The Effects of Nanoclay on the Mechanical Properties, Carvacrol Release and Degradation of a PLA/PBAT Blend
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation
2.3. Characterization Techniques
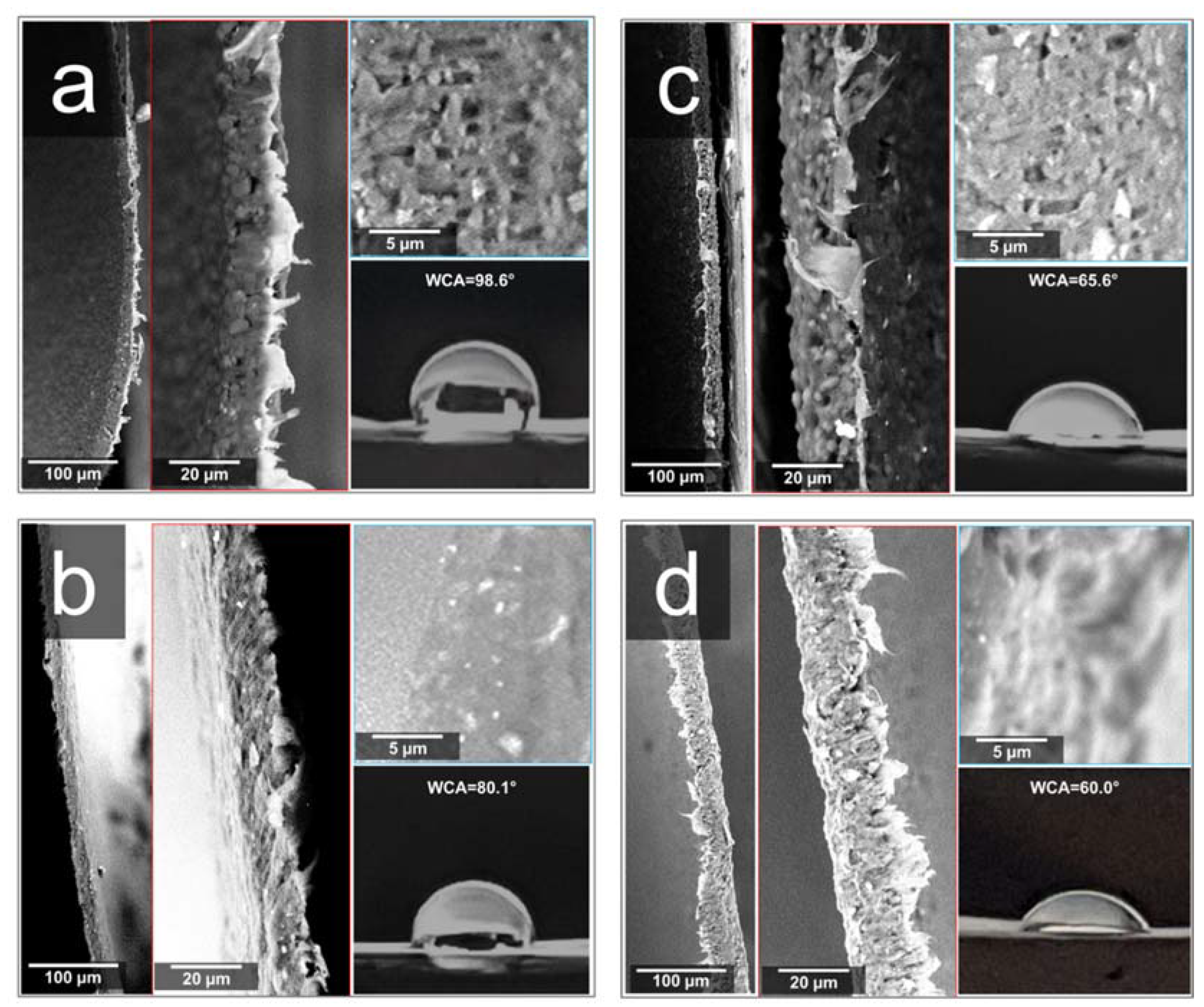
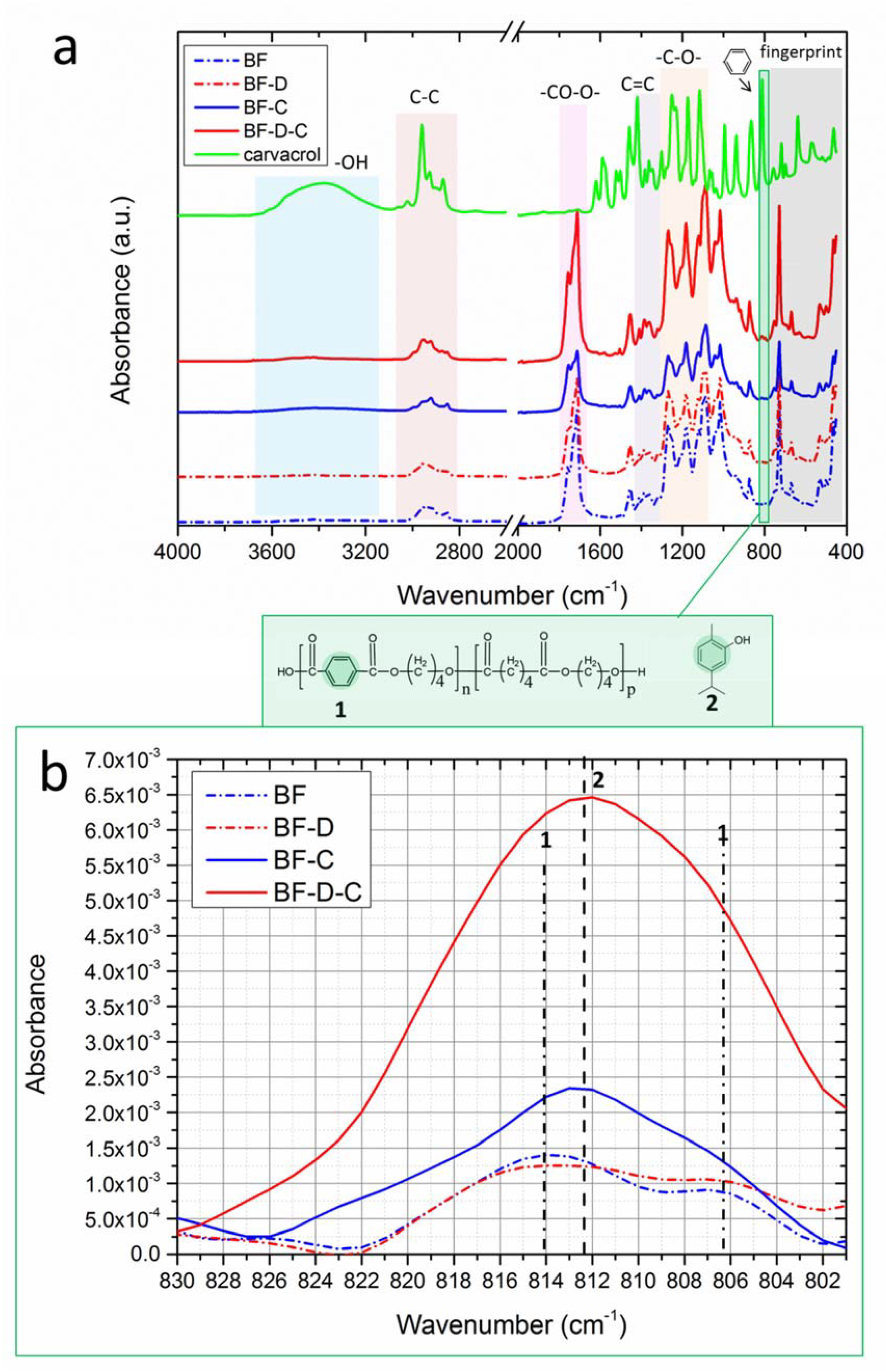
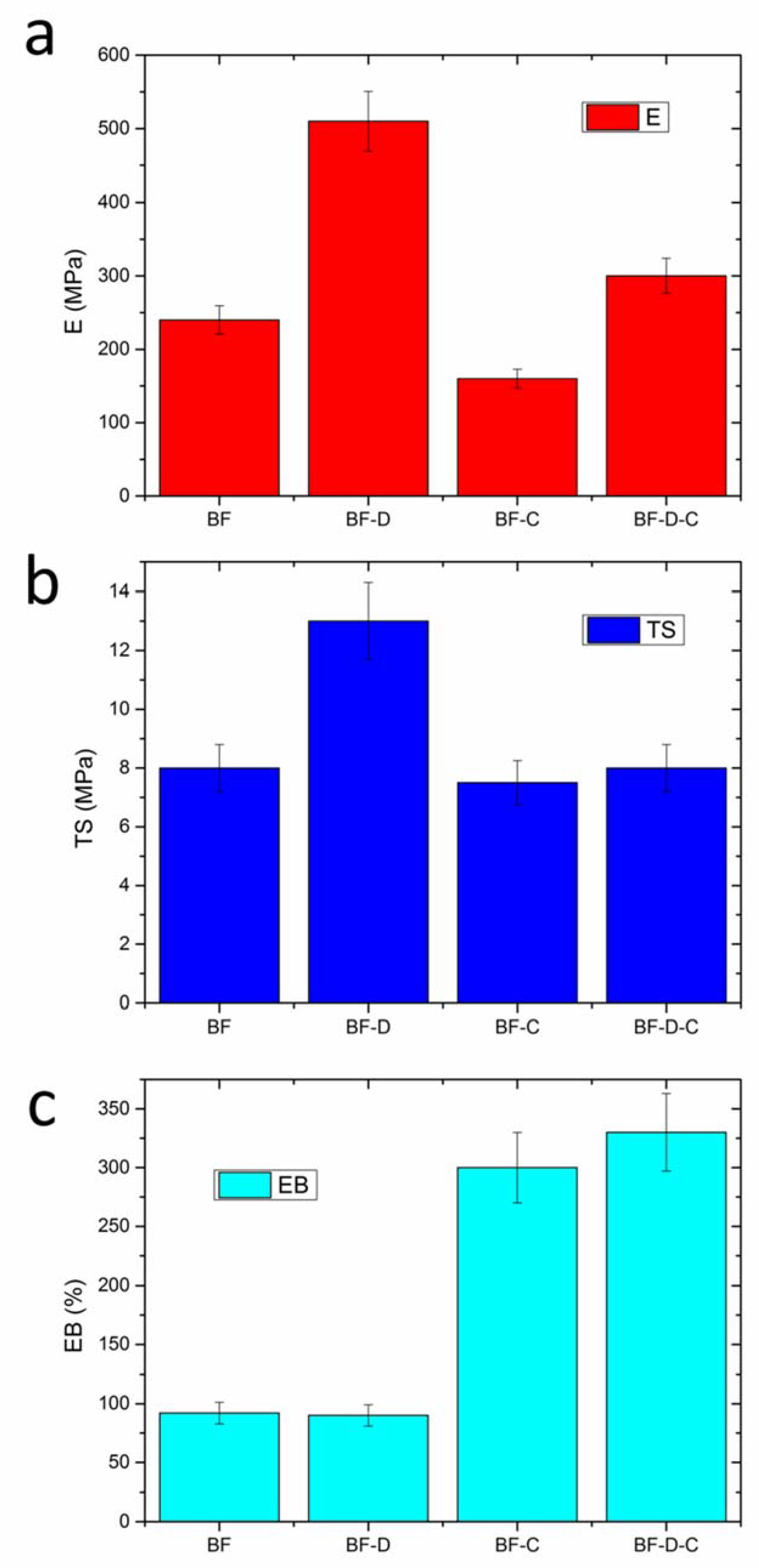
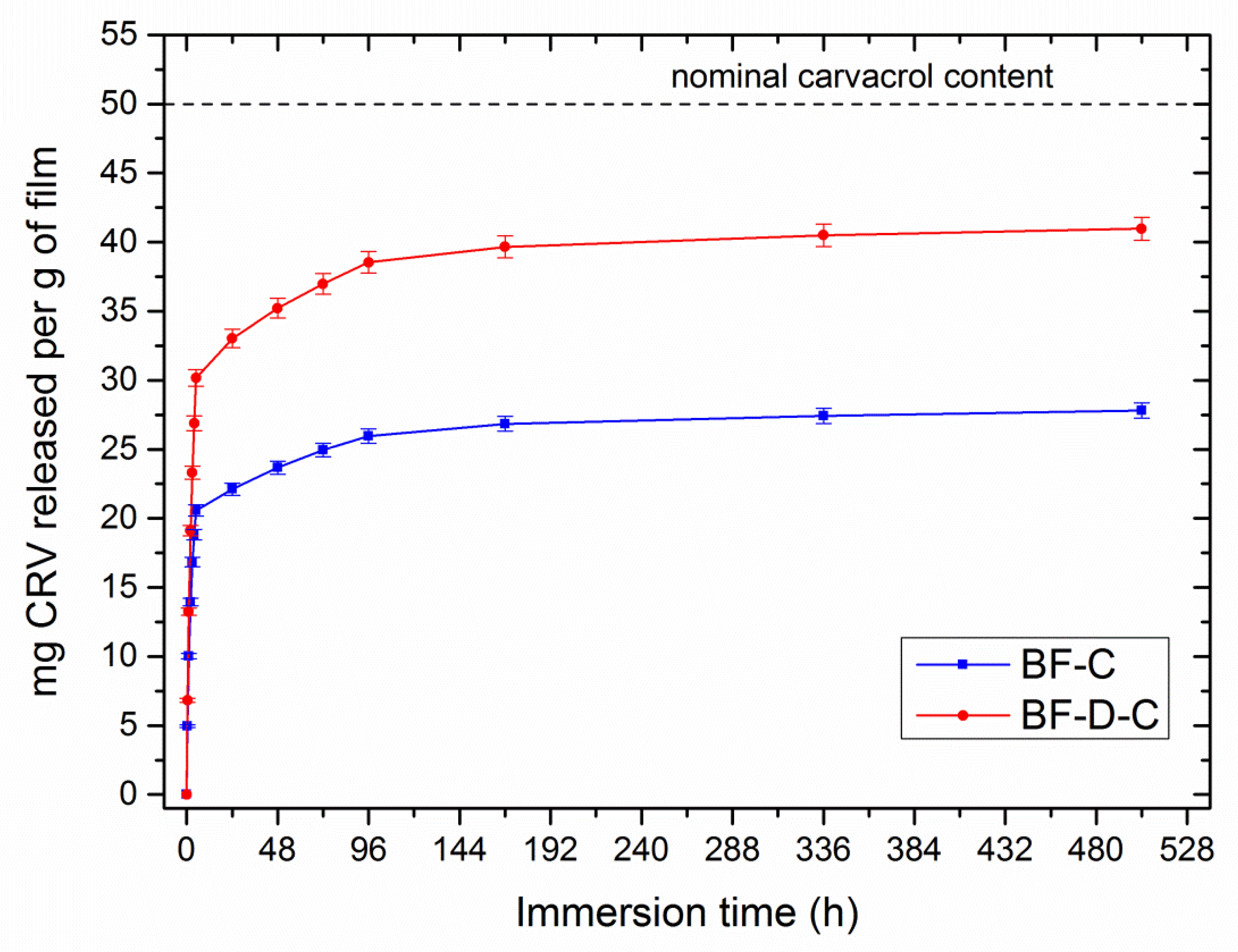
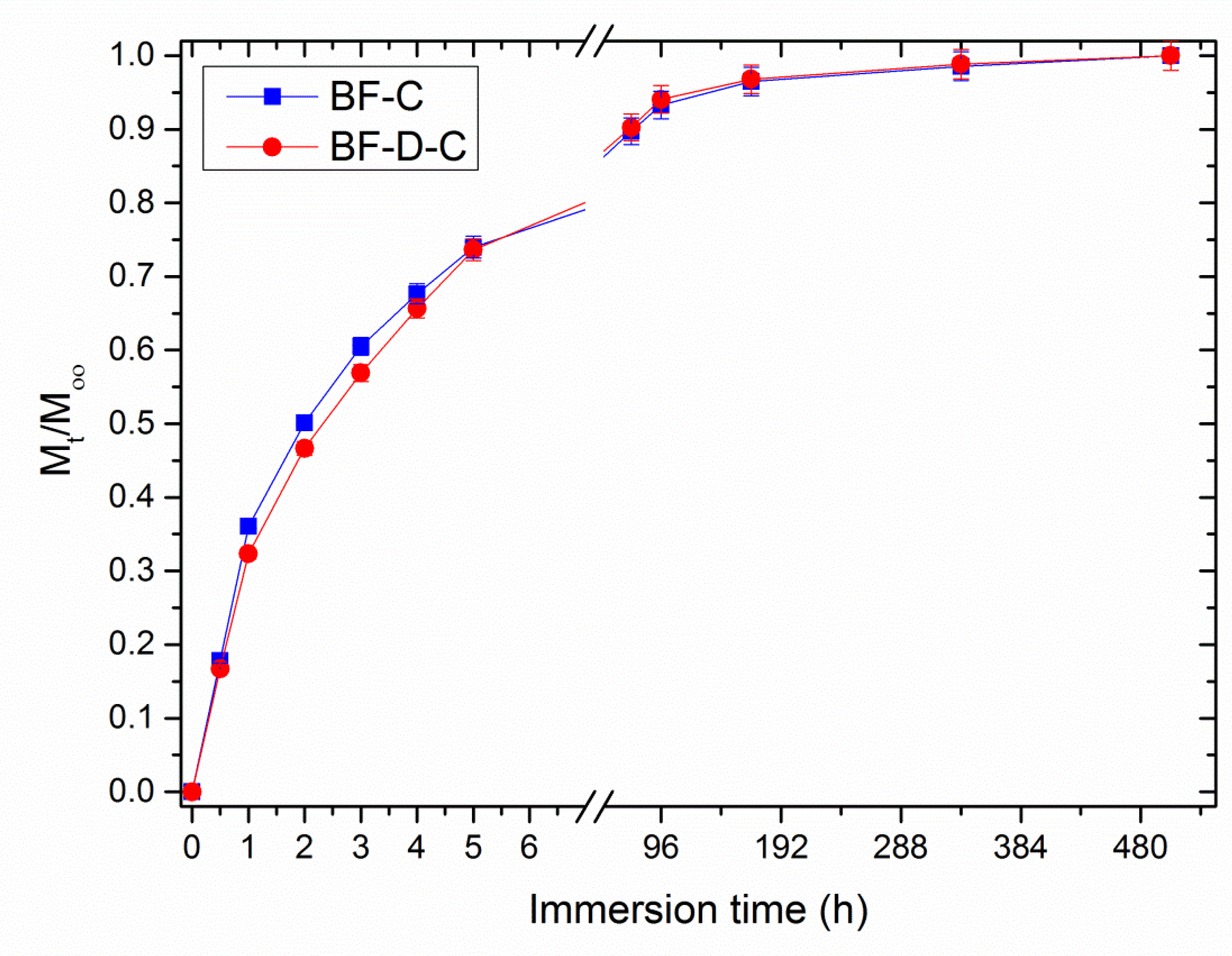
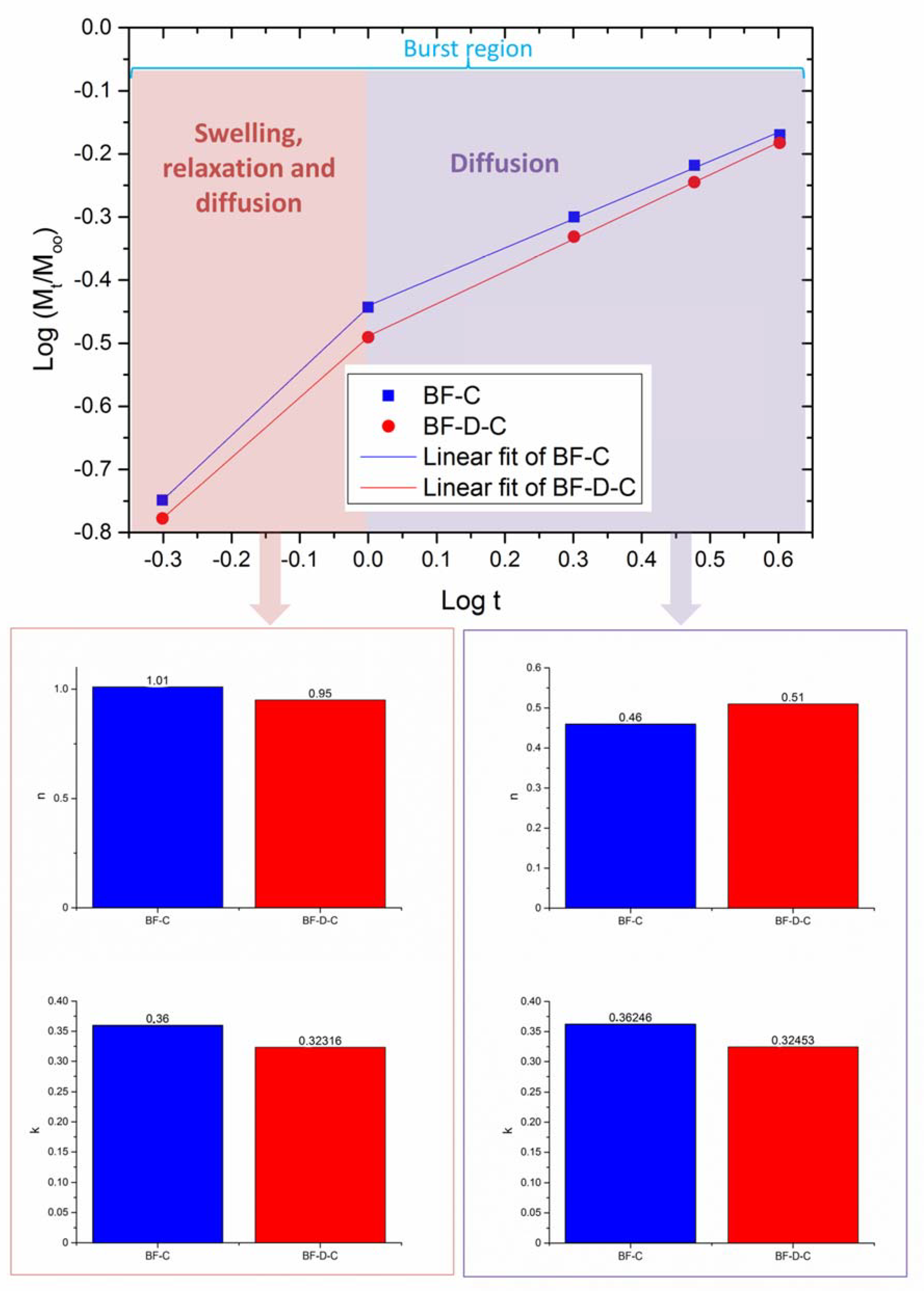
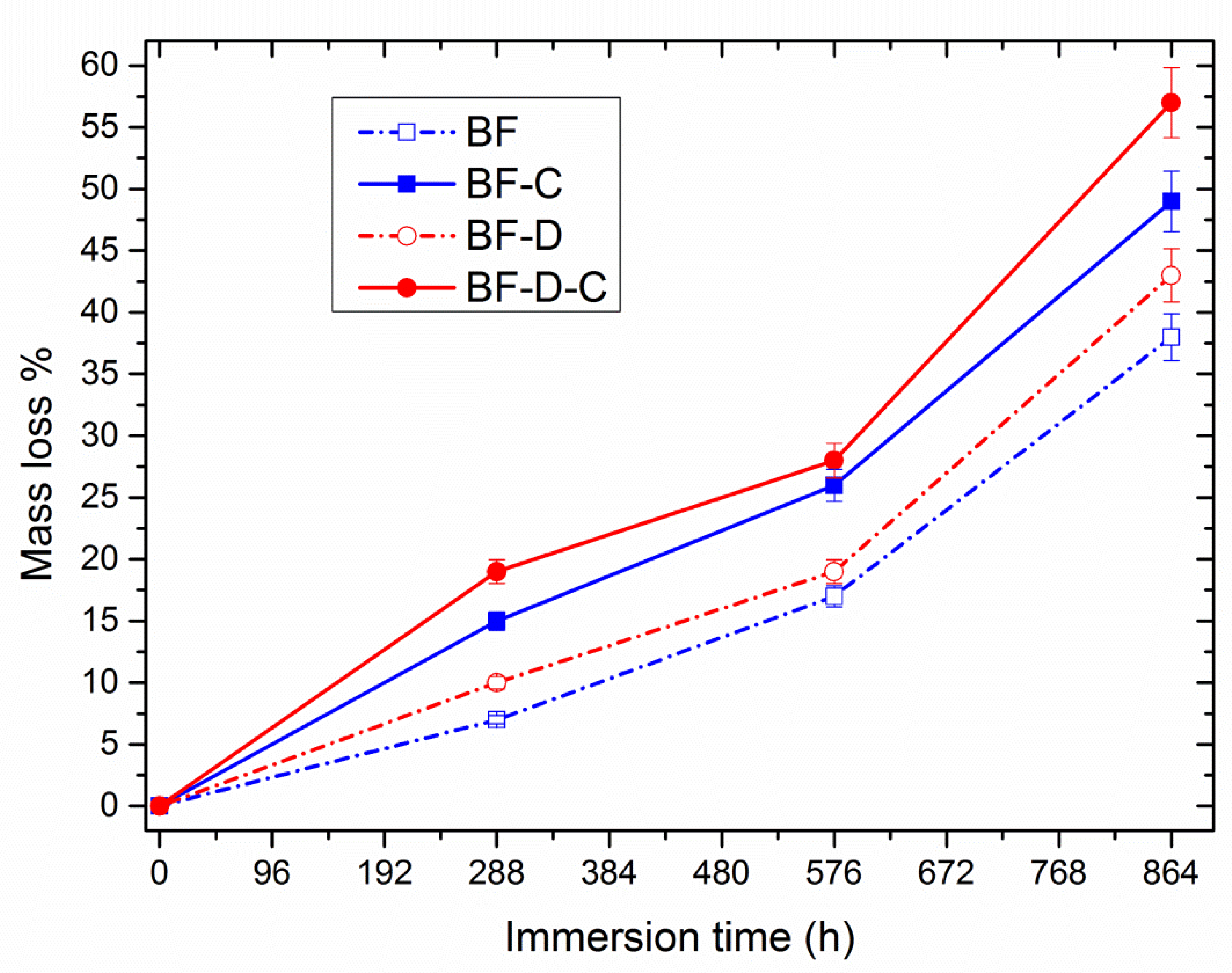
3. Results and Discussion
- (i)
- Carvacrol tends to volatilize, especially at the high temperatures required for melt-processing;
- (ii)
- A certain aliquot of carvacrol remains entrapped inside the matrix.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Scaffaro, R.; Sutera, F.; Botta, L. Biopolymeric bilayer films produced by co-extrusion film blowing. Polym. Test. 2018, 65, 35–43. [Google Scholar] [CrossRef]
- Bourtoom, T.; Chinnan, M.S. Preparation and properties of rice starch-chitosan blend biodegradable film. LWT Food Sci. Technol. 2008, 41, 1633–1641. [Google Scholar] [CrossRef]
- Claro, P.I.C.; Neto, A.R.S.; Bibbo, A.C.C.; Mattoso, L.H.C.; Bastos, M.S.R.; Marconcini, J.M. Biodegradable blends with potential use in packaging: A comparison of PLA/Chitosan and PLA/Cellulose Acetate films. J. Polym. Environ. 2016, 24, 363–371. [Google Scholar] [CrossRef]
- Imran, M.; Revol-Junelles, A.-M.; Martyn, A.; Tehrany, E.A.; Jacquot, M.; Linder, M.; Desobry, S. Active Food Packaging Evolution: Transformation from Micro- to Nanotechnology. Crit. Rev. Food Sci. Nutr. 2010, 50, 799–821. [Google Scholar] [CrossRef] [PubMed]
- Salwa, H.N.; Sapuan, S.M.; Mastura, M.T.; Zuhri, M.Y.M. Green bio composites for food packaging. Int. J. Recent Technol. Eng. 2019, 8, 450–459. [Google Scholar]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Touchaleaume, F.; Martin-Closas, L.; Angellier-Coussy, H.; Chevillard, A.; Cesar, G.; Gontard, N.; Gastaldi, E. Performance and environmental impact of biodegradable polymers as agricultural mulching films. Chemosphere 2016, 144, 433–439. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M. Development of expert system for biobased polymer material selection: Food packaging application. J. Food Sci. Technol. 2015, 52, 6445–6454. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Sutera, F.; Gulino, E.F.; Morreale, M. Degradation and recycling of films based on biodegradable polymers: A short review. Polymers 2019, 11, 651. [Google Scholar] [CrossRef]
- Scaffaro, R.; Botta, L.; Maio, A.; Mistretta, M.C.; La Mantia, F.P. Effect of Graphene Nanoplatelets on the Physical and Antimicrobial Properties of Biopolymer-Based Nanocomposites. Materials 2016, 9, 351. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Lo Re, G.; Parisi, A.; Busacca, A. Advanced piezoresistive sensor achieved by amphiphilic nanointerfaces of graphene oxide and biodegradable polymer blends. Compos. Sci. Technol. 2018, 156, 166–176. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A. Integrated ternary bionanocomposites with superior mechanical performance via the synergistic role of graphene and plasma treated carbon nanotubes. Compos. Part B Eng. 2019, 168, 550–559. [Google Scholar]
- Scaffaro, R.; Maio, A.; Nostro, A. Poly(lactic acid)/carvacrol-based materials: Preparation, physicochemical properties, and antimicrobial activity. Appl. Microbiol. Biotechnol. 2020, 104, 1823–1835. [Google Scholar] [PubMed]
- Sharma, R.; Jafari, S.M.; Sharma, S. Antimicrobial bio-nanocomposites and their potential applications in food packaging. Food Control 2020, 112, 107086. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Marino, A.; Nostro, A. Antimicrobial additives for poly(lactic acid) materials and their applications: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 7739–7756. [Google Scholar] [CrossRef]
- Arruda, L.C.; Magaton, M.; Bretas, R.E.S.; Ueki, M.M. Influence of chain extender on mechanical, thermal and morphological properties of blown films of PLA/PBAT blends. Polym. Test. 2015, 43, 27–37. [Google Scholar] [CrossRef]
- Briassoulis, D.; Giannoulis, A. Evaluation of the functionality of bio-based food packaging films. Polym. Test. 2018, 69, 39–51. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Ceraulo, M.; Mistretta, M.C.; Morreale, M. Rheological behaviour, mechanical properties and processability of biodegradable polymer systems for film blowing. J. Polym. Environ. 2018, 26, 749–755. [Google Scholar] [CrossRef]
- Frine, V.-C.; Hector, A.-P.; Manuel, N.-D.S.; Estrella, N.-D.; Antonio, G.J. Development and Characterization of a Biodegradable PLA Food Packaging Hold Monoterpene-Cyclodextrin Complexes against Alternaria alternata. Polymers 2019, 11, 1720. [Google Scholar] [CrossRef]
- Chen, R.; Abdelwahab, M.A.; Misra, M.; Mohanty, A.K. Biobased Ternary Blends of Lignin, Poly(Lactic Acid), and Poly(Butylene Adipate-co-Terephthalate): The Effect of Lignin Heterogeneity on Blend Morphology and Compatibility. J. Polym. Environ. 2014, 22, 439–448. [Google Scholar] [CrossRef]
- Yang, C.; Tang, H.; Wang, Y.; Liu, Y.; Wang, J.; Shi, W.; Li, L. Development of PLA-PBSA based biodegradable active film and its application to salmon slices. Food Packag. Shelf Life 2019, 22, 100393. [Google Scholar] [CrossRef]
- Gregorova, A.; Riedl, E.; Sedlarik, V.; Stelzer, F. Effect of 4,4′-methylenediphenyl diisocyanate on thermal and mechanical properties of Bioflex/lactic acid polycondensate blends. Asia Pac. J. Chem. Eng. 2012, 7, S317–S323. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Lopresti, F. Effect of graphene and fabrication technique on the release kinetics of carvacrol from polylactic acid. Compos. Sci. Technol. 2019, 169, 60–69. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; D’Arrigo, M.; Marino, A.; Nostro, A. Efficacy of poly(lactic acid)/carvacrol electrospun membranes against Staphylococcus aureus and Candida albicans in single and mixed cultures. Appl. Microbiol. Biotechnol. 2018, 102, 4171–4181. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Lopresti, F. Processing, structure, property relationships and release kinetics of electrospun PLA/Carvacrol membranes. Eur. Polym. J. 2018, 100, 165–171. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Improvement of thermal stability, rheological and mechanical properties of PLA, PBAT and their blends by reactive extrusion with functionalized epoxy. Polym. Degrad. Stab. 2012, 97, 1898–1914. [Google Scholar] [CrossRef]
- Maio, A.; Giallombardo, D.; Scaffaro, R.; Palumbo Piccionello, A.; Pibiri, I. Synthesis of a fluorinated graphene oxide--silica nanohybrid: Improving oxygen affinity. RSC Adv. 2016, 6, 46037–46047. [Google Scholar] [CrossRef]
- Maio, A.; Scaffaro, R.; Lentini, L.; Palumbo Piccionello, A.; Pibiri, I. Perfluorocarbons–graphene oxide nanoplatforms as biocompatible oxygen reservoirs. Chem. Eng. J. 2018, 334, 54–65. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Lopresti, F.; Giallombardo, D.; Botta, L.; Bondì, M.L.; Agnello, S. Synthesis and self-assembly of a PEGylated-graphene aerogel. Compos. Sci. Technol. 2016, 128, 193–200. [Google Scholar] [CrossRef]
- Rapisarda, M.; La Mantia, F.P.; Ceraulo, M.; Mistretta, M.C.; Giuffrè, C.; Pellegrino, R.; Valenti, G.; Rizzarelli, P. Photo-oxidative and soil burial degradation of irrigation tubes based on biodegradable polymer blends. Polymers 2019, 11, 1489. [Google Scholar] [CrossRef]
- Mukherjee, T.; Tobin, M.J.; Puskar, L.; Sani, M.-A.; Kao, N.; Gupta, R.K.; Pannirselvam, M.; Quazi, N.; Bhattacharya, S. Chemically imaging the interaction of acetylated nanocrystalline cellulose (NCC) with a polylactic acid (PLA) polymer matrix. Cellulose 2017, 24, 1717–1729. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Botta, L.; Gulino, E.F.; Gulli, D. Tunable release of Chlorhexidine from Polycaprolactone-based filaments containing graphene nanoplatelets. Eur. Polym. J. 2019, 110, 221–232. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A. Influence of oxidation level of graphene oxide on the mechanical performance and photo-oxidation resistance of a polyamide 6. Polymers 2019, 11, 857. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Maio, A. A green method to prepare nanosilica modified graphene oxide to inhibit nanoparticles re-aggregation during melt processing. Chem. Eng. J. 2017, 308, 1034–1047. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Gulino, E.F.; Micale, G.D.M. PLA-based functionally graded laminates for tunable controlled release of carvacrol obtained by combining electrospinning with solvent casting. React. Funct. Polym. 2020, 148, 104490. [Google Scholar] [CrossRef]
- Requena, R.; Vargas, M.; Chiralt, A. Obtaining antimicrobial bilayer starch and polyester-blend films with carvacrol. Food Hydrocoll. 2018, 83, 118–133. [Google Scholar] [CrossRef]
- Armentano, I.; Fortunati, E.; Burgos, N.; Dominici, F.; Luzi, F.; Fiori, S.; Jiménez, A.; Yoon, K.; Ahn, J.; Kang, S.; et al. Bio-based PLA_PHB plasticized blend films: Processing and structural characterization. LWT Food Sci. Technol. 2015, 64, 980–988. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Re, G.L.; Morreale, M.; Scaffaro, R.; La Mantia, F.P. Biodegradation paths of Mater-Bi®/kenaf biodegradable composites. J. Appl. Polym. Sci. 2013, 129, 3198–3208. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Gulino, E.F.; Pitarresi, G. Lignocellulosic fillers and graphene nanoplatelets as hybrid reinforcement for polylactic acid: Effect on mechanical properties and degradability. Compos. Sci. Technol. 2020, 190, 108008. [Google Scholar] [CrossRef]
- Vey, E.; Rodger, C.; Meehan, L.; Booth, J.; Claybourn, M.; Miller, A.F.; Saiani, A. The impact of chemical composition on the degradation kinetics of poly(lactic-co-glycolic) acid copolymers cast films in phosphate buffer solution. Polym. Degrad. Stab. 2012, 97, 358–365. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A. Optimization of two-step techniques engineered for the preparation of polyamide 6 graphene oxide nanocomposites. Compos. Part B Eng. 2019, 165, 55–64. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Rayón, E.; López, J.; Kenny, J.M. PLA-PHB/cellulose based films: Mechanical, barrier and disintegration properties. Polym. Degrad. Stab. 2014, 107, 139–149. [Google Scholar] [CrossRef]
- Seoane, I.T.; Manfredi, L.B.; Cyras, V.P.; Torre, L.; Fortunati, E.; Puglia, D. Effect of Cellulose Nanocrystals and Bacterial Cellulose on Disintegrability in Composting Conditions of Plasticized PHB Nanocomposites. Polymers 2017, 9, 561. [Google Scholar] [CrossRef] [PubMed]
| Sample | Carvacrol wt.% 1 | D72T wt.% 1 | Screw Speed (rpm) | T Profile (°C) | BUR 2 | Thickness (μm) |
|---|---|---|---|---|---|---|
| BF | - | - | 60 | 120-130-140-150-160 | 4 | 16 ± 3 |
| BF-C | 5 | - | 60 | 120-130-140-150-160 | 3.9 | 20 ± 2 |
| BF-D | - | 5 | 60 | 120-130-140-150-160 | 4.1 | 16 ± 2 |
| BF-D-C | 5 | 5 | 60 | 120-130-140-150-160 | 4 | 21 ± 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scaffaro, R.; Maio, A.; Gulino, E.F.; Morreale, M.; La Mantia, F.P. The Effects of Nanoclay on the Mechanical Properties, Carvacrol Release and Degradation of a PLA/PBAT Blend. Materials 2020, 13, 983. https://doi.org/10.3390/ma13040983
Scaffaro R, Maio A, Gulino EF, Morreale M, La Mantia FP. The Effects of Nanoclay on the Mechanical Properties, Carvacrol Release and Degradation of a PLA/PBAT Blend. Materials. 2020; 13(4):983. https://doi.org/10.3390/ma13040983
Chicago/Turabian StyleScaffaro, Roberto, Andrea Maio, Emmanuel Fortunato Gulino, Marco Morreale, and Francesco Paolo La Mantia. 2020. "The Effects of Nanoclay on the Mechanical Properties, Carvacrol Release and Degradation of a PLA/PBAT Blend" Materials 13, no. 4: 983. https://doi.org/10.3390/ma13040983
APA StyleScaffaro, R., Maio, A., Gulino, E. F., Morreale, M., & La Mantia, F. P. (2020). The Effects of Nanoclay on the Mechanical Properties, Carvacrol Release and Degradation of a PLA/PBAT Blend. Materials, 13(4), 983. https://doi.org/10.3390/ma13040983









