Developing Nanostructured Ti Alloys for Innovative Implantable Medical Devices
Abstract
1. Introduction
2. SPD Processing of Nanostructured Titanium Materials
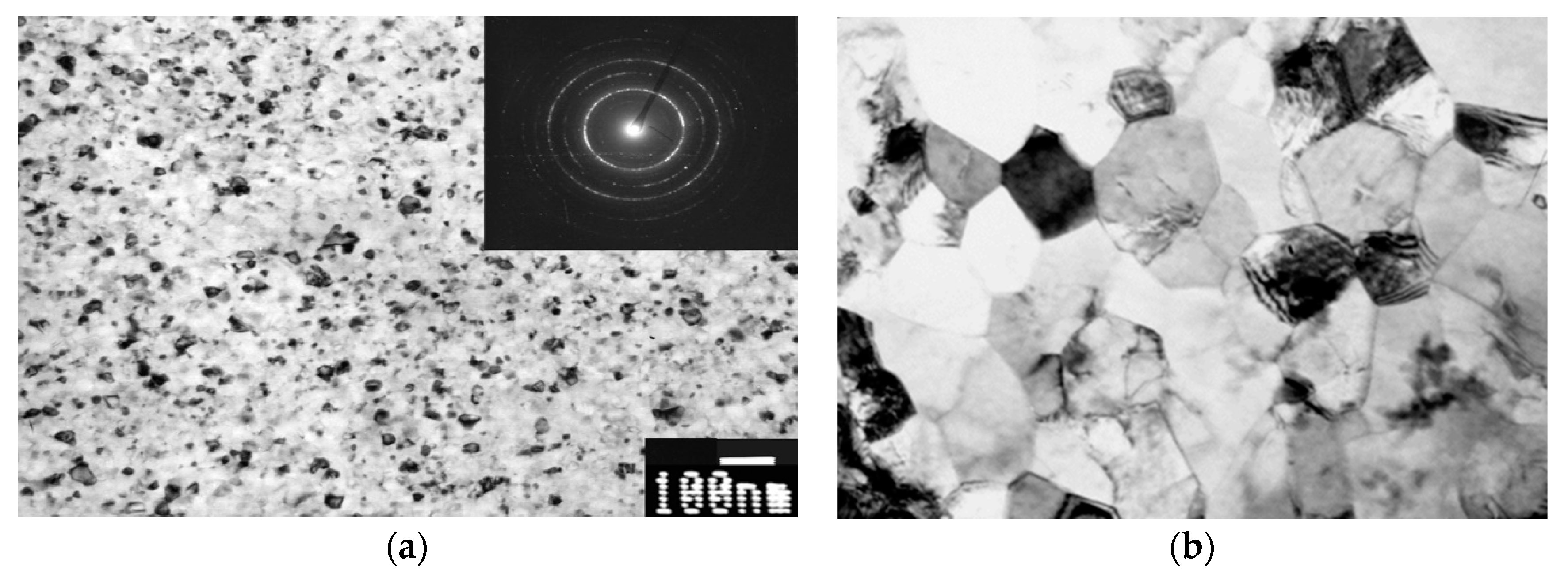
2.1. Commercially Pure Ti
2.2. Titanium Alloys
2.3. Nanostructured NiTi Shape Memory Alloys
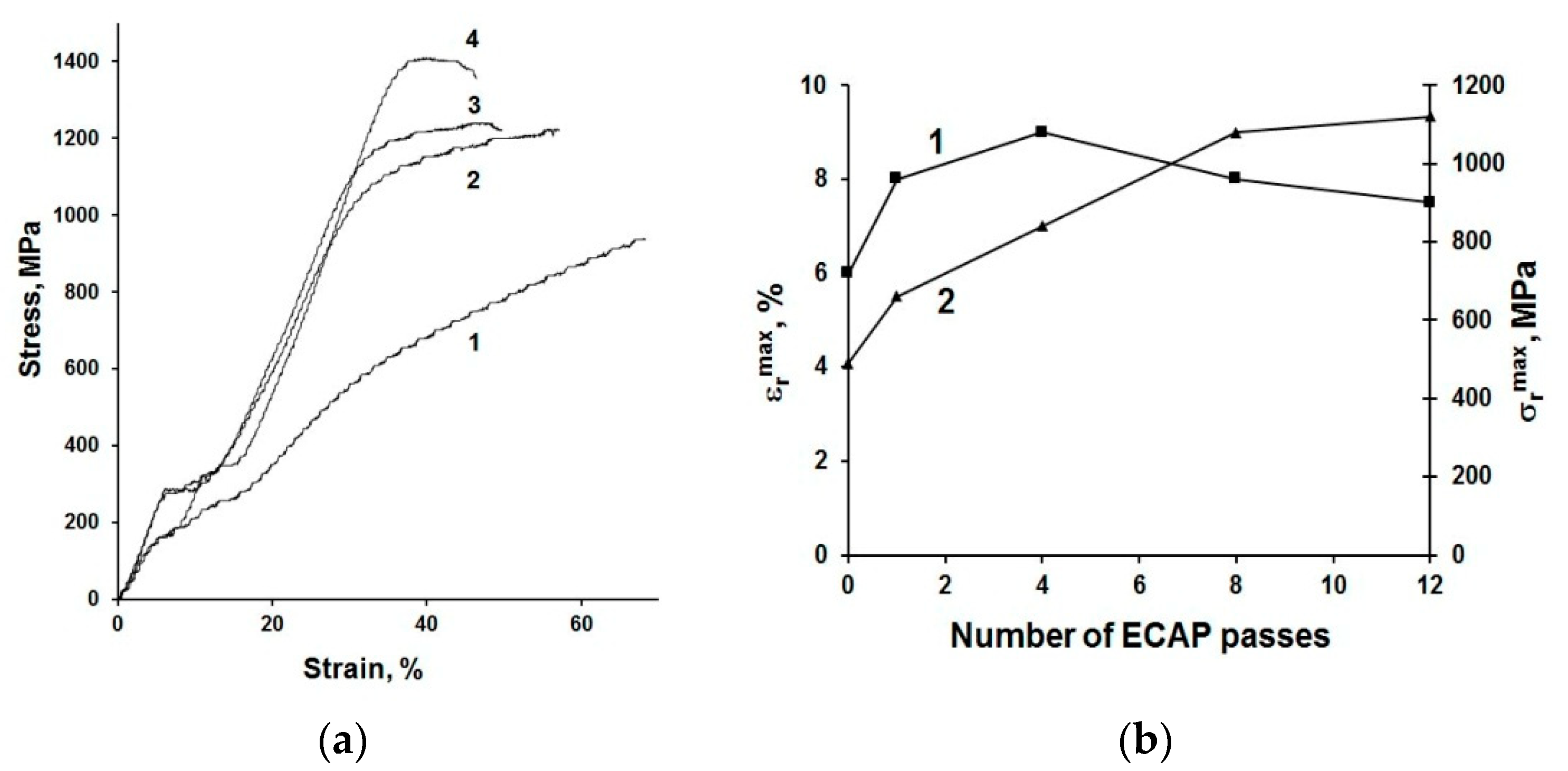
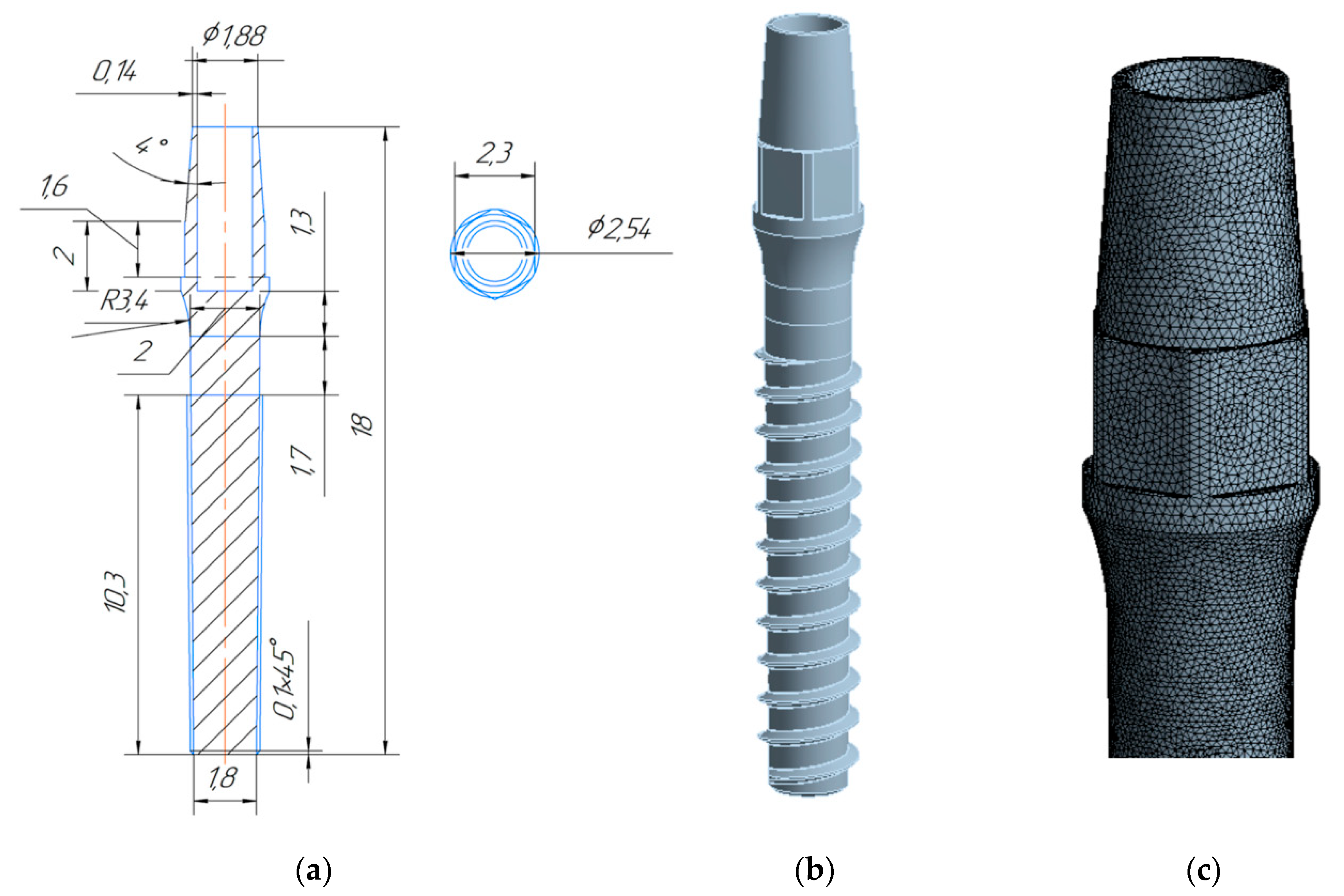
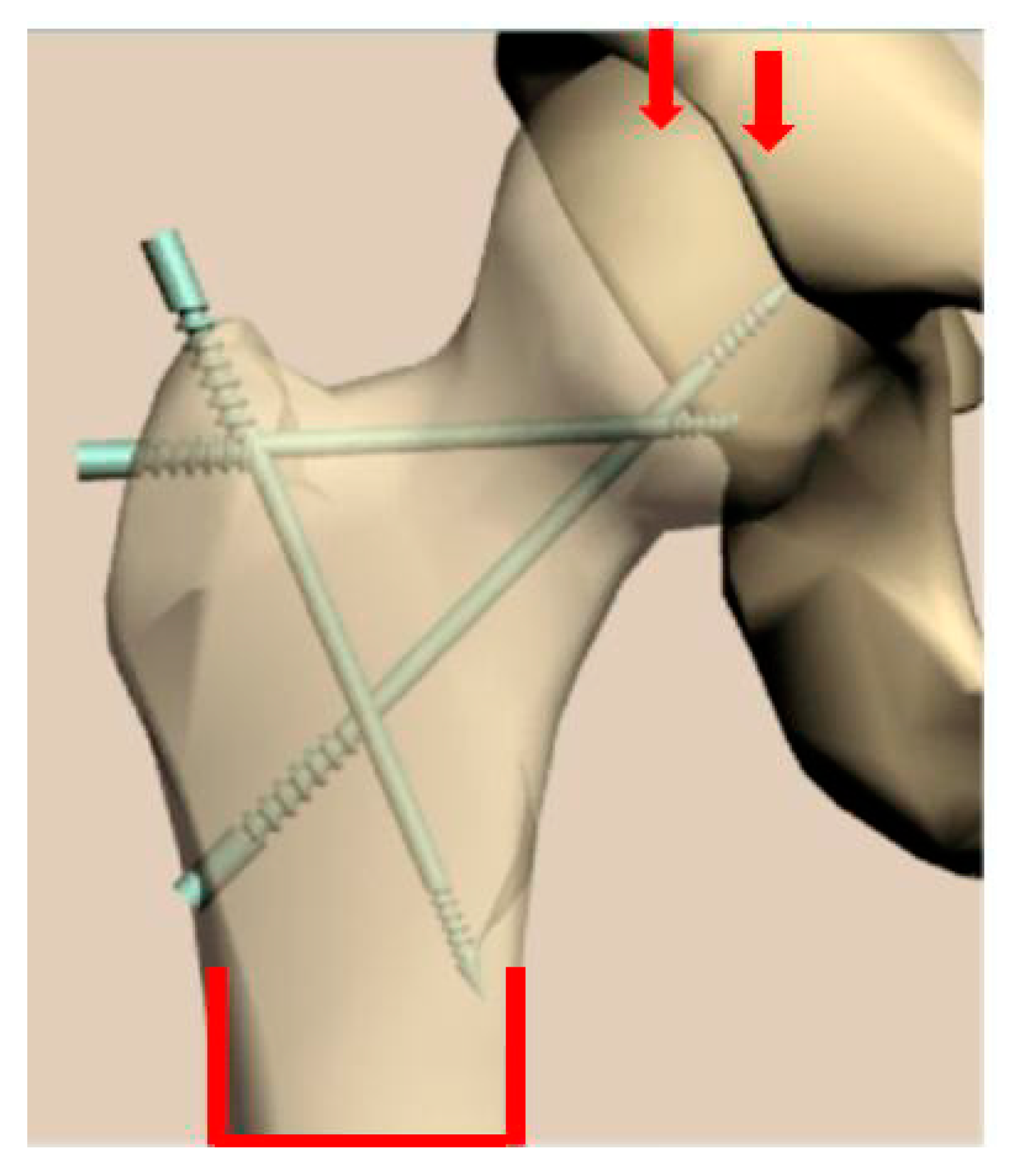
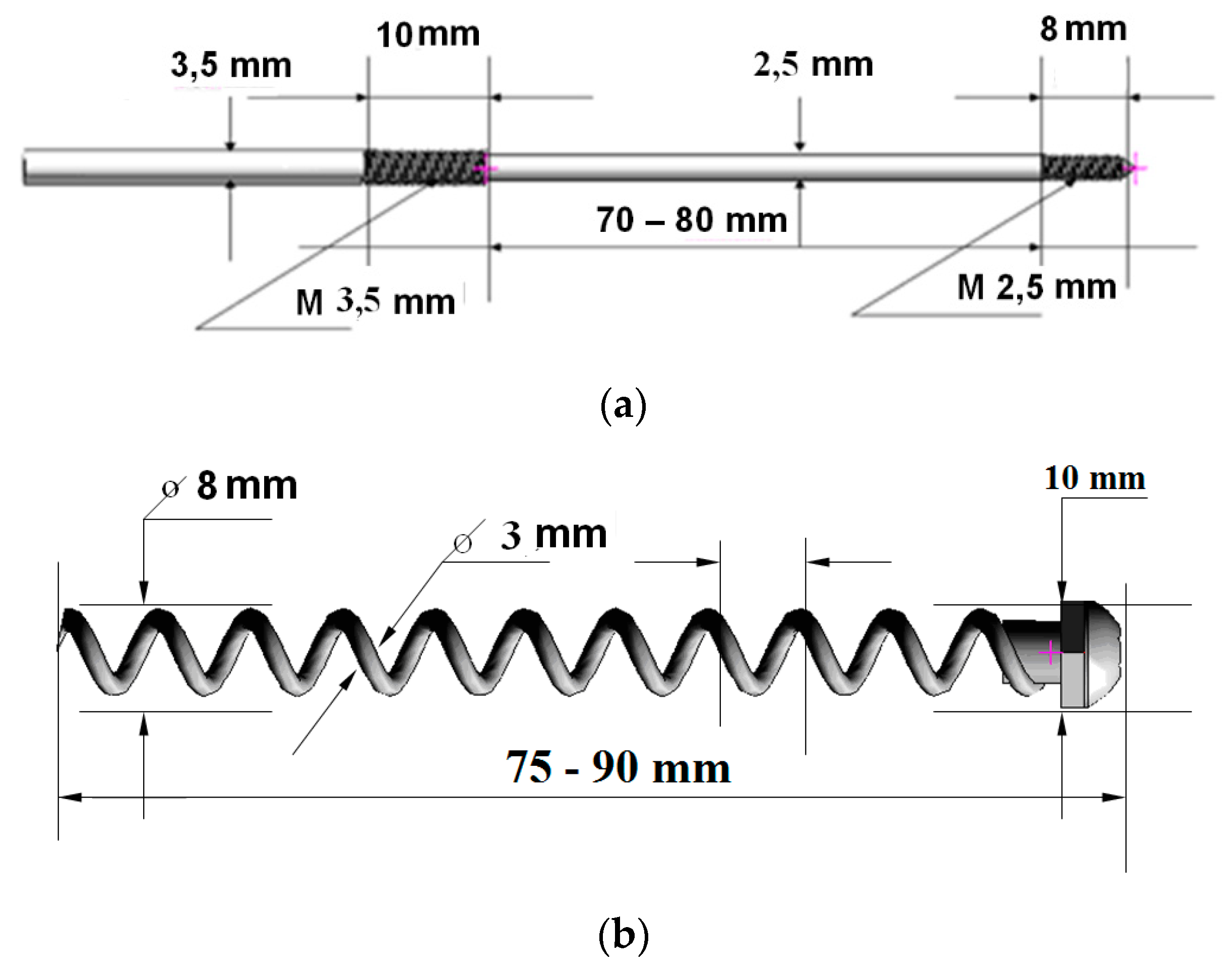
3. Design of Miniaturized Implants
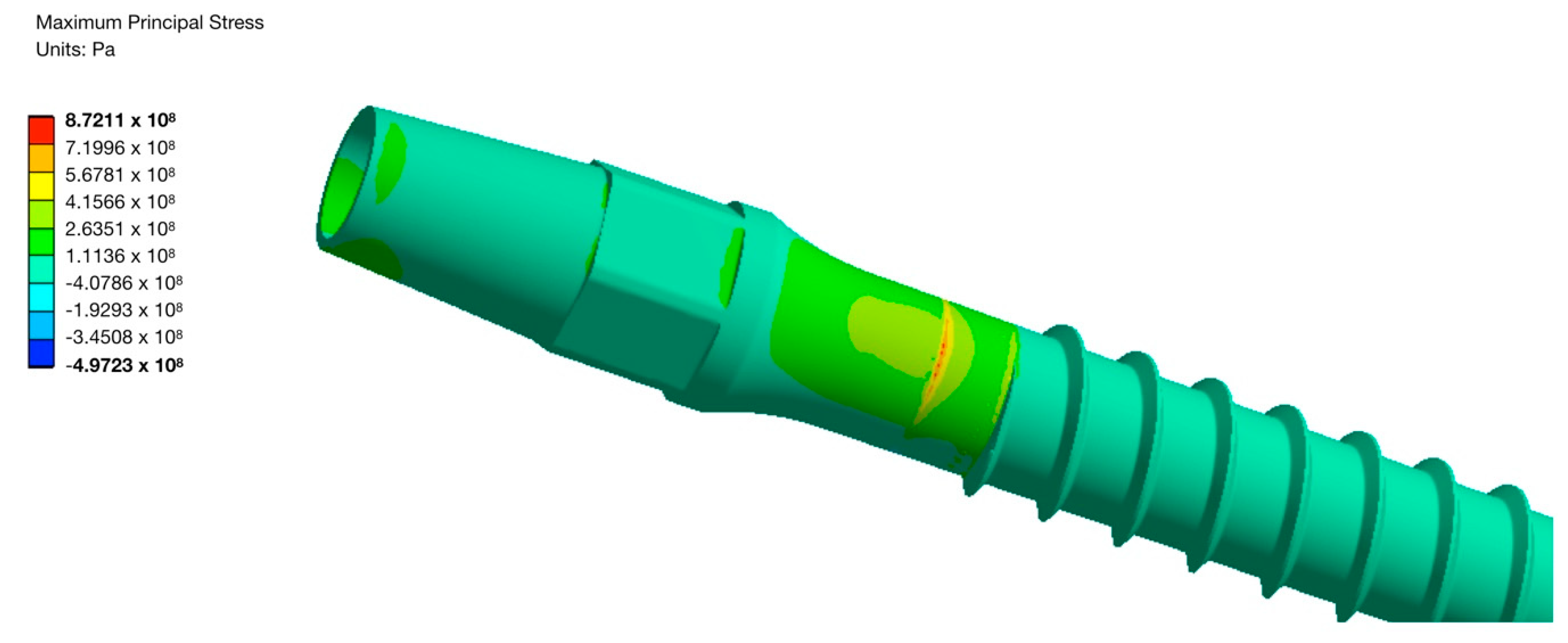
4. Fabrication and Tests of Medical Nanoimplants
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hanawa, T. Overview of metals and applications. In Metals for Biomedical Devices; Elsevier BV: Amsterdam, The Netherlands, 2010; pp. 3–24. [Google Scholar]
- Froes, F.H.; Qian, M. Titanium in Medical and Dental Applications, 1st ed.; Woodhead Publishing: Duxford, UK, 2018. [Google Scholar]
- Valiev, R.; Islamgaliev, R.; Alexandrov, I. Bulk nanostructured materials from severe plastic deformation. Prog. Mater. Sci. 2000, 45, 103–189. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Estrin, Y.; Horita, Z.; Langdon, T.G.; Zehetbauer, M.J.; Zhu, Y.T. Producing bulk ultrafine-grained materials by severe plastic deformation: Ten years later. JOM 2016, 68, 1216–1226. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Estrin, Y.; Horita, Z.; Langdon, T.G.; Zehetbauer, M.J.; Zhu, Y.T. Fundamentals of superior properties in bulk nanoSPD materials. Mater. Res. Lett. 2016, 4, 1–21. [Google Scholar] [CrossRef]
- Whang, S.H. Nanostructured Metals and Alloys: Processing, Microstructure, Mechanical Properties and Applications, 1st ed.; Woodhead Publishing Limited: Cambridge, UK, 2011. [Google Scholar]
- Rosochowski, A. Severe Plastic Deformation Technology; Whittles Publishing: Scotland, UK, 2017. [Google Scholar]
- Estrin, Y.; Vinogradov, A. Extreme grain refinement by severe plastic deformation: A wealth of challenging science. Acta Mater. 2013, 61, 782–817. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Langdon, T.G. Principles of equal-channel angular pressing as a processing tool for grain refinement. Prog. Mater. Sci. 2006, 51, 881–981. [Google Scholar] [CrossRef]
- Rack, H.J.; Qazi, J.; Allard, L.; Valiev, R.Z. Thermal Stability of Severe Plastically Deformed VT-6 (Ti-6Al-4V). Mater. Sci. Forum 2008, 584, 893–898. [Google Scholar] [CrossRef]
- Takizawa, Y.; Masuda, T.; Fujimitsu, K.; Kajita, T.; Watanabe, K.; Yumoto, M.; Otagiri, Y.; Horita, Z. Scaling up of High-Pressure Sliding (HPS) for Grain Refinement and Superplasticity. Met. Mater. Trans. A 2016, 47, 4669–4681. [Google Scholar] [CrossRef]
- Fakhretdinova, E.I.; Raab, G.I.; Valiev, R.Z. Modeling of Metal Flow during Processing by Multi-ECAP-Conform. Adv. Eng. Mater. 2015, 17, 1723–1727. [Google Scholar] [CrossRef]
- Valiev, R.Z. Nanostructuring of metals by severe plastic deformation for advanced properties. Nat. Mater. 2004, 3, 511–516. [Google Scholar] [CrossRef]
- Polyakova, V.; Semenova, I.; Valiev, R. Influence of annealing on the structure and mechanical properties of ultrafine-grained alloy Ti-6Al-7Nb, processed by severe plastic deformation. Mater. Sci. Forum 2011, 667–669, 943–948. [Google Scholar] [CrossRef]
- Semenova, I.; Yakushina, E.; Nurgaleeva, V.; Valiev, R. Nanostructuring of Ti-aloys by SPD processing to achieve superior fatigue properties. Int. J. Mat. Res. 2009, 100, 1691–1696. [Google Scholar] [CrossRef]
- Lowe, T.C.; Valiev, R.Z. Frontiers of bulk nanostructured metals in biomedical applications. In Advanced Biomaterials and Biodevices; Tiwari, A., Nordin, A.N., Eds.; Wiley-Scrivener Publ.: Beverly, MA, USA, 2014; pp. 3–52. [Google Scholar]
- Valiev, R.Z.; Sabirov, I.; Zemtsova, E.G.; Parfenov, E.V.; Dluhoš, L.; Lowe, T.C. Nanostructured pure Ti for development of miniturized biomedical implants. In Titanium in Medical and Dental Applications; Froes, F., Qian, M., Eds.; Woodhead Publishing: Duxford, UK, 2018; pp. 393–418. [Google Scholar]
- Zemtsova, E.; Arbenin, A.; Valiev, R.Z.; Smirnov, V.M. Modern techniques of surface geometry modification for the implants based on titanium and its alloys used for improvement of the biomedical characteristics. In Titanium in Medical and Dental Applications; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 115–145. [Google Scholar]
- Valiev, R.Z.; Semenova, I.P.; Latysh, V.V.; Rack, H.; Lowe, T.C.; Petruzelka, J.; Dluhos, L.; Hrusak, D.; Sochova, J. Nanostructured Titanium for Biomedical Applications. Adv. Eng. Mater. 2008, 10, B15–B17. [Google Scholar] [CrossRef]
- Estrin, Y.; Lapovok, R.; Medvedev, A.E.; Kasper, C.; Ivanová, E.; Lowe, T.C. Mechanical performance and cell response of pure titanium with ultrafine-grained structure produced by severe plastic deformation. In Titanium in Medical and Dental Applications; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 419–454. [Google Scholar]
- Dyakonov, G.; Mironov, S.; Semenova, I.P.; Valiev, R.Z.; Semiatin, S.L. Microstructure evolution and strengthening mechanisms in commercial-purity titanium subjected to equal-channel angular pressing. Mater. Sci. Eng. A 2017, 701, 289–301. [Google Scholar] [CrossRef]
- Brunette, D.M.; Tengvall, P.; Textor, M.; Thomsen, P. Titanium in Medicine; Springer-Verlag: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Gunderov, D.; Polyakov, A.; Semenova, I.; Raab, G.; Churakova, A.; Gimaltdinova, E.; Sabirov, I.; Segurado, J.; Sitdikov, V.; Alexandrov, I.; et al. Evolution of microstructure, macrotexture and mechanical properties of commercially pure Ti during ECAP-conform processing and drawing. Mater. Sci. Eng. A 2013, 562, 128–136. [Google Scholar] [CrossRef]
- Mishnaevsky, L.; Levashov, E.; Valiev, R.Z.; Segurado, J.; Sabirov, I.; Enikeev, N.; Prokoshkin, S.; Solov’Yov, A.V.; Korotitskiy, A.; Gutmanas, E.; et al. Nanostructured titanium-based materials for medical implants: Modeling and development. Mater. Sci. Eng. R: Rep. 2014, 81, 1–19. [Google Scholar] [CrossRef]
- Boyer, R.; Welsch, G.; Collings, E. Materials Properties Handbook: Titanium Alloys; ASM International: Materials Park, OH, USA, 1998. [Google Scholar]
- Petruželka, J.; Dluhoš, L.; Hrušák, D.; Sochová, J. Nanostructured titanium - application in dental implants. Trans. VSB Tech. Univ. Ostrava 2006, 52, 177–186. [Google Scholar]
- Faghihi, S.; Azari, F.; Zhilyaev, A.; Szpunar, J.; Vali, H.; Tabrizian, M. Cellular and molecular interactions between MC3T3-E1 pre-osteoblasts and nanostructured titanium produced by high-pressure torsion. Biomater. 2007, 28, 3887–3895. [Google Scholar] [CrossRef]
- Estrin, Y.; Ivanova, E.P.; Michalska, A.; Truong, V.K.; Lapovok, R.; Boyd, R. Accelerated stem cell attachment to ultrafine grained titanium. Acta Biomater. 2011, 7, 900–906. [Google Scholar] [CrossRef]
- Nie, F.L.; Zheng, Y.F.; Wei, S.C.; Wang, D.S.; Yu, Z.T.; Salimgareeva, G.K.; Polyakov, A.V.; Valiev, R.Z. In vitro and in vivo studies on nanocrystalline Ti fabricated by equal channel angular pressing with microcrystalline CP Ti as control. J. Biomed. Mater. Res. – Part A 2013, 101A, 1694–1707. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.; Asokamani, R.; Gogia, A. Ti based biomaterials, the ultimate choice for orthopaedic implants – A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Nakai, M.; Niinomi, M.; Akahori, T.; Ohtsu, N.; Nishimura, H.; Toda, H.; Fukui, H.; Ogawa, M. Surface hardening of biomedical Ti–29Nb–13Ta–4.6Zr and Ti–6Al–4V ELI by gas nitriding. Mater. Sci. Eng. A 2008, 486, 193–201. [Google Scholar] [CrossRef]
- Saitova, L.; Höppel, H.W.; Göken, M.; Semenova, I.; Valiev, R. Cyclic deformation behavior and fatigue lives of ultrafine-grained Ti-6AL-4V ELI alloy for medical use. Int. J. Fatigue 2009, 31, 322–331. [Google Scholar] [CrossRef]
- Semenova, I.P.; Saitova, L.R.; Raab, G.I.; Korshunov, A.I.; Zhu, Y.T.; Lowe, T.C.; Valiev, R.Z. Microstructural features and mechanical properties of the Ti-6Al-4V ELI alloy processed by severe plastic deformation. Mater. Sci. Forum 2006, 503–504, 757–762. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Zhilyaev, A.P.; Langdon, T.G. Bulk Nanostructured Materials: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Hoseini, M.; Pourian, M.H.; Bridier, F.; Vali, H.; Szpunar, J.A.; Bocher, P. Thermal stability and annealing behaviour of ultrafine grained commercially pure titanium. Mater. Sci. Eng. A 2012, 532, 58–63. [Google Scholar] [CrossRef]
- Zháňal, P.; Václavová, K.; Hadzima, B.; Harcuba, P.; Stráský, J.; Janeček, M.; Polyakova, V.; Semenova, I.P.; Hájek, M.; Hajizadeh, K. Thermal stability of ultrafine-grained commercial purity Tiand Ti–6Al–7Nb alloy investigated by electrical resistance, microhardness and scanning electron microscopy. Mater. Sci. Eng. A 2016, 651, 886–892. [Google Scholar]
- Bartha, K.; Zháňal, P.; Stráský, J.; Čížek, J.; Dopita, M.; Lukáč, F.; Harcuba, P.; Hájek, M.; Polyakova, V.; Semenova, I.P.; et al. Lattice defects in severely deformed biomedical Ti-6Al-7Nb alloy and thermal stability of its ultra-fine grained microstructure. J. Alloys Compd. 2019, 788, 881–890. [Google Scholar] [CrossRef]
- Zherebtsov, S.; Salishchev, G.; Galeyev, R.; Maekawa, K. Mechanical Properties of Ti–6Al–4V Titanium Alloy with Submicrocrystalline Structure Produced by Severe Plastic Deformation. Mater. Trans. 2005, 46, 2020–2025. [Google Scholar]
- Niinomi, M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2008, 1, 30–42. [Google Scholar]
- Steinemann, S.G. Titanium—The material of choice? Periodontology 2000 1998, 17, 7–21. [Google Scholar]
- Raabe, D.; Sander, B.; Friák, M.; Ma, D.; Neugebauer, J. Theory-guided bottom-up design of β-titanium alloys as biomaterials based on first principles calculations: Theory and experiments. Acta Mater. 2007, 55, 4475–4487. [Google Scholar] [CrossRef]
- Hou, F.; Li, S.; Hao, Y.; Yang, R. Nonlinear elastic deformation behaviour of Ti–30Nb–12Zr alloys. Scr. Mater. 2010, 63, 54–57. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Zhang, J.Y.; Vermaut, P.; Choudhuri, D.; Alam, T.; Mantri, S.A.; Svec, P.; Gloriant, T.; Jacques, P.J.; Banerjee, R.; et al. Strengthening strategy for a ductile metastable β-titanium alloy using low-temperature aging. Mater. Res. Lett. 2017, 5, 547–553. [Google Scholar] [CrossRef]
- Najdahmadi, A.; Zarei-Hanzaki, A.; Farghadani, E. Mechanical properties enhancement in Ti–29Nb–13Ta–4.6Zr alloy via heat treatment with no detrimental effect on its biocompatibility. Mater. Des. 2014, 54, 786–791. [Google Scholar] [CrossRef]
- Xu, W.; Wu, X.; Figueiredo, R.B.; Stoica, M.; Calin, M.; Eckert, J.; Langdon, T.G.; Xia, K. Nanocrystalline body-centred cubic beta-titanium alloy processed by high-pressure torsion. Int. J. Mater. Res. 2009, 100, 1662–1667. [Google Scholar] [CrossRef]
- Zafari, A.; Wei, X.; Xu, W.; Xia, K. Formation of nanocrystalline β structure in metastable beta Ti alloy during high pressure torsion: The role played by stress induced martensitic transformation. Acta Mater. 2015, 97, 146–155. [Google Scholar] [CrossRef]
- Xu, W.; Wu, X.; Calin, M.; Stoica, M.; Eckert, J.; Xia, K. Formation of an ultrafine-grained structure during equal-channel angular pressing of a β-titanium alloy with low phase stability. Scr. Mater. 2009, 60, 1012–1015. [Google Scholar] [CrossRef]
- Polyakov, A.V.; Semenova, I.P.; Ivanov, E.; Valiev, R.Z. Ultra-fine grained β-type TNZT ELI alloy with high strength and low elastic modulus. IOP Conf. Ser.: Mater. Sci. Eng 2019, 461, 012077. [Google Scholar] [CrossRef]
- Xie, K.; Wang, Y.-B.; Zhao, Y.; Chang, L.; Wang, G.; Chen, Z.; Cao, Y.; Liao, X.; Lavernia, E.J.; Valiev, R.Z.; et al. Nanocrystalline β-Ti alloy with high hardness, low Young’s modulus and excellent in vitro biocompatibility for biomedical applications. Mater. Sci. Eng. C 2013, 33, 3530–3536. [Google Scholar] [CrossRef]
- Stráský, J.; Janeĉek, M.; Semenova, I.; Čížek, J.; Bartha, K.; Harcuba, P.; Polyakova, V.; Gatina, S. Microstructure and lattice defects in ultrafine grained biomedical α+β and metastable β Ti alloys. In Titanium in Medical and Dental Applications; Froes, F., Qian, M., Eds.; Woodhead Publishing: Duxford, UK, 2018; pp. 455–475. [Google Scholar]
- Valiev, R.Z. Superior Strength in Ultrafine-Grained Materials Produced by SPD Processing. Mater. Trans. 2014, 55, 13–18. [Google Scholar] [CrossRef]
- Janeček, M.; Čížek, J.; Stráský, J.; Bartha, K.; Hruška, P.; Polyakova, V.; Gatina, S.; Semenova, I. Microstructure evolution in solution treated Ti15Mo alloy processed by high pressure torsion. Mater. Charact. 2014, 98, 233–240. [Google Scholar] [CrossRef]
- Bartha, K.; Stráský, J.; Polyakova, V.; Stráská, J.; Nejezchlebová, J.; Seiner, H.; Semenova, I.; Janeček, M. Microhardness and microstructure evolution of ultra-fine grained Ti-15Mo and TIMETAL LCB alloys prepared by high pressure torsion. Mater. Sci. Eng. A 2017, 682, 220–228. [Google Scholar]
- Gatina, S.; Semenova, I.; Janeček, M.; Stráský, J. Effect of high pressure torsion on the aging kinetics of β-titanium Ti-15Mo alloy. IOP Conf. Series: Mater. Sci. Eng. 2014, 63, 012068. [Google Scholar] [CrossRef]
- Bartha, K.; Stráský, J.; Veverková, A.; Barriobero-Vila, P.; Lukáč, F.; Doležal, P.; Sedlák, P.; Polyakova, V.; Semenova, I.P.; Janeček, M. Effect of the High-Pressure Torsion (HPT) and Subsequent Isothermal Annealing on the Phase Transformation in Biomedical Ti15Mo Alloy. Metals 2019, 9, 1194. [Google Scholar] [CrossRef]
- Bartha, K.; Veverková, A.; Stráský, J.; Veselý, J.; Minárik, P.; Correa, C.; Polyakova, V.; Semenova, I.; Janeček, M. Effect of the severe plastic deformation by ECAP on microstructure and phase transformations in Ti-15Mo alloy. Mater. Today Commun. 2020, 22, 100811. [Google Scholar] [CrossRef]
- Yilmazer, H.; Niinomi, M.; Nakai, M.; Hieda, J.; Todaka, Y.; Akahori, T.; Miyazaki, T. Heterogeneous structure and mechanical hardness of biomedical -type Ti–29Nb–13Ta–4.6Zr subjected to high-pressure torsion. J. Mech. Behav. Biomed. Mater. 2012, 10, 235–245. [Google Scholar] [CrossRef]
- Yilmazer, H.; Niinomi, M.; Nakai, M.; Cho, K.; Hieda, J.; Todaka, Y.; Miyazaki, T. Mechanical properties of a medical β-type titanium alloy with specific microstructural evolution through high-pressure torsion. Mater. Sci. Eng. C 2013, 33, 2499–2507. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, L.; Xue, X.; Lu, W.; Qin, J.; Zhang, D. Microstructure evolution and mechanical properties of a Ti–35Nb–3Zr–2Ta biomedical alloy processed by equal channel angular pressing (ECAP). Mater. Sci. Eng. C 2013, 33, 4551–4561. [Google Scholar] [CrossRef]
- Otsuka, K.; Ren, X. Physical metallurgy of Ti–Ni-based shape memory alloys. Prog. Mater. Sci. 2005, 50, 511–678. [Google Scholar] [CrossRef]
- Brailovski, V.; Prokoshkin, S.; Terriault, P.; Trochu, F. Shape Memory Alloys: Fundamentals, Modeling and Applications; E´cole de technologie supe´rieure (ETS): Montreal, QC, Canada, 2003; p. 851. [Google Scholar]
- Pushin, V.G.; Stolyarov, V.V.; Valiev, R.Z.; Kourov, N.I.; Kuranova, N.N.; Prokofiev, E.A.; Yurchenko, L.I. Features of structure and phase transformation in shape memory TiNi-based alloys after severe plastic deformation. Ann. Chim. Sci. Mater. 2002, 27, 77–88. [Google Scholar] [CrossRef]
- Valiev, R.; Gunderov, D.; Prokofiev, E.; Pushin, V.; Zhu, Y. Nanostruturing of TiNi alloy by SPD processing for advanced properties. Mater. Trans. 2008, 49, 97–101. [Google Scholar] [CrossRef]
- Malard, B.; Pilch, J.; Sittner, P.; Delville, R.; Curfs, C. In situ investigation of the fast microstructure evolution during electropulse treatment of cold drawn NiTi wires. Acta Mater. 2011, 59, 1542–1556. [Google Scholar] [CrossRef]
- Burow, J.; Frenzel, J.; Somsen, C.; Prokofiev, E.; Valiev, R.; Eggeler, G. Grain Nucleation and Growth in Deformed NiTi Shape Memory Alloys: An In Situ TEM Study. Shape Mem. Superelasticity 2017, 3, 347–360. [Google Scholar] [CrossRef]
- Waitz, T.; Kazykhanov, V.; Karnthaler, H. Martensitic phase transformations in nanocrystalline NiTi studied by TEM. Acta Mater. 2004, 52, 137–147. [Google Scholar] [CrossRef]
- Prokoshkin, S.D.; Khmelevskaya, I.; Dobatkin, S.; Trubitsyna, I.; Tatyanin, E.; Stolyarov, V.; Prokofiev, E. Alloy composition, deformation temperature, pressure and post-deformation annealing effects in severely deformed Ti–Ni based shape memory alloys. Acta Mater. 2005, 53, 2703–2714. [Google Scholar] [CrossRef]
- Stolyarov, V.V.; Prokofiev, E.A.; Prokoshkin, S.D.; Dobatkin, S.V.; Trubitsyna, I.B.; Khmelevskaya, I.Y.; Pushin, V.G.; Valiev, R.Z. Structural features, techanical properties, and the shape-memory effect in TiNi alloys subjected to equal-channel angular pressing. Phys. Met. Metall. 2005, 100, 608–618. [Google Scholar]
- Tong, Y.; Guo, B.; Chen, F.; Tian, B.; Li, L.; Zheng, Y.; Prokofiev, E.; Gunderov, D.V.; Valiev, R.Z. Thermal cycling stability of ultrafine-grained TiNi shape memory alloys processed by equal channel angular pressing. Scr. Mater. 2012, 67, 1–4. [Google Scholar] [CrossRef]
- Prokofiev, E.; Burow, J.; Frenzel, J.; Gunderov, D.; Eggeler, G.; Valiev, R. Phase transformations and functional properties of NiTi alloy with ultrafine-grained structure. Mater. Sci. Forum 2011, 667–669, 1059–1064. [Google Scholar] [CrossRef]
- Semenova, I.P.; Klevtsov, G.V.; Klevtsova, N.A.; Dyakonov, G.; Matchin, A.A.; Valiev, R.Z. Nanostructured Titanium for Maxillofacial Mini-Implants. Adv. Eng. Mater. 2016, 18, 1216–1224. [Google Scholar] [CrossRef]
- ANSYS Workbench. Available online: https://cae-expert.ru/product/ansys-workbench (accessed on 24 October 2019).
- Timplant ®-Dental Implants. Available online: http://www.timplant.cz/en/ (accessed on 24 October 2019).
- Minasov, T.B.; Bakusov, L.M.; Nasyrov, R.V. Intratissular Tension in the Segments of the Musculoskeletal System; LAP LAMBERT Acad. Publ.: Saarbrucken, Germany, 2012. [Google Scholar]
- Minasov, T.B.; Minasov, B.S. The effectiveness of combined therapy of postmenopausal osteoporosis using dual-action medicine. Traumatol. Orthop. 2011, 4, 92–94. [Google Scholar]
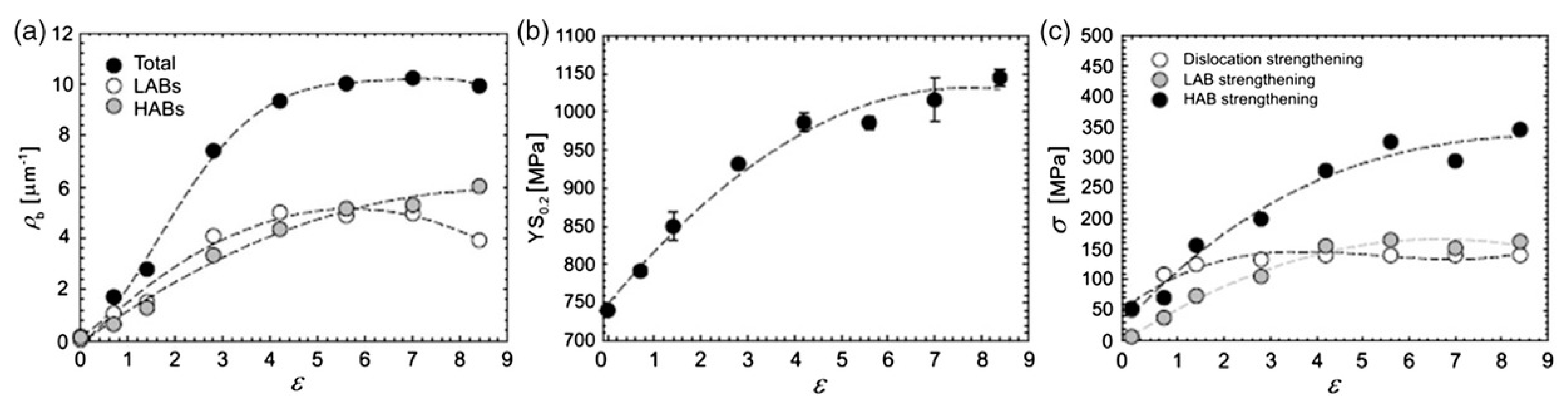
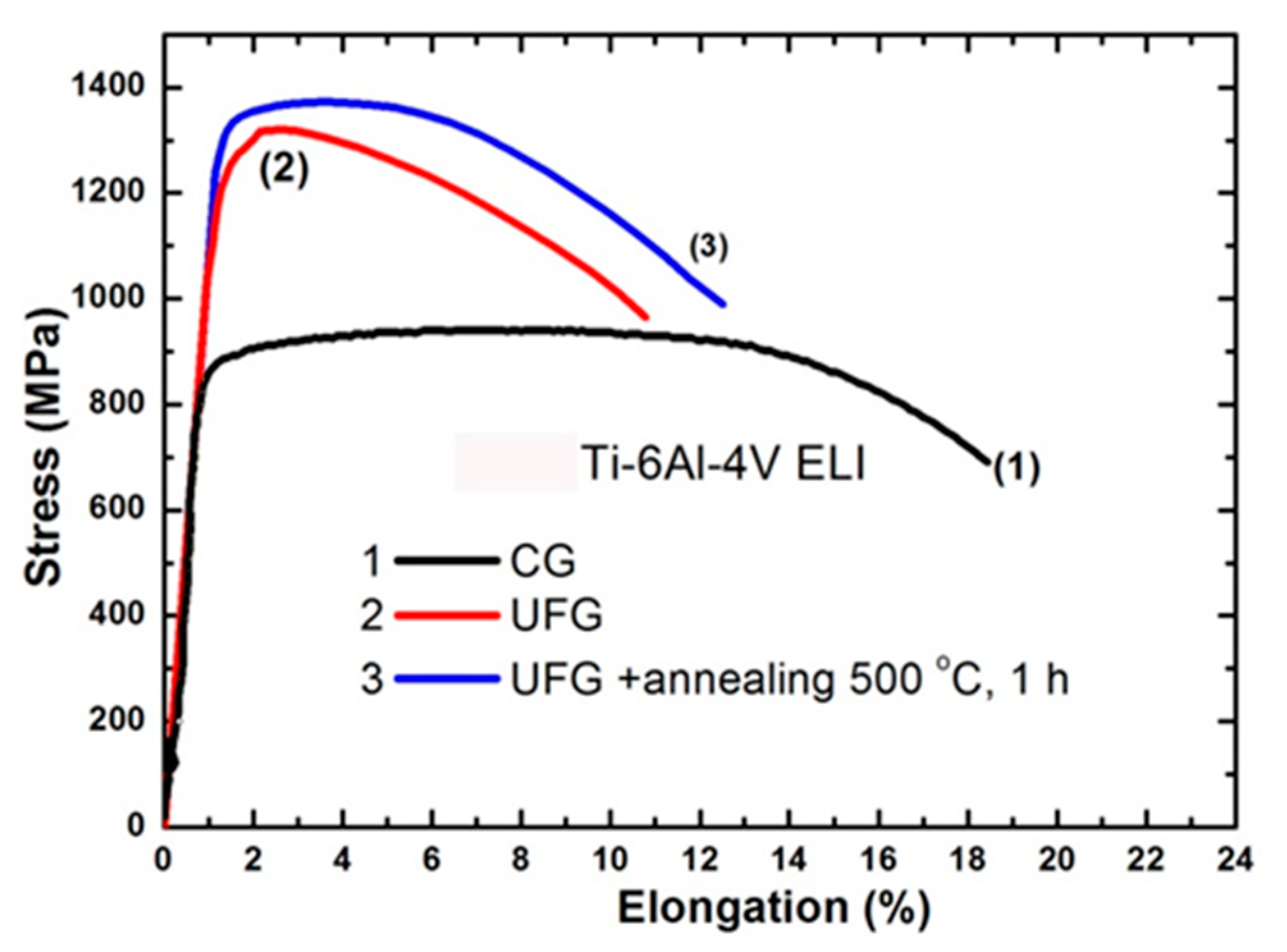
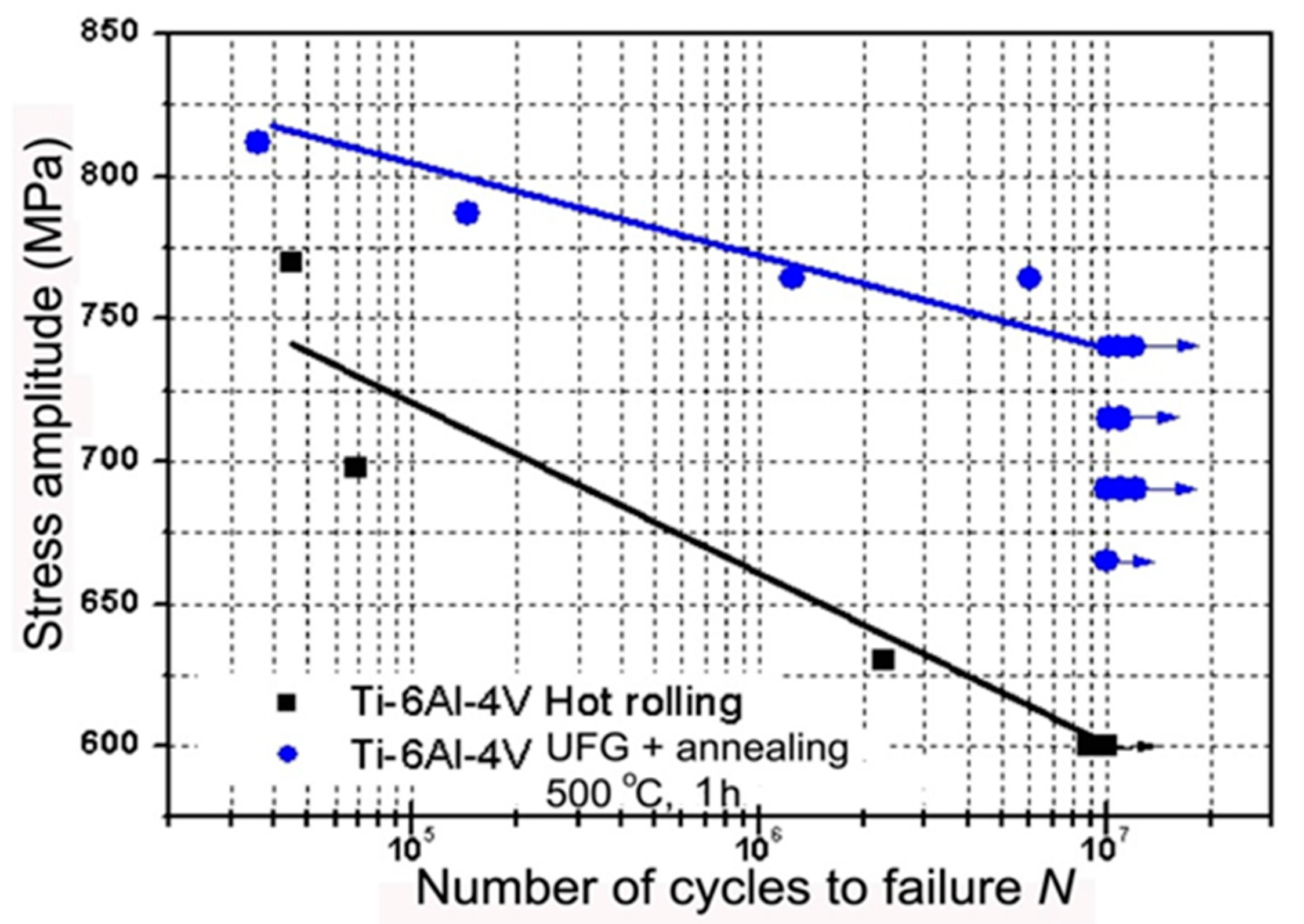
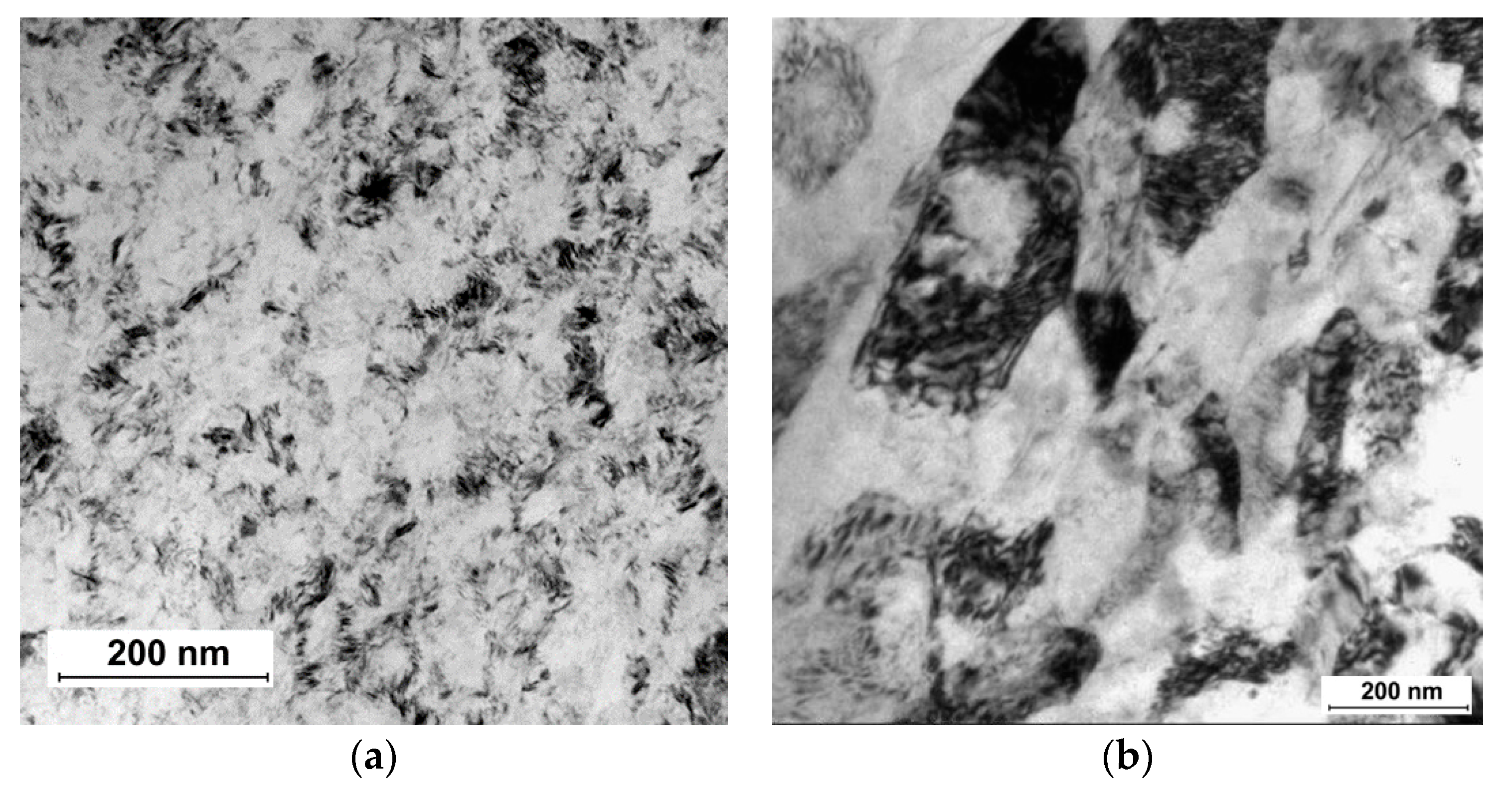

| State | Processing | UTS, MPa | YS, MPa | Elongation, % | Reduction Area, % | Fatigue Strength at 106 Cycles |
|---|---|---|---|---|---|---|
| 1 | Initial CG Ti | 700 | 530 | 25 | 52 | 340 |
| 2 | nanoTi | 1240 | 1200 | 12 | 42 | 620 |
| 3 | Annealed Ti-6Al-4V ELI | 940 | 840 | 16 | 45 | 530 |
| Material | Opening Angle of the Jaws, ° | Opening of the Jaws at Reversible Shape Memory Effect, mm | Max Rated Force of the Clipping Device, H |
|---|---|---|---|
| CG | <110 | 2 | 0.44 |
| UFG | 160 | 4 | 0.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valiev, R.Z.; Prokofiev, E.A.; Kazarinov, N.A.; Raab, G.I.; Minasov, T.B.; Stráský, J. Developing Nanostructured Ti Alloys for Innovative Implantable Medical Devices. Materials 2020, 13, 967. https://doi.org/10.3390/ma13040967
Valiev RZ, Prokofiev EA, Kazarinov NA, Raab GI, Minasov TB, Stráský J. Developing Nanostructured Ti Alloys for Innovative Implantable Medical Devices. Materials. 2020; 13(4):967. https://doi.org/10.3390/ma13040967
Chicago/Turabian StyleValiev, Ruslan Z., Egor A. Prokofiev, Nikita A. Kazarinov, Georgy I. Raab, Timur B. Minasov, and Josef Stráský. 2020. "Developing Nanostructured Ti Alloys for Innovative Implantable Medical Devices" Materials 13, no. 4: 967. https://doi.org/10.3390/ma13040967
APA StyleValiev, R. Z., Prokofiev, E. A., Kazarinov, N. A., Raab, G. I., Minasov, T. B., & Stráský, J. (2020). Developing Nanostructured Ti Alloys for Innovative Implantable Medical Devices. Materials, 13(4), 967. https://doi.org/10.3390/ma13040967






