Effect of Graphene Oxide Modified with Organic Amine on the Aging Resistance, Rolling Loss and Wet-Skid Resistance of Solution Polymerized Styrene-Butadiene Rubber
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Synthesis of PPD-GO
2.3. The Preparation of SSBR Composite
2.4. Characterization
3. Results and Discussion
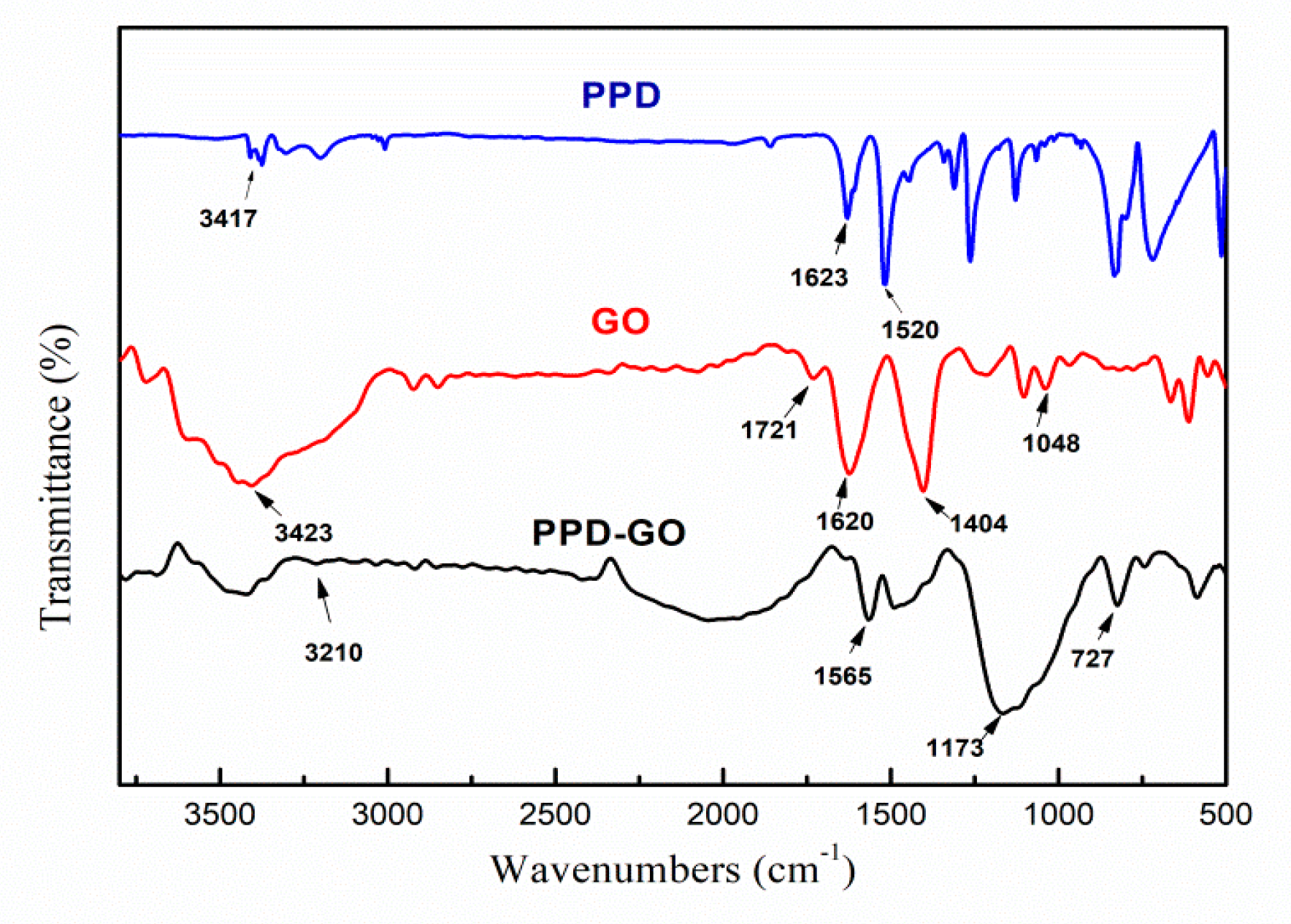
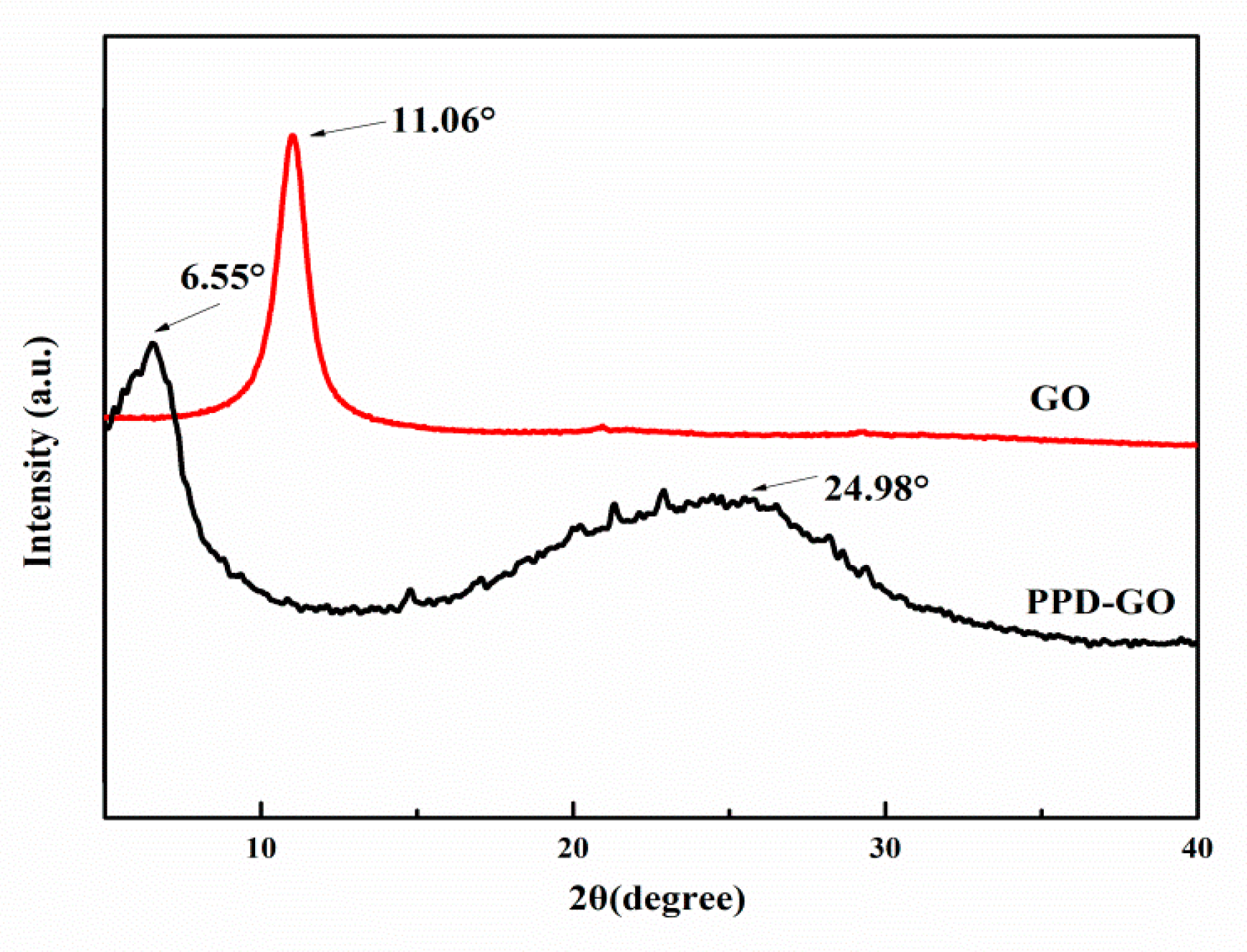
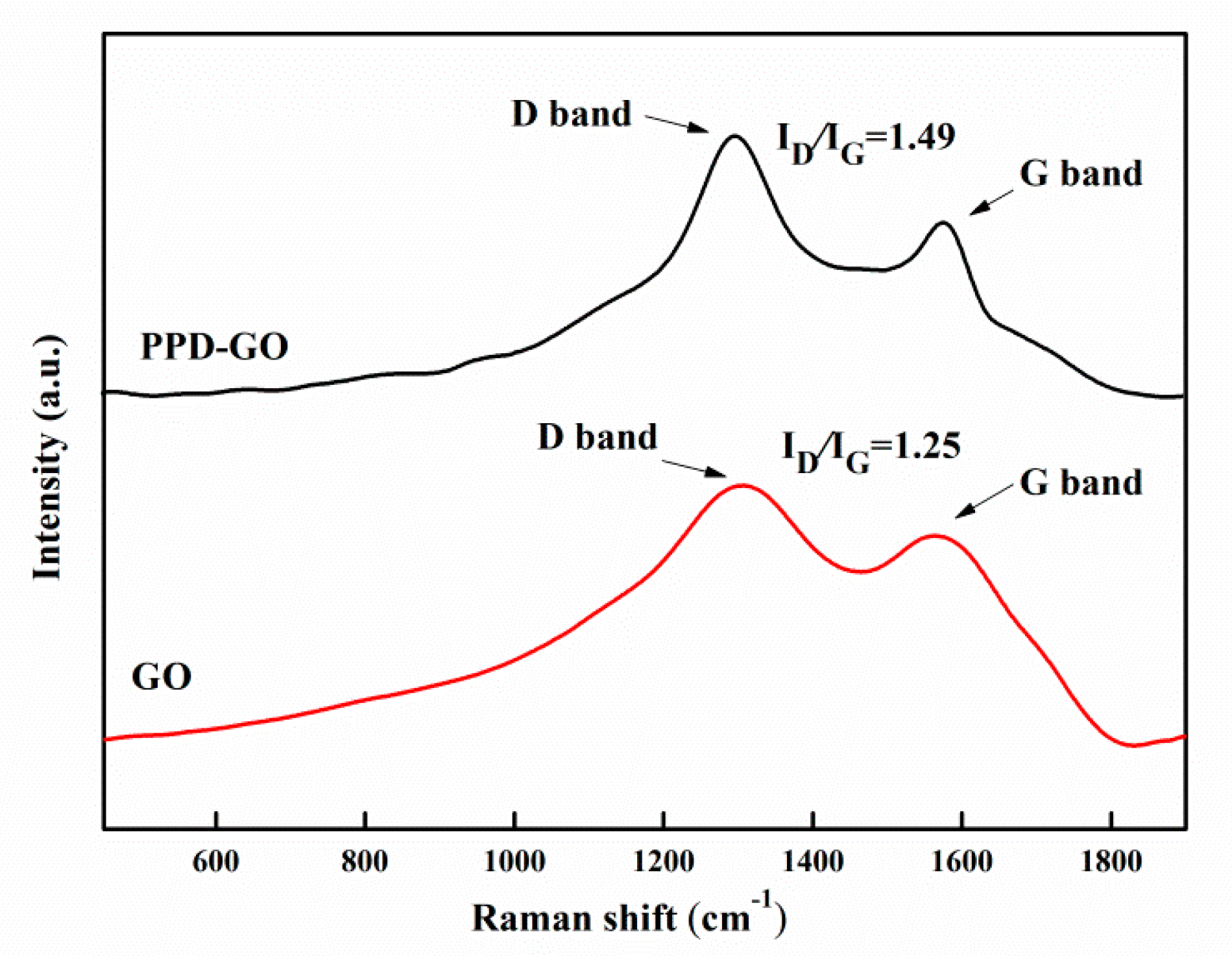
3.1. The Characterization of Structure of PPD-GO
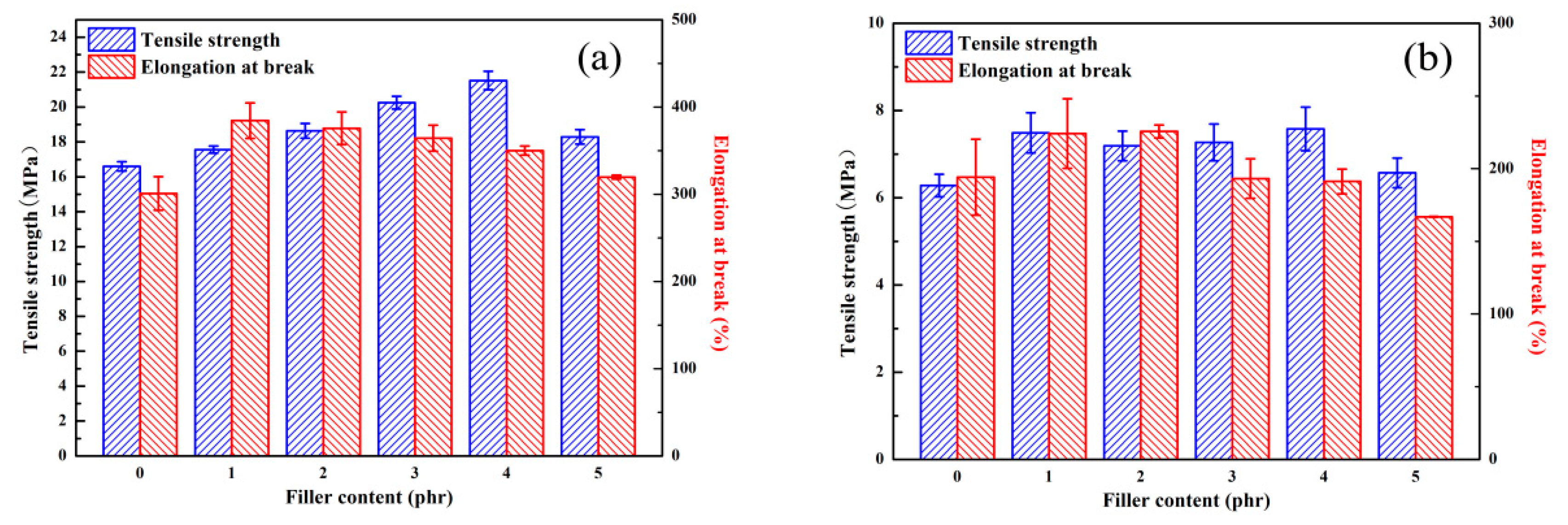
3.2. The Mechanical Properties of SSBR Composites Before and After Aging.
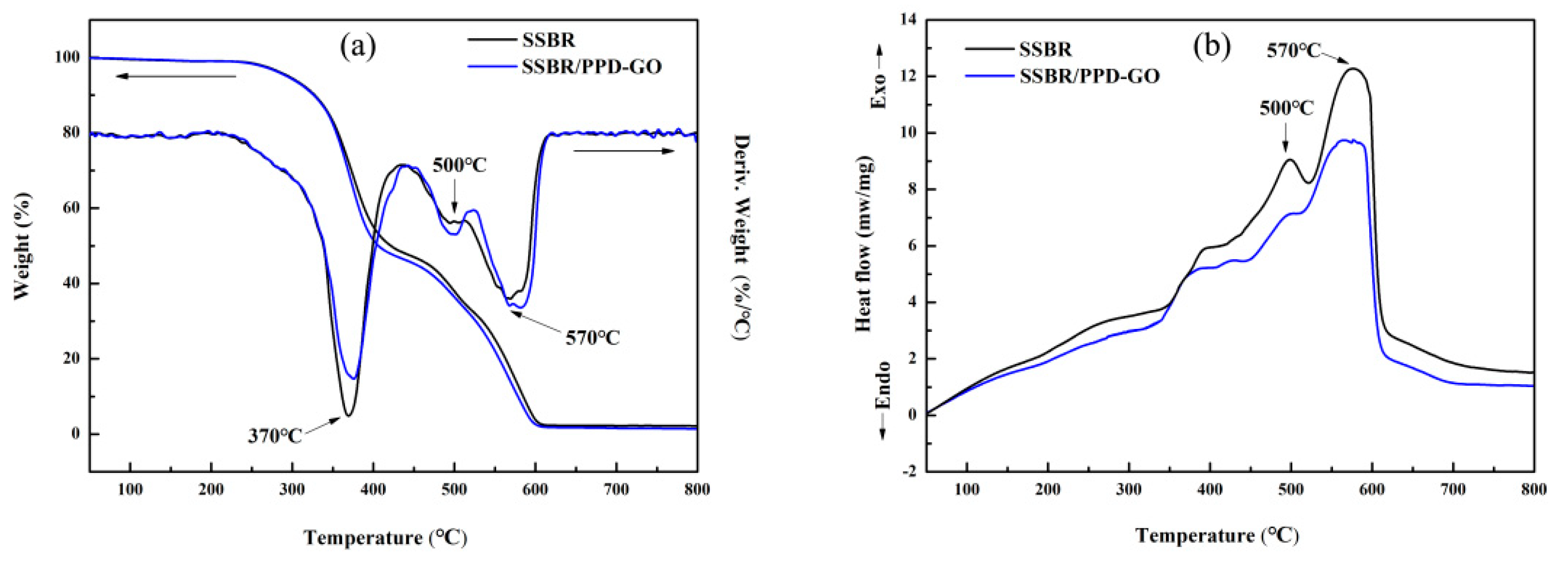
3.3. The Thermal Properties of SSBR Composites
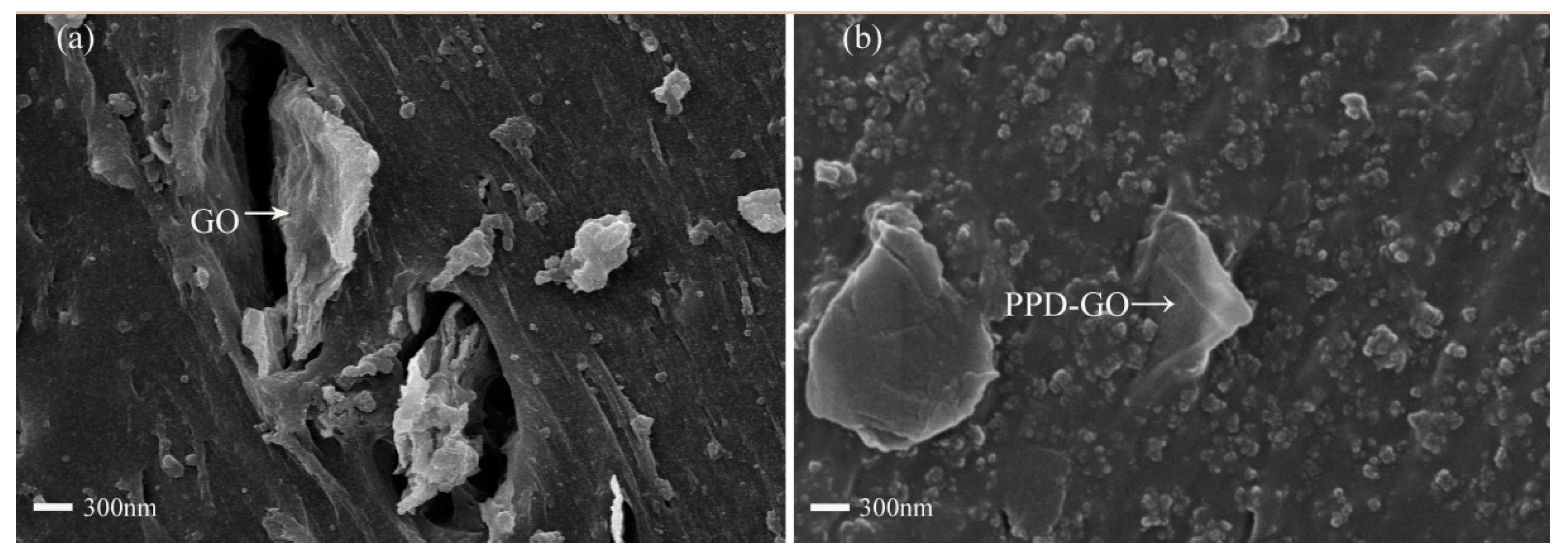
3.4. The Micromorphology of SSBR Composites
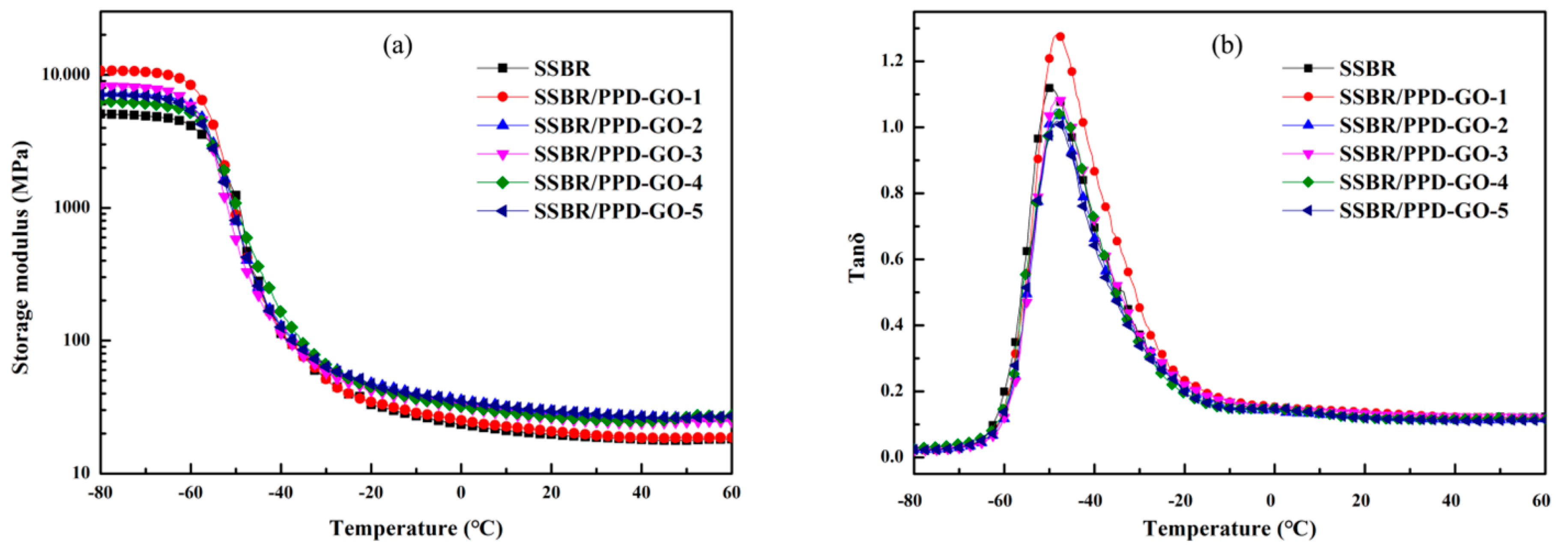
3.5. The Dynamic Mechanical Properties of SSBR Composites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xiao, L.; Zhao, S. Study on structure and properties of SSBR/SiO2 co-coagulated rubber and SSBR filled with nanosilica composites. J. Appl. Polym. Sci. 2008, 109, 3900–3907. [Google Scholar]
- Li, H.; Sun, J.; Song, Y.; Zheng, Q. The mechanical and viscoelastic properties of SSBR vulcanizates filled with organically modified montmorillonite and silica. J. Mater. Sci. 2009, 44, 1881–1888. [Google Scholar] [CrossRef]
- Ge, G.; Sun, A.; Li, S.; Huang, Z. Effect of silane coupling agent Si69 on the hot air aging properties of SSBR/SiO2 vulcanizates. World Rubber Ind. 2013, 40, 7–11. [Google Scholar]
- Luo, K.; Zheng, W.; Zhao, X.; Wang, X.; Wu, S. Effects of antioxidant functionalized silica on reinforcement and anti-aging for solution-polymerized styrene butadiene rubber: Experimental and molecular simulation study. Mater. Des. 2018, 154, 312–325. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, C.; Hu, J.; He, X.; Zhang, R. Damping analysis of some inorganic particles on poly(butyl-methacrylate). Materials 2018, 11, 992. [Google Scholar] [CrossRef]
- Zhong, R.; Zhang, Z.; Zhao, H.; He, X.; Wang, X.; Zhang, R. Improving thermo-oxidative stability of nitrile rubber composites by functional graphene oxide. Materials 2018, 11, 921. [Google Scholar] [CrossRef]
- Tropin, T.V.; Gunnar, S.; Jürn, W.P.S.; Christoph, S. Heat capacity measurements and modeling of polystyrene glass transition in a wide range of cooling rates. J. Non-Cryst. Solids 2015, 409, 63–75. [Google Scholar] [CrossRef]
- Scherillo, G.; Marino, L.; Giovanna, G.B.; Zhan, Y.H.; Xia, H.S.; Giuseppe, M.; Luigi, A. Tailoring assembly of reduced graphene oxide nanosheets to control gas barrier properties of natural rubber nanocomposites. Acs Appl. Mater. Interfaces 2014, 6, 2230–2234. [Google Scholar] [CrossRef]
- Li, T.; Shi, Z.; He, X.; Jiang, P.; Lu, X.; Zhang, R.; Wang, X. Aging-resistant functionalized ldh–sas/nitrile-butadiene rubber composites: preparation and study of aging kinetics/anti-aging mechanism. Materials 2018, 11, 836. [Google Scholar] [CrossRef]
- He, X.; Li, T.; Shi, Z.; Wang, X.; Xue, F.; Wu, Z.; Che, Q. Thermal-oxidative aging behavior of nitrile-butadiene rubber/functional LDHs composites. Polym. Degrad. Stab. 2016, 133, 219–226. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, Z.; Kong, L.; Huang, M.; Li, P. Natural rubber nanocomposite reinforced with nano silica. Polym. Eng. Sci. 2008, 48, 1674–1677. [Google Scholar] [CrossRef]
- Das, A.; Stöckelhuber, K.W.; Jurk, R.; Saphiannikova, M.; Fritzsche, J.; Lorenz, H.H.; Klüppel, M.; Heinrich, G. Modified and unmodified multiwalled carbon nanotubes in high performance solution-styrene–butadiene and butadiene rubber blends. Polymer 2008, 49, 5276–5283. [Google Scholar] [CrossRef]
- Yoo, Y.; Cui, L.; Yoon, P.J.; Paul, D.R. Morphology and mechanical properties of rubber toughened amorphous polyamide/mmt nanocomposites. Macromolecules 2009, 43, 615–624. [Google Scholar] [CrossRef]
- Mishra, S.; Shimpi, N.G.; Mali, A.D. Surface modification of montmorillonite (MMT) using column chromatography technique and its application in silicone rubber nanocomposites. Macromol. Res. 2012, 20, 44–50. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.B.; Evmenenko, G.; Nguyen, S.B.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: preparation, functionalization and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.; Potts, J.; Ruoff, R.S. Graphene and graphene oxide: synthesis, properties and applications. Cheminform 2010, 22, 3906–3924. [Google Scholar]
- Mao, Y.; Wen, S.; Chen, Y.; Zhang, F.; Panine, P.; Chan, T.; Zhang, L.; Liang, Y.; Liu, L. High performance graphene oxide based rubber composites. Sci. Rep. 2013, 3, 2508. [Google Scholar] [CrossRef]
- Li, C.; Feng, C.; Peng, Z.; Gong, W.; Kong, L. Ammonium-assisted green fabrication of graphene/natural rubber latex composite. Polym. Compos. 2013, 34, 88–95. [Google Scholar] [CrossRef]
- Song, S.H.; Jeong, H.K.; Kang, Y.G. Preparation and characterization of exfoliated graphite and its styrene butadiene rubber nanocomposites. J. Ind. Eng. Chem. 2010, 16, 1059–1065. [Google Scholar] [CrossRef]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Piner, R.D.; Nguyen, S.; Ruoff, R. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 2006, 44, 3342–3347. [Google Scholar] [CrossRef]
- Niyogi, S.; Bekyarova, E.; Itkis, M.; McWilliams, J.; Hamon, M.; Haddon, R. Solution Properties of Graphite and Graphene. J. Am. Chem. Soc. 2006, 128, 7720–7721. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, J.; Zhang, X.; Liu, Z.; Wan, X.; Tain, J.; Wang, T.; Chen, Y. Synthesis, characterization and optical limiting property of covalently oligothiophene-functionalized graphene material. Carbon 2009, 47, 3113–3121. [Google Scholar] [CrossRef]
- Tang, Z.; Kang, H.; Shen, Z.; Guo, B.; Zhang, L.; Jia, D. Grafting of polyester onto graphene for electrically and thermally conductive composites. Macromolecules 2012, 45, 3444–3451. [Google Scholar] [CrossRef]
- Veca, L.M.; Lu, F.; Meziani, M.; Cao, L.; Zhang, P.; Qi, G.; Qu, L.; Shrestha, M.; Sun, Y. Polymer functionalization and solubilization of carbon nanosheets. Chem. Commun. 2009, 18, 2565–2567. [Google Scholar] [CrossRef]
- Liu, X.; Kuang, W.; Guo, B. Preparation of rubber/graphene oxide composites with in-situ interfacial design. Polymer 2015, 56, 553–562. [Google Scholar] [CrossRef]
- Ma, H.L.; Zhang, H.; Hu, Q.; Li, W.; Jiang, Z.; Yu, Z.; Dasari, A. Functionalization and reduction of graphene oxide with p-phenylene diamine for electrically conductive and thermally stable polystyrene composites. ACS Appl. Mater. Interfaces 2012, 4, 1948–1953. [Google Scholar] [CrossRef]
- Caliman, C.C.; Mesquita, A.; Cipriano, D.; Freitas, J.; Cotta, A.; Macedo, W.; Porto, A. One-pot synthesis of amine-functionalized graphene oxide by microwave-assisted reactions: An outstanding alternative for supporting materials in supercapacitors. Rsc Adv. 2018, 8, 6136–6145. [Google Scholar] [CrossRef]
- Zhou, J.; Wei, L.; Zheng, J.; Huang, G. The synthesis of graphene-based antioxidants to promote anti-thermal properties of styrene-butadiene rubber. Rsc Adv. 2017, 7, 53596–53603. [Google Scholar] [CrossRef]
- Zhong, B.; Dong, H.; Luo, Y.; Zhang, D.; Zhang, D.; Jia, Z.; Jia, Z.; Jia, D.; Liu, F. Simultaneous reduction and functionalization of graphene oxide via antioxidant for highly aging resistant and thermal conductive elastomer composites. Compos. Sci. Technol. 2017, 151, 156–163. [Google Scholar] [CrossRef]
- Li, G.Y.; Koenig, J. FTIR imaging of oxidation of polyisoprene. 2. The role of N-phenyl-N′-dimethyl-butyl-p-phenylenediamine antioxidant. Polym. Degrad. Stab. 2003, 81, 377–385. [Google Scholar] [CrossRef]
- Hashim, A.S.; Kohjiya, S. Curing of epoxidized natural rubber with p-phenylenediamine. J. Polym. Sci. Pol. Chem. 1994, 32, 1149–1157. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, J.; Li, N.; Shi, M.; Ma, H.; Yan, B.; Wang, W.; Huang, W.; Ye, M. Amino-functionalization of graphene sheets and the fabrication of their nanocomposites. Polym. Compos. 2010, 31, 1987–1994. [Google Scholar] [CrossRef]
- Hung, W.S.; Tsou, C.; Guzman, M.; An, Q.; Liu, L.; Zhang, M.; Hu, C.C.; Lee, K.R.; Lai, J.Y. Cross-linking with diamine monomers to prepare composite graphene oxide-framework membranes with varying spacing. Chem. Mater. 2014, 26, 2983–2990. [Google Scholar] [CrossRef]
- Hussein, A.; Sarkar, S.; Oh, D.; Lee, K.; Kim, B. Epoxy/p-phenylenediamine functionalized graphene oxide composites and evaluation of their fracture toughness and tensile properties. J. Appl. Polym. Sci. 2016, 133, 34. [Google Scholar] [CrossRef]
- Li, B.; Zhou, L.; Wu, D.; Peng, H.; Yan, K.; Zhou, Y.; Liu, Z. Photochemical chlorination of graphene. ACS Nano 2011, 5, 5957–5961. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Hu, X.; Feng, A.; Jiang, F.; Song, L. p -Phenylenediamine strengthened graphene oxide for the fabrication of superhydrophobic surface. Mater. Des. 2017, 127, 22–29. [Google Scholar] [CrossRef]
- Song, Y.D.; Gao, Y.; Rong, H.; Wen, H.; Sha, Y.; Zhang, H.; Liu, H.; Liu, Q. Functionalization of graphene oxide with naphthalenediimide diamine for high-performance cathode materials of lithium-ion batteries. Sustain. Energy Fuels 2013, 2, 803–810. [Google Scholar]
- Liao, R.; Tang, Z.; Lei, Y.; Guo, B. Polyphenol-reduced graphene oxide: mechanism and derivatization. J. Phys. Chem. C 2011, 115, 20740–20746. [Google Scholar] [CrossRef]
- Rui, Z.; He, X.; Lai, Z.; Yang, D. Effect of some inorganic particles on the softening dispersion of the dynamics of butyl rubber. Polym. Bull. 2016, 74, 1–13. [Google Scholar]
- Zhang, R.; He, X.; Yu, H. Why tanδ of poly (butyl acrylate) and poly (ethyl acrylate) with little double bonds are becoming asymmetric? Polymer 2014, 55, 4720–4727. [Google Scholar] [CrossRef]
- Zhang, R.; He, X.; Rodrigues, A.; Guo, Q. Softening dynamics of polymer blends and composites investigated by differentia spectra of dynamic mechanical analysis. Adv. Polym. Technol. 2017, 37, 2504–2509. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, S.; Peng, J.; Liu, L. The filler–rubber interface and reinforcement in styrene butadiene rubber composites with graphene/silica hybrids: A quantitative correlation with the constrained region. Compos. Part A Appl. Sci. Manuf. 2016, 86, 19–30. [Google Scholar] [CrossRef]

| Sample | SSBR | ZnO | Stearic Acid | Sulfur | TBBS | Carbon Black | PPD-GO |
|---|---|---|---|---|---|---|---|
| SSBR | 100 | 2.18 | 0.73 | 1.27 | 1.00 | 50.00 | 0 |
| PPD-GO1 | 100 | 2.18 | 0.73 | 1.27 | 1.00 | 50.00 | 1.00 |
| PPD-GO2 | 100 | 2.18 | 0.73 | 1.27 | 1.00 | 50.00 | 2.00 |
| PPD-GO3 | 100 | 2.18 | 0.73 | 1.27 | 1.00 | 50.00 | 3.00 |
| PPD-GO4 | 100 | 2.18 | 0.73 | 1.27 | 1.00 | 50.00 | 4.00 |
| PPD-GO5 | 100 | 2.18 | 0.73 | 1.27 | 1.00 | 50.00 | 5.00 |
| GO | PPD | PPD-GO | |||
|---|---|---|---|---|---|
| Wavenumber(cm−1) | Group | Wavenumber(cm−1) | Group | Wavenumber(cm−1) | Group |
| 3423 | -OH | 3417 | -NH | 3210 | H-bond |
| 1721 | C=O | 1623, 1520 | N-H of NH2 | 1173 | C−N |
| 1620 | C=C | - | - | 727 | N-H of C-NH- |
| 1404 | -COOH | - | - | - | - |
| 1048 | C-O-C | - | - | - | - |
| Sample | SSBR | PPD-GO1 | PPD-GO2 | PPD-GO3 | PPD-GO4 | PPD-GO5 | |
|---|---|---|---|---|---|---|---|
| tensile strength (Mpa) elongation at break (%) | before aging | 16.6 ± 0.3 | 17.6 ± 0.2 | 18.6 ± 0.4 | 20.3 ± 0.4 | 21.5 ± 0.5 | 18.3 ± 0.4 |
| after aging | 6.3 ± 0.3 | 7.5 ± 0.5 | 7.9 ± 0.3 | 7.3 ± 0.4 | 7.6 ± 0.5 | 6.6 ± 0.3 | |
| before aging | 301 ± 19 | 384 ± 20 | 375 ± 18 | 364 ± 14 | 350 ± 5 | 319 ± 2 | |
| after aging | 194 ± 26 | 224 ± 23 | 225 ± 4 | 193 ± 13 | 181 ± 8 | 166 ± 2 | |
| Sample | SSBR | PPD-GO1 | PPD-GO2 | PPD-GO3 | PPD-GO4 | PPD-GO5 |
|---|---|---|---|---|---|---|
| 0 °C | 0.145 | 0.153 | 0.145 | 0.146 | 0.148 | 0.147 |
| ±0.0002 | ±0.0001 | ±0.0002 | ±0.0001 | ±0.0001 | ±0.0002 | |
| 60 °C | 0.123 | 0.119 | 0.116 | 0.120 | 0.115 | 0.112 |
| ±0.0001 | ±0.00023 | ±0.0002 | ±0.0003 | ±0.0001 | ±0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, S.; Lu, X.; Zhao, H.; Chen, S.; Cai, S.; He, X.; Zhang, R. Effect of Graphene Oxide Modified with Organic Amine on the Aging Resistance, Rolling Loss and Wet-Skid Resistance of Solution Polymerized Styrene-Butadiene Rubber. Materials 2020, 13, 1025. https://doi.org/10.3390/ma13051025
Wan S, Lu X, Zhao H, Chen S, Cai S, He X, Zhang R. Effect of Graphene Oxide Modified with Organic Amine on the Aging Resistance, Rolling Loss and Wet-Skid Resistance of Solution Polymerized Styrene-Butadiene Rubber. Materials. 2020; 13(5):1025. https://doi.org/10.3390/ma13051025
Chicago/Turabian StyleWan, Songhan, Xiaobin Lu, Hongguo Zhao, Songbo Chen, Shuwei Cai, Xianru He, and Rui Zhang. 2020. "Effect of Graphene Oxide Modified with Organic Amine on the Aging Resistance, Rolling Loss and Wet-Skid Resistance of Solution Polymerized Styrene-Butadiene Rubber" Materials 13, no. 5: 1025. https://doi.org/10.3390/ma13051025
APA StyleWan, S., Lu, X., Zhao, H., Chen, S., Cai, S., He, X., & Zhang, R. (2020). Effect of Graphene Oxide Modified with Organic Amine on the Aging Resistance, Rolling Loss and Wet-Skid Resistance of Solution Polymerized Styrene-Butadiene Rubber. Materials, 13(5), 1025. https://doi.org/10.3390/ma13051025





