Using BiVO4/CuO-Based Photoelectrocatalyzer for 4-Nitrophenol Degradation
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Apparatus
2.3. Preparation of BiVO4/CuO
3. Results and Discussion
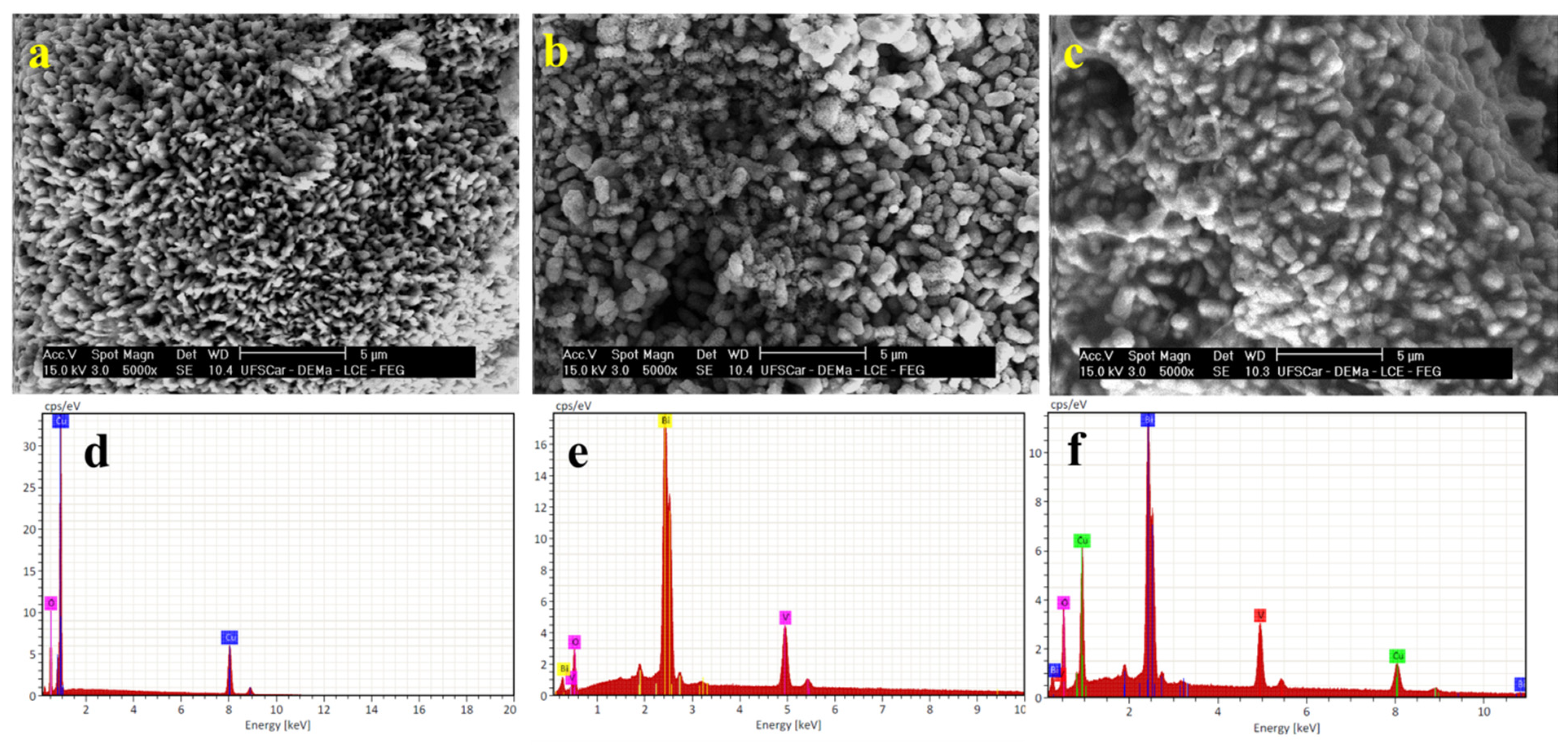
3.1. Characterization of BiVO4/CuO
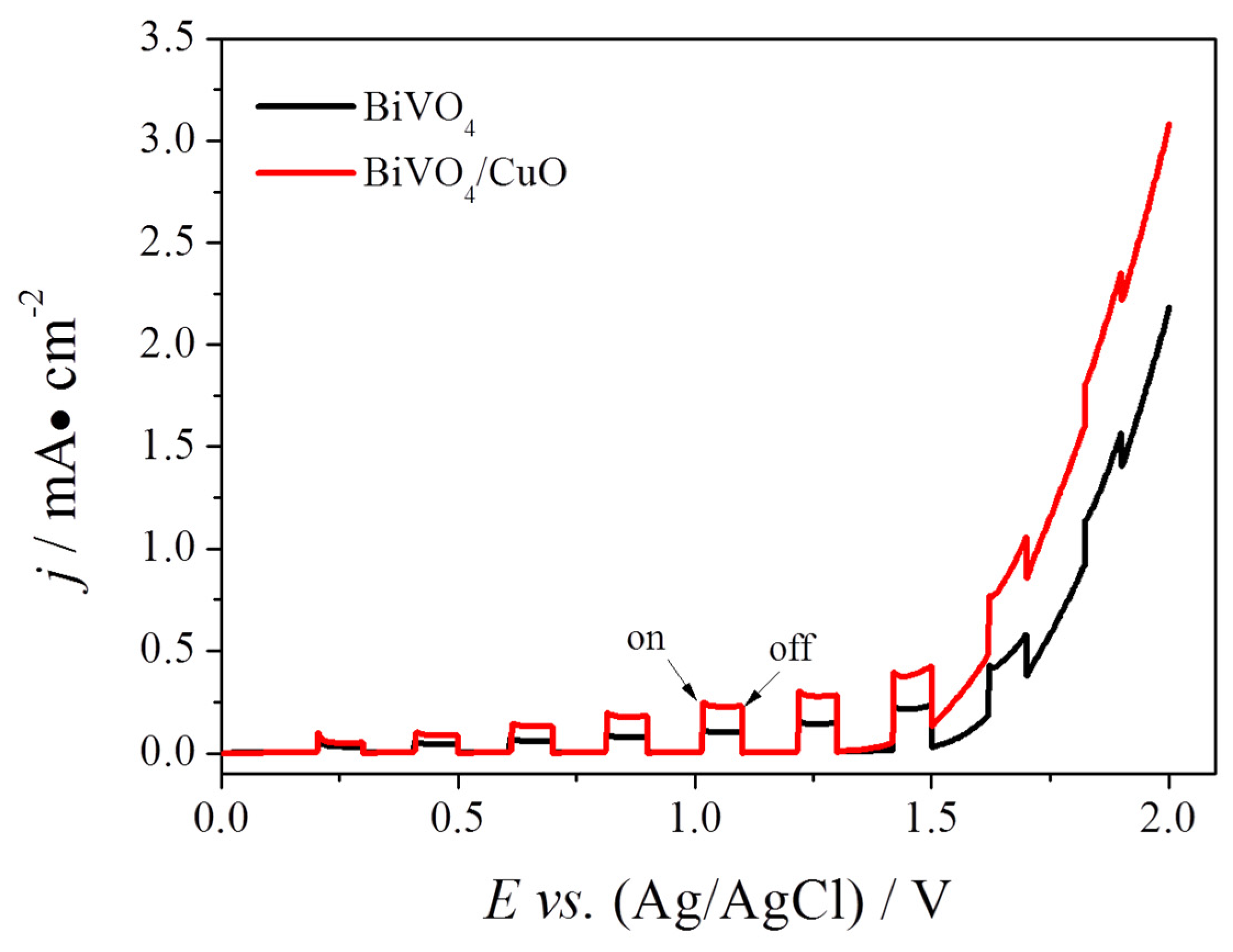
3.2. Photoelectrocatalytic Performance of FTO/BiVO4/CuO
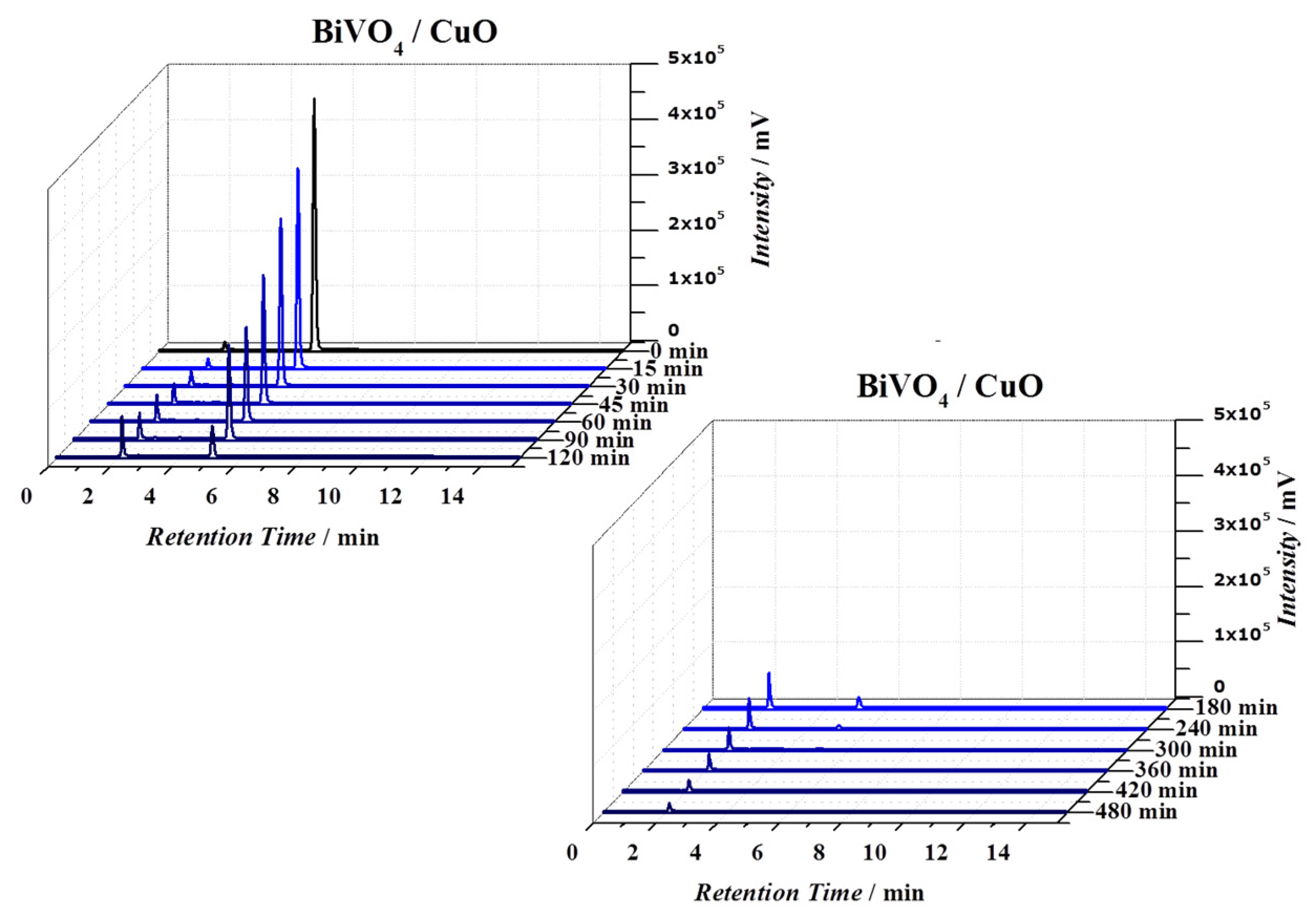
3.3. Photoelectrochemical Degradation of 4-Nitrophenol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Severini, S.; Sorrentino, A. Efficiency and coordination in the EU agri-food systems. Agric. Food Econ. 2017, 5, 15. [Google Scholar] [CrossRef]
- Agula, C.; Akudugu, M.A.; Mabe, F.N.; Dittoh, S. Promoting ecosystem-friendly irrigation farm management practices for sustainable livelihoods in Africa: The Ghanaian experience. Agric. Food Econ. 2018, 6, 13. [Google Scholar] [CrossRef]
- Flister, L.; Galushko, V. The impact of wheat market liberalization on the seed industry’s innovative capacity: An assessment of Brazil’s experience. Agric. Food Econ. 2016, 4, 11. [Google Scholar] [CrossRef]
- Ragasa, C.; Thornsbury, S.; Joshi, S. Dynamics of EU food safety certification: A survival analysis of firm decisions. Agric. Food Econ. 2017, 5, 11. [Google Scholar] [CrossRef][Green Version]
- Honfoga, B.G.; N’tandou-Bonzitou, G.; Vodouhè, R.S.; Bellon, M.R.; Hounhouigan, J.D. Assessing the role of market integration in the consumption of traditional foods in Benin: A joint price instability coefficient and diet composition approach. Agric. Food Econ. 2018, 6, 2. [Google Scholar] [CrossRef]
- Fonta, W.M.; Sanfo, S.; Kedir, A.M.; Thiam, D.R. Estimating farmers’ willingness to pay for weather index-based crop insurance uptake in West Africa: Insight from a pilot initiative in Southwestern Burkina Faso. Agric. Food Econ. 2018, 6, 11. [Google Scholar] [CrossRef]
- Daam, M.A.; Chelinho, S.; Niemeyer, J.C.; Owojori, O.J.; de Silva, P.; Sousa, J.P.; van Gestel, C.A.M.; Rombke, J. Environmental risk assessment of pesticides in tropical terrestrial ecosystems: Test procedures, current status and future perspectives. Ecotoxicol. Environ. Saf. 2019, 181, 534–547. [Google Scholar] [CrossRef]
- Cryder, Z.; Greenberg, L.; Richards, J.; Wolf, D.; Luo, Y.Z.; Gan, J. Fiproles in urban surface runoff: Understanding sources and causes of contamination. Environ. Pollut. 2019, 250, 754–761. [Google Scholar] [CrossRef]
- Jacques, M.T.; Bornhorst, J.; Soares, M.V.; Schwerdtle, T.; Garcia, S.; Avila, D.S. Reprotoxicity of glyphosate-based formulation in Caenorhabditis elegans is not due to the active ingredient only. Environ. Pollut. 2019, 252, 1854–1862. [Google Scholar] [CrossRef]
- Vale, R.L.; Netto, A.M.; Xavier, B.T.D.; Barreto, M.D.P.; da Silva, J.P.S. Assessment of the gray water footprint of the pesticide mixture in a soil cultivated with sugarcane in the northern area of the State of Pernambuco. Braz. J. Cleaner Prod. 2019, 234, 925–932. [Google Scholar] [CrossRef]
- Bila, D.M.; Dezotti, M. Endocrine disrupters in the environment: Part 1—Effects and consequences. Quim. Nova 2007, 30, 651–666. [Google Scholar] [CrossRef]
- Vilela, C.L.S.; Bassin, J.P.; Peixoto, R.S. Water contamination by endocrine disruptors: Impacts, microbiological aspects and trends for environmental protection. Environ. Pollut. 2018, 235, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, D.M.; Beaucham, C.C.; Kurtz, K.; Musolin, K. Assessing occupational exposure to sea lamprey pesticides. Int. J. Occup. Environ. Health 2015, 21, 151–160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Loos, R.; Locoro, G.; Contini, S. Occurrence of polar organic contaminants in the dissolved water phase of the Danube River and its major tributaries using SPE-LC-MS2 analysis. Water Res. 2010, 44, 2325–2335. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Wang, B.; Hu, X.K. Effect of inoculation of Burkholderia sp. strain SJ98 on bacterial community dynamics and para-nitrophenol, 3-methyl-4-nitrophenol, and 2-chloro-4-nitrophenol degradation in soil. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.; Fernandes, C.; Pereira, C.; Rebelo, S.L.H.; Pereira, M.F.R.; Freire, C. Gold-supported magnetically recyclable nanocatalysts: A sustainable solution for the reduction of 4-nitrophenol in water. RSC Adv. 2015, 5, 5131–5141. [Google Scholar] [CrossRef]
- Baptistella, L.H.B.; Giacomini, R.A.; Imamura, P.M. Synthesis of analgesics paracetamol and phenacetin and the sweetener dulcin: A project for undergraduate organic chemistry laboratory. Quim. Nova 2003, 26, 284–286. [Google Scholar] [CrossRef][Green Version]
- Korzeniewska, E.; Harnisz, M. Relationship between modification of activated sludge wastewater treatment and changes in antibiotic resistance of bacteria. Sci. Total Environ. 2018, 639, 304–315. [Google Scholar] [CrossRef]
- la Farre, M.; Oubina, A.; Marco, M.P.; Ginebreda, A.; Tirapu, L.; Barcelo, D. Evaluation of 4-nitrophenol ELISA kit for assessing the origin of organic pollution in wastewater treatment works. Environ. Sci. Technol. 1999, 33, 3898–3904. [Google Scholar] [CrossRef]
- Salvatierra-Stamp, V.; Muniz-Valencia, R.; Jurado, J.M.; Ceballos-Magana, S.G. Hollow fiber liquid phase microextraction combined with liquid chromatography-tandem mass spectrometry for the analysis of emerging contaminants in water samples. Microchem. J. 2018, 140, 87–95. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Brillas, E. Applied photoelectrocatalysis on the degradation of organic pollutants in wastewaters. J. Photochem. Photobiol. C 2017, 31, 1–35. [Google Scholar] [CrossRef]
- Orimolade, B.O.; Koiki, B.A.; Peleyeju, G.M.; Arotiba, O.A. Visible light driven photoelectrocatalysis on a FTO/BiVO4/BiOI anode for water treatment involving emerging pharmaceutical pollutants. Electrochim. Acta 2019, 307, 285–292. [Google Scholar] [CrossRef]
- Meghlaoui, F.Z.; Merouani, S.; Hamdaoui, O.; Bouhelassa, M.; Ashokkumar, M. Rapid catalytic degradation of refractory textile dyes in Fe(II)/chlorine system at near neutral pH: Radical mechanism involving chlorine radical anion (Cl-2(center dot-))-mediated transformation pathways and impact of environmental matrices. Sep. Purif. Technol. 2019, 227, 1–11. [Google Scholar] [CrossRef]
- Zwiener, C.; Frimmel, F.H. Oxidative treatment of pharmaceuticals in water. Water Res. 2000, 34, 1881–1885. [Google Scholar] [CrossRef]
- Punniyamurthy, T.; Rout, L. Recent advances in copper-catalyzed oxidation of organic compounds. Coord. Chem. Rev. 2008, 252, 34–154. [Google Scholar] [CrossRef]
- Prado, T.M.; Carrico, A.; Cincotto, F.H.; Fatibello-Filho, O.; Moraes, F.C. Bismuth vanadate/graphene quantum dot: A new nanocomposite for photoelectrochemical determination of dopamine. Sens. Actuators B 2019, 285, 248–253. [Google Scholar] [CrossRef]
- Scherrer, P. Nachrichten von der Gesellschaft der Wissenschaften zu Göttingen. Math. Phys. Kl. 1918, 2, 98–100. [Google Scholar]
- Priscilla, S.J.; Sivaji, K.; Vimaladevi, L. Synthesis and characterization of Na doped cupric oxide (CuO) nanoparticles. In 61st Dae-Solid State Physics Symposium; Bhattacharya, S., Singh, S., Eds.; AIP: Bhubaneswar, India, 2017. [Google Scholar]
- Abdullahi, S.S.; Güner, S.; Musa, Y.K.I.M.; Adamu, B.I.; Abdulhamid, M.I. Sımple method for the determınatıon of band gap of a nanopowdered sample usıng Kubelka Munk theory. NAMP J. 2016, 35, 241–246. [Google Scholar]
- Assadi, Z.; Emtiazi, G.; Zarrabi, A. Opto-electronic and antibacterial activity investigations of mono-dispersed nanostructure copper oxide prepared by a novel method: Reduction of reactive oxygen species (ROS). J. Mater. Sci. Mater. Electron. 2018, 29, 1798–1807. [Google Scholar] [CrossRef]
- Liu, J.; Huang, X.; Li, Y.; Sulieman, K.M.; He, X.; Sun, F. Self-Assembled CuO monocrystalline nanoarchitectures with controlled dimensionality and morphology. Cryst. Growth Des. 2006, 6, 1690–1696. [Google Scholar] [CrossRef]
- Karunakaran, C.; Kalaivani, S. Synthesis of nanoparticulate in-doped BiVO4 for enhanced visible-light photocatalytic degradation of dye. Int. J. Appl. Ceram. Technol. 2015, 12, 711–721. [Google Scholar] [CrossRef]
- Nogueira, A.E.; Lopes, O.F.; Neto, A.B.S.; Ribeiro, C. Enhanced Cr(VI) photoreduction in aqueous solution using Nb2O5/CuO heterostructures under UV and visible irradiation. Chem. Eng. J. 2017, 312, 220–227. [Google Scholar] [CrossRef]
- Ogunbayo, T.B.; Antunes, E.; Nyokong, T. Investigation of homogeneous photosensitized oxidation activities of palladium and platinum octasubstituted phthalocyanines: Oxidation of 4-nitrophenol. J. Mol. Catal. Chem. 2011, 334, 123–129. [Google Scholar] [CrossRef]
- Abdullah, H.; Kuo, D.-H. Utilization of photocatalytic hydrogen evolved (Zn,Sn)(O,S) nanoparticles to reduce 4-nitrophenol to 4-aminophenol. Int. J. Hydrog. Energy 2019, 44, 191–201. [Google Scholar] [CrossRef]
- Alamelu, K.; Ali, B.M.J. TiO2-Pt composite photocatalyst for photodegradation and chemical reduction of recalcitrant organic pollutants. J. Environ. Chem. Eng. 2018, 6, 5720–5731. [Google Scholar]
- Karlova, P.; Gelbicova, T.; Sedlacek, I. Substrate interactions between 4-nitrophenol and 4-nitrotoluene during biodegradation of their mixture. Desalin. Water Treat. 2016, 57, 2759–2765. [Google Scholar] [CrossRef]
- Yi, S.; Zhuang, W.Q.; Wu, B.; Tay, S.T.L.; Tay, J.H. Biodegradation of p-nitrophenol by aerobic granules in a sequencing batch reactor. Environ. Sci. Technol. 2006, 40, 2396–2401. [Google Scholar] [CrossRef]



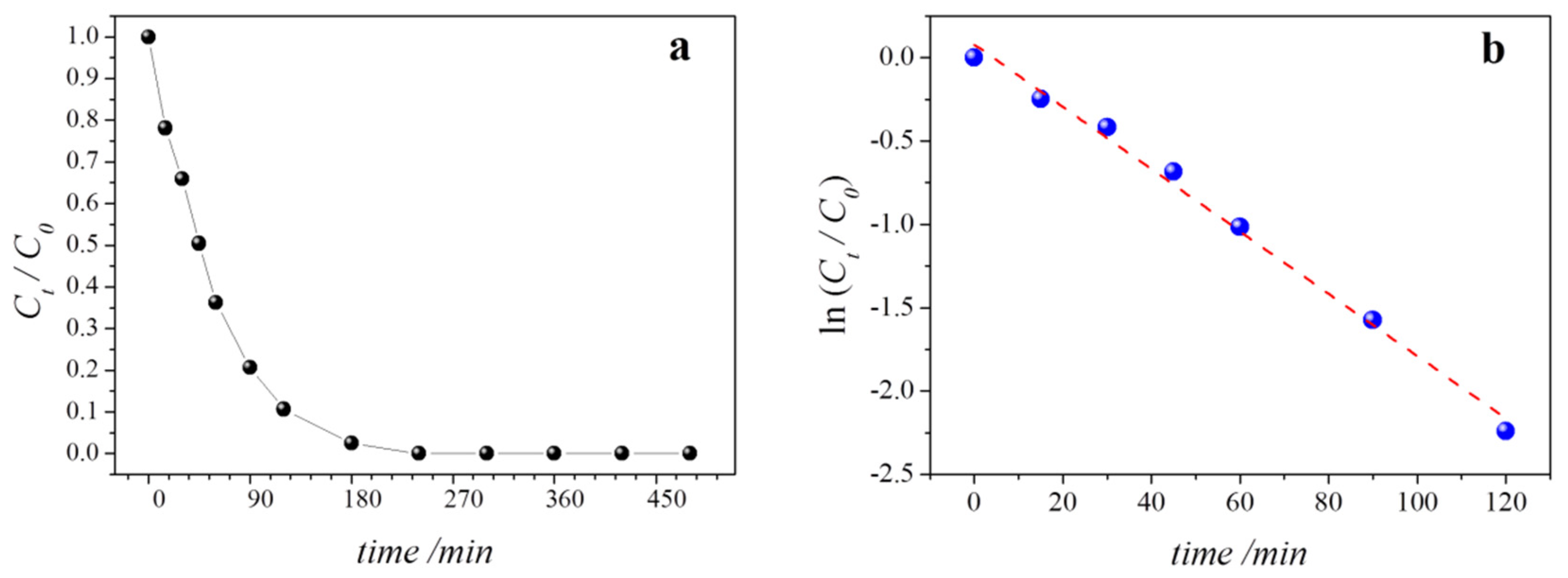
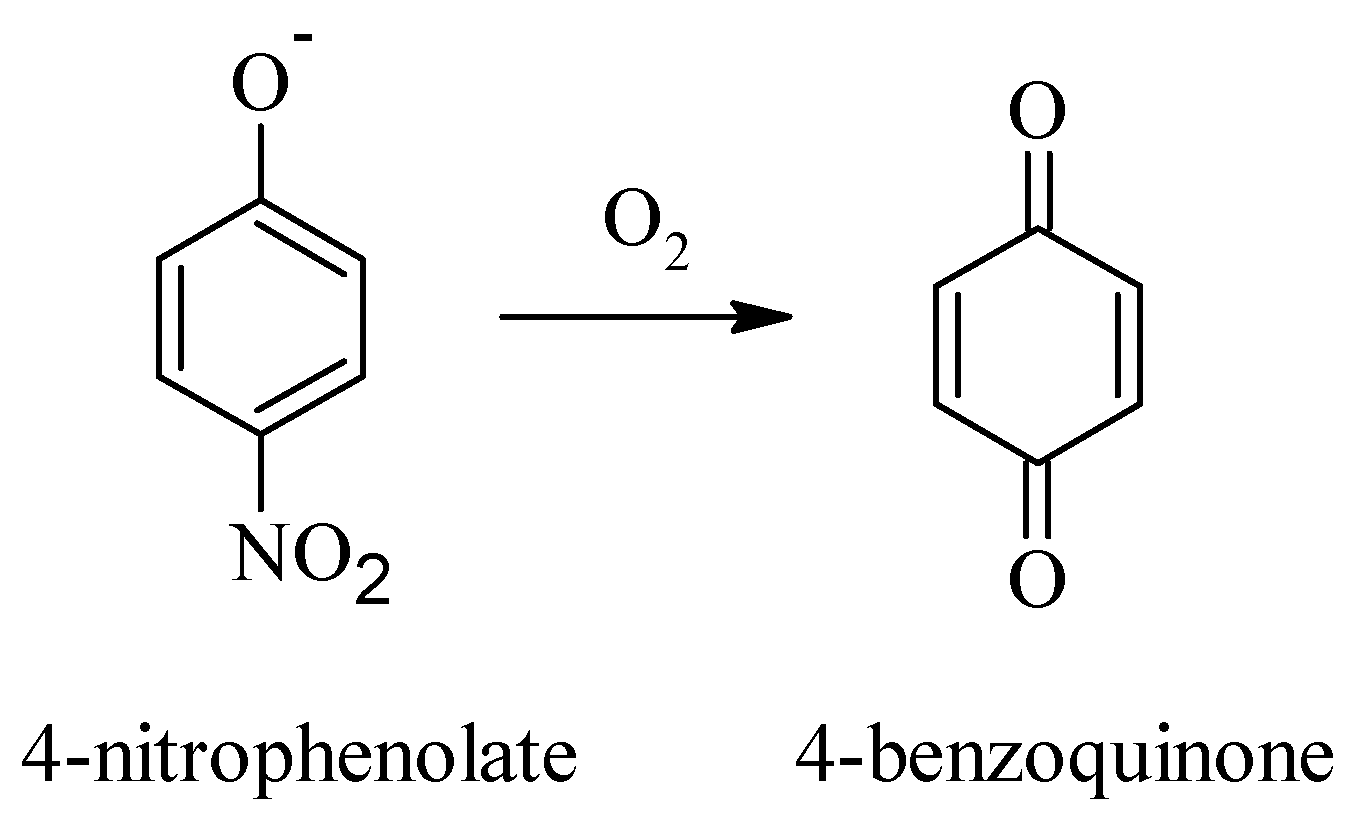

| Degradation Time/min | Nitrate/mg∙L−1 |
|---|---|
| 0 | 0.39 |
| 15 | 0.38 |
| 30 | 0.36 |
| 45 | 0.36 |
| 60 | 0.38 |
| 90 | 0.38 |
| 120 | 0.40 |
| 180 | 0.41 |
| 240 | 0.41 |
| 300 | 0.45 |
| 360 | 0.44 |
| 420 | 0.48 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martimiano do Prado, T.; Lindo Silva, F.; Grosseli, G.; Sergio Fadini, P.; Fatibello-Filho, O.; Cruz de Moraes, F. Using BiVO4/CuO-Based Photoelectrocatalyzer for 4-Nitrophenol Degradation. Materials 2020, 13, 1322. https://doi.org/10.3390/ma13061322
Martimiano do Prado T, Lindo Silva F, Grosseli G, Sergio Fadini P, Fatibello-Filho O, Cruz de Moraes F. Using BiVO4/CuO-Based Photoelectrocatalyzer for 4-Nitrophenol Degradation. Materials. 2020; 13(6):1322. https://doi.org/10.3390/ma13061322
Chicago/Turabian StyleMartimiano do Prado, Thiago, Fernando Lindo Silva, Guilherme Grosseli, Pedro Sergio Fadini, Orlando Fatibello-Filho, and Fernando Cruz de Moraes. 2020. "Using BiVO4/CuO-Based Photoelectrocatalyzer for 4-Nitrophenol Degradation" Materials 13, no. 6: 1322. https://doi.org/10.3390/ma13061322
APA StyleMartimiano do Prado, T., Lindo Silva, F., Grosseli, G., Sergio Fadini, P., Fatibello-Filho, O., & Cruz de Moraes, F. (2020). Using BiVO4/CuO-Based Photoelectrocatalyzer for 4-Nitrophenol Degradation. Materials, 13(6), 1322. https://doi.org/10.3390/ma13061322