Investigation of the Space Charge and DC Breakdown Behavior of XLPE/α-Al2O3 Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of α-Al2O3
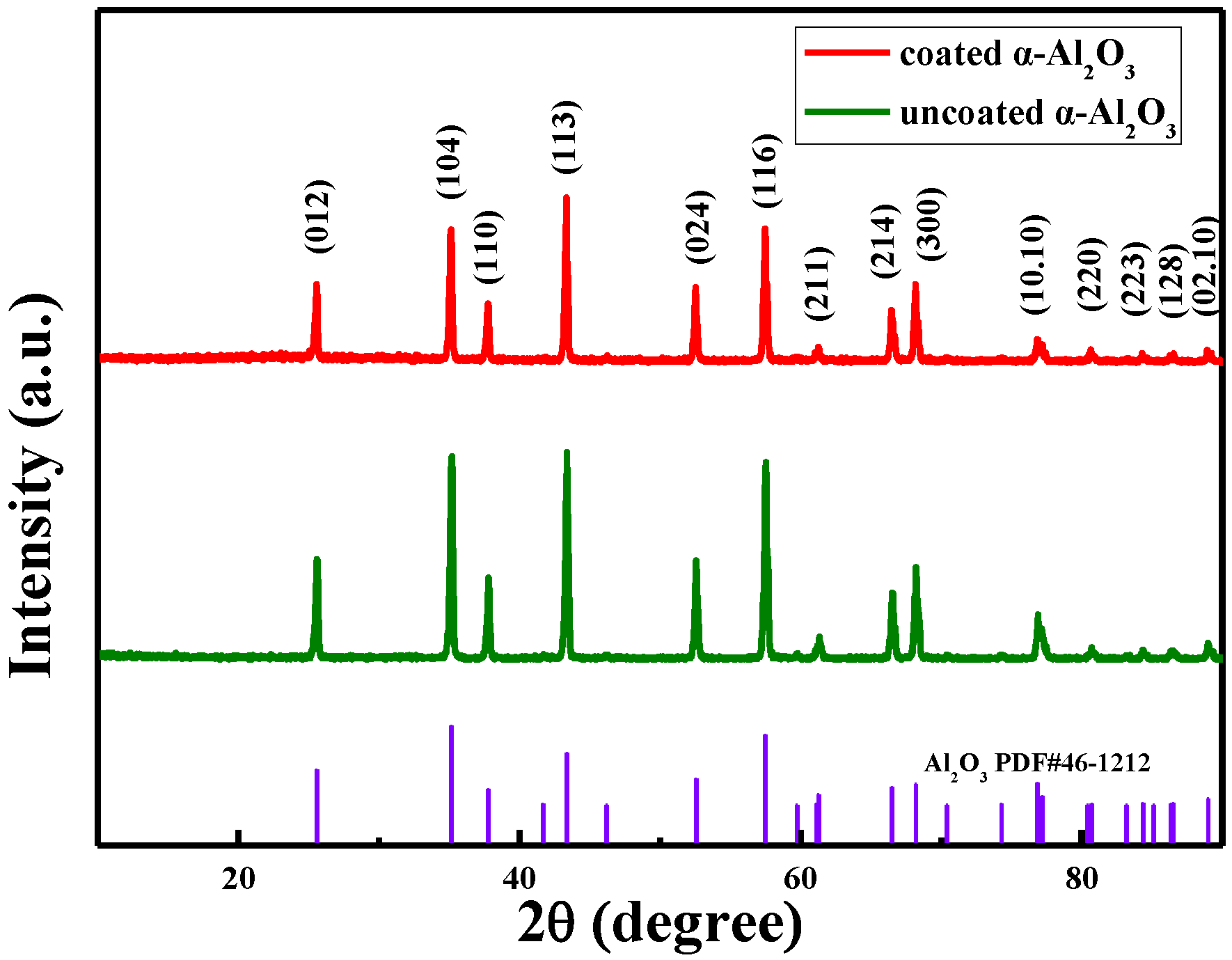
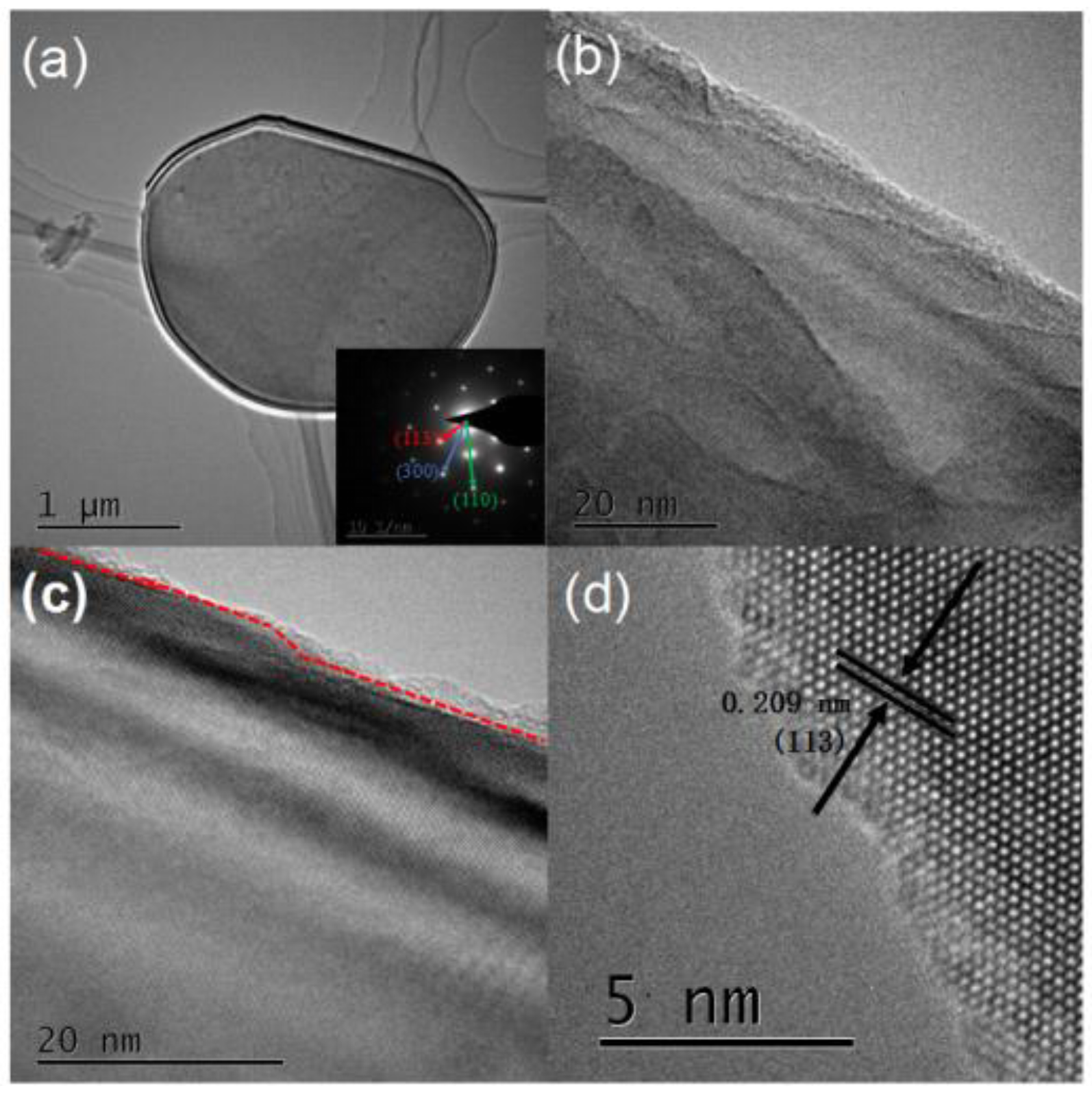
2.3. Characterization of α-Al2O3
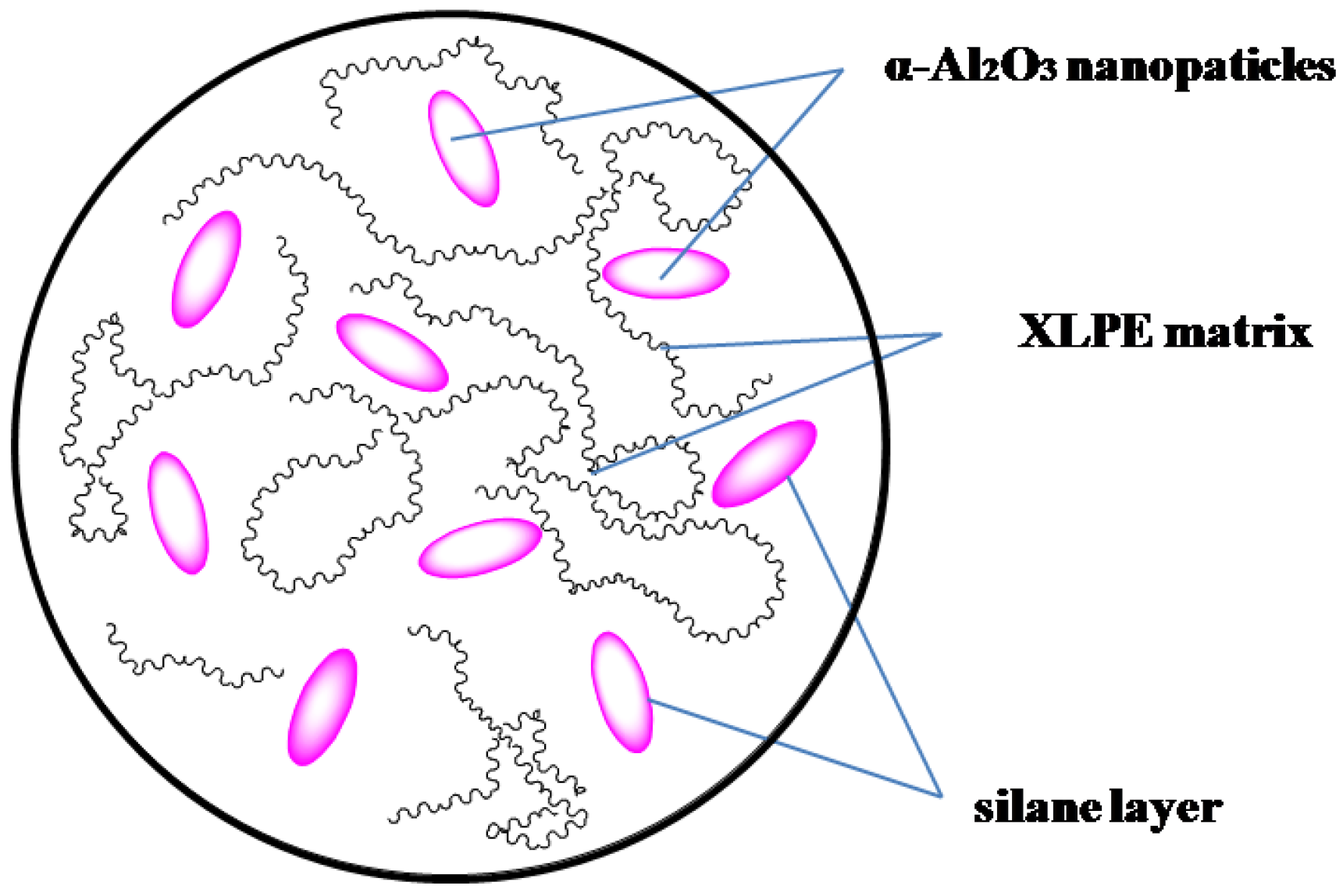
2.4. Preparation of XLPE/α-Al2O3 Nanocomposites
2.5. Electrical Properties Testing of XLPE/α-Al2O3 Nanocomposites
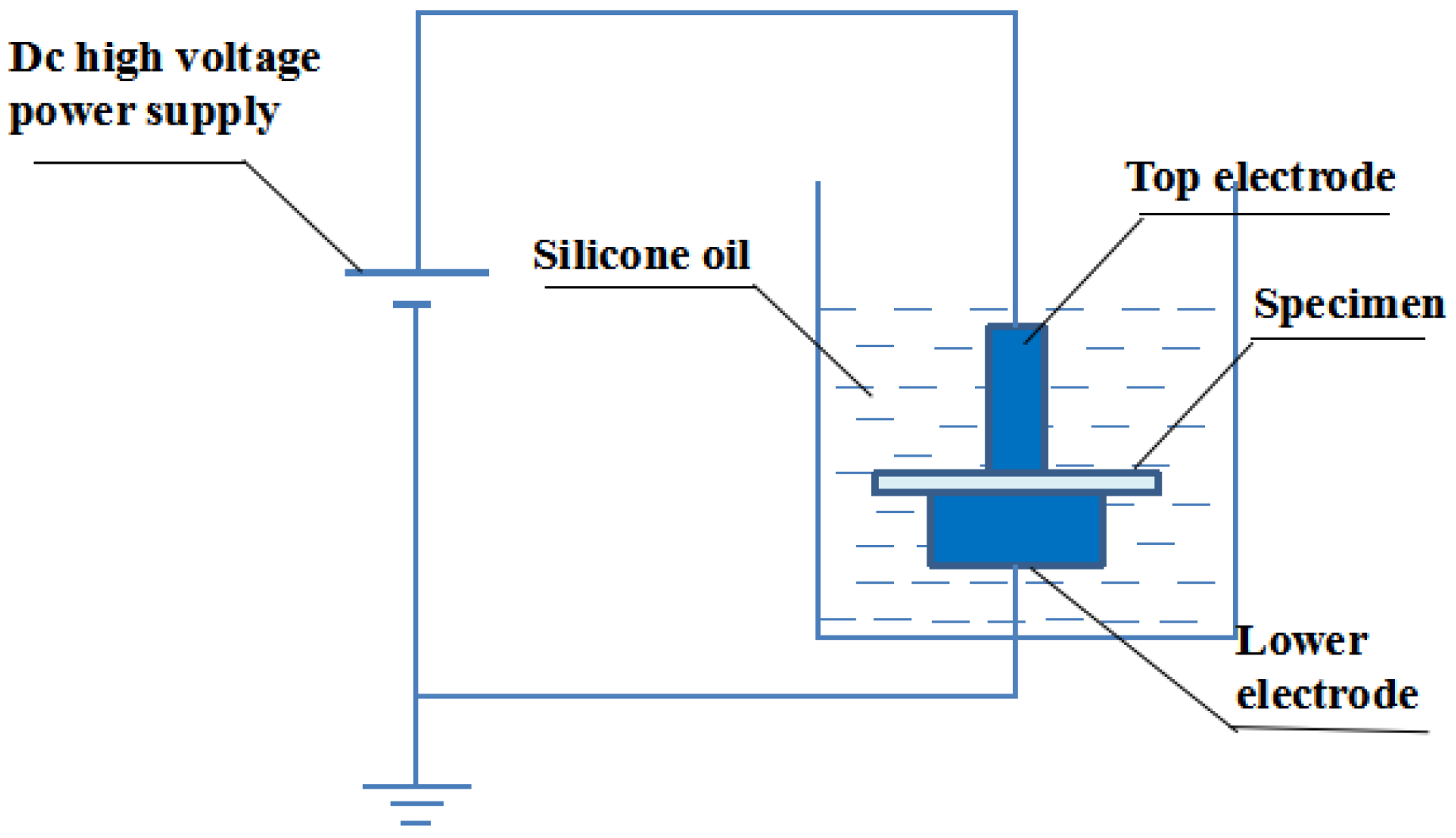
2.5.1. Voltage Breakdown Testing
2.5.2. Pulsed Electro-Acoustic Method
3. Results and Discussion
3.1. Characterization Results of α-Al2O3 Nanosheets
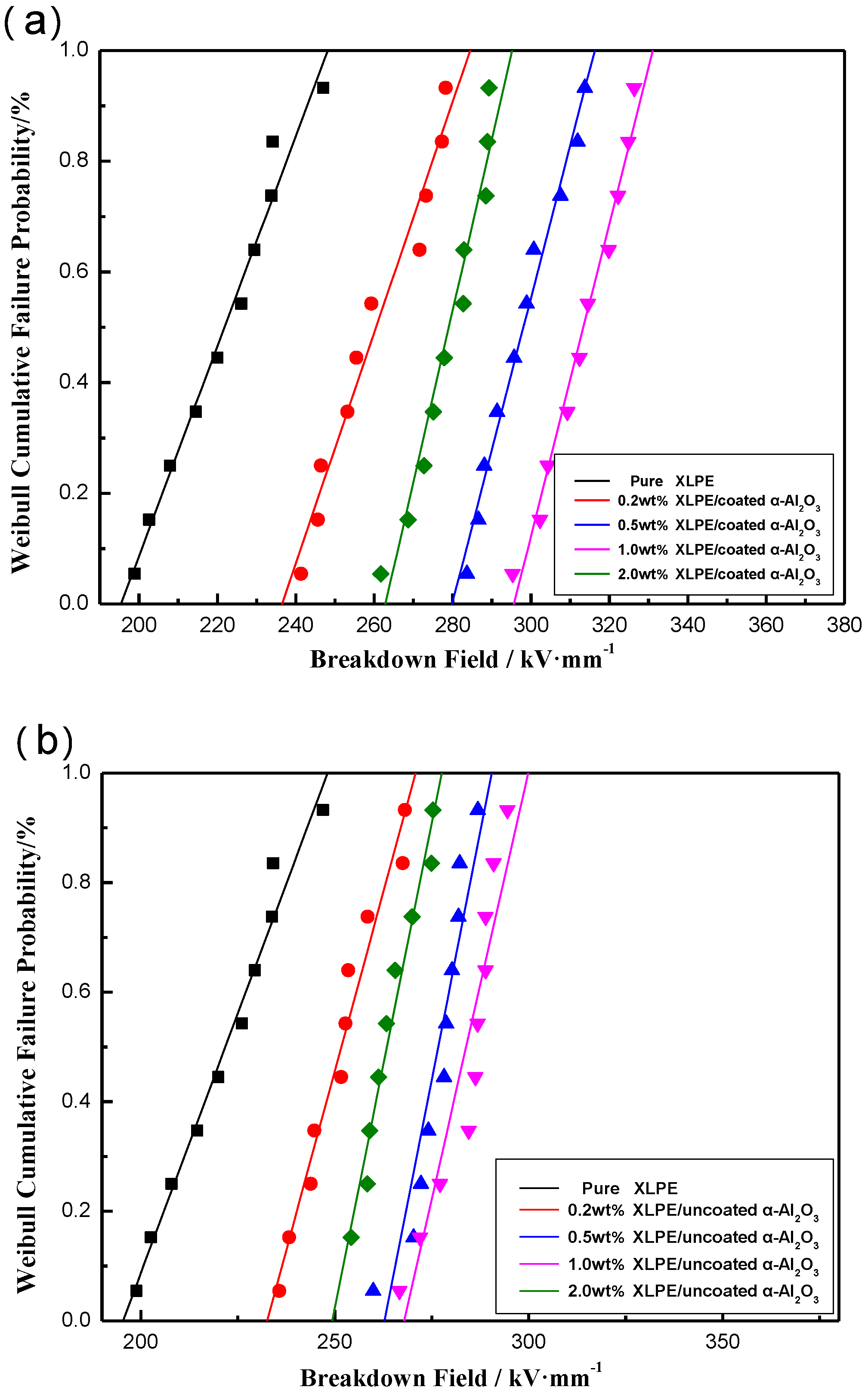
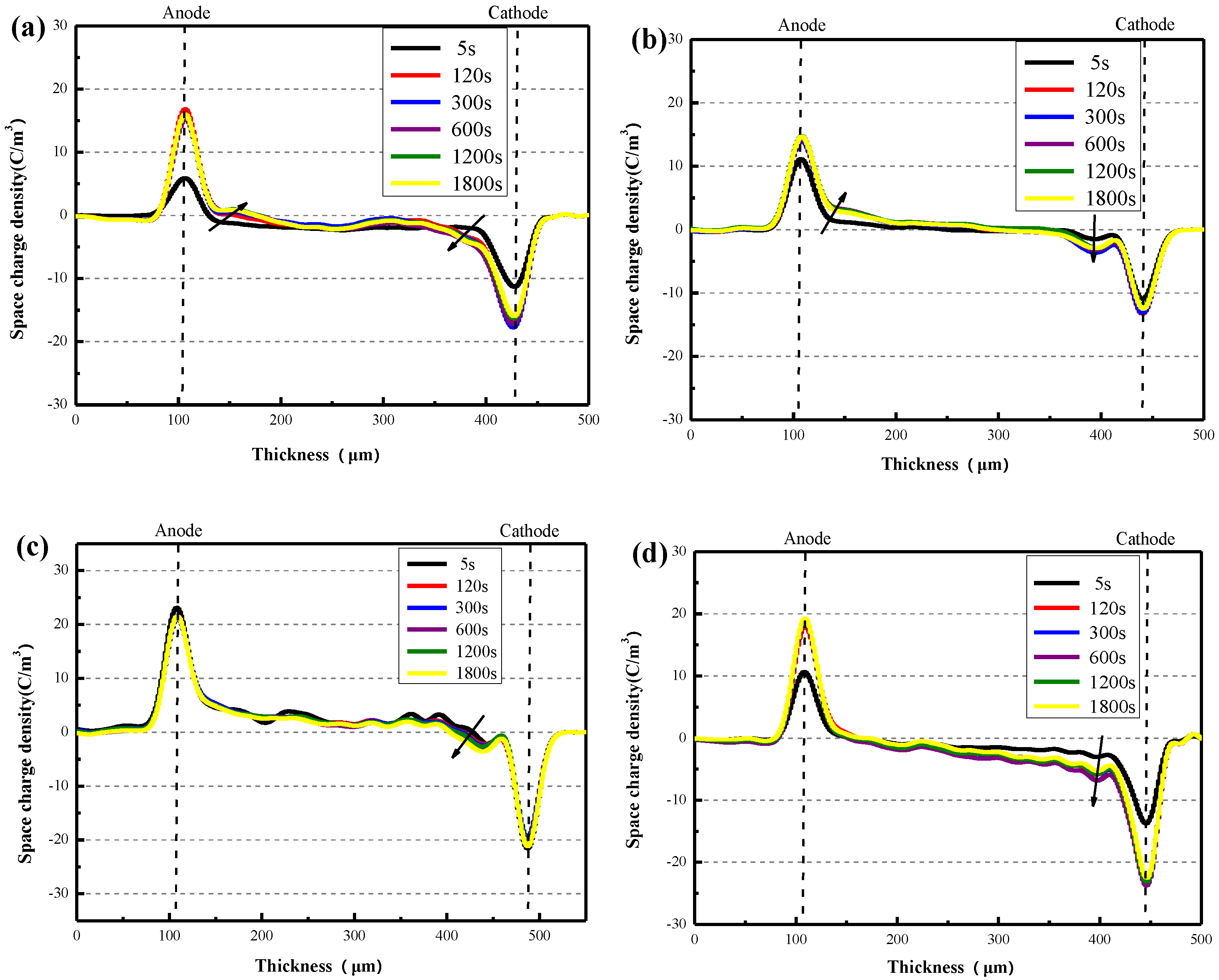
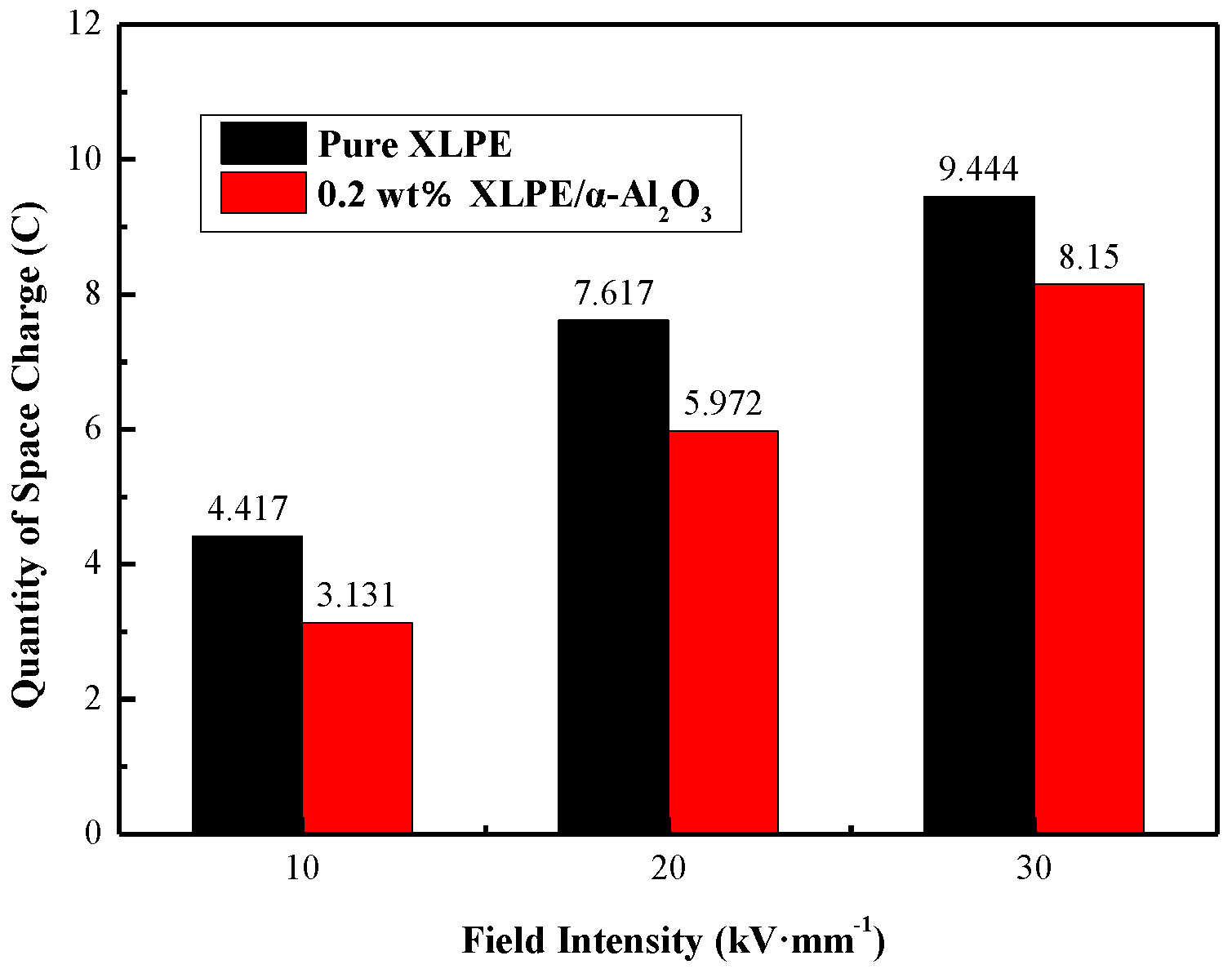
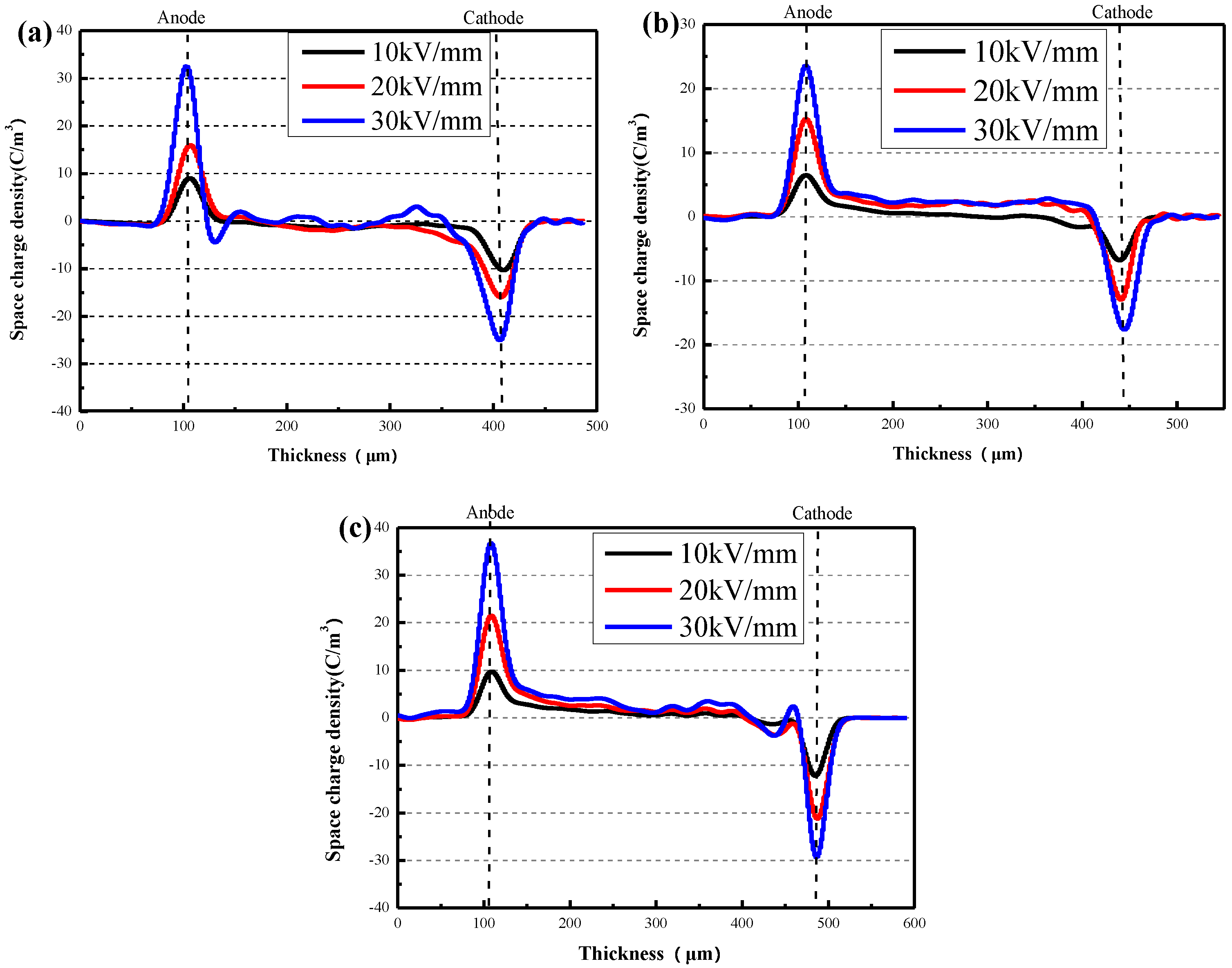
3.2. Electrical Properties Testing Results of XLPE/α-Al2O3 Nanocomposites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Plesa, I.; Notingher, P.V.; Stancu, C.; Wiesbrock, F.; Schlogl, S. Polyethylene Nanocomposites for Power Cable Insulations. Polymers 2019, 11, 24. [Google Scholar]
- Chen, G.; Xu, Z. Charge trapping and detrapping in polymeric materials. J. Appl. Phys. 2009, 106, 123707. [Google Scholar] [CrossRef]
- Boggs, S. A rational consideration of space charge. IEEE Electr. Insul. Mag. 2004, 20, 22–27. [Google Scholar] [CrossRef]
- Imburgia, A.; Miceli, R.; Sanseverino, E.R.; Romano, P.; Viola, F. Review of space charge measurement systems: Acoustic, thermal and optical methods. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 3126. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, H.; Li, C.; Duan, S.; Jiang, Y.; Yang, J.; Han, B.; Zhao, H. Crosslinked polyethylene/polypyrrole nanocomposites with improved direct current electrical characteristics. Polym. Test. 2018, 71, 223–230. [Google Scholar] [CrossRef]
- Cheng, Y.; Bai, L.; Yu, G.; Zhang, X. Effect of Particles Size on Dielectric Properties of Nano-ZnO/LDPE Composites. Materials 2018, 12, 5. [Google Scholar] [CrossRef]
- Tian, F.; Lei, Q.; Wang, X.; Wang, Y. Effect of deep trapping states on space charge suppression in polyethylene/ZnO nanocomposite. Appl. Phys. Lett. 2011, 99, 142903. [Google Scholar] [CrossRef]
- Lau, K.; Vaughan, A.; Chen, G.; Hosier, I.; Holt, A.; Ching, K. On the Space Charge and DC Breakdown Behavior of Polyethylene/Silica Nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 340–351. [Google Scholar] [CrossRef]
- Chi, X.; Cheng, L.; Liu, W.; Zhang, X.; Li., S. Characterization of Polypropylene Modified by Blending Elastomer and Nano-Silica. Materials 2018, 11, 1321. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Xiao, K. Investigation of the electrical properties of XLPE/SiC nanocomposites. Polym. Test. 2016, 50, 145–151. [Google Scholar] [CrossRef]
- Zha, J.; Dang, Z.; Song, H.; Yin, Y.; Chen, G. Dielectric properties and effect of electrical aging on space charge accumulation in polyimide/ TiO2 nanocomposite films. J. Appl. Phys. 2010, 108, 094113. [Google Scholar] [CrossRef]
- Pallon, L.K.H.; Hoang, A.T.; Pourrahimi, A.M.; Hedenqvist, M.S.; Nilsson, F.; Gubanski, S.; Gedde, U.W.; Olsson, R.T. The impact of MgO nanoparticle interface in ultrainsulating polyethylene nanocomposites for high voltage DC cables. J. Mater. Chem. A 2016, 4, 8590–8601. [Google Scholar] [CrossRef]
- Hayase, Y.; Aoyama, H.; Tanaka, Y.; Takada, T.; Murata, Y. Space Charge Formation in LDPE/MgO Nano-composite Thin Film under Ultra-high DC Electric Stress. IEEJ Trans. Fundam. Mater. 2006, 126, 1084–1089. [Google Scholar] [CrossRef]
- Gupta, K.; Jana, P.C.; Meikap, A.K.; Nath, T.K. Synthesis of La0.67Sr0.33MnO3 and polyaniline nanocomposite with its electrical and magneto-transport properties. J. Appl. Phys. 2010, 107, 073704. [Google Scholar] [CrossRef]
- Han, B.; Wang, X.; Sun, Z.; Yang, J.; Lei, Q. Space charge suppression induced by deep traps in polyethylene/zeolite nanocomposite. Appl. Phys. Lett. 2013, 102, 012902. [Google Scholar] [CrossRef]
- Wang, S.; Zha, J.; Li, W.; Dang, Z. Distinctive electrical properties in sandwich-structured Al2O3/low density polyethylene nanocomposites. Appl. Phys. Lett. 2016, 108, 092902. [Google Scholar] [CrossRef]
- Wang, S.; Zha, J.; Wu, Y.; Ren, L.; Dang, Z.; Wu, J. Preparation, Microstructure and Properties of Polyethylene/Alumina nanocomposites for HVDC insulation. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 3350–3356. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nguyen, Q.D.; Wirges, W.; Gerhard, R.; Serdyuk, Y.V.; Gubanski, S.M. Open-circuit Thermally Stimulated Currents in LDPE/Al2O3 Nanocomposite. In Proceedings of the 2016 IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Toronto, ON, Canada, 16–19 October 2016; pp. 611–614. [Google Scholar]
- Suchanek, L.W. Hydrothermal Synthesis of Alpha Alumina (a-Al2O3) Powders: Study of the Processing Variables and Growth Mechanisms. J. Am. Ceram. Soc. 2010, 93, 399–412. [Google Scholar] [CrossRef]
- Lule, Z.; Ju, H.; Kim, J. Effffect of surface-modifified Al2O3 on the thermomechanical properties of polybutylene succinate/Al2O3 composites. Ceram. Int. 2018, 44, 13530–13537. [Google Scholar] [CrossRef]
- Cao, J.; Wang, H.; Cao, C.; Li, H.; Xiao, L.; Qian, Q.; Huang, B.; Chen, Q. Simultaneously enhanced mechanical properties and thermal properties of ultrahigh-molecular-weight polyethylene with polydopamine-coated α-alumina platelets. Polym. Int. 2019, 68, 151–159. [Google Scholar] [CrossRef]
- Qi, B.L.; Lee, B.I.; Chen, S.; Samuels, W.D.; Exarhos, G.J. High-Dielectric-Constant Silver-Epoxy Composites as Embedded Dielectrics. Adv. Mater. 2005, 17, 1777–1781. [Google Scholar] [CrossRef]
- Xu, J.; Wong, C.P. Characterization and properties of an organic–inorganic dielectric nanocomposite for embedded decoupling capacitor applications. Compos. Part A 2007, 38, 13–19. [Google Scholar] [CrossRef]
- Zeng, X.; Yao, Y.; Gong, Z.; Wang, F.; Sun, R.; Xu, J.; Wong, C.P. Ice-Templated Assembly Strategy to Construct 3D Boron Nitride Nanosheet Networks in Polymer Composites for Thermal Conductivity Improvement. Small 2015, 11, 6205–6213. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.P.M.; Nguyen, N.T.; Vu, T.L.; Dao, T.H.; Dinh, L.C.; Nguyen, H.L.; Hoang, T.H.; Le, T.S.; Pham, T.D. Synthesis, Characterization, and Modifification of Alumina Nanoparticles for Cationic Dye Removal. Materials 2019, 12, 450. [Google Scholar] [CrossRef]
- Jin, X.; Gao, L. Size Control of -Al2O3 Platelets Synthesized in Molten Na2SO4 Flux. J. Am. Ceram. Soc. 2004, 87, 533–540. [Google Scholar] [CrossRef]
- Hashimoto, S.; Yamaguchi, A. Synthesis of α-Al2O3 platelets using sodium sulfate flux. J. Mater. Res. 1999, 14, 4667–4672. [Google Scholar] [CrossRef]
- Li, H.; Wang, R.; Hu, H.; Liu, W. Surface modifification of self-healing poly(urea-formaldehyde) microcapsules using silane-coupling agent. Appl. Surf. Sci. 2008, 255, 1894–1990. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, F.; Zhang, C.; Wang, L.; Wang, M.; Qin, M.; Hu, G.; Kong, J. Enhanced dielectric tunability of Ba0.6Sr0.4TiO3/Poly(vinylidene fluoride) composites via interface modifification by silane coupling agent. Compos. Sci. Technol. 2016, 129, 93–100. [Google Scholar] [CrossRef]
- Xu, H.; Dang, Z.; Jiang, M.; Yao, S.; Bai, J. Enhanced dielectric properties and positive temperature coefficient effect in the binary polymer composites with surface modified carbon black. J. Mater. Chem. 2008, 18, 229–234. [Google Scholar] [CrossRef]
- Dong, W.; Wang, X.; Tian, B.; Liu, Y.; Jiang, Z.; Li, Z.; Zhou, W. Use of Grafted Voltage Stabilizer to Enhance Dielectric Strength of Cross-Linked Polyethylene. Polymers 2019, 11, 176. [Google Scholar] [CrossRef]
- Murakami., Y.; Nemoto, M.; Okuzumi, S.; Masuda, S.; Nagao, M. DC Conduction and Electrical Breakdown of MgO/LDPE Nanocomposite. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 33–39. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, J.; Li, S.; Zhong, L. Origin of thickness dependent dc electrical breakdown in dielectrics. Appl. Phys. Lett. 2012, 100, 222904. [Google Scholar] [CrossRef]
- Bodega, R.; Morshuis, P.H.F.; Smit, J.J. Space charge measurements on multi-dielectrics by means of the pulsed electroacoustic method. IEEE Trans. Dielectr. Electr. Insul. 2006, 13, 272–281. [Google Scholar] [CrossRef]
- Maeno, T.; Futami, T.; Kushibe, H.; Takada, T.; Cooke, C.M. Measurement of Spatial Charge Distribution in Thick Dielectrics Using the Pulsed Electroacoustic Method. IEEE Trans. Dielectr. Electr. Insul. 1988, 23, 433–439. [Google Scholar] [CrossRef]
- Gao, C.; He, D.; Wang, P.; Wang, W. A Study on the Space Charge Characteristics of AC Sliced XLPE Cables. IEEE ACCESS 2019, 7, 20531–20537. [Google Scholar] [CrossRef]
- Marco, D.D.; Drissi, K.; Delhote, N.; Tantot, O.; Geffroy, P.M.; Verdeyme, S.; Chartier, T. Dielectric properties of pure alumina from 8 GHz to 73 GHz. J. Eur. Ceram. Soc. 2016, 36, 3355–3361. [Google Scholar] [CrossRef]
- Jarvid, M.; Johansson, A.; Englund, V.; Lundin, A.; Gubanski, S.; Müller, C.; Andersson, M.R. High electron affinity: A guiding criterion for voltage stabilizer design. J. Mater. Chem. A 2015, 3, 7273–7286. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Xing, Z.; Zhao, S.; Cui, Y.; Li, G.; Wei, Y.; Lei, Q.; Hao, C. Investigation of the Space Charge and DC Breakdown Behavior of XLPE/α-Al2O3 Nanocomposites. Materials 2020, 13, 1333. https://doi.org/10.3390/ma13061333
Guo X, Xing Z, Zhao S, Cui Y, Li G, Wei Y, Lei Q, Hao C. Investigation of the Space Charge and DC Breakdown Behavior of XLPE/α-Al2O3 Nanocomposites. Materials. 2020; 13(6):1333. https://doi.org/10.3390/ma13061333
Chicago/Turabian StyleGuo, Xiangjin, Zhaoliang Xing, Shiyi Zhao, Yingchao Cui, Guochang Li, Yanhui Wei, Qingquan Lei, and Chuncheng Hao. 2020. "Investigation of the Space Charge and DC Breakdown Behavior of XLPE/α-Al2O3 Nanocomposites" Materials 13, no. 6: 1333. https://doi.org/10.3390/ma13061333
APA StyleGuo, X., Xing, Z., Zhao, S., Cui, Y., Li, G., Wei, Y., Lei, Q., & Hao, C. (2020). Investigation of the Space Charge and DC Breakdown Behavior of XLPE/α-Al2O3 Nanocomposites. Materials, 13(6), 1333. https://doi.org/10.3390/ma13061333





