Recycled Glass as a Substitute for Quartz Sand in Silicate Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Lime
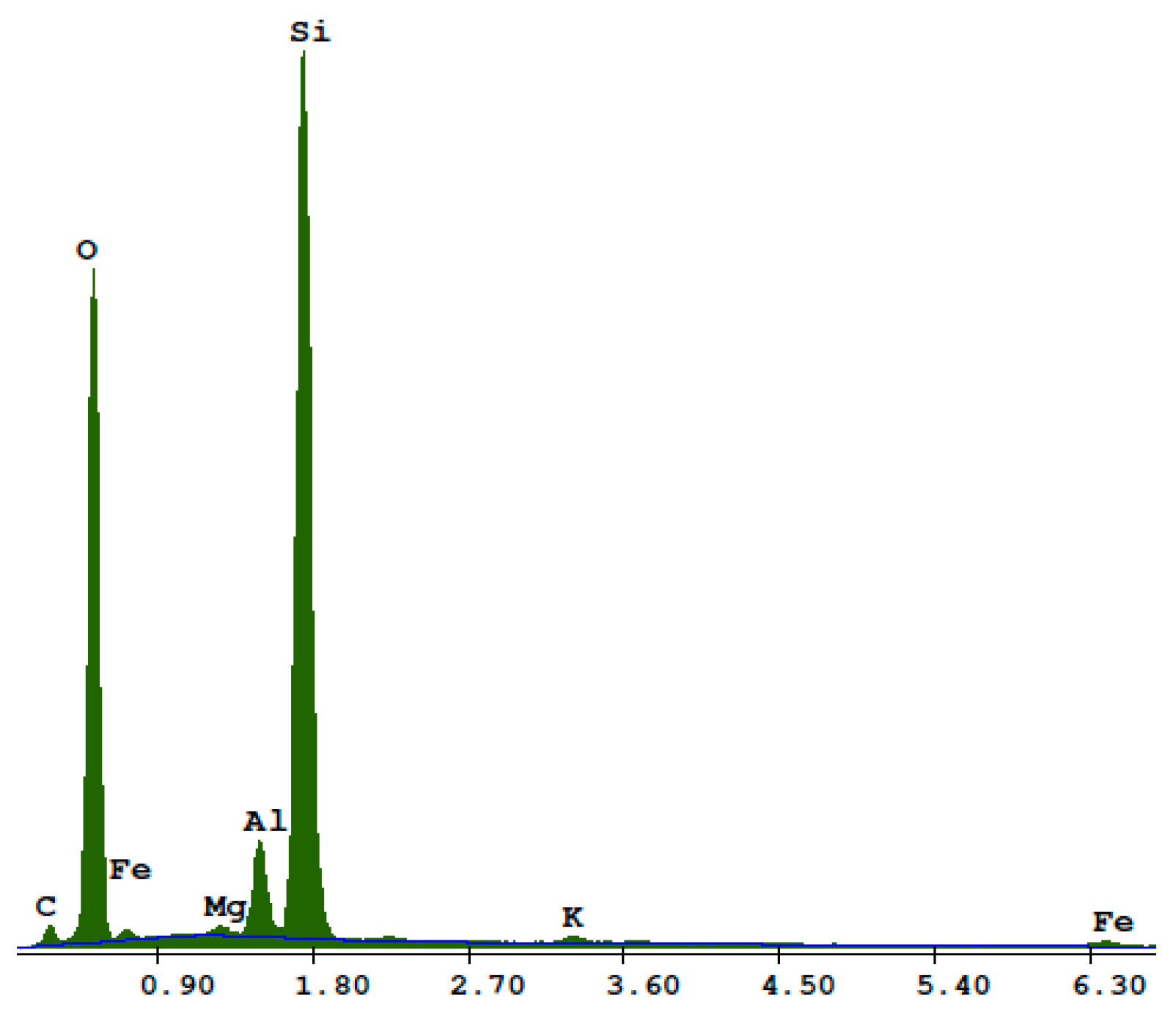
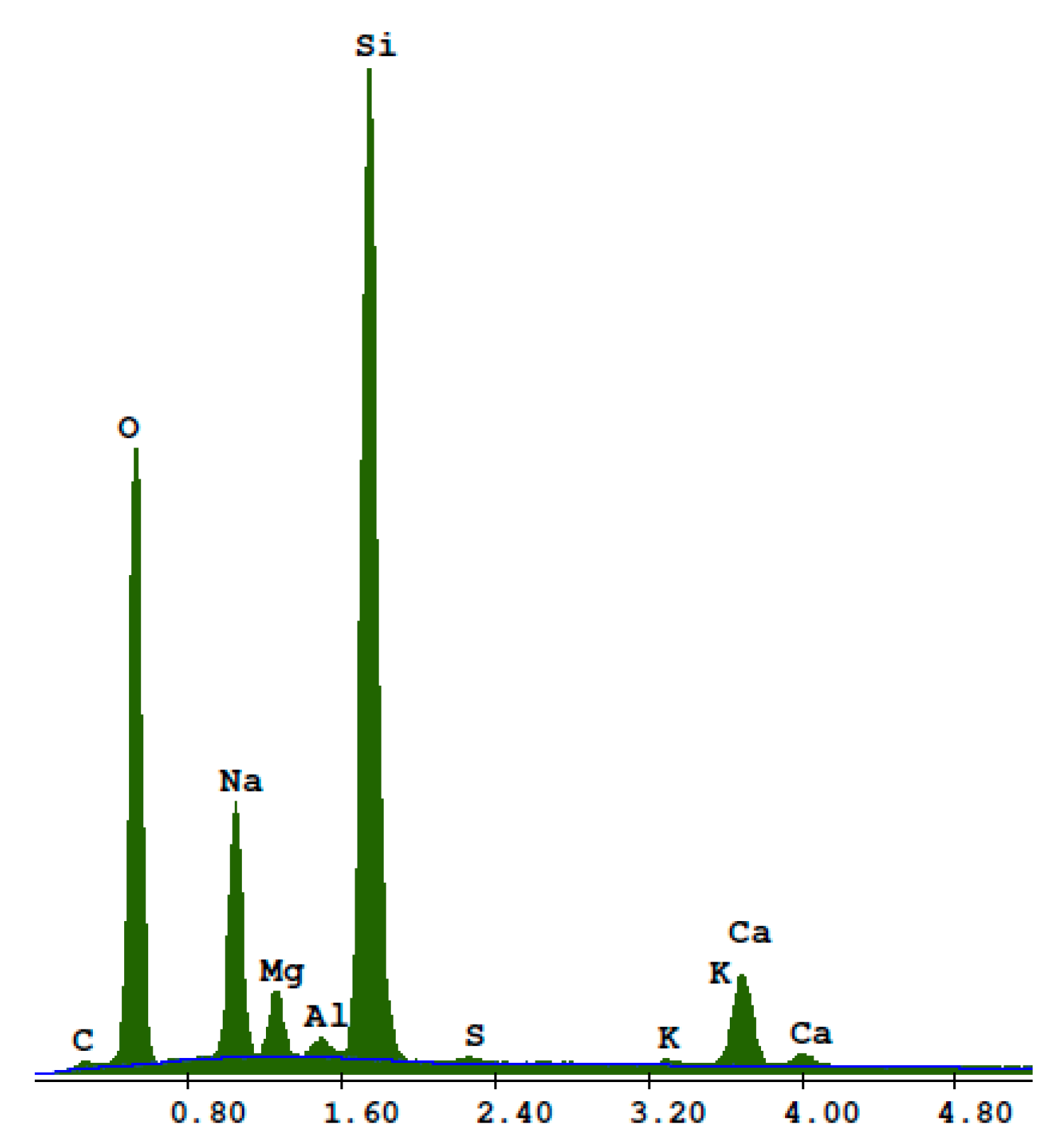
2.2. Sand
2.3. Recycled Waste Glass
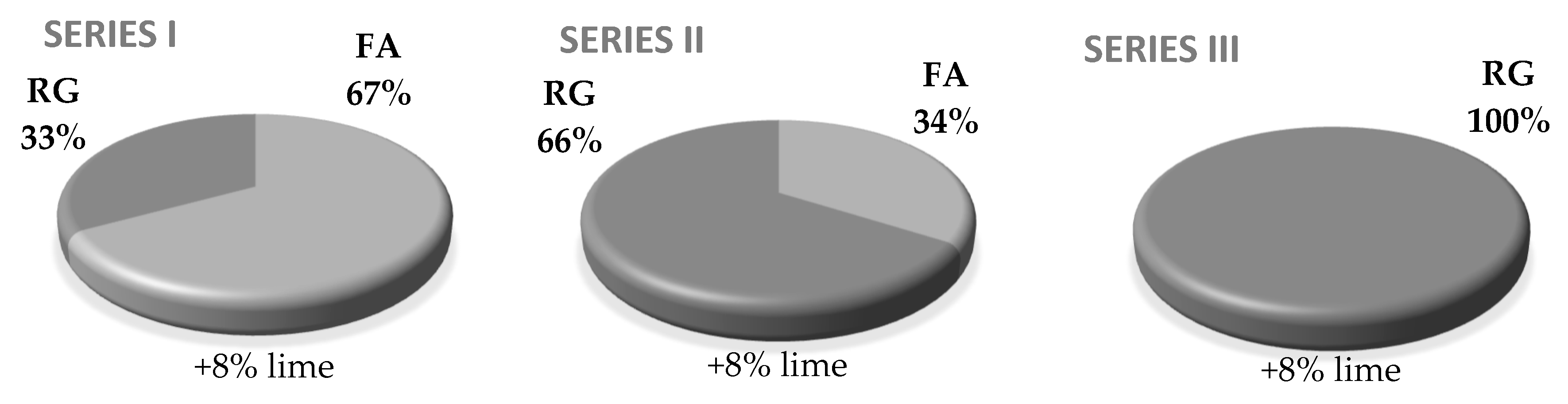
2.4. Preparing the Sand-Lime Specimens with the Addition of Waste Glass
2.5. Testing Methods
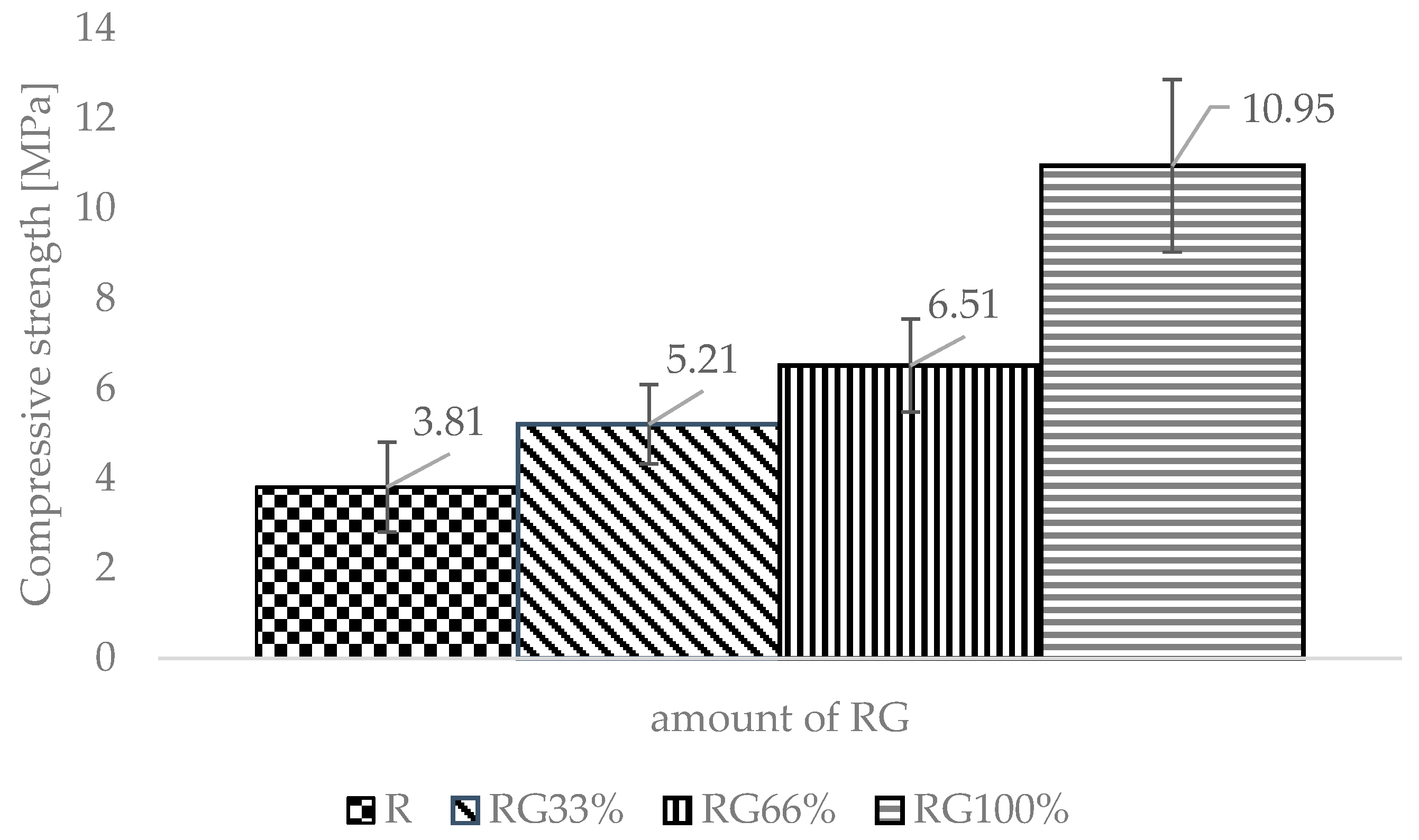
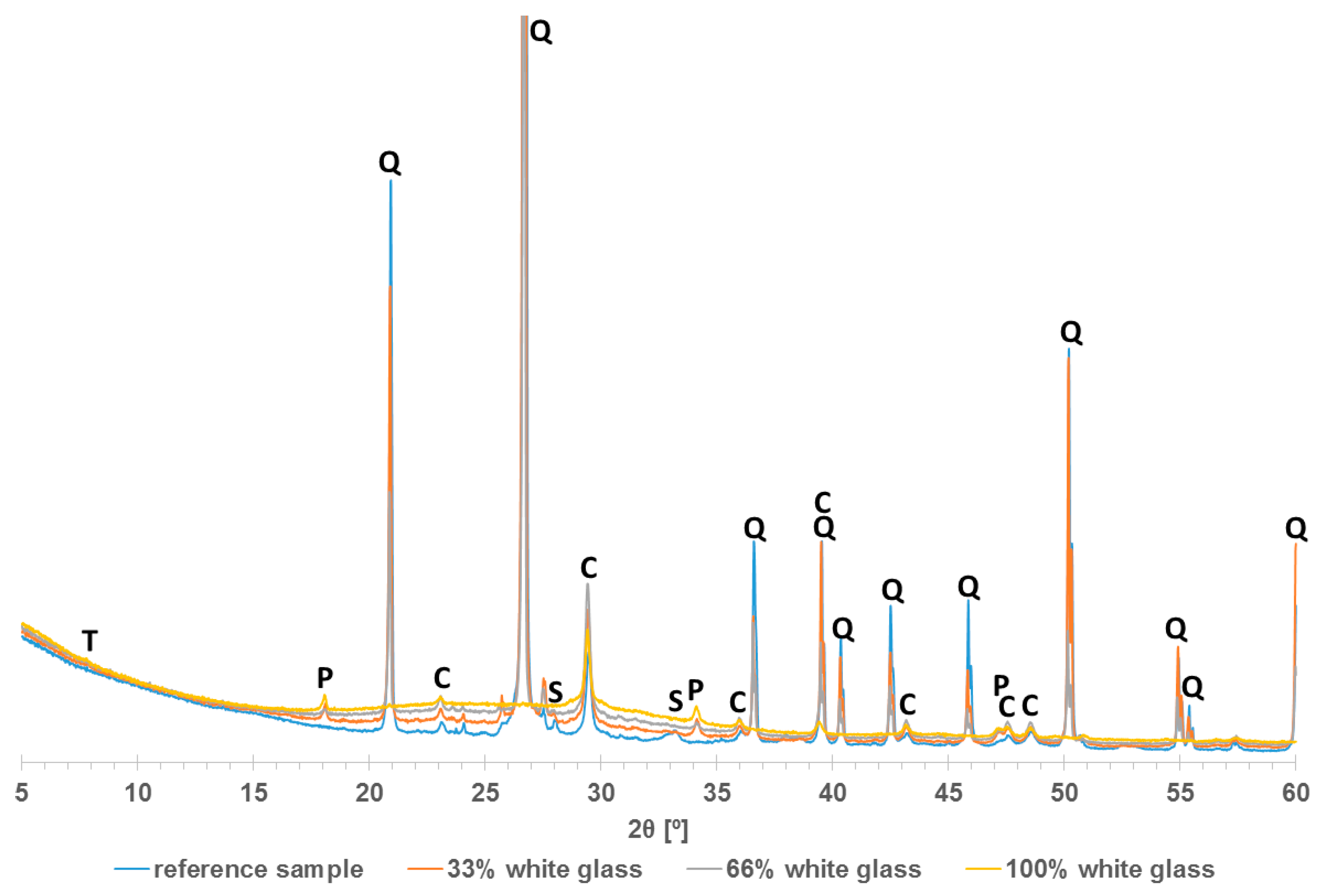
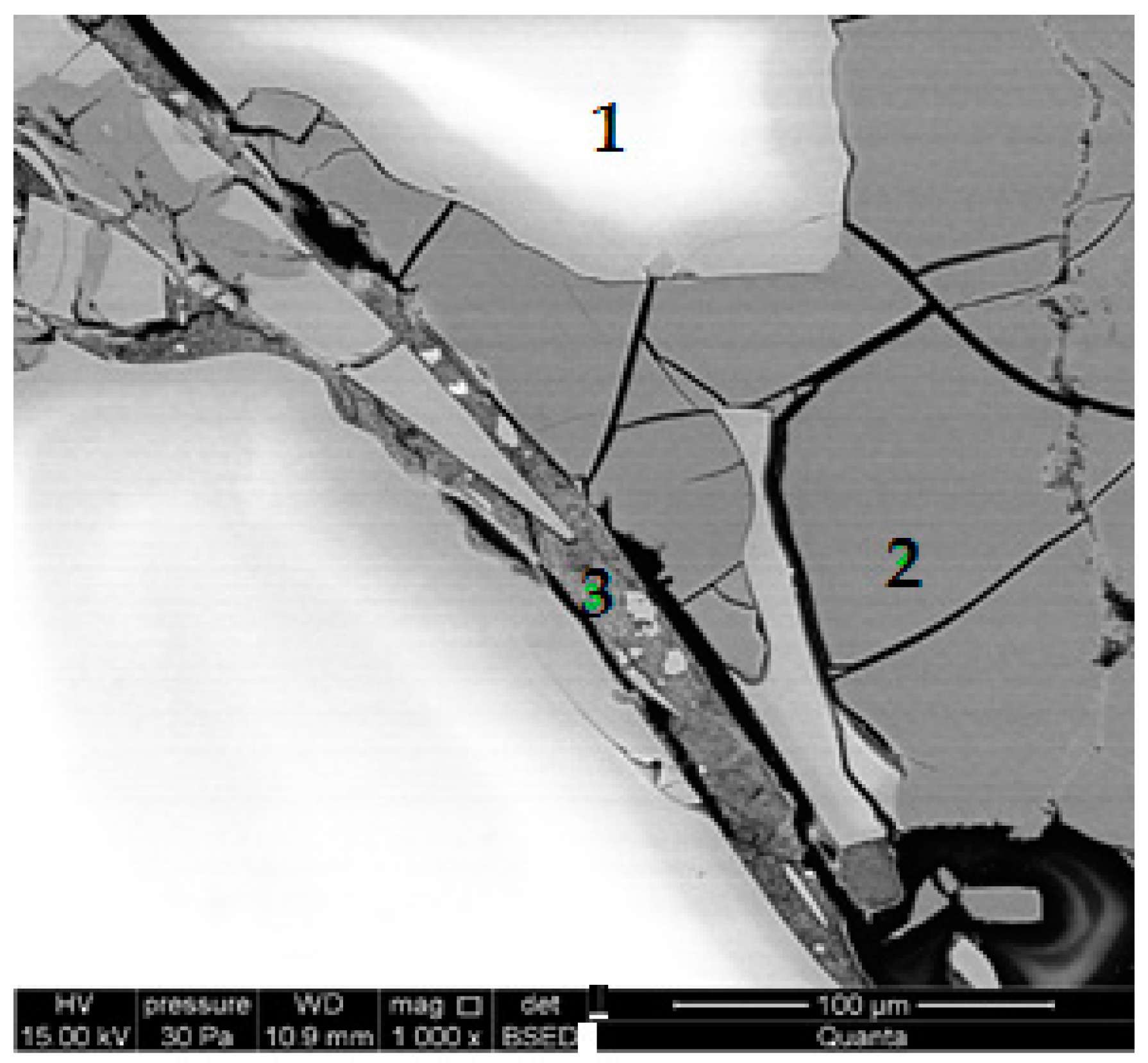
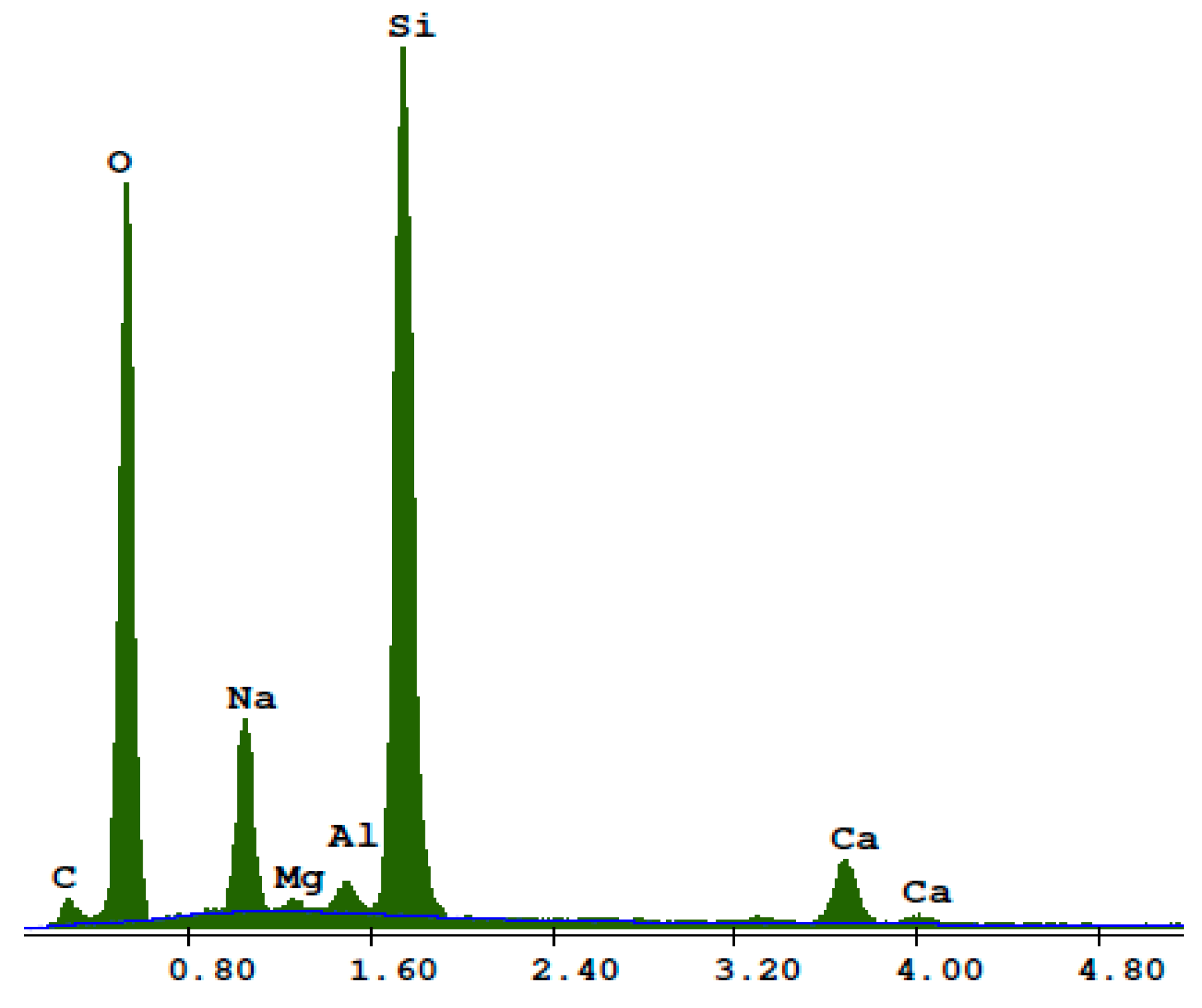
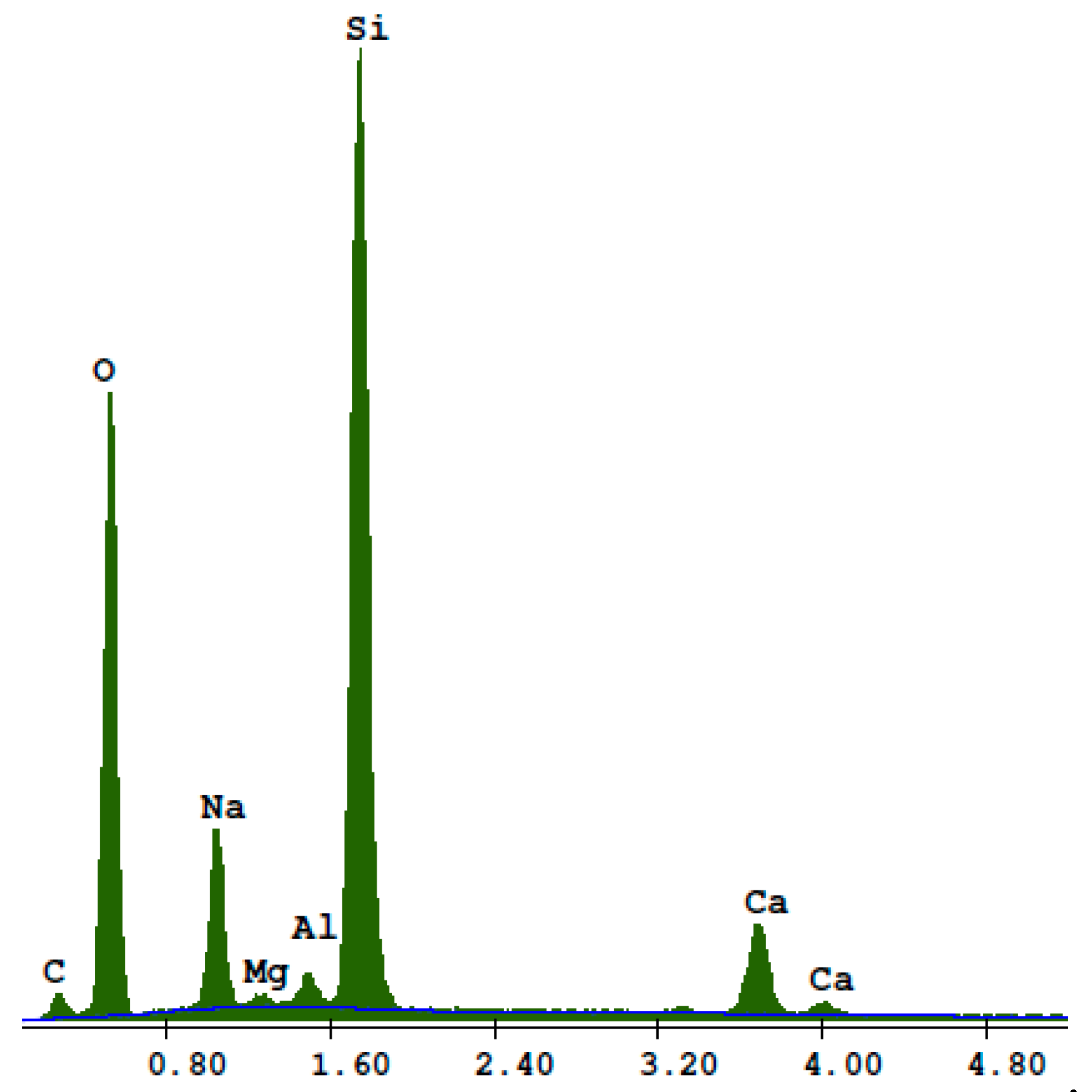
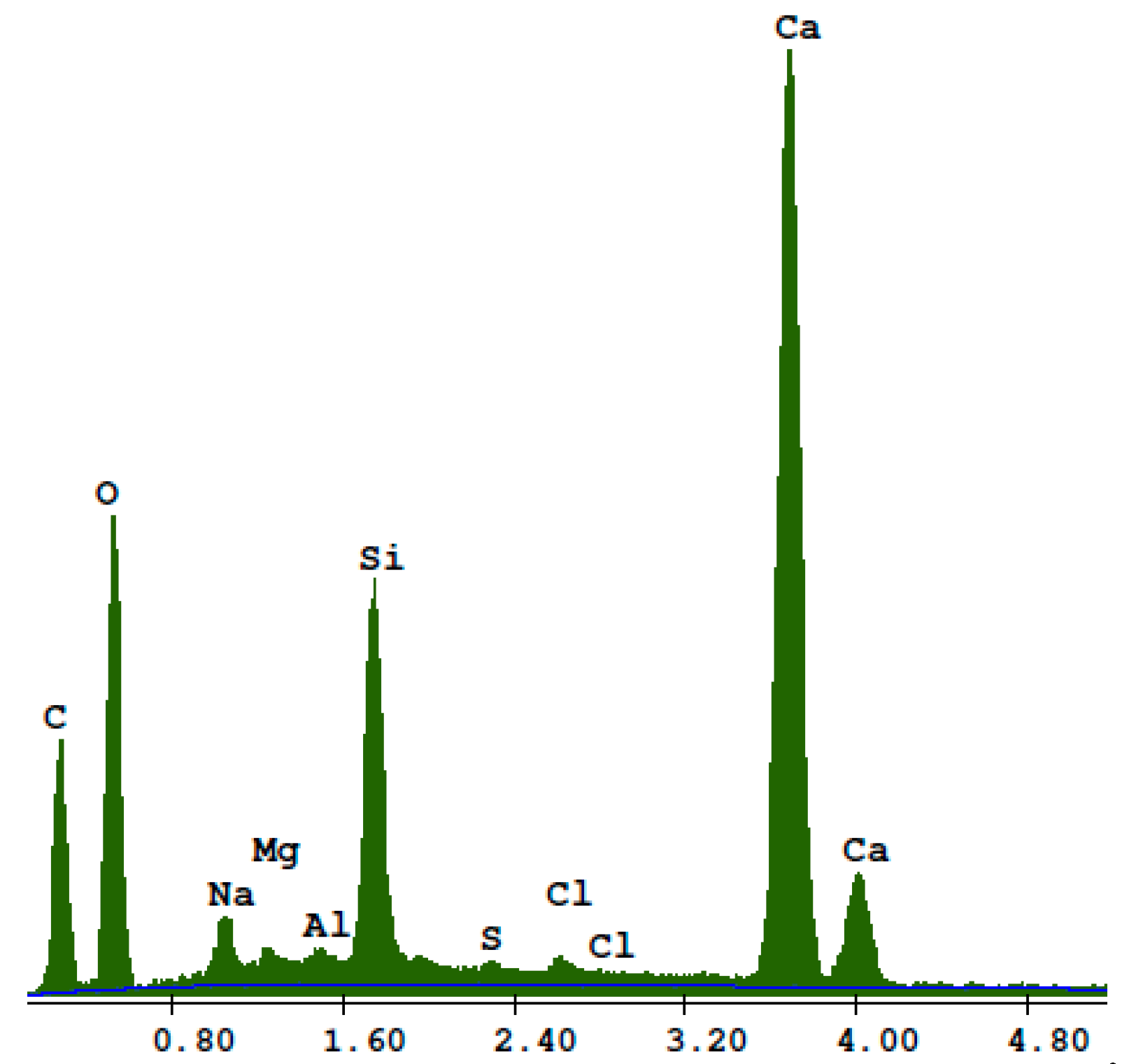
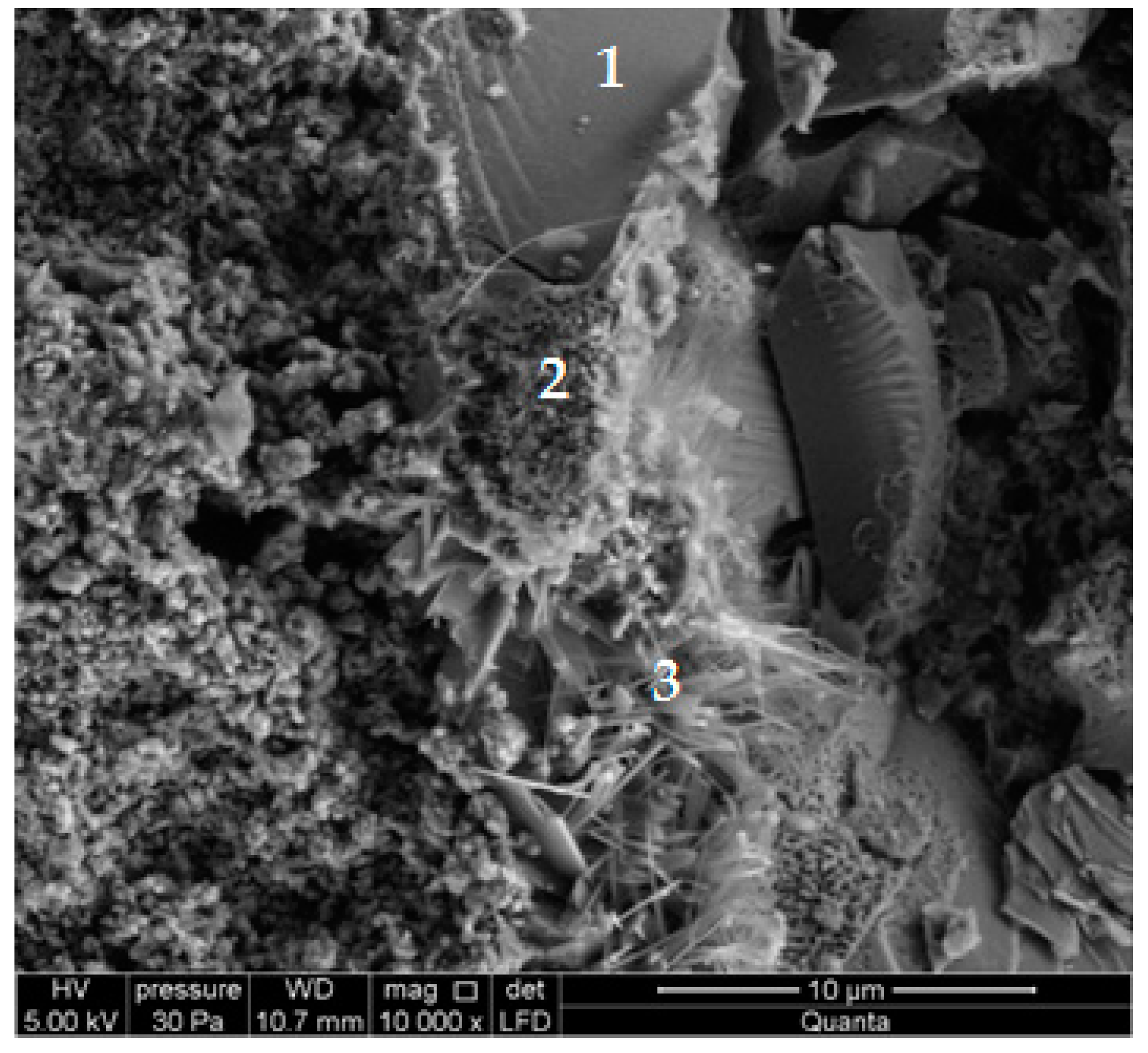
3. Results
4. Conclusions
- RG can be used as a substitute for quartz sand in sand-lime products. A noteworthy increase in compressive strength compared with the reference specimen indicated that it was possible to completely replace the quartz sand in the silicate mix with the recycled waste aggregate. The larger compressive strength was obtained while reducing the density. This fits the current trends in the development of building materials, which are based on both economic and construction considerations: less material consumption, easier transport, the possibility of building taller buildings, and decreasing the cross-section of structural elements.
- For the strength properties and density, the point of view for the silicates production, completely replacing the quartz sand with waste glass was the most advantageous. This operation allowed for obtaining a silicate with a 287% higher compressive strength with a decrease in density by more than 15% compared to a traditional silicate.
- The increase in the content of RG in sand-lime products caused a significant increase in water absorption.
- Research on the microstructure showed that the exchange of quartz sand for recycled waste glass did not influence the formation of the C-S-H phase and tobermorite in autoclaved silicate products.
- The properties of small silicate samples were analyzed in this research. To better understand the silicates’ properties in practice, further research will be carried out on larger volume samples. Comparing the impact of different types of glass on the sand-lime properties is also planned.
Author Contributions
Funding
Acknowledgments

Conflicts of Interest
References
- Rok, B. Rok w zamkniętym obiegu. In Gospodarka Obiegu Zamkniętego; Karwacka, M., Ed.; Koalicja na Rzecz Gospodarki Obiegu Zamkniętego Reconomy: Warsaw, Poland, 2017; pp. 10–14. [Google Scholar]
- Dales, J.H. Pollution. Property and Prices: An Essay in Policy-Making and Economics; Edward Elgar Publishing: Toronto, ON, Canada, 2002. [Google Scholar]
- Kuśnierz, A. Glass recycling. Sci. Works Inst. Ceram. Constr. Mater. 2010, 6, 22–33. [Google Scholar]
- Ibrahim, S.; Meawad, A. Assessment of waste packaging glass bottles as supplementary cementitious materials. Constr. Build. Mater. 2018, 182, 451–458. [Google Scholar] [CrossRef]
- Du, H.; Tan, K.H. Properties of high volume glass powder concrete Cem. Concr. Compos. 2017, 75, 22–29. [Google Scholar] [CrossRef]
- Shao, Y.; Lefort, T.; Moras, S.; Rodriguez, D. Studies on concrete containing ground waste glass. Cem. Concr. Res. 2000, 30, 91–100. [Google Scholar] [CrossRef]
- Meawad, A.; Ibrahim, S. Novel bifunctional dispersing agents from waste PET packaging materials and interaction with cement. Waste Manag. 2019, 85, 563–573. [Google Scholar] [CrossRef]
- Nergis, D.D.B.N.; Abdullah, M.M.A.B.; Sandu, A.V.; Vizureanu, P. XRD and TG-DTA study of new alkali activated materials based on fly ash with sand and glass powder. Materials 2020, 13, 343. [Google Scholar] [CrossRef]
- Ling, T.C.; Poon, C.S. Feasible use of recycled CRT funnel glass as heavyweight fine aggregate in barite concrete. J. Clean. Prod. 2012, 33, 42–49. [Google Scholar] [CrossRef]
- Lee, G.; Poon, C.S.; Wong, Y.L.; Ling, T.C. Effects of recycled fine glass aggregates on the properties of dry-mixed concrete blocks. Constr. Build. Mater. 2013, 38, 638–643. [Google Scholar] [CrossRef]
- Topçu, İ.B.; Canbaz, M. Properties of concrete containing waste glass. Cem. Concr. Res. 2004, 34, 267–274. [Google Scholar] [CrossRef]
- Soliman, N.A.; Arezki, T.H. Using glass sand as an alternative for quartz sand in UHPC. Constr. Build. Mater. 2017, 145, 243–252. [Google Scholar] [CrossRef]
- Mostofinejad, D.; Hosseini, S.M.; Nosouhian, F.; Ozbakkaloglu, T.; Tehrania, B.N. Durability of concrete containing recycled concrete coarse and fine aggregates and milled waste glass in magnesium sulfate environment. J. Build. Eng. 2020. [Google Scholar] [CrossRef]
- Ling, T.C.; Poon, C.S. Feasible use of large volumes of GGBS in 100% recycled glass architectural mortar. Cem. Concr. Compos. 2014, 53, 350–356. [Google Scholar] [CrossRef]
- Guo, M.Z.; Maury-Ramirez, A.; Poon, C.S. Versatile photocatalytic functions of self-compacting architectural glass mortars and their inter-relationship. Mater. Des. 2015, 88, 1260–1268. [Google Scholar] [CrossRef]
- Guo, M.Z.; Chen, Z.; Ling, T.C.; Poo, C.S. Effects of recycled glass on properties of architectural mortar before and after exposure to elevated temperatures. J. Clean. Prod. 2015, 101, 158–164. [Google Scholar] [CrossRef]
- Ling, C.; Poon, C.S. Properties of architectural mortar prepared with recycled glass with different particle sizes. Mater. Des. 2011, 32, 2675–2684. [Google Scholar] [CrossRef]
- Ling, T.C.; Poon, C.S. Effects of particle size of treated CRT funnel glass on properties of cement mortar. Mater. Struct. 2013, 46, 25–34. [Google Scholar] [CrossRef]
- Zhao, H.; Poon, C.S.; Ling, T.C. Utilizing recycled cathode ray tube funnel glass sand as river sand replacement in the high-density concrete. J. Clean. Prod. 2013, 51, 184–190. [Google Scholar] [CrossRef]
- Park, S.B.; Lee, B.C.; Kim, J.H. Studies on mechanical properties of concrete containing waste glass aggregate. Cem. Concr. Res. 2004, 34, 2181–2189. [Google Scholar] [CrossRef]
- Ling, T.C.; Poon, C.S. A comparative study on the feasible use of recycled beverage and CRT funnel glass as fine aggregate in cement mortar. J. Clean. Prod. 2012, 29–30, 46–52. [Google Scholar] [CrossRef]
- Uttam, K.; Balfors, B.; Faith-Ell, C. Green public procurement (GPP) of construction and building materials. In Eco-Efficient Construction and Building Materials; Pacheco-Torgal, F., Cabeza, L.F., Labrincha, J., de Magalhães, A., Eds.; Woodhead Publishing: Cambridge, UK, 2014; pp. 166–195. [Google Scholar] [CrossRef]
- Manzone, F.; Rebaudengo, M.; Lorenzo Zaccaro, V. The Italian Response to Sustainability in Built Environment: The Match between Law and Technical Assessment. In Proceedings of the Third International Congress on Information and Communication Technology, London, UK, 27–28 February 2018; Yang, X.-S., Sherratt, S., Dey, N., Joshi, A., Eds.; Springer Nature Singapore Pte Ltd. Publishing: London, UK, 2019. [Google Scholar] [CrossRef]
- Stępień, A.; Sitarz, M.; Leśniak, M. A sustainable autoclaved material made of glass sand buildings. Buildings 2019, 9, 232. [Google Scholar] [CrossRef]
- Li, J.; Lv, Y.; Jiao, X.; Sun, P.; Li, J.; Wuri, L.; Zhang, T.C. Electrolytic manganese residue based autoclaved bricks with Ca(OH)2 and thermal-mechanical activated K-feldspar additions. Constr. Build. Mater. 2020, 230. [Google Scholar] [CrossRef]
- Fang, Y.; Gu, Y.; Kang, Q.; Wen, Q.; Dai, P. Utilization of copper tailing for autoclaved sand–lime brick. Constr. Build. Mater. 2011, 25, 867–872. [Google Scholar] [CrossRef]
- Komisarczyk, K.; Czapik, P.; Komisarczyk, K. Quartz bentonite sandmix in sand-lime products. Open Eng. 2019, 9, 363–373. [Google Scholar] [CrossRef]
- PN-EN 933-1:2012 Tests for Geometrical Properties of Aggregates—Part 1: Determination of Particle Size Distribution—Sieving Method; Comite Europeen de Normalisation: Warsaw, Poland, 2012.
- PN-EN-1097-6: 2013-11 Tests for Mechanical and Physical Properties of Aggregates—Part 6: Determination of Particle Density and Water Absorption; Comite Europeen de Normalisation: Warsaw, Poland, 2013.
- PN-EN 772-1+A1:2015-10 Methods of Test for Masonry Units—Part 1: Determination of Compressive Strength; Comite Europeen de Normalisation: Warsaw, Poland, 2015.
- PN-EN 772-21:2011 Methods of Test for Masonry Units—Part 21: Determination of Water Absorption of Clay and Calcium Silicate Masonry Units by Cold Water Absorption; Comite Europeen de Normalisation: Warsaw, Poland, 2011.
- Powęzka, A.; Szulej, J.; Ogrodnik, P. Effect of high temperatures on the impact strength of concrete based on recycled aggregate made of heat-resistant cullet. Materials 2020, 13, 465. [Google Scholar] [CrossRef] [PubMed]




| Functional Features of Burnt Lime | Declared Value |
|---|---|
| CaO + MgO (%) | ≥91 |
| MgO (%) | ≤2.0 |
| CO2 (%) | ≤3.0 |
| SO3 (%) | ≤0.5 |
| Screening through a 0.09 mm sieve (%) | ≥90 |
| Reactivity at 60 °C | ≤2.0 |
| Sieve Size | Series I RG33% | Series II RG66% | Series III RG100% | |||
|---|---|---|---|---|---|---|
| FA (g) | RG (g) | FA (g) | RG (g) | FA (g) | RG (g) | |
| 0 | 0.11 | 0.05 | 0.05 | 0.11 | 0 | 0.16 |
| 0.063 | 4.56 | 2.24 | 2.31 | 4.49 | 0 | 6.8 |
| 0.125 | 57.38 | 28.26 | 29.12 | 56.52 | 0 | 85.64 |
| 0.25 | 155.36 | 76.52 | 78.84 | 153.04 | 0 | 231.88 |
| 0.5 | 49.45 | 24.35 | 25.09 | 48.71 | 0 | 73.8 |
| 1.0 | 1.15 | 0.57 | 0.58 | 1.14 | 0 | 1.72 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borek, K.; Czapik, P.; Dachowski, R. Recycled Glass as a Substitute for Quartz Sand in Silicate Products. Materials 2020, 13, 1030. https://doi.org/10.3390/ma13051030
Borek K, Czapik P, Dachowski R. Recycled Glass as a Substitute for Quartz Sand in Silicate Products. Materials. 2020; 13(5):1030. https://doi.org/10.3390/ma13051030
Chicago/Turabian StyleBorek, Katarzyna, Przemysław Czapik, and Ryszard Dachowski. 2020. "Recycled Glass as a Substitute for Quartz Sand in Silicate Products" Materials 13, no. 5: 1030. https://doi.org/10.3390/ma13051030
APA StyleBorek, K., Czapik, P., & Dachowski, R. (2020). Recycled Glass as a Substitute for Quartz Sand in Silicate Products. Materials, 13(5), 1030. https://doi.org/10.3390/ma13051030






