Structure-Property Relationships in Hybrid Cellulose Nanofibrils/Nafion-Based Ionic Polymer-Metal Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of Hybrid Membranes
2.2. Thermogravimetric Analysis (TGA)
2.3. Scanning Electron Microscopy
2.4. Tensile Testing
2.5. Sputter Coating
2.6. Statistical Analysis
3. Results
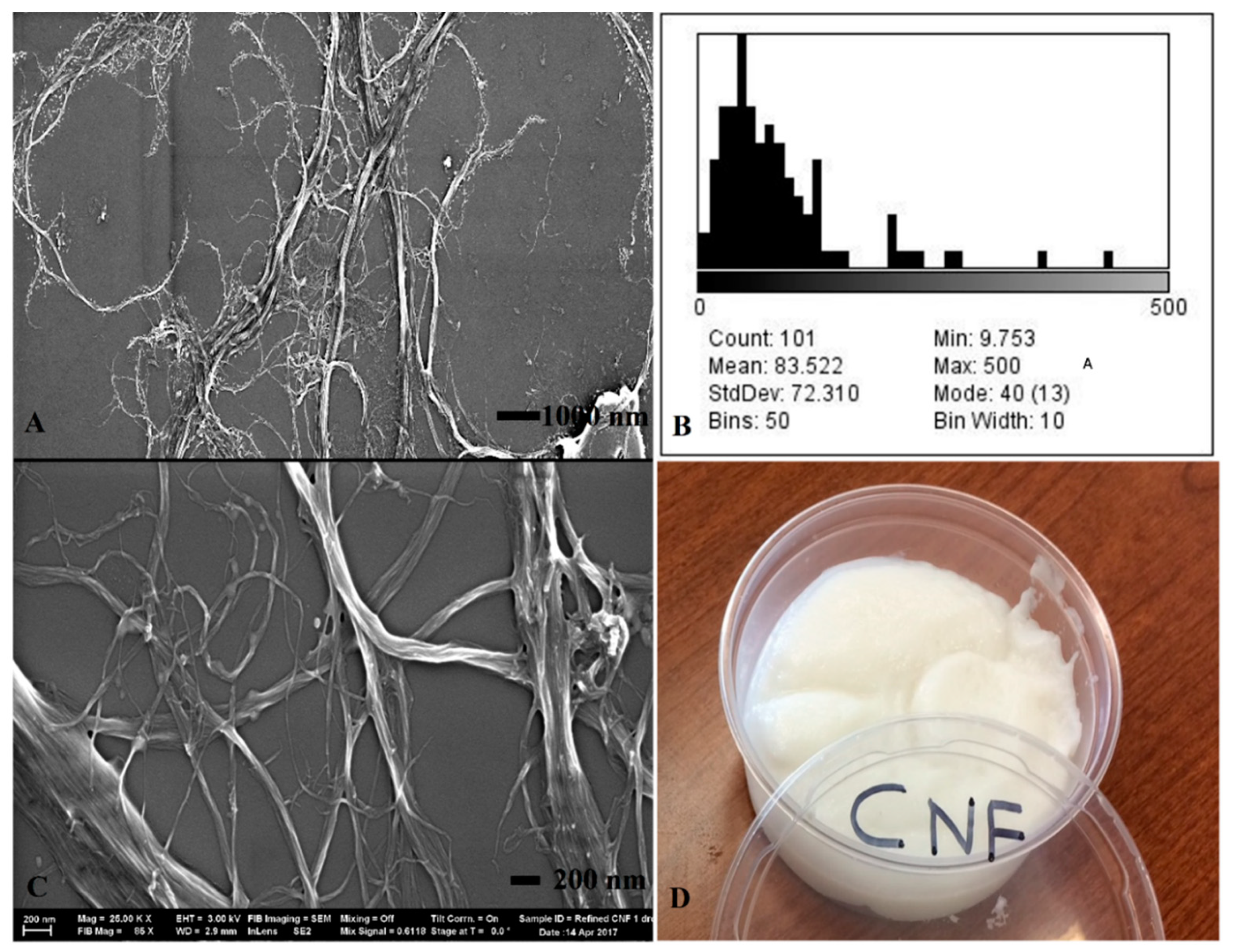
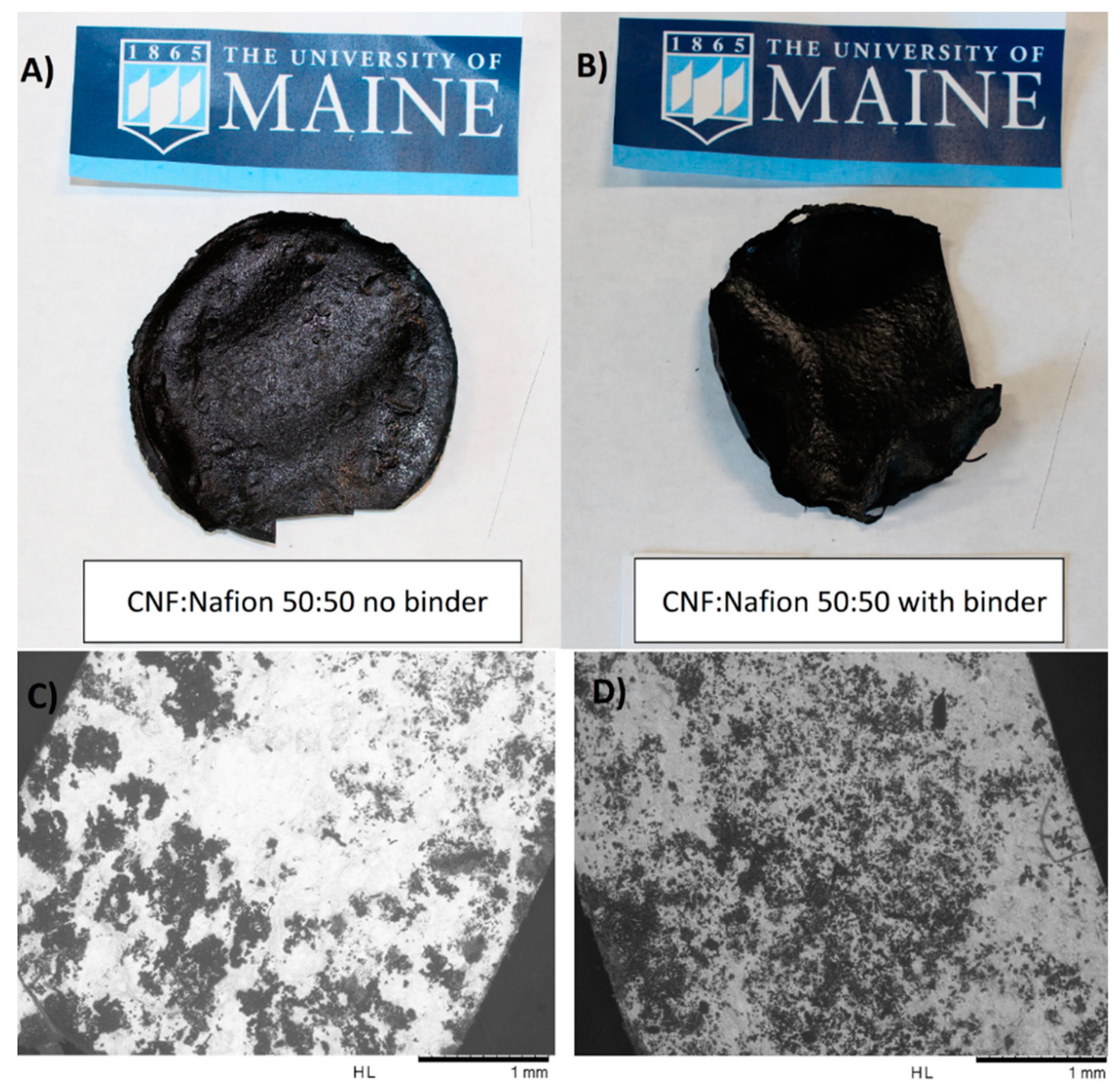
3.1. Morphology and Microstructure
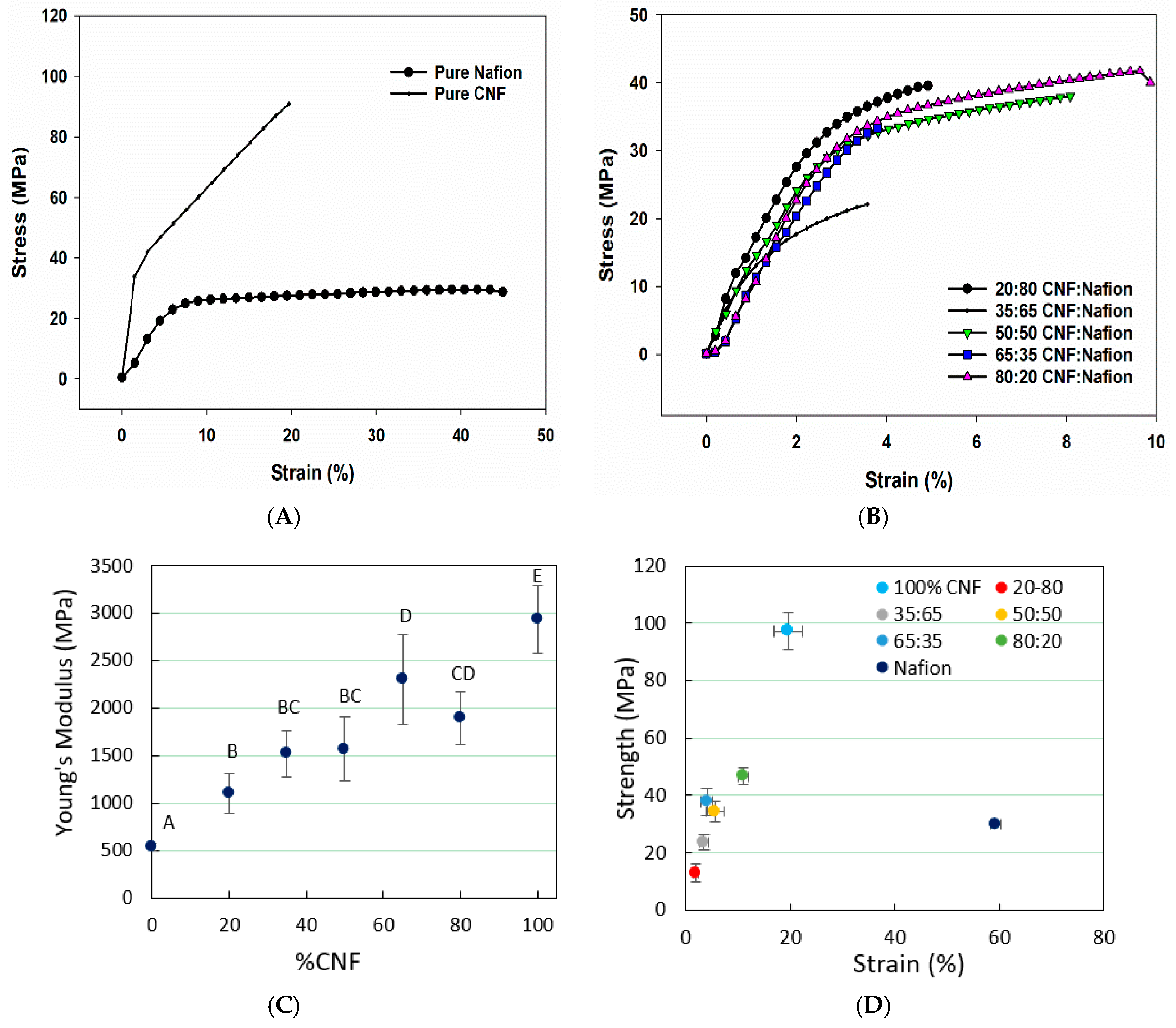
3.2. Mechanical Properties
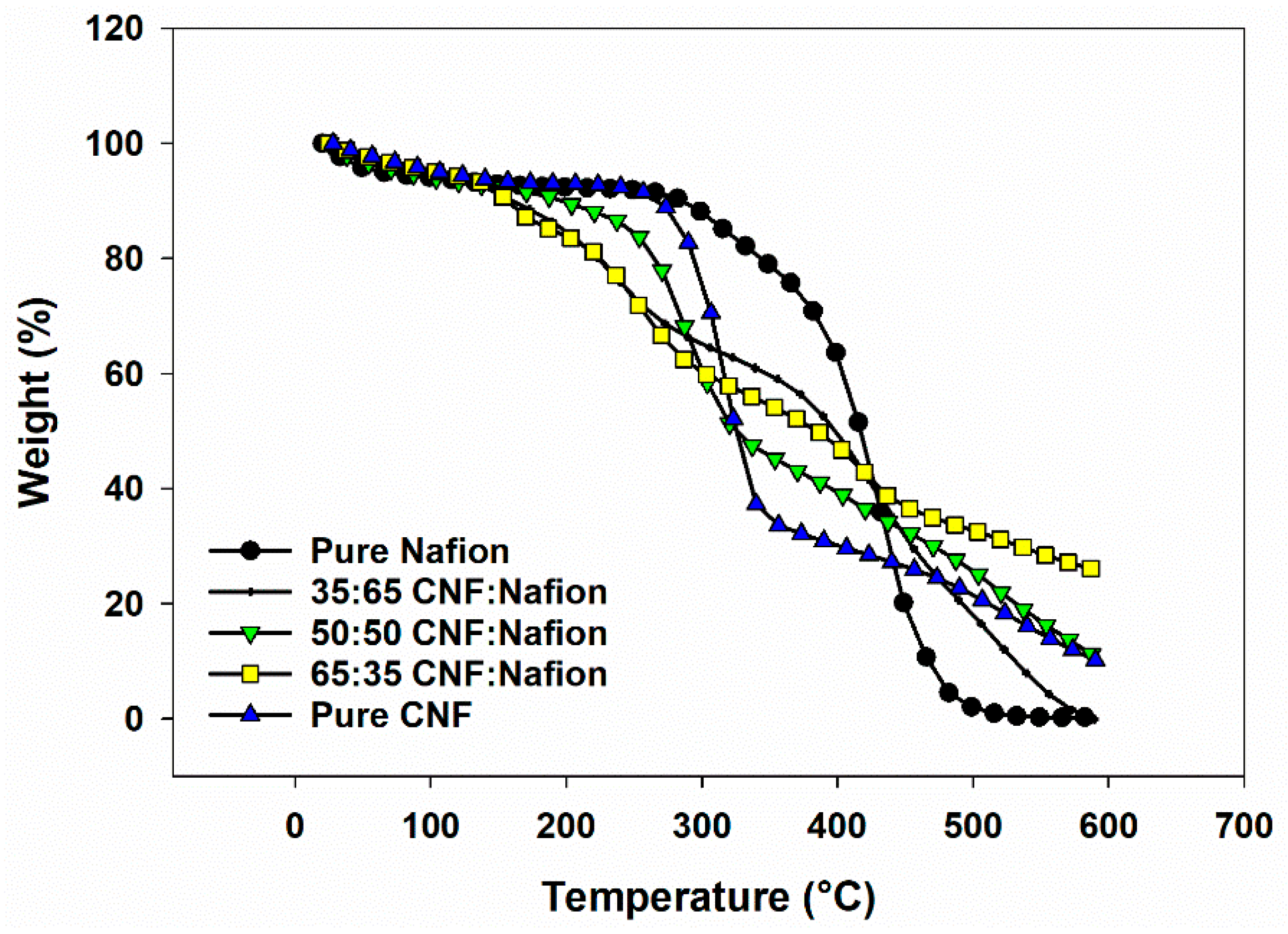
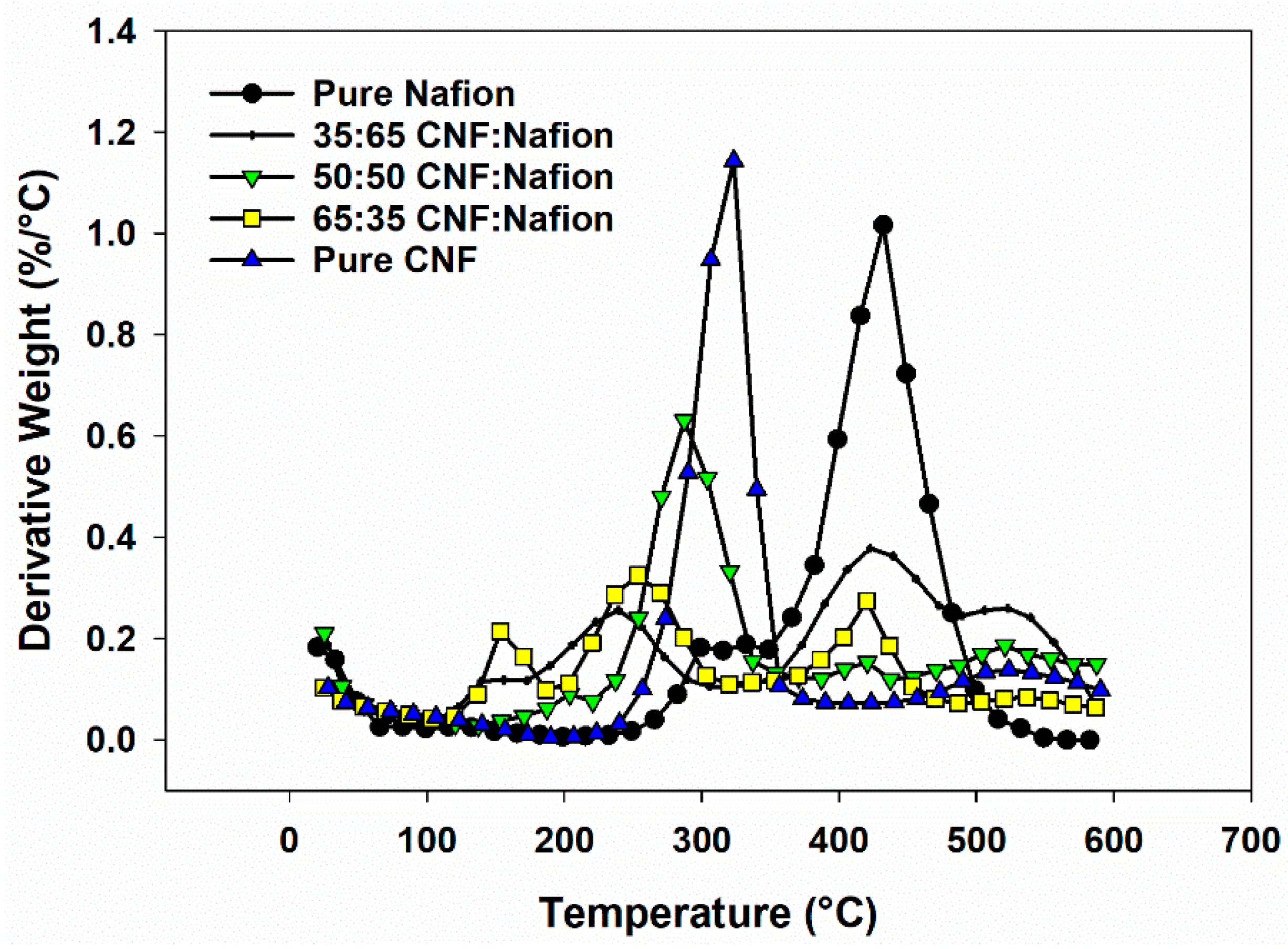
3.3. Thermal Stability
3.4. Sputter Coating
3.5. Chemical Plating by Oxidation-Reduction
3.6. Ionic Conductivity
3.7. Incorporation of Additives
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shahinpoor, M.; Kim, K.J. The effect of surface-electrode resistance on the performance of ionic polymer-metal composite (IPMC) artificial muscles. Smart Mater. Struct. 2000, 9, 543–551. [Google Scholar] [CrossRef]
- Shahinpoor, M.; Kim, K.J. Ionic polymer-metal composites: I. Fundamentals. Smart Mater. Struct. 2001, 10, 819–833. [Google Scholar] [CrossRef]
- Shahinpoor, M.; Kim, K.J. Ionic polymer–metal composites: IV. Industrial and medical applications. Smart Mater. Struct. 2005, 14, 197–214. [Google Scholar] [CrossRef]
- Shen, Q.; Wang, T.; Kim, K.J. A biomimetic underwater vehicle actuated by waves with ionic polymer–metal composite soft sensors. Bioinspir. Biomim. 2015, 10, 55007. [Google Scholar] [CrossRef]
- Vinh, N.D.; Kim, H.-M. Ocean-based electricity generating system utilizing the electrochemical conversion of wave energy by ionic polymer-metal composites. Electrochem. Commun. 2017, 75, 64–68. [Google Scholar] [CrossRef]
- Aureli, M.; Prince, C.; Porfiri, M.; Peterson, S.D. Energy harvesting from base excitation of ionic polymer metal composites in fluid environments. Smart Mater. Struct. 2010, 19, 015003. [Google Scholar] [CrossRef]
- Yong Jung, S.; Young Ko, S.; Park, J.-O.; Park, S. Enhanced ionic polymer metal composite actuator with porous nafion membrane using zinc oxide particulate leaching method. Smart Mater. Struct. 2015, 24, 37007. [Google Scholar] [CrossRef]
- Ru, J.; Wang, Y.; Chang, L.; Chen, H.; Li, D. Preparation and characterization of water-soluble carbon nanotube reinforced Nafion membranes and so-based ionic polymer metal composite actuators. Smart Mater. Struct. 2016, 25, 095006. [Google Scholar] [CrossRef]
- Kaal, W.; Herold, S. Electroactive Polymer Actuators in Dynamic Applications. IEEE/ASME Trans. Mechatron. 2011, 16, 24–32. [Google Scholar] [CrossRef]
- Leary, S.P.; Bar-Cohen, Y. Electrical impedence of ionic polymeric metal composites. In Smart Structures and Materials; Bar-Cohen, Y., Ed.; International Society for Optics and Photonics: Bellingham, WA, USA, 1999; Volume 3669, pp. 1–6. [Google Scholar]
- Bar-Cohen, Y. Electroactive Polymer (EAP) Actuators as Artificial Muscles: Reality, Potential, and Challenges; SPIE Press: Bellingham, WA, USA, 2004; ISBN 9780819452979. [Google Scholar]
- Asaka, K.; Oguro, K.; Nishimura, Y.; Mizuhata, M.; Takenaka, H. Bending of Polyelectrolyte Membrane–Platinum Composites by Electric Stimuli I. Response Characteristics to Various Waveforms. Polym. J. 1995, 27, 436–440. [Google Scholar] [CrossRef]
- Napoli, L.; Lavorante, M.; Franco, J.; Sanguinetti, A.; Fasoli, H. Effects on Nafion® 117 Membrane using Different Strong Acids in Various Concentrations. J. New Mater. Electrochem. Syst. 2013, 16, 151–156. [Google Scholar] [CrossRef]
- Kreuer, K.D. On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J. Memb. Sci. 2001, 185, 29–39. [Google Scholar] [CrossRef]
- Zengin, T. Fabrication, Characterization and Modeling of Electroactive Polymer based Smart Structures for a Biological-like Artificial Muscle. Ph.D. Thesis, Université Paris 6–Pierre et Marie Curie, Paris, France, 2012. [Google Scholar]
- Neburchilov, V.; Martin, J.; Wang, H.; Zhang, J. A review of polymer electrolyte membranes for direct methanol fuel cells. J. Power Sources 2007, 169, 221–238. [Google Scholar] [CrossRef]
- Kraytsberg, A.; Ein-Eli, Y. Review of Advanced Materials for Proton Exchange Membrane Fuel Cells. Energy Fuels 2014, 28, 7303–7330. [Google Scholar] [CrossRef]
- Hwang, T.; Palmre, V.; Nam, J.; Lee, D.-C.; Kim, K.J. A new ionic polymer–metal composite based on Nafion/poly(vinyl alcohol- co -ethylene) blends. Smart Mater. Struct. 2015, 24, 105011. [Google Scholar] [CrossRef]
- Hong, W.; Almomani, A.; Montazami, R. Electrochemical and morphological studies of ionic polymer metal composites as stress sensors. Measurement 2017, 95, 128–134. [Google Scholar] [CrossRef]
- Xia, R.; Zhou, H.; Wu, R.; Wu, W.-P.; Xia, R.; Zhou, H.; Wu, R.; Wu, W.-P. Nanoindentation Investigation of Temperature Effects on the Mechanical Properties of Nafion® 117. Polymers 2016, 8, 344. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Mattoso, L.H.C.; Wood, D.; Williams, T.G.; Avena-Bustillos, R.J.; McHugh, T.H. Nanocomposite Edible Films from Mango Puree Reinforced with Cellulose Nanofibers. J. Food Sci. 2009, 74, 31–35. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Yadav, M.; Chiu, F.-C. Cellulose nanocrystals reinforced κ-carrageenan based UV resistant transparent bionanocomposite films for sustainable packaging applications. Carbohydr. Polym. 2019, 211, 181–194. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Tayeb, P.; Joyce, M.; Tyagi, P.; Kehoe, M.; Dimic-Misic, K.; Pal, L. Rheology of nanocellulose-rich aqueous suspensions: A review. BioResources 2017, 12, 9556–9661. [Google Scholar]
- Chinga-Carrasco, G.; Syverud, K. Computer-assisted quantification of the multi-scale structure of films made of nanofibrillated cellulose. J. Nanopart. Res. 2010, 12, 841–851. [Google Scholar] [CrossRef]
- Tayeb, A.H.; Amini, E.; Ghasemi, S.; Tajvidi, M. Cellulose Nanomaterials—Binding Properties and Applications: A Review. Molecules 2018, 23, 2684. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, G.; Atifi, S.; Vignolini, S.; Hamad, W.Y. Flexible Photonic Cellulose Nanocrystal Films. Adv. Mater. 2016, 28, 10042–10047. [Google Scholar] [CrossRef] [PubMed]
- Gadim, T.D.O.; Vilela, C.; Loureiro, F.J.A.; Silvestre, A.J.D.; Freire, C.S.R.; Figueiredo, F.M.L. Nafion® and nanocellulose: A partnership for greener polymer electrolyte membranes. Ind. Crops Prod. 2016, 93, 212–218. [Google Scholar] [CrossRef]
- Jiang, G.; Zhang, J.; Qiao, J.; Jiang, Y.; Zarrin, H.; Chen, Z.; Hong, F. Bacterial nanocellulose/Nafion composite membranes for low temperature polymer electrolyte fuel cells. J. Power Sources 2015, 273, 697–706. [Google Scholar] [CrossRef]
- Shen, Q.; Trabia, S.; Stalbaum, T.; Palmre, V.; Kim, K.; Oh, I.-K. A multiple-shape memory polymer-metal composite actuator capable of programmable control, creating complex 3D motion of bending, twisting, and oscillation. Sci. Rep. 2016, 6, 24462. [Google Scholar] [CrossRef]
- Tayeb, A.H.; Tajvidi, M. Sustainable Barrier System via Self-Assembly of Colloidal Montmorillonite and Cross-linking Resins on Nanocellulose Interfaces. ACS Appl. Mater. Interfaces 2018, 11, 1604–1615. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Johna, L. Ion Exchange Capacity of Nafion and Nafion Composites. Langmuir 2000, 16, 2866–2871. [Google Scholar] [CrossRef]
- Xu, C.; Zhu, S.; Xing, C.; Li, D.; Zhu, N.; Zhou, H. Isolation and Properties of Cellulose Nanofibrils from Coconut Palm Petioles by Different Mechanical Process. PLoS ONE 2015, 10, e0122123. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, L.B.; Rodríguez, E.S.; Wladyka-Przybylak, M.; Vázquez, A. Thermal degradation and fire resistance of unsaturated polyester, modified acrylic resins and their composites with natural fibres. Polym. Degrad. Stab. 2006, 91, 255–261. [Google Scholar] [CrossRef]
- Yildirim, N.; Shaler, S.; Yildirim, N.; Shaler, S. A Study on Thermal and Nanomechanical Performance of Cellulose Nanomaterials (CNs). Materials 2017, 10, 718. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Wilkie, C.A.; Moore, R.B.; Mauritz, K.A. TGA–FTi.r. investigation of the thermal degradation of Nafion® and Nafion®/[silicon oxide]-based nanocomposites. Polymer 1998, 39, 5961–5972. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Z.; Liu, Y.; Lu, H.; Leng, J. The quintuple-shape memory effect in electrospun nanofiber membranes. Smart Mater. Struct. 2013, 22, 85020. [Google Scholar] [CrossRef]
- Lee, S.G.; Park, H.C.; Pandita, S.D.; Yoo, Y. Performance improvement of IPMC (ionic polymer metal composites) for a flapping actuator. J. Control. Autom. Syst. 2006, 4, 748–755. [Google Scholar]
- Kim, K.J.; Shahinpoor, M. Ionic polymer metal composites: II. Manufacturing techniques. Smart Mater. Struct. 2003, 12, 65–79. [Google Scholar] [CrossRef]
- Kuwertz, R.; Kirstein, C.; Turek, T.; Kunz, U. Influence of acid pretreatment on ionic conductivity of Nafion® membranes. J. Memb. Sci. 2016, 500, 225–235. [Google Scholar] [CrossRef]
- Jiang, G.; Qiao, J.; Hong, F. Application of phosphoric acid and phytic acid-doped bacterial cellulose as novel proton-conducting membranes to PEMFC. Int. J. Hydrog. Energy 2012, 37, 9182–9192. [Google Scholar] [CrossRef]
- Ben Azouz, K.; Ramires, E.C.; Van den Fonteyne, W.; El Kissi, N.; Dufresne, A. Simple Method for the Melt Extrusion of a Cellulose Nanocrystal Reinforced Hydrophobic Polymer. ACS Macro Lett. 2012, 1, 236–240. [Google Scholar] [CrossRef]
- Roman, M.; Winter, W.T. Effect of Sulfate Groups from Sulfuric Acid Hydrolysis on the Thermal Degradation Behavior of Bacterial Cellulose. Biomacromolecules 2004, 5, 1671–1677. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noonan, C.; Tajvidi, M.; Tayeb, A.H.; Shahinpoor, M.; Tabatabaie, S.E. Structure-Property Relationships in Hybrid Cellulose Nanofibrils/Nafion-Based Ionic Polymer-Metal Composites. Materials 2019, 12, 1269. https://doi.org/10.3390/ma12081269
Noonan C, Tajvidi M, Tayeb AH, Shahinpoor M, Tabatabaie SE. Structure-Property Relationships in Hybrid Cellulose Nanofibrils/Nafion-Based Ionic Polymer-Metal Composites. Materials. 2019; 12(8):1269. https://doi.org/10.3390/ma12081269
Chicago/Turabian StyleNoonan, Colin, Mehdi Tajvidi, Ali H. Tayeb, Mohsen Shahinpoor, and Seyed Ehsan Tabatabaie. 2019. "Structure-Property Relationships in Hybrid Cellulose Nanofibrils/Nafion-Based Ionic Polymer-Metal Composites" Materials 12, no. 8: 1269. https://doi.org/10.3390/ma12081269
APA StyleNoonan, C., Tajvidi, M., Tayeb, A. H., Shahinpoor, M., & Tabatabaie, S. E. (2019). Structure-Property Relationships in Hybrid Cellulose Nanofibrils/Nafion-Based Ionic Polymer-Metal Composites. Materials, 12(8), 1269. https://doi.org/10.3390/ma12081269






