Laminated Wallboard Panels Made with Cellulose Nanofibrils as a Binder: Production and Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
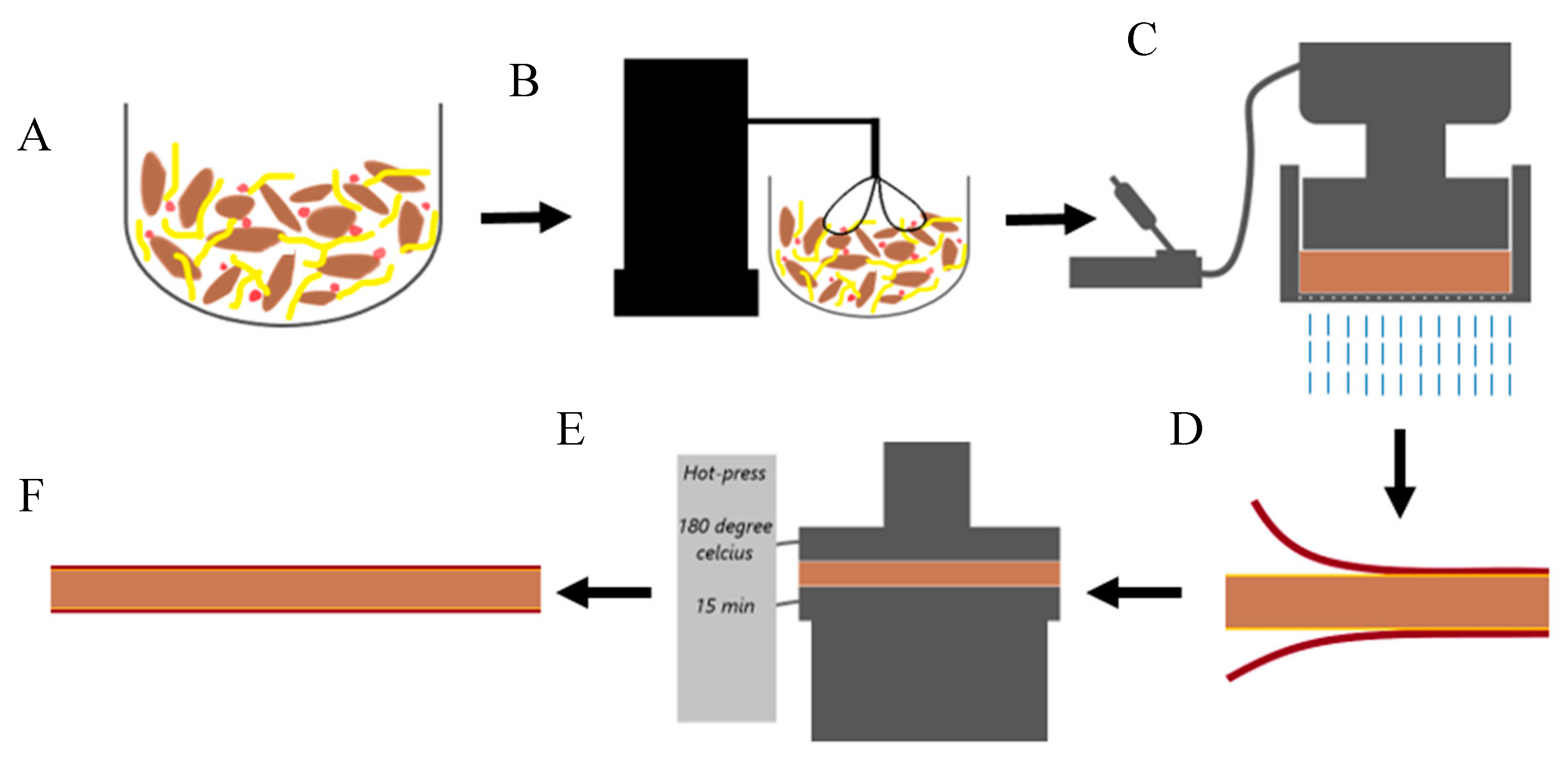
2.2. Panel Formation
2.3. Characterization
3. Results and Discussions
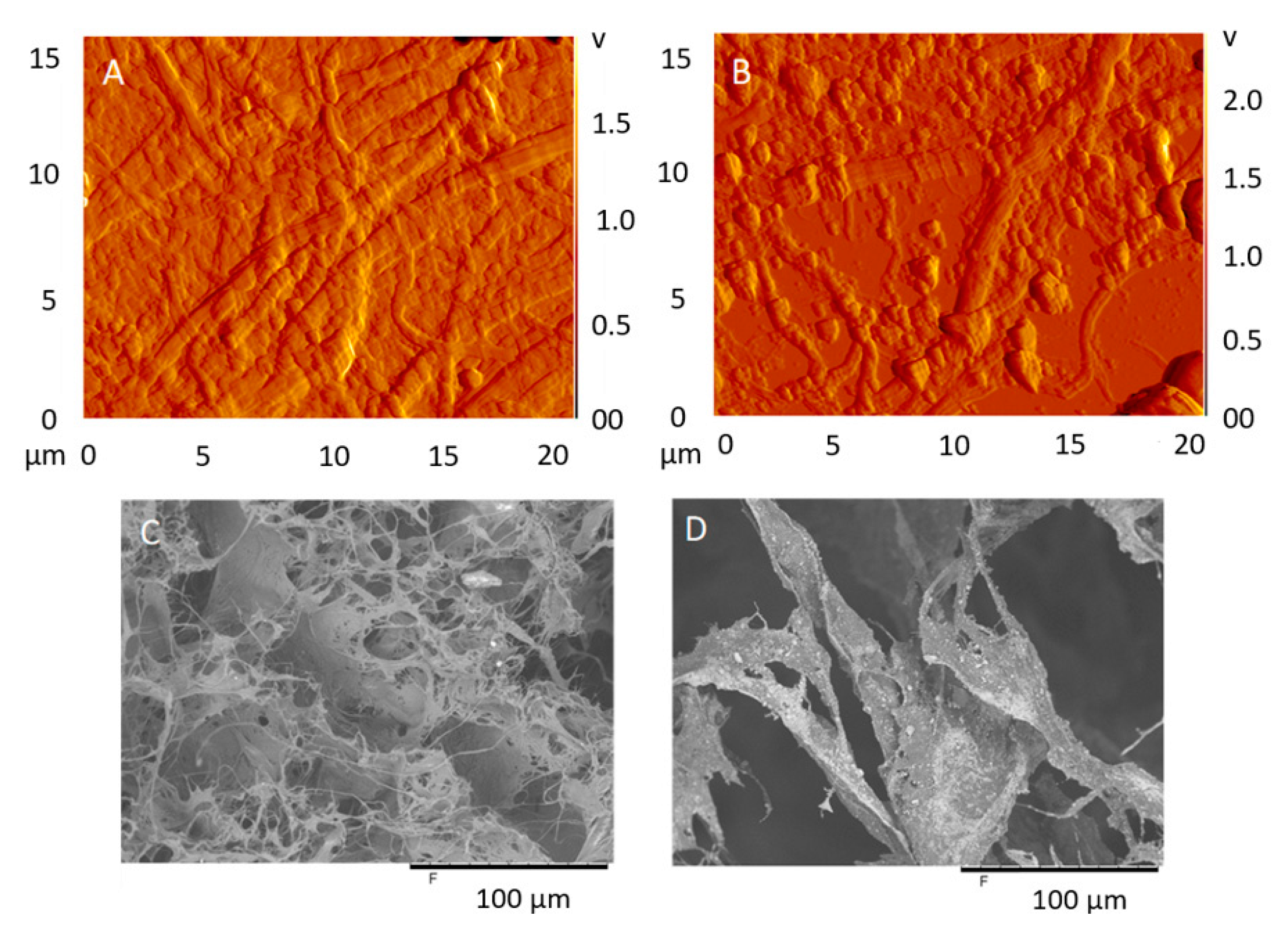
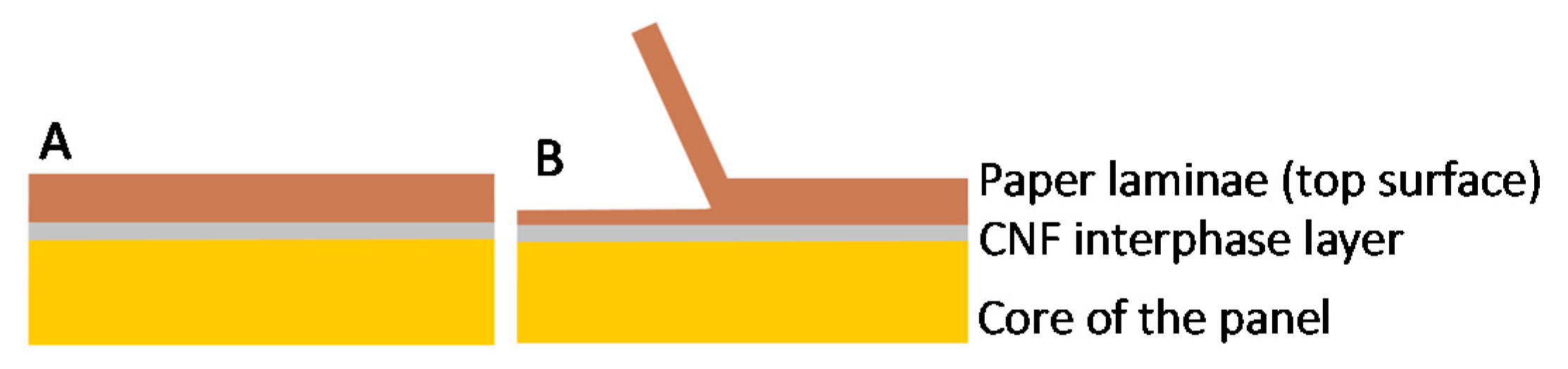
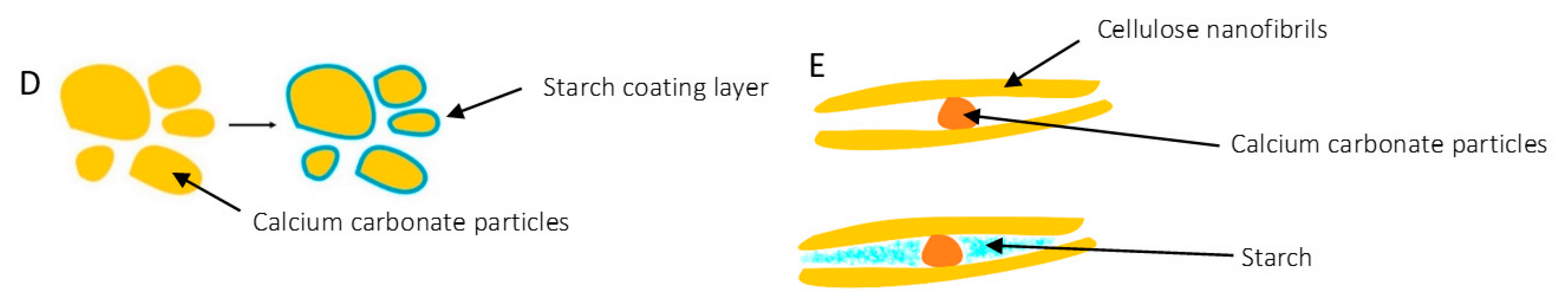
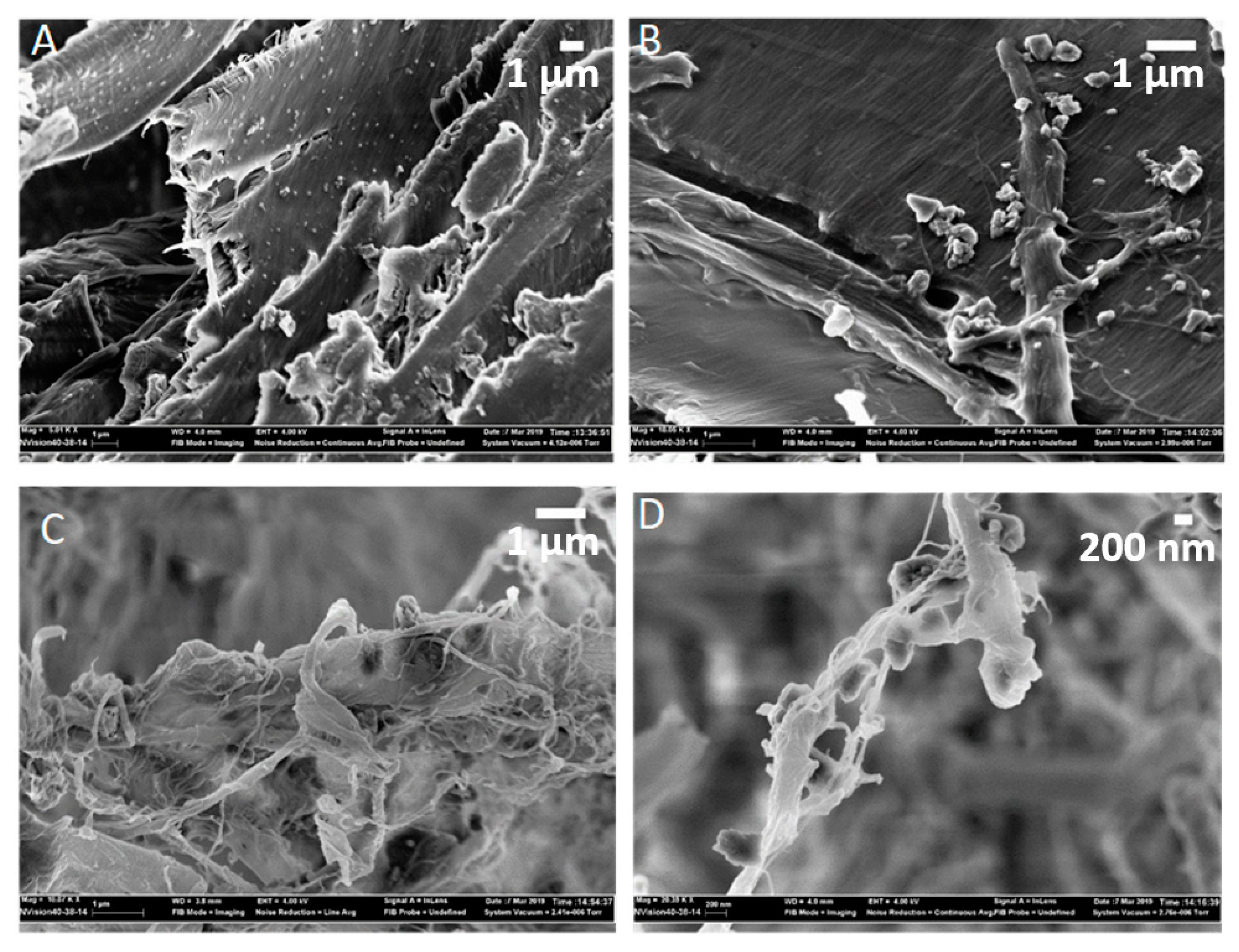
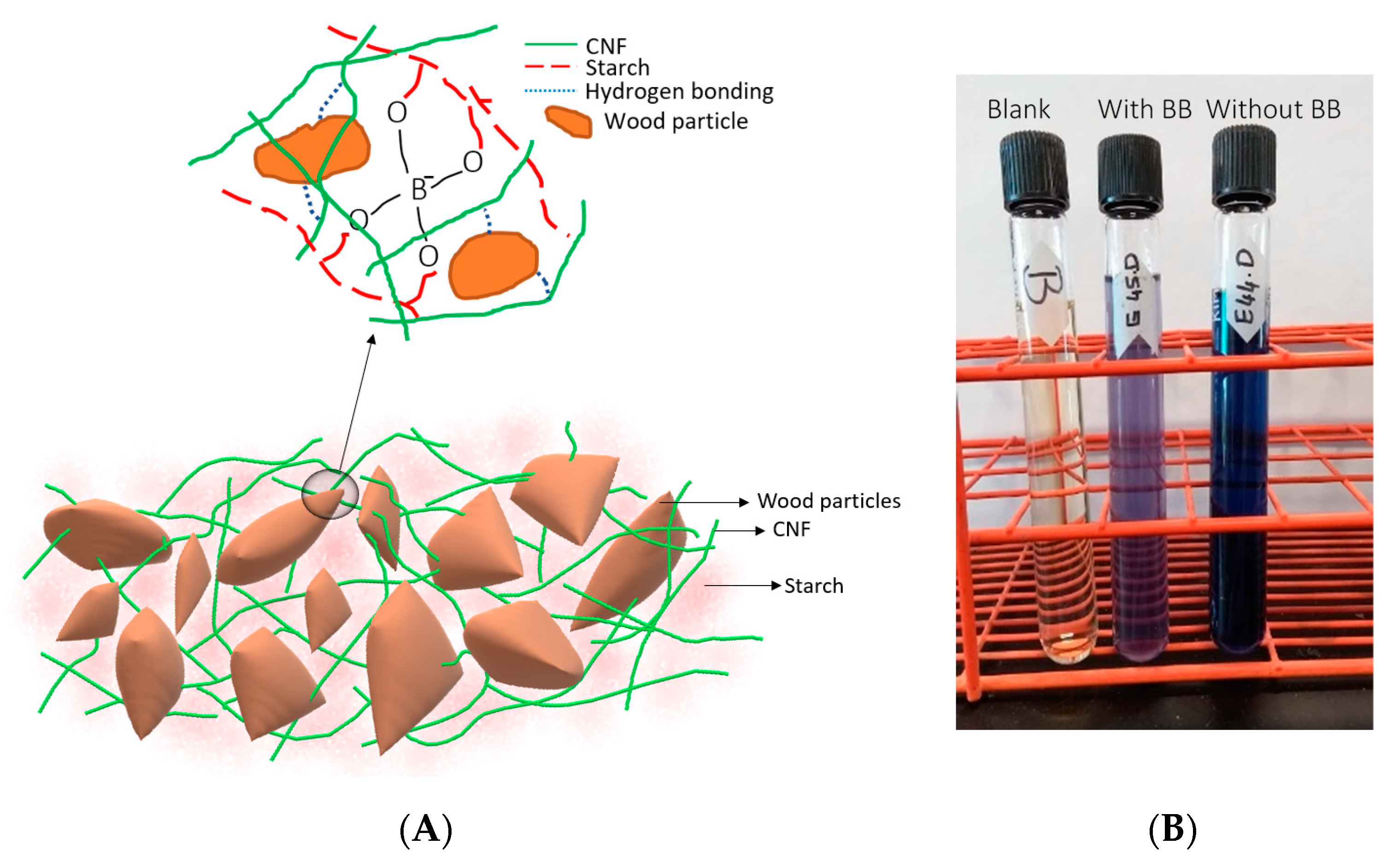
3.1. Morphology and Surface Characterization
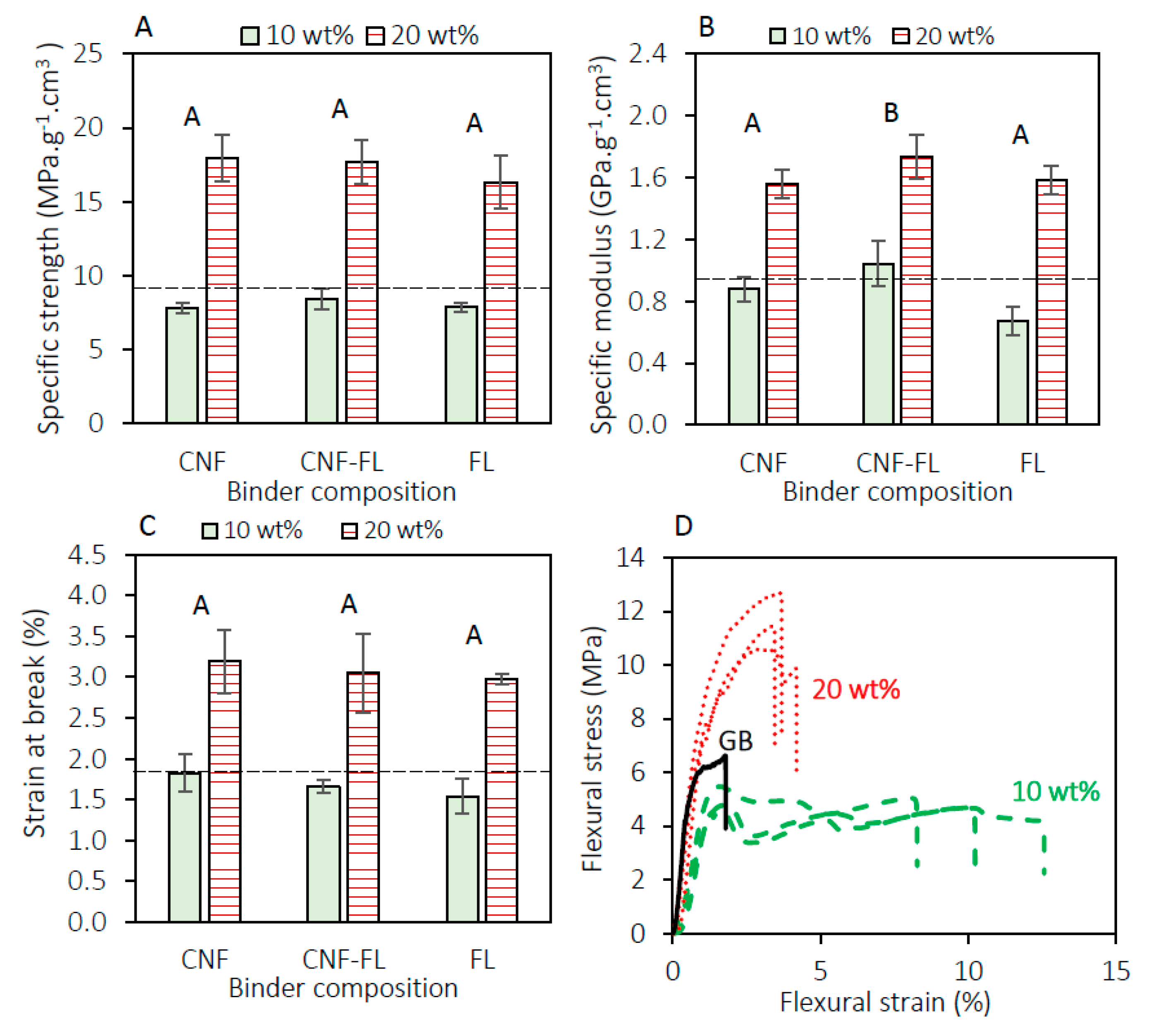
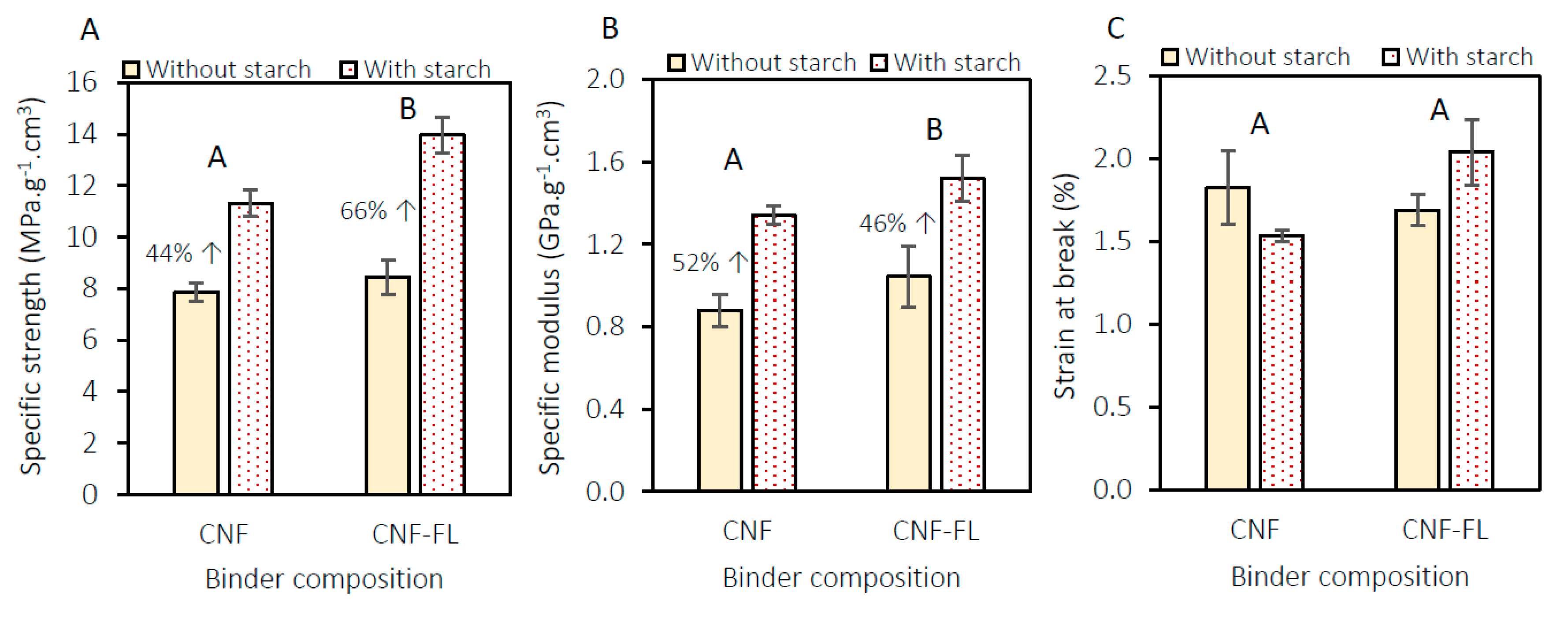
3.2. Mechanical Characterization of the Panels
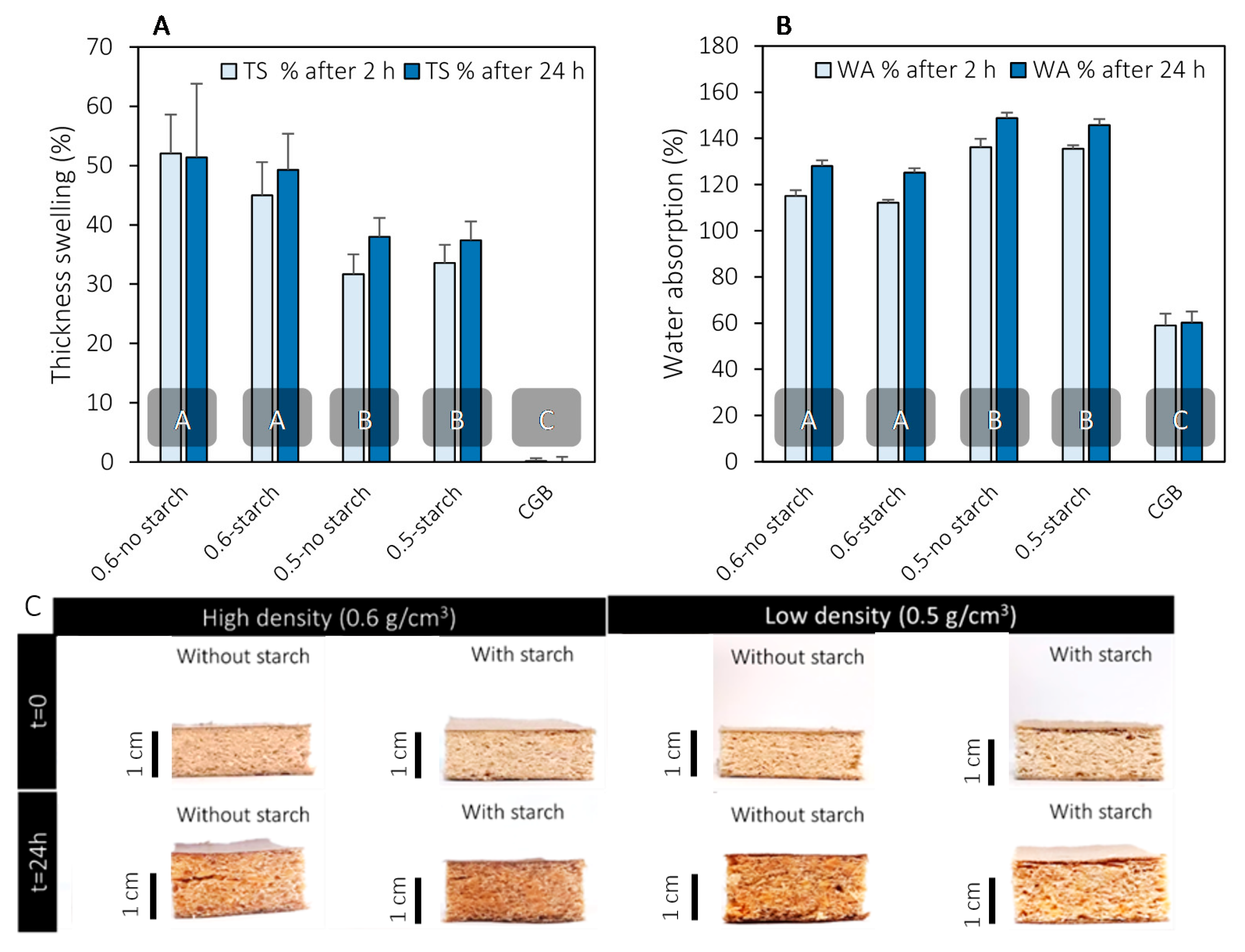
3.3. Thickness Swelling and Water Absorption
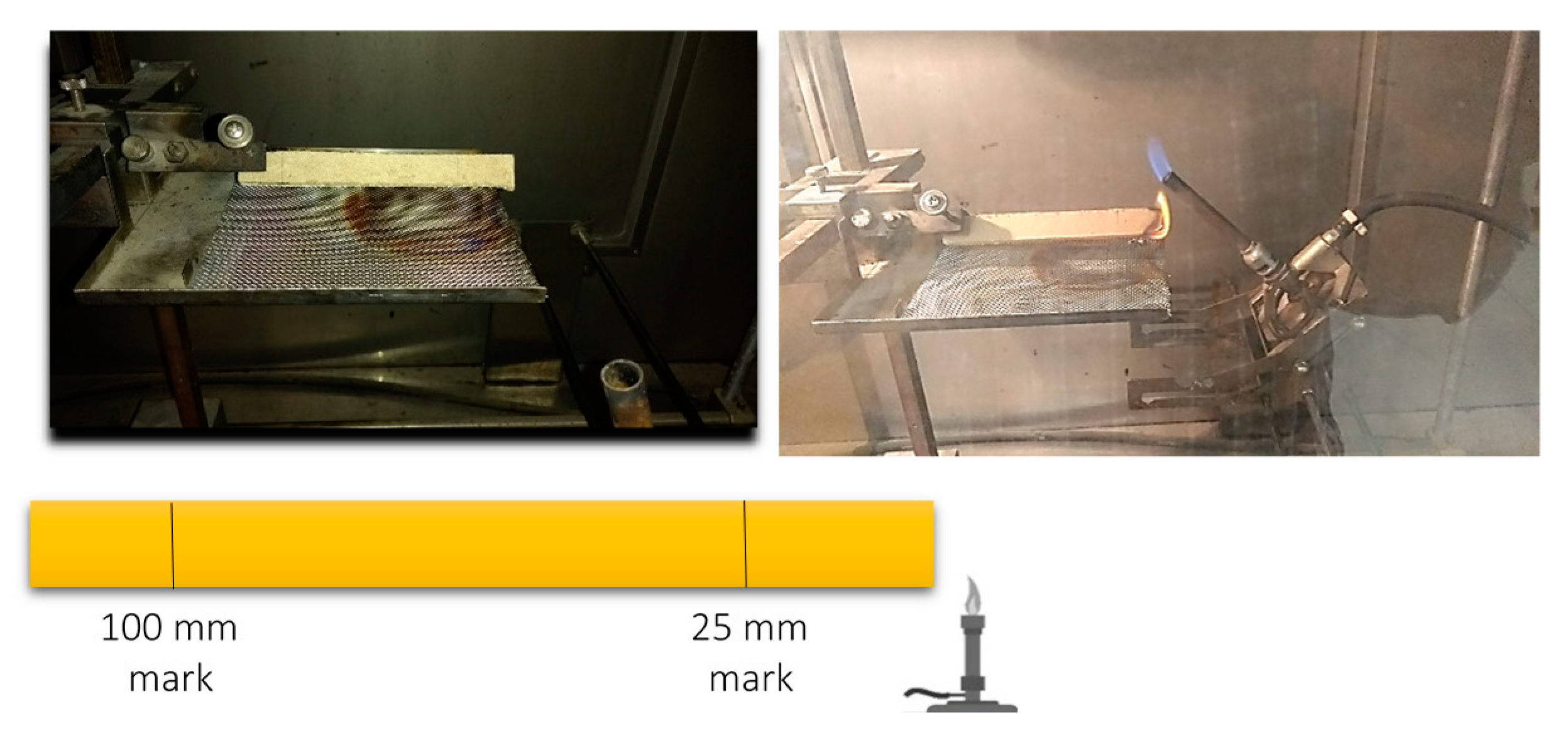
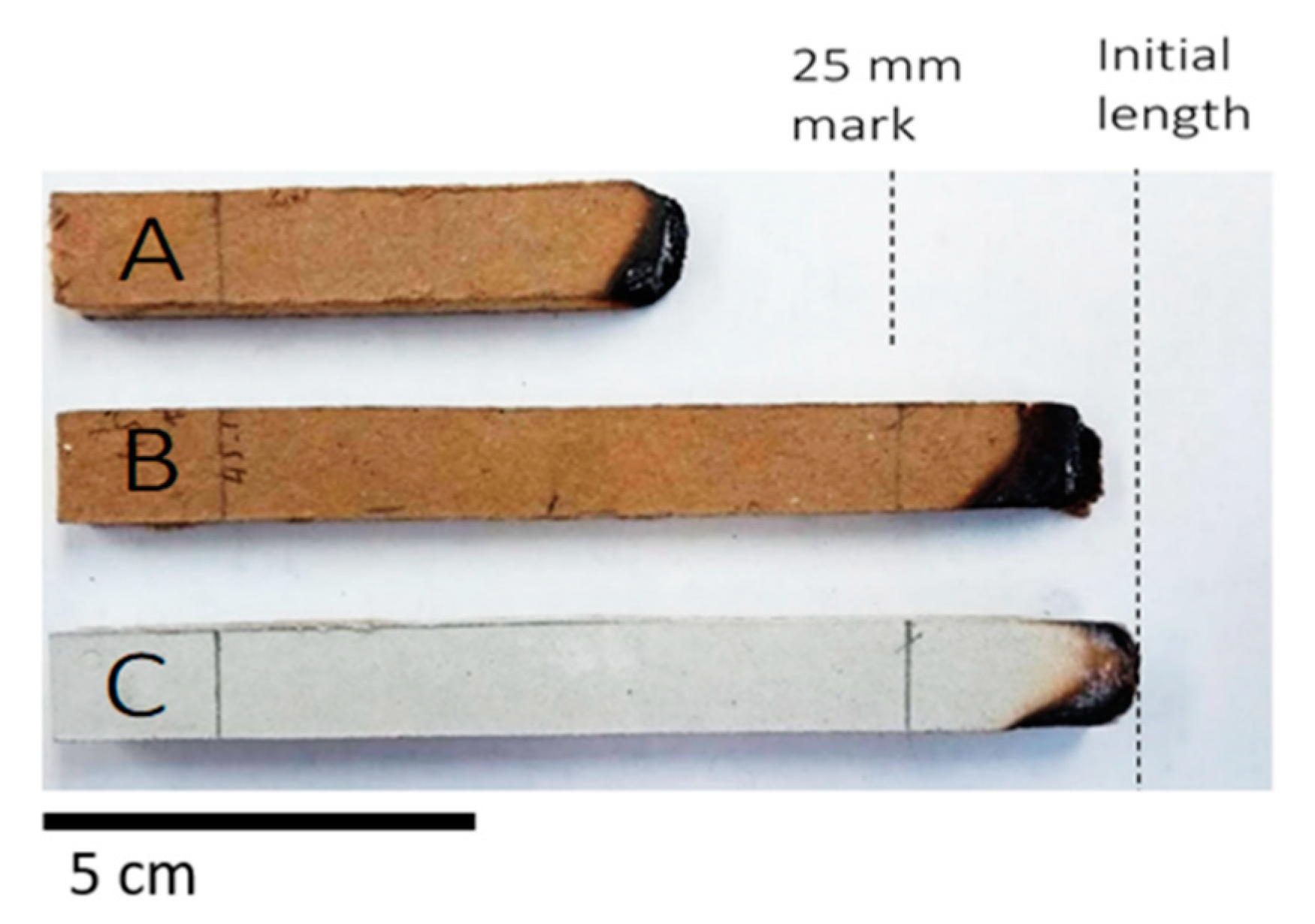
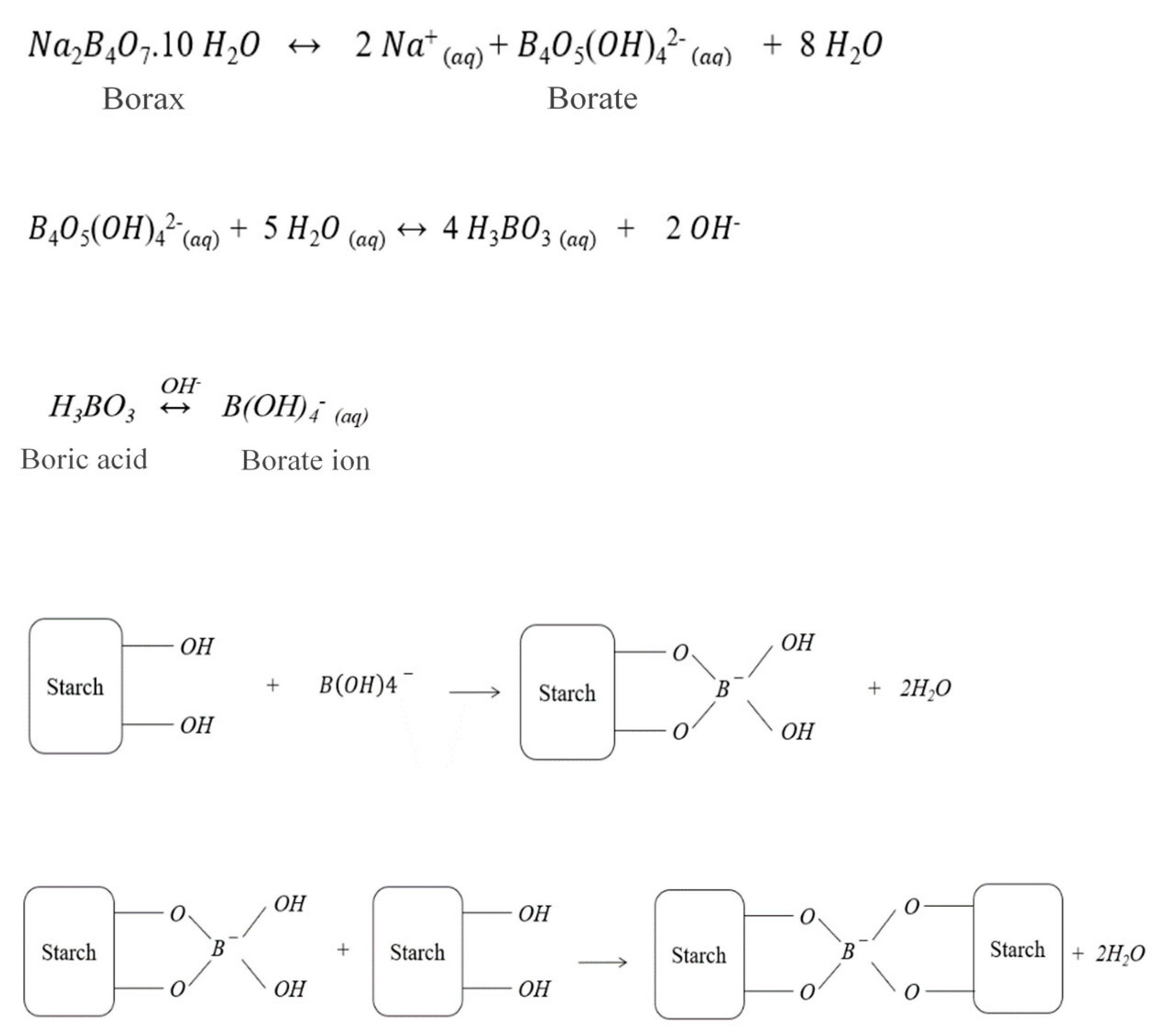
3.4. Burning Characteristics and Effect of the Fire Retardant
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Norström, E.; Demircan, D.; Fogelström, L.; Khabbaz, F.; Malmström, E. Green binders for wood adhesives. In Applied Adhesive Bonding in Science and Technology; InTech: London, UK, 2018. [Google Scholar]
- Birkeland, M.J.; Frihart, C.R. From the lab to commercial reality with biobased adhesives for wood. In Proceedings of the WCTE 2016 World Conference on Timber Engineering, Vienna, Austria, 22–25 August 2016. [Google Scholar]
- Amini, E.; Tajvidi, M.; Gardner, D.J.; Bousfield, D.W. Utilization of cellulose nanofibrils as a binder for particleboard manufacture. BioResources 2017, 12, 4093–4110. [Google Scholar] [CrossRef]
- Cowie, J.; Bilek, E.T.; Wegner, T.H.; Shatkin, J.A. Market projections of cellulose nanomaterial-enabled products-Part 2: Volume estimates. TAPPI J. 2014, 13, 57–69. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S.N. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Mondal, S. Review on nanocellulose polymer nanocomposites. Polym. Plast. Technol. Eng. 2018, 57, 1377–1391. [Google Scholar] [CrossRef]
- Gardner, D.J.; Oporto, G.S.; Mills, R.; Samir, M.A.S.A. Adhesion and surface issues in cellulose and nanocellulose. J. Adhes. Sci. Technol. 2008, 22, 545–567. [Google Scholar] [CrossRef]
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Microfibrillated cellulose, a new cellulose product: Properties, uses, and commercial potential. J. Appl. Polym. Sci. Appl. Polym. Symp. 1983, 37, 815–827. [Google Scholar]
- Herrick, F.W.; Caseiber, R.L.; Hamilton, J.K.; Sandberg, K.R. Microfibrillated cellulose: Morphology and accessibility. J. Appl. Polym. Sci. Appl. Polym. Symp. 1983, 37, 797–813. [Google Scholar]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crops Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Wetterling, J.; Sahlin, K.; Mattsson, T.; Westman, G.; Theliander, H. Electroosmotic dewatering of cellulose nanocrystals. Cellulose 2018, 25, 2321–2329. [Google Scholar] [CrossRef]
- Peng, Y.; Gardner, D.J.; Han, Y. Drying cellulose nanofibrils: In search of a suitable method. Cellulose 2012, 19, 91–102. [Google Scholar] [CrossRef]
- Leng, W.; Hunt, J.F.; Tajvidi, M. Effects of density, cellulose nanofibrils addition ratio, pressing method, and particle size on the bending properties of wet-formed particleboard. BioResources 2017, 12, 4986–5000. [Google Scholar] [CrossRef]
- Leng, W.; Hunt, J.F.; Tajvidi, M. Screw and nail withdrawal strength and water soak properties of wet-formed cellulose nanofibrils bonded particleboard. BioResources 2016, 12, 7692–7710. [Google Scholar]
- Hunt, J.F.; Leng, W.; Tajvidi, M. Vertical density profile and internal bond strength of wet-formed particleboard bonded with cellulose nanofibrils. Wood Fiber Sci. 2017, 49, 1–11. [Google Scholar]
- Yousefi Shivyari, N.; Tajvidi, M.; Bousfield, D.W.; Gardner, D.J. Production and characterization of laminates of paper and cellulose nanofibrils. ACS Appl. Mater. Interfaces 2016, 8, 25520–25528. [Google Scholar] [CrossRef]
- Sun, W.; Tajvidi, M.; Hunt, C.G.; McIntyre, G.; Gardner, D.J. Fully bio-based hybrid composites made of wood, fungal mycelium and cellulose nanofibrils. Sci. Rep. 2019, 9, 3766. [Google Scholar] [CrossRef]
- Lázaro, D.; Puente, E.; Peña, J.; Alvear, D. Gypsum board failure model based on cardboard behaviour. Fire Mater. 2018, 42, 221–233. [Google Scholar] [CrossRef]
- Ndukwe, I.; Yuan, Q. Drywall (gyproc plasterboard) recycling and reuse as a compost-bulking agent in Canada and North America: A review. Recycling 2016, 1, 311–320. [Google Scholar] [CrossRef]
- Miller, A.K.; Eric, J.E.; Allen, J. Health Hazard Evaluation Report; Center to Protect Workers’ Rights: Washington, DC, USA, 1997. [Google Scholar]
- Nindiyasari, F.; Griesshaber, E.; Zimmermann, T.; Manian, A.P.; Randow, C.; Zehbe, R.; Fernandez-Diaz, L.; Ziegler, A.; Fleck, C.; Schmahl, W.W. Characterization and mechanical properties investigation of the cellulose/gypsum composite. J. Compos. Mater. 2016, 50, 657–672. [Google Scholar] [CrossRef]
- Jang, Y.C.; Townsend, T. Sulfate leaching from recovered construction and demolition debris fines. Adv. Environ. Res. 2001, 5, 203–217. [Google Scholar] [CrossRef]
- Amini, E.N.; Tajvidi, M.; Bousfield, D.W.; Gardner, D.J.; Shaler, S.M. Dewatering behavior of a wood-cellulose nanofibril particulate system. Sci. Rep. 2019, 9, 14584. [Google Scholar] [CrossRef] [PubMed]
- Hrncirova, M.; Pospisil, J.; Spilacek, M. Size analysis of solid particles using laser diffraction and sieve analysis. Eng. Mech. 2013, 20, 309–318. [Google Scholar]
- Delgado, A.V.; González-Caballero, F.; Hunter, R.J.; Koopal, L.K.; Lyklema, J. Measurement and interpretation of electrokinetic phenomena (IUPAC Technical Report). Pure Appl. Chem. 2005, 77, 1753–1805. [Google Scholar] [CrossRef]
- Kaszuba, M.; Corbett, J.; Watson, F.M.N.; Jones, A. High-concentration zeta potential measurements using light-scattering techniques. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 4439–4451. [Google Scholar] [CrossRef] [PubMed]
- ASTM C473-16: Standard Test Methods for Physical Testing of Gypsum Panel Products; ASTM: West Conshohocken, PA, USA, 2016.
- ASTM D1037-12: Standard Test Methods for Evaluating Properties of Wood-Based Fiber and Particle Panel Materials; ASTM: West Conshohocken, PA, USA, 2012.
- ASTM D635-18: American Society for Testing and Materials Standard Test Method for Rate of Burning and/or Extent and Time of Burning of Plastics in a Horizontal Position; ASTM: West Conshohocken, PA, USA, 2018.
- Nakamura, S.; Satoh, H.; Ohtsubo, K.N.I. Development of formulae for estimating amylose content, amylopectin chain length distribution, and resistant starch content based on the iodine absorption curve of rice starch. Biosci. Biotechnol. Biochem. 2015, 79, 443–455. [Google Scholar] [CrossRef]
- Barhoum, A.; Rahier, H.; Abou-Zaied, R.E.; Rehan, M.; Dufour, T.; Hill, G.; Dufresne, A. Effect of cationic and anionic surfactants on the application of calcium carbonate nanoparticles in paper coating. ACS Appl. Mater. Interfaces 2014, 6, 2734–2744. [Google Scholar] [CrossRef]
- Kao-Walter, S.; Stahle, P.; Hägglund, R. Fracture toughness of a laminated composite. Eur. Struct. Integr. Soc. 2003, 32, 355–364. [Google Scholar]
- Tomasiewicz, R. The Adhesion of Paperboard to the Gypsum Core of Wallboard: An Investigation of Adhesive Bond Quality in Response to Paper Production Variables and Relative Humidity. Master’s Thesis, North Carolina State University, Raleigh, NC, USA, 2003. [Google Scholar]
- Tajvidi, M.; Gardner, D.J.; Bousfield, D.W. Cellulose nanomaterials as binders: Laminate and particulate systems. J. Renew. Mater. 2016, 4, 365–376. [Google Scholar] [CrossRef]
- ANSI A208.1-2016 Particleboard. 2016. Available online: https://www.compositepanel.org/education-resources/store/standards/ansi-a2081-particleboard.html (accessed on 5 February 2020).
- Fan, H.; Wang, X.; Liu, J.; Xu, B. Surface modification of ground calcium carbonate with starch, sodium stearate, and hexametaphosphate. BioResources 2016, 11, 957–964. [Google Scholar] [CrossRef]
- Sang, Y.; McQuaid, M.; Englezos, P. Pre-flocculation of precipitated calcium carbonate filler by cationic starch for highly filled mechanical grade paper. BioResources 2012, 7, 354–373. [Google Scholar]
- Hubbe, M.A.; Gill, R.A. Fillers for papermaking: A review of their properties, usage practices, and their mechanistic role. BioResources 2016, 11, 2886–2963. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, Q.; Deng, Y.; Ragauskas, A. Improvement of paper strength with starch modified clay. J. Appl. Polym. Sci. 2005, 97, 44–50. [Google Scholar] [CrossRef]
- Deng, Y.; Jones, P.; McLain, L.; Ragauskas, A.J. Starch-modified fillers for linerboard and paper grades: A perspective review. TAPPI J. 2010, 9, 31–36. [Google Scholar] [CrossRef]
- Diop, C.I.K.; Tajvidi, M.; Bilodeau, M.A.; Bousfield, D.W.; Hunt, J.F. Evaluation of the incorporation of lignocellulose nanofibrils as sustainable adhesive replacement in medium density fiberboards. Ind. Crops Prod. 2017, 109, 27–36. [Google Scholar] [CrossRef]
- Bennett, P.D.; Bilodeau, M.A.; Johnson, D.A.; Paradis, M.A.; Spender, J.M.; Chiboroski, P.S.; Christiansen, C.D.; Custer, M.W.; Golden, M.A.; Hargreaves, D.A.; et al. Fire Resistant Fibrous Composite Articles. U.S. Patent US2008/0250741A1, 16 October 2008. [Google Scholar]
- Yu, L.; Cai, J.; Li, H.; Lu, F.; Qin, D.; Fei, B. Effects of boric acid and/or borax treatments on the fire resistance of bamboo filament. BioResources 2017, 12, 5296–5307. [Google Scholar] [CrossRef]
- Kandola, B.K.; Horrocks, A.R.; Price, D.; Coleman, G.V. Flame-retardant treatments of cellulose and their influence on the mechanism of cellulose pyrolysis. J. Macromol. Sci. Part C 1996, 36, 721–794. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Winandy, J.E. Chemical mechanism of fire retardance of boric acid on wood. Wood Sci. Technol. 2004, 38, 375–389. [Google Scholar] [CrossRef]
- Wing, R.E.; Maiti, S.; Doane, W.M. Factors affecting release of butylate from calcium ion-modified starch-borate matrices. J. Control. Release 1987, 5, 79–89. [Google Scholar] [CrossRef]
- Nagieb, Z.A.; Nassar, M.A.; El-Meligy, M.G. Effect of addition of boric acid and borax on fire-retardant and mechanical properties of urea formaldehyde saw dust composites. Int. J. Carbohydr. Chem. 2011, 2011, 146763. [Google Scholar] [CrossRef]
- Pedieu, R.; Koubaa, A.; Riedl, B.; Wang, X.M.; Deng, J. Fire-retardant properties of wood particleboards treated with boric acid. Eur. J. Wood Wood Prod. 2012, 70, 191–197. [Google Scholar] [CrossRef]
- Awada, H.; Montplaisir, D.; Daneault, C. The development of a composite based on cellulose fibres and polyvinyl alcohol in the presence of boric acid. BioResources 2014, 9, 3439–3448. [Google Scholar] [CrossRef]
- Yin, Y.; Li, J.; Liu, Y.; Li, Z. Starch crosslinked with poly(vinyl alcohol) by boric acid. J. Appl. Polym. Sci. 2005, 96, 1394–1397. [Google Scholar] [CrossRef]
- Tan, X.-M.; Huang, N.-Y.; Xie, H.-Q. Complexation between borate ion and hydroxyl groups of phenol-formaldehyde resol resin. J. Wuhan Univ. Technol. Sci. Ed. 2002, 17, 14–18. [Google Scholar]
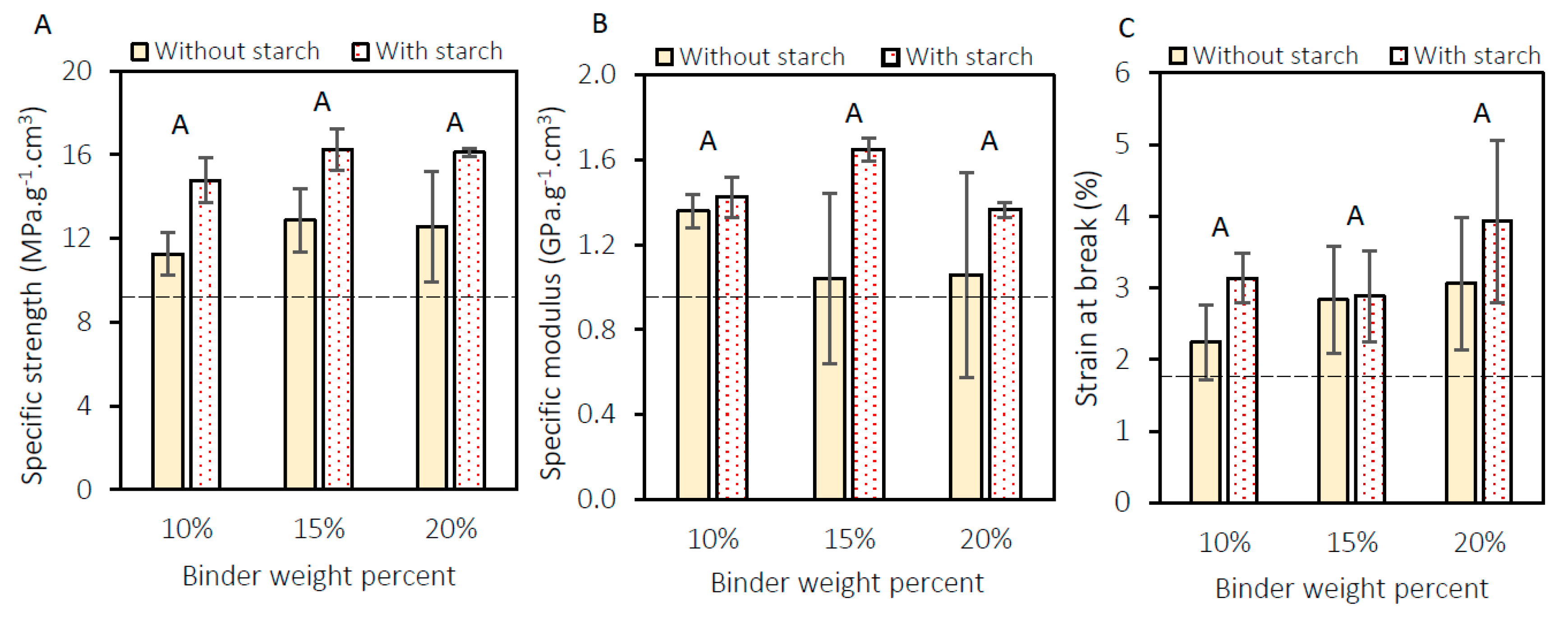
| Treatment | Specific Strength (MPa·g−1·cm3) | Specific Modulus (GPa·g−1·cm3) | Strain at Break (%) | Absorbance at 620 nm |
|---|---|---|---|---|
| Without fire retardant | 16.0 ± 1.0 | 1.3 ± 0.2 | 3.9 ± 1.9 | 1.59 |
| With fire retardant | 16.8 ± 0.8 | 1.6 ± 0.2 | 3.4 ± 1.0 | 0.86 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafez, I.; Tajvidi, M. Laminated Wallboard Panels Made with Cellulose Nanofibrils as a Binder: Production and Properties. Materials 2020, 13, 1303. https://doi.org/10.3390/ma13061303
Hafez I, Tajvidi M. Laminated Wallboard Panels Made with Cellulose Nanofibrils as a Binder: Production and Properties. Materials. 2020; 13(6):1303. https://doi.org/10.3390/ma13061303
Chicago/Turabian StyleHafez, Islam, and Mehdi Tajvidi. 2020. "Laminated Wallboard Panels Made with Cellulose Nanofibrils as a Binder: Production and Properties" Materials 13, no. 6: 1303. https://doi.org/10.3390/ma13061303
APA StyleHafez, I., & Tajvidi, M. (2020). Laminated Wallboard Panels Made with Cellulose Nanofibrils as a Binder: Production and Properties. Materials, 13(6), 1303. https://doi.org/10.3390/ma13061303




