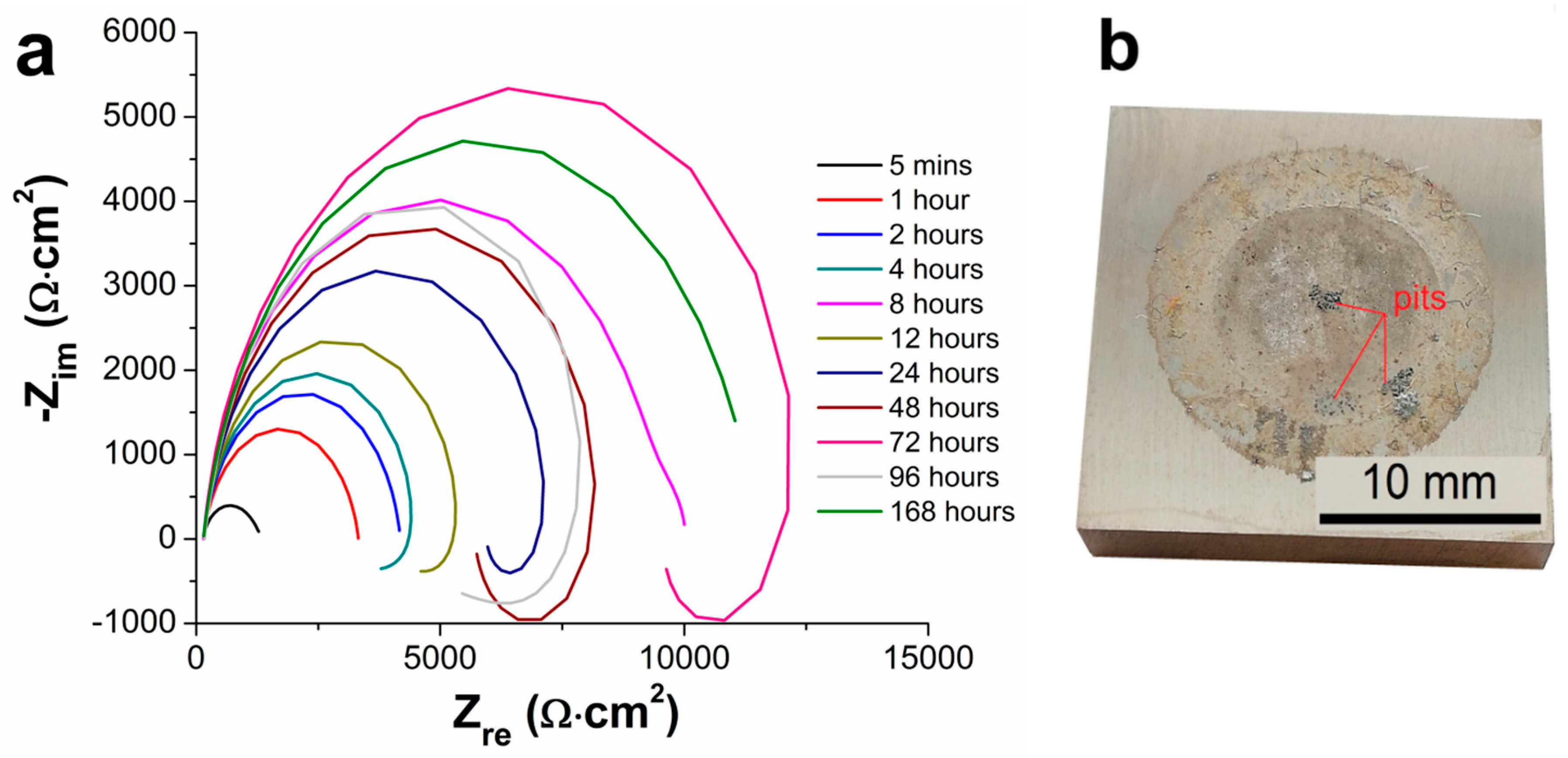
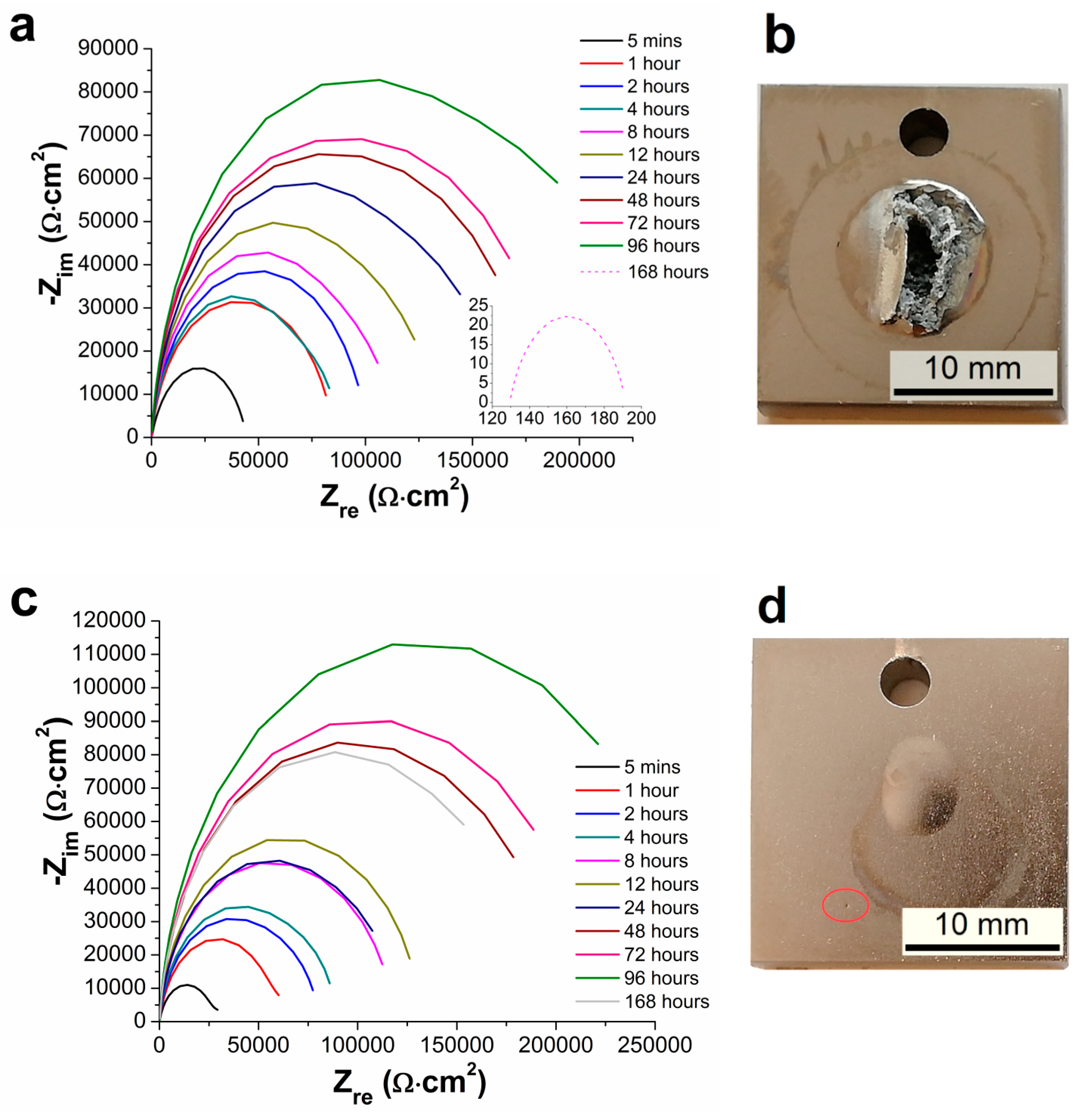
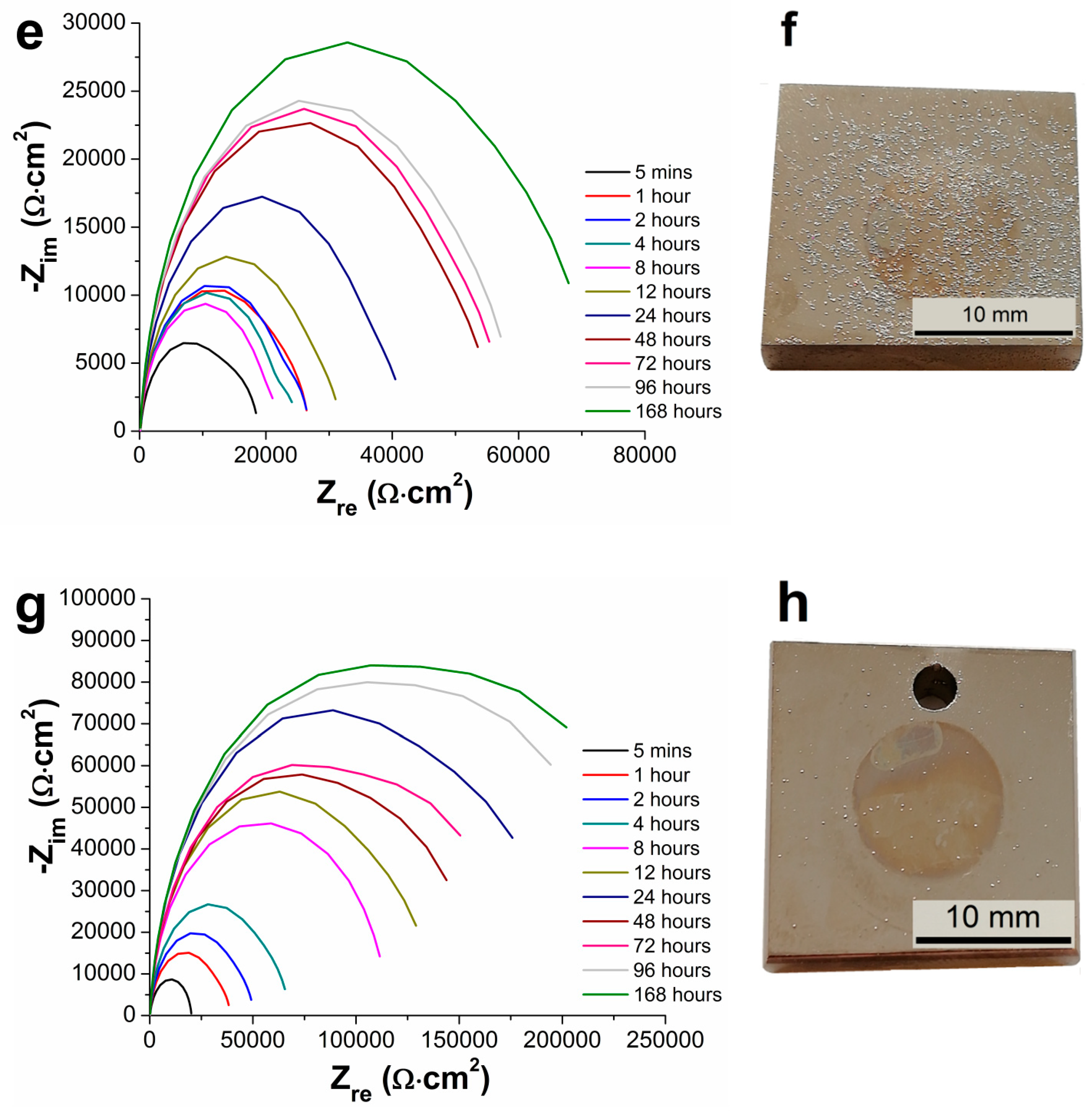
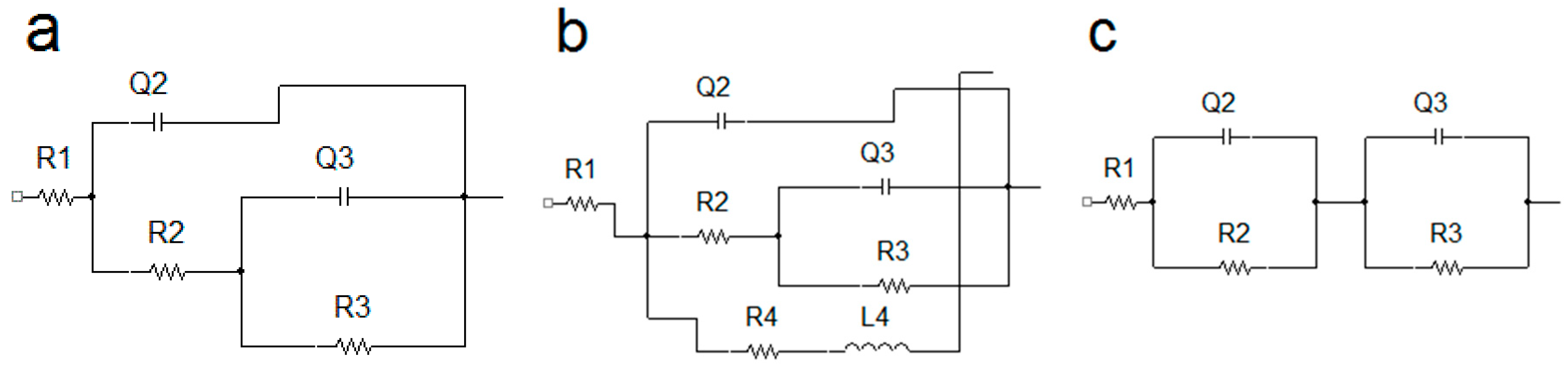
3.5. Electrochemical Impedance Spectroscopy Characteristics
3.6. Corrosion Behavior during Immersion Tests
3.7. Neutral Salt Spray Exposure
4. Conclusions
- Ni-P coatings with final thicknesses of 25, 50, 75 and 100 µm deposited on AZ91 magnesium alloy were formed by two layers—inner low-phosphorus Ni-P layer with the average P content of 5.7 wt.% and outer high-phosphorus Ni-P layer with the average P content of 11.5 wt.%.
- Low-phosphorus inner layer has the average microhardness of 620 ± 20 HV 0.025, and the outer high-phosphorus top layer has the average microhardness of 580 ± 10 HV 0.025.
- Deposited coatings significantly improved the corrosion resistance of the AZ91 magnesium alloy.
- Potentiodynamic tests in 0.1 M NaCl solution showed that the Ni-P duplex coatings deposited on AZ91 alloy improved the alloy electrochemical corrosion properties; a significant shift of Ecorr from −1563 mV to ~−430 mV was observed. The value of icorr decreased from 6.289 µA·cm−2 to 0.358 µA·cm−2. All coatings exhibited similar electrochemical characteristics, regardless of the coating thickness.
- From the results of long-term EIS measurements, the highest value of Rp = 250 167 Ω·cm2 in 0.1 M NaCl solution after 168 h for 100 µm thick coating was determined. All layers offered the same protection against the corrosion regardless of the coating thickness, except the coating with the thickness of 75 µm reaching slightly lower values of the polarization resistance.
- The immersion tests in 3.5% NaCl solution and 10% HCl solution and salt spray tests showed that with increasing thickness of the duplex Ni-P coating, the exposition time characterizing the resistance of specimens against the corrosion was increasing. In the case of the immersion test in 10% NaOH, the exposition time reached up to 1000 h for all coated samples.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Friedrich, H.; Mordike, B.L. Magnesium Technology: Metallurgy, Design Data, Applications; Springer: New York, NY, USA, 2006; ISSN 978-3-540-20599-9. [Google Scholar]
- Buchtík, M.; Kosár, P.; Wasserbauer, J.; Tkacz, J.; Dolezal, P. Characterization of Electroless Ni–P Coating Prepared on a Wrought ZE10 Magnesium Alloy. Coatings 2018, 8, 96. [Google Scholar] [CrossRef]
- Liu, H.; Cao, F.; Song, G.-L.; Zheng, D.; Shi, Z.; Dargusch, M.; Atrens, A. Review of the atmospheric corrosion of magnesium alloys. J. Mater. Sci. Technol. 2019, 35, 2003–2016. [Google Scholar] [CrossRef]
- Gupta, M.; Nai, M.L.S. Magnesium, Magnesium Alloys, and Magnesium Composites; John Wiley: New York, NY, USA, 2011; ISSN 978-0-470-49417-2. [Google Scholar]
- Song, G.-L. Corrosion Prevention of Magnesium Alloys; Elsevier BV: Oxford, UK; Cambridge, UK; Philadelphia, PA, USA; New Delhi, India, 2013; p. 978. [Google Scholar]
- Anthony, E.H.; Johannes, M.C.M.; Mikhail, L.Z.; Rudolph, G.B. Active Protective Coatings; Springer: New York, NY, USA, 2016; ISSN 978-9401775380. [Google Scholar]
- Riedel, W. Electroless Nickel Plating; Reprinted; ASM International: Cleveland, OH, USA, 1991; p. 320. ISSN 9780904477122. [Google Scholar]
- Parkinson, R. Properties and Applications of Electroless Nickel; Nickel Development Institute: Toronto, ON, Canada, 1997. [Google Scholar]
- Mallory, G.O.; Hajdu, J.B. Electroless Coating: Fundamentals and Applications; Knoyes Publishing: William Andrew, UK, 2009; p. 575. [Google Scholar]
- Sudagar, J.; Lian, J.; Sha, W. Electroless nickel, alloy, composite and nano coatings—A critical review. J. Alloys Compd. 2013, 571, 183–204. [Google Scholar] [CrossRef]
- Agarwala, R.C.; Agarwala, V. Electroless alloy/composite coatings: A review. Sadhana 2003, 28, 475–493. [Google Scholar] [CrossRef]
- Lo, P.-H.; Tsai, W.-T.; Lee, J.-T.; Hung, M.-P. Role of phosphorus in the electrochemical behavior of electroless Ni-P alloys in 3.5 wt.% NaCl solutions. Surf. Coat. Technol. 1994, 67, 27–34. [Google Scholar] [CrossRef]
- Gu, C.; Lian, J.; Li, G.; Niu, L.; Jiang, Z. High corrosion-resistant Ni–P/Ni/Ni–P multilayer coatings on steel. Surf. Coat. Technol. 2005, 197, 61–67. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, Z.; Li, G.; Jiang, Q.; Li, G. Electroless Ni–Sn–P coating on AZ91D magnesium alloy and its corrosion resistance. Surf. Coat. Technol. 2008, 202, 2570–2576. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, Z.; Li, G.; Jiang, Q.; Li, G. Electroless Ni-P/Ni-B duplex coatings for improving the hardness and the corrosion resistance of AZ91D magnesium alloy. Appl. Surf. Sci. 2008, 254, 4949–4955. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, N.; He, J.; Jiang, Z.; Jiang, Q.; Li, G. Electroless deposition of Ni–W–P coating on AZ91D magnesium alloy. Appl. Surf. Sci. 2007, 253, 5116–5121. [Google Scholar] [CrossRef]
- Zhang, J. Corrosion Behavior of Electroless Ni-P/Ni-B Coating on Magnesium Alloy AZ91D in NaCl Environment. Int. J. Electrochem. Sci. 2016, 11, 10053–10066. [Google Scholar] [CrossRef]
- Czerwinski, F. Magnesium Alloys: Design, Processing and Properties; InTech: Rijeka, Croatia, 2011; ISBN 978-953-307-520-4. [Google Scholar]
- Sun, C.; Guo, X.; Wang, S.-H.; Guo, J.-C.; Ding, W. Homogenization pretreatment and electroless Ni–P plating on AZ91D magnesium alloy. Trans. Nonferrous Met. Soc. China 2014, 24, 3825–3833. [Google Scholar] [CrossRef]
- Zhang, W.; He, J.; Jiang, Z.; Jiang, Q.; Li, G. Electroless Ni–P layer with a chromium-free pretreatment on AZ91D magnesium alloy. Surf. Coat. Technol. 2007, 201, 4594–4600. [Google Scholar] [CrossRef]
- Tkacz, J.; Slouková, K.; Minda, J.; Drábiková, J.; Fintová, S.; Dolezal, P.; Wasserbauer, J. Influence of the Composition of the Hank’s Balanced Salt Solution on the Corrosion Behavior of AZ31 and AZ61 Magnesium Alloys. Metals 2017, 7, 465. [Google Scholar] [CrossRef]
- Ambat, R.; Zhou, W. Electroless nickel-plating on AZ91D magnesium alloy: Effect of substrate microstructure and plating parameters. Surf. Coat. Technol. 2004, 179, 124–134. [Google Scholar] [CrossRef]
- Zarebidaki, A.; Mahmoudikohani, H.; Aboutalebi, M.-R. Microstructure and corrosion behavior of electrodeposited nano-crystalline nickel coating on AZ91 Mg alloy. J. Alloy. Compd. 2014, 615, 825–830. [Google Scholar] [CrossRef]
- Wang, H.-L.; Liu, L.-Y.; Dou, Y.; Zhang, W.-Z.; Jiang, W.-F. Preparation and corrosion resistance of electroless Ni-P/SiC functionally gradient coatings on AZ91D magnesium alloy. Appl. Surf. Sci. 2013, 286, 319–327. [Google Scholar] [CrossRef]
- Shu, X.; Wang, Y.; Liu, C.; Aljaafari, A.; Gao, W. Double-layered Ni-P/Ni-P-ZrO 2 electroless coatings on AZ31 magnesium alloy with improved corrosion resistance. Surf. Coat. Technol. 2015, 261, 161–166. [Google Scholar] [CrossRef]
- Duncan, R.N. The metallurgical structure of electroless nickel deposits: Effect on coating properties. Plat. Surface Finish. 1996, 8, 65–69. [Google Scholar]
- Mainier, F.B.; Fonseca, M.P.C.; Tavares, S.S.M.; Pardal, J.M. Quality of Electroless Ni-P (Nickel-Phosphorus) Coatings Applied in Oil Production Equipment with Salinity. J. Mater. Sci. Chem. Eng. 2013, 1, 1–8. [Google Scholar]
- Sampath, K.P.; Kesavan, N.P. Studies on crystallization of electroless Ni-P deposits. J. Mater. Process. Technol. 1996, 56, 511–520. [Google Scholar] [CrossRef]
- Czagany, M.; Baumli, P. Effect of pH on the characteristics of electroless Ni-P coatings. J. Min. Met. Sect. B Met. 2017, 53, 327–332. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Corrosion Mechanisms of Magnesium Alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Březina, M.; Minda, J.; Dolezal, P.; Krystýnová, M.; Fintová, S.; Zapletal, J.; Wasserbauer, J.; Ptáček, P. Characterization of Powder Metallurgy Processed Pure Magnesium Materials for Biomedical Applications. Metals 2017, 7, 461. [Google Scholar] [CrossRef]
- Lampke, T.; Leopold, A.; Dietrich, D.; Alisch, G.; Wielage, B. Correlation between structure and corrosion behaviour of nickel dispersion coatings containing ceramic particles of different sizes. Surf. Coat. Technol. 2006, 201, 3510–3517. [Google Scholar] [CrossRef]
- Fini, M.H.; Amadeh, A. Improvement of wear and corrosion resistance of AZ91 magnesium alloy by applying Ni–SiC nanocomposite coating via pulse electrodeposition. Trans. Nonferrous Met. Soc. China 2013, 23, 2914–2922. [Google Scholar] [CrossRef]
- Alishahi, M.; Monirvaghefi, S.M.; Saatchi, A.; Hosseini, S. The effect of carbon nanotubes on the corrosion and tribological behavior of electroless Ni–P–CNT composite coating. Appl. Surf. Sci. 2012, 258, 2439–2446. [Google Scholar] [CrossRef]
- Zeng, R.; Zhang, J.; Huang, W.; Dietzel, W.; Kainer, K.; Blawert, C.; Ke, W. Review of studies on corrosion of magnesium alloys. Trans. Nonferrous Met. Soc. China 2006, 16, s763–s771. [Google Scholar] [CrossRef]
- Orel, Z.C.; Hutchins, M.; Mcmeeking, G. The electrochromic properties of hydrated nickel oxide films formed by colloidal and anodic deposition. Sol. Energy Mater. Sol. Cells 1993, 30, 327–337. [Google Scholar] [CrossRef]
- Zeller, R.L.; Salvati, L. Effects of Phosphorus on Corrosion Resistance of Electroless Nickel in 50% Sodium Hydroxide. Corrosion 1994, 50, 457–467. [Google Scholar] [CrossRef]
- Chen, B.-H.; Hong, L.; Ma, Y.; Ko, T.-M. Effects of Surfactants in an Electroless Nickel-Plating Bath on the Properties of Ni−P Alloy Deposits. Ind. Eng. Chem. Res. 2002, 41, 2668–2678. [Google Scholar] [CrossRef]
- Balaraju, J.; Narayanan, T.S.; Seshadri, S. Electroless Ni–P composite coatings. J. Appl. Electrochem. 2003, 33, 807–816. [Google Scholar] [CrossRef]
- Fan, L.; Tang, F.; Reis, S.T.; Chen, G.; Koenigstein, M.L. Corrosion Resistances of Steel Pipes Internally Coated with Enamel. Corrosion 2017, 73, 1335–1345. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, X.; Liu, B.; Liu, J.; Han, X.; Deng, Y.; Hu, W.; Zhong, C. One-Step Fabrication and Localized Electrochemical Characterization of Continuous Al-Alloyed Intermetallic Surface Layer on Magnesium Alloy. Coatings 2018, 8, 148. [Google Scholar] [CrossRef]
- Song, G.-L.; Atrens, A.; Wu, X.; Zhang, B. Corrosion behaviour of AZ21, AZ501 and AZ91 in sodium chloride. Corros. Sci. 1998, 40, 1769–1791. [Google Scholar] [CrossRef]
- Fan, L.; Reis, S.T.; Chen, G.; Koenigstein, M. Corrosion Resistance of Pipeline Steel with Damaged Enamel Coating and Cathodic Protection. Coatings 2018, 8, 185. [Google Scholar] [CrossRef]
- Song, Y.; Shan, D.; Han, E.-H. High corrosion resistance of electroless composite plating coatings on AZ91D magnesium alloys. Electrochim. Acta 2008, 53, 2135–2143. [Google Scholar] [CrossRef]
- Chen, M.-A.; Cheng, N.; Li, J.-M.; Liu, S. Improvement to corrosion resistance of Ni–P coating on MAO magnesium alloy by BTESPT. Surf. Eng. 2012, 28, 491–497. [Google Scholar] [CrossRef]
- Moore, R.L.; Salvati, J.L. Investigation of the correlation between surface properties and surface chemistry of electroless nickel coatings. Thin Solid Films. 1990, 193, 1–574. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, S.T.; Dryfe, R. A study of corrosion performance of electroless Ni-P and Ni-W-P coatings on AZ91D magnesium alloy. Mater. Werkst. 2011, 42, 833–837. [Google Scholar] [CrossRef]
- Wang, L.L.; Chen, H.J.; Hao, L.; Lin, A.; Gan, F.X. Electrochemical corrosion behavior of electroless Ni-P coating in NaCl and H2SO4 solutions. Mater. Corros. 2010, 62, 1003–1007. [Google Scholar] [CrossRef]
| Al | Zn | Mn | Si | Fe | Ni | Zr | Mg | |
|---|---|---|---|---|---|---|---|---|
| Content | 8.80 | 0.81 | 0.32 | 0.01 | <0.01 | <0.01 | 0.01 | Balance |
| Process | Parameters | Conditions |
|---|---|---|
| Grinding | SiC paper | No. 1200 |
| Alkaline degreasing | Soil releasing agents | pH < 12, higher temp., 20 min |
| Acid pickling | Acid bath | 25 °C, 5 s |
| Low-phosphorus electroless nickel bath | NiSO4·6H2O NaH2PO2·H2O complexing agent activator | 1.5/2 h (14/18 µm) 60 °C pH 6.8 ± 0.1 |
| High-phosphorus electroless nickel bath | Industrial NiChem HP 1151 (Atotech) | 1.5/3/5.5/8.5 h 90 °C pH 4.7 ± 0.1 |
| Thickness of Ni-P Coating [µm] | Ecorr [mV] | icorr [µA·cm−2] |
|---|---|---|
| AZ91 alloy | −1563 | 6.289 |
| 25 | −425 | 0.358 |
| 50 | −434 | 0.371 |
| 75 | −431 | 0.365 |
| 100 | −410 | 0.457 |
| Samples | Rp [ Ω·cm2] | |||||
| 5 Minutes | 1 Hour | 2 Hours | 4 Hours | 8 Hours | 12 Hours | |
| AZ91 alloy | 1207 | 3167 | 2588 | 2152 | 9880 | 3685 |
| Ni-P 25 µm | 47,787 | 85,110 | 101,112 | 86,969 | 114,169 | 134,782 |
| Ni-P 50 µm | 36,730 | 70,723 | 80,356 | 88,790 | 118,200 | 131,146 |
| Ni-P 75 µm | 18,694 | 26,688 | 26,591 | 24,794 | 22,212 | 31,847 |
| Ni-P 100 µm | 20,136 | 38,920 | 50,203 | 67,388 | 115,983 | 138,065 |
| Samples | Rp [ Ω·cm2] | |||||
| 24 Hours | 48 Hours | 72 Hours | 96 Hours | 168 Hours | − | |
| AZ91 alloy | 5,786 | 5,543 | 9,011 | 1,294 | 11,342 | − |
| Ni-P 25 µm | 164,038 | 181,489 | 190,141 | 233,570 | 63 | − |
| Ni-P 50 µm | 120,846 | 201,011 | 214,179 | 264,982 | 218,359 | − |
| Ni-P 75 µm | 42,016 | 55,873 | 57,294 | 59,391 | 72,095 | − |
| Ni-P 100 µm | 199,510 | 159,432 | 173,856 | 242,660 | 250,167 | − |
| Thickness of Duplex Ni-P Coating [µm] | Environment | |||
|---|---|---|---|---|
| 10% HCl Solution | 3.5% NaCl Solution | 10% NaOH Solution | Exposition Time NSS | |
| 25 | 2 | 264 | 1000 | 96 |
| 50 | 5 | 408 | 1000 | 149 |
| 75 | 11 | 552 | 1000 | 332 |
| 100 | 24 | 792 | 1000 | 430 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasserbauer, J.; Buchtík, M.; Tkacz, J.; Fintová, S.; Minda, J.; Doskočil, L. Improvement of AZ91 Alloy Corrosion Properties by Duplex NI-P Coating Deposition. Materials 2020, 13, 1357. https://doi.org/10.3390/ma13061357
Wasserbauer J, Buchtík M, Tkacz J, Fintová S, Minda J, Doskočil L. Improvement of AZ91 Alloy Corrosion Properties by Duplex NI-P Coating Deposition. Materials. 2020; 13(6):1357. https://doi.org/10.3390/ma13061357
Chicago/Turabian StyleWasserbauer, Jaromír, Martin Buchtík, Jakub Tkacz, Stanislava Fintová, Jozef Minda, and Leoš Doskočil. 2020. "Improvement of AZ91 Alloy Corrosion Properties by Duplex NI-P Coating Deposition" Materials 13, no. 6: 1357. https://doi.org/10.3390/ma13061357
APA StyleWasserbauer, J., Buchtík, M., Tkacz, J., Fintová, S., Minda, J., & Doskočil, L. (2020). Improvement of AZ91 Alloy Corrosion Properties by Duplex NI-P Coating Deposition. Materials, 13(6), 1357. https://doi.org/10.3390/ma13061357






