Deterioration Process of Concrete Exposed to Internal Sulfate Attack
Abstract
:1. Introduction
2. Research Significance
3. Materials and Mixes
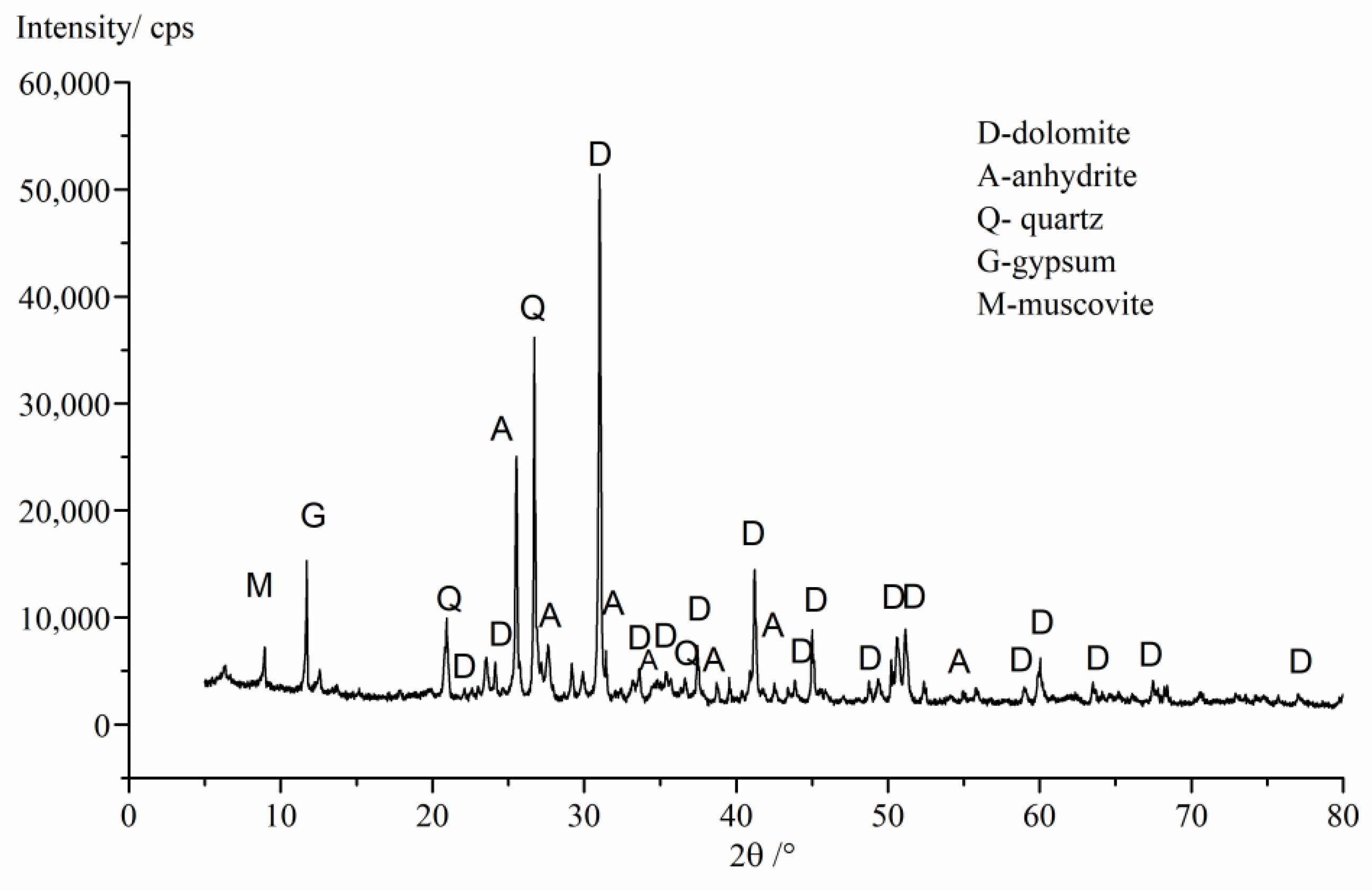
3.1. Raw Materials
3.2. Mix Proportion
4. Methods
4.1. Expansion Test on Concrete
4.2. Compressive Strength of Concrete
4.3. Ultrasonic Test on Prism Concrete
4.4. XRD and Thermogravimetry–Differential Scanning Calorimetry (TG–DSC) Analysis on Mortar
4.5. Microstructure Examination on Concrete
4.6. Dissolution of WP in Concrete
5. Results
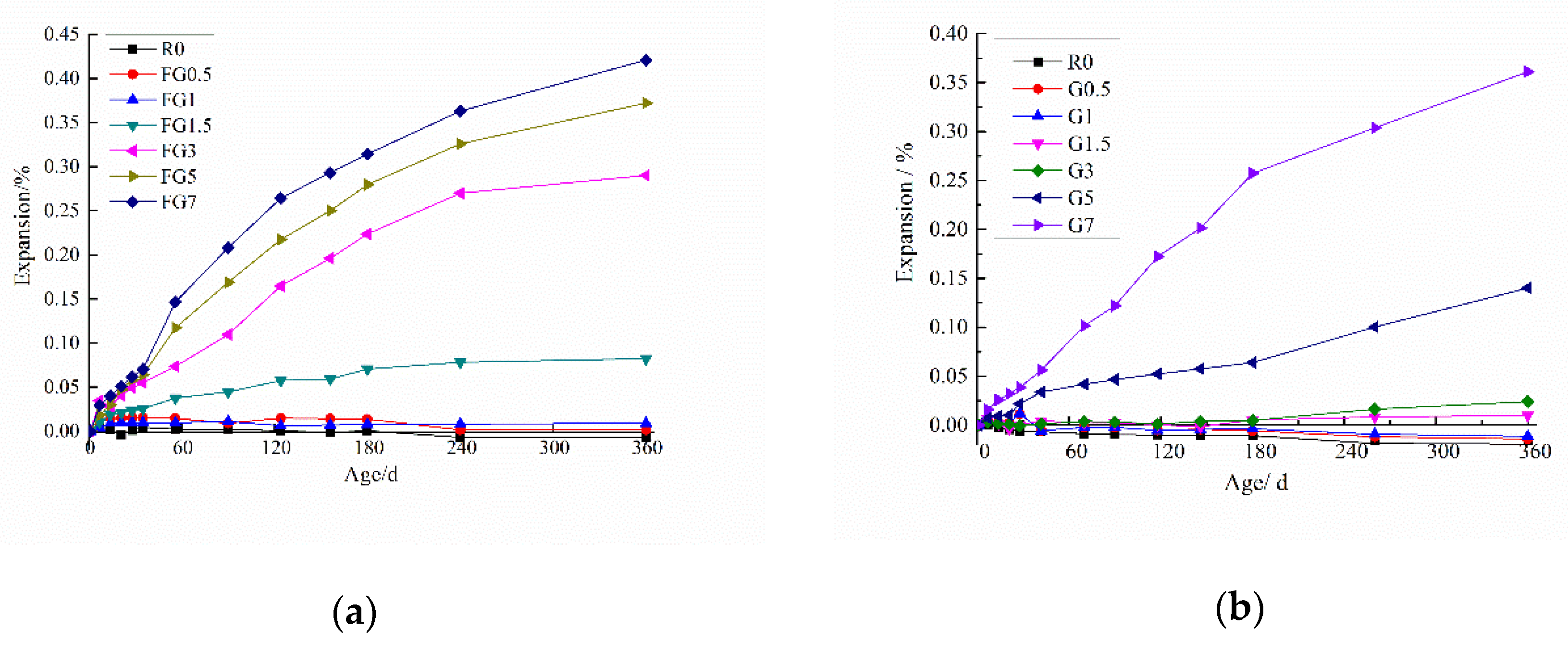
5.1. Expansion Measurements
5.2. Compressive Strength
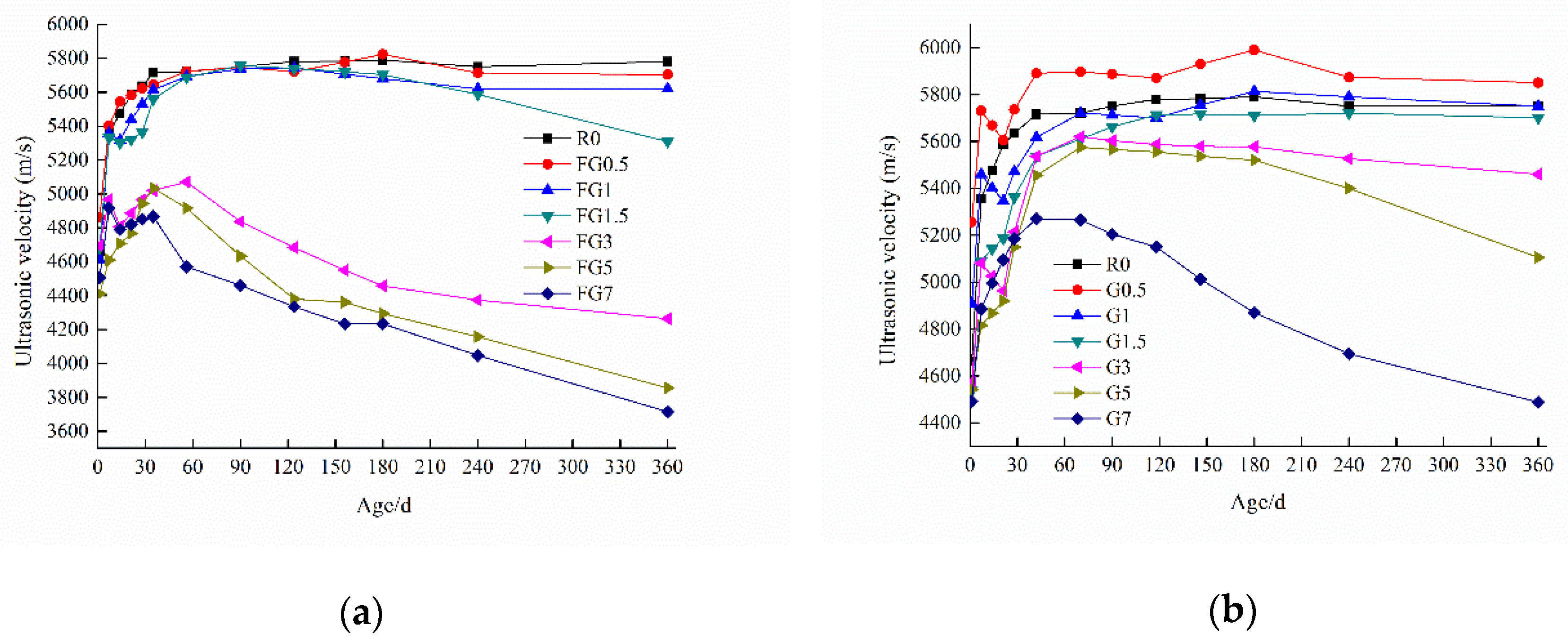
5.3. Ultrasonic Test
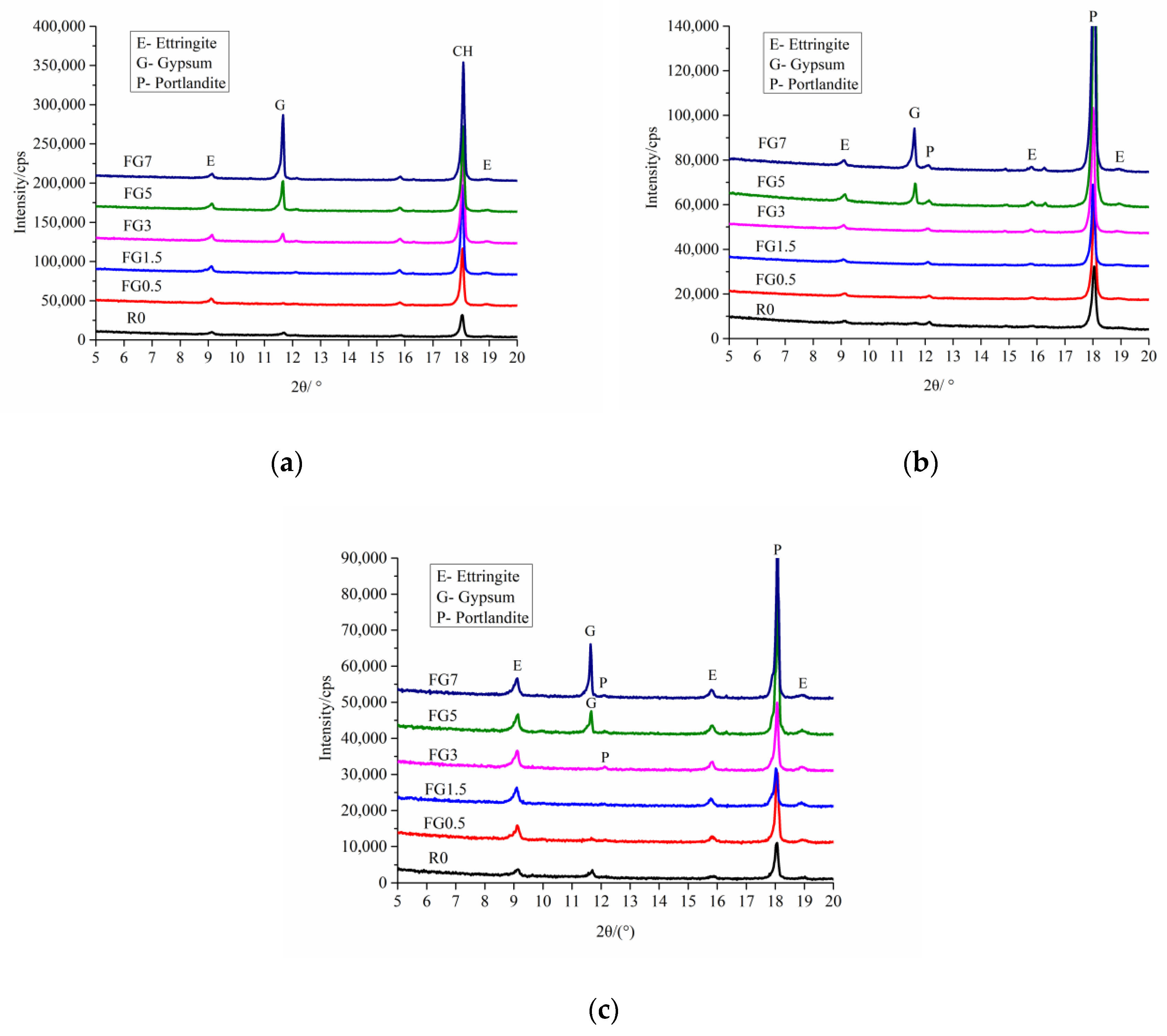
5.4. Mineral Composition Examination
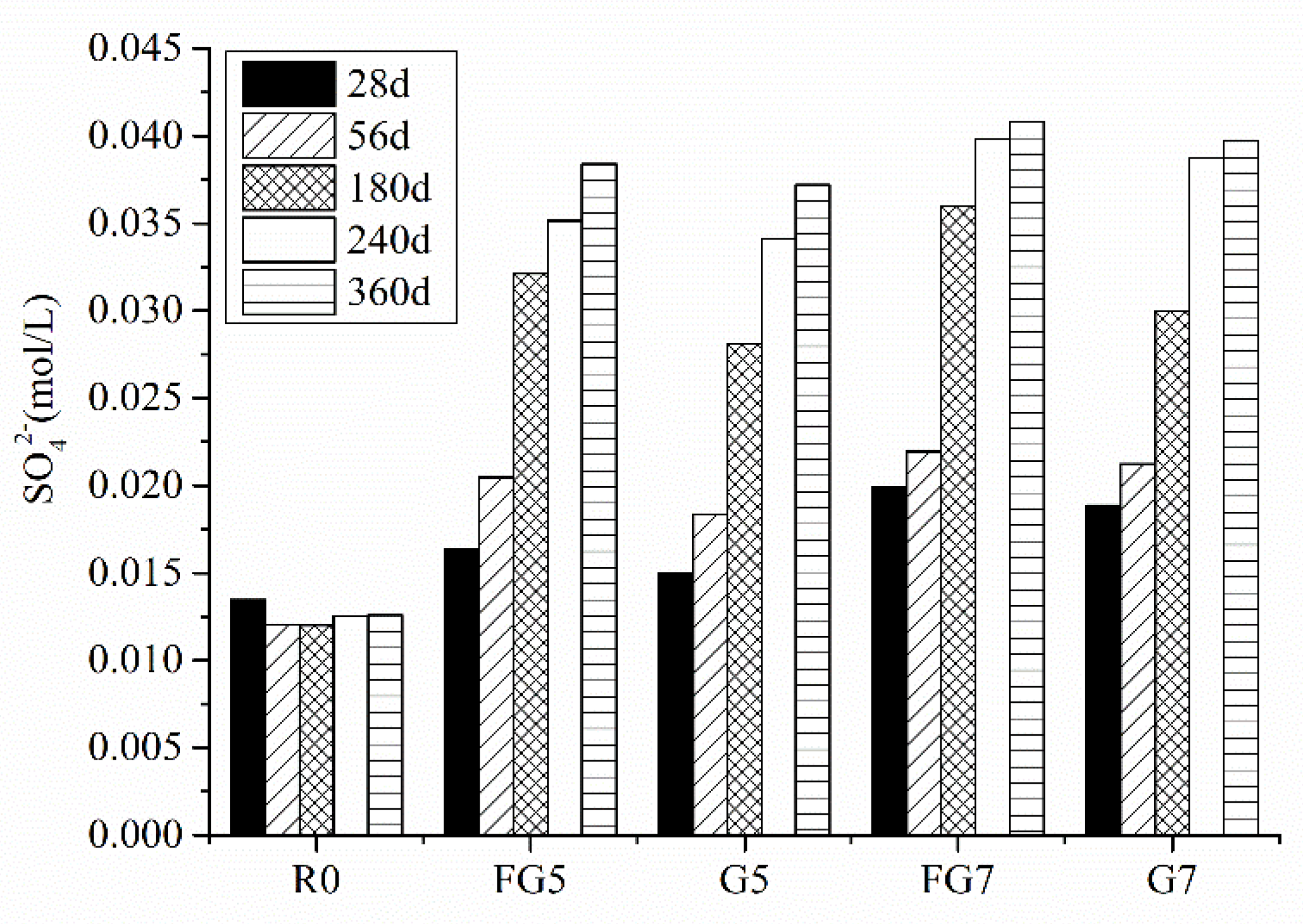
5.5. WP Dissolution in Concrete
5.6. Microstructure
6. Conclusions
- With an increase in the SO3 content in aggregate, the concrete expansion increased, and the compressive strength decreased. However, if the SO3 content was lower than 1.5% and 3% in fine aggregate and coarse aggregate, respectively. Although, the expansion was not obvious, and the compressive strength of samples was above the initial strength of the reference specimen. The critical values of SO3 content in fine and coarse aggregate were found to be 1.25% and 3%, respectively. At values above these harmful expansion and large strength loss appear.
- SO42− dissolved more easily when the particle size of aggregate was smaller. When the SO3 content in the sand was less than 1.5% at 56 days, all the gypsum was dissolved. That was the reason why the expansion of specimens with a smaller SO3 content (1.25% for fine aggregate and 3% for coarse aggregate) was not significant.
- The concentration of sulfate ions in the pore solution of concrete was not enough to form a new phase of gypsum when the SO3 content was less than 7% in aggregate. For the internal sulfate attack, the main reaction product is ettringite, which causes the expansion of specimens.
- The fracture mode of internal sulfate attack was the crack extending from gypsum particles to paste, which made the aggregate separate from the paste.
- In this paper, only TG–DSC was used to analyze the content of major phases, and no other methods were combined. This paper only studied the changes in compressive strength and expansion performance of concrete containing gypsum aggregates for 360 days. To study whether it is safe to use gypsum aggregates in concrete, other durability tests are needed. In addition, there has been lack of evaluation on methods that can be used to determine the safe dosage of gypsum aggregate in concrete at home and abroad, which will be the focus of our follow-up work.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hime, W.G.; Mather, B. “Sulfate attack,” or is it. Cem. Concr. Res. 1999, 29, 789–791. [Google Scholar] [CrossRef]
- Skalny, J.; Marchand, J.; Odler, I. Sulfate Attack on Concrete; Spon Press: London, UK, 2002. [Google Scholar]
- Leemann, A.; Löser, R. Analysis of concrete in a vertical ventilation shaft exposed to sulfate-containing groundwater for 45 years. Cem. Concr. Compos. 2011, 33, 74–83. [Google Scholar] [CrossRef]
- Ma, B.; Gao, X.; Byars, E.A.; Zhou, Q. Thaumasite formation in a tunnel of Bapanxia Dam in Western China. Cem. Concr. Res. 2006, 36, 716–722. [Google Scholar] [CrossRef]
- Gesoglu, M.; Guneyisi, E.; Nahhab, A.H.; Yazıcı, H. The effect of aggregates with high gypsum content on the performance of ultra-high strength concretes and Portland cement mortars. Constr. Build. Mater. 2016, 110, 346–354. [Google Scholar] [CrossRef]
- Crammond, N. The thaumasite form of sulfate attack in the UK. Cem. Concr. Compos. 2003, 25, 809–818. [Google Scholar] [CrossRef]
- Janković, K.; Miličić, L.; Stanković, S.; Šušić, N. Investigation of the mortar and concrete resistance in aggressive solutions. Tehnički Vjesnik 2014, 21, 173–176. [Google Scholar]
- Fu, Y.; Gu, P.; Xie, P.; Beaudoin, J. A kinetic study of delayed ettringite formation in hydrated portland cement paste. Cem. Concr. Res. 1995, 25, 63–70. [Google Scholar] [CrossRef]
- Clark, B.A.; Brown, P.W. The formation of ettringite from tricalcium aluminate and magnesium sulphate. Adv. Cem. Res. 2000, 12, 137–142. [Google Scholar] [CrossRef]
- Tian, B.; Cohen, M.D. Expansion of Alite Paste Caused by Gypsum Formation during Sulfate Attack. J. Mater. Civ. Eng. 2000, 12, 24–25. [Google Scholar] [CrossRef]
- Bellmann, F.; Moser, B.; Stark, J. Influence of sulfate solution concentration on the formation of gypsum in sulfate resistance test specimen. Cem. Concr. Res. 2006, 36, 358–363. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Sulfate attack research—Whither now? Cem. Concr. Res. 2001, 31, 845–851. [Google Scholar] [CrossRef]
- Scholtzová, E.; Kucková, L.; Kožíšek, J.; Palkova, H.; Tunega, D. Experimental and computational study of thaumasite structure. Cem. Concr. Res. 2014, 59, 66–72. [Google Scholar] [CrossRef]
- Kohler, S.; Heinz, D.; Urbonas, L. Effect of ettringite on thaumasite formation. Cem. Concr. Res. 2006, 36, 697–706. [Google Scholar] [CrossRef]
- Oliveira, I.; Cavalaro, S.H.P.; Aguado, A. New kinetic model to quantify the internal sulfate attack in concrete. Cem. Concr. Res. 2013, 43, 95–104. [Google Scholar] [CrossRef]
- Samarai, M.A. The disintegration of concrete containing sulphate-contaminated aggregates. Mag. Concr. Res. 1976, 28, 130–142. [Google Scholar] [CrossRef]
- Crammond, N. Examination of mortar bars containing varying percentages of coarsely crystalline gypsum as aggregate. Cem. Concr. Res. 1984, 14, 225–230. [Google Scholar] [CrossRef]
- Qoku, E.; Bier, T.; Westphal, T. Phase assemblage in ettringite-forming cement pastes: A X-ray diffraction and thermal analysis characterization. J. Build. Eng. 2017, 12, 37–50. [Google Scholar] [CrossRef]












| SiO2 | CaO | Al2O3 | Fe2O3 | MgO | Na2O | SO3 |
|---|---|---|---|---|---|---|
| 19.46 | 63.23 | 4.53 | 2.80 | 2.41 | 0.13 | 3.31 |
| SiO2 | CaO | Al2O3 | Fe2O3 | MgO | K2O | Na2O | SO3 | LOI |
|---|---|---|---|---|---|---|---|---|
| 15.83 | 17.21 | 6.71 | 4.64 | 11.59 | 2.87 | 0.26 | 18.03 | 21.08 |
| SiO2 | CaO | Al2O3 | MgO | Fe2O3 | SO3 | K2O | Na2O | TiO2 | LOI |
|---|---|---|---|---|---|---|---|---|---|
| 0.19 | 35.43 | 0.08 | 4.05 | 0.02 | 51.36 | 0.01 | 0.01 | 0.01 | 12.79 |
| No. | SO3 by Weight of Aggregate/% | Cement | Water | Fine Aggregate | Coarse Aggregate | ||
|---|---|---|---|---|---|---|---|
| River Sand | Gypsum Sand | Limestone | Gypsum Stone | ||||
| R0 | 0 | 420 | 189 | 735 | 0 | 1110 | 0 |
| G0.5 | 0.5 | 420 | 189 | 735 | 0 | 1058 | 52 |
| G1 | 1 | 1006 | 104 | ||||
| G1.5 | 1.5 | 954 | 156 | ||||
| G3 | 3 | 798 | 312 | ||||
| G5 | 5 | 590 | 520 | ||||
| G7 | 7 | 382 | 728 | ||||
| FG0.5 | 0.5 | 420 | 189 | 683 | 52 | 1110 | 0 |
| FG1 | 1 | 631 | 104 | ||||
| FG1.5 | 1.5 | 579 | 156 | ||||
| FG3 | 3 | 423 | 312 | ||||
| FG5 | 5 | 215 | 520 | ||||
| FG7 | 7 | 7 | 728 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Huang, B.; Yuan, Y.; Deng, M. Deterioration Process of Concrete Exposed to Internal Sulfate Attack. Materials 2020, 13, 1336. https://doi.org/10.3390/ma13061336
Chen W, Huang B, Yuan Y, Deng M. Deterioration Process of Concrete Exposed to Internal Sulfate Attack. Materials. 2020; 13(6):1336. https://doi.org/10.3390/ma13061336
Chicago/Turabian StyleChen, Weifeng, Bei Huang, Yuexue Yuan, and Min Deng. 2020. "Deterioration Process of Concrete Exposed to Internal Sulfate Attack" Materials 13, no. 6: 1336. https://doi.org/10.3390/ma13061336
APA StyleChen, W., Huang, B., Yuan, Y., & Deng, M. (2020). Deterioration Process of Concrete Exposed to Internal Sulfate Attack. Materials, 13(6), 1336. https://doi.org/10.3390/ma13061336




