Highly Fluorescent Distyrylnaphthalene Derivatives as a Tool for Visualization of Cellular Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Synthesis
2.2. Cell Cultures and Reagents
2.3. Cell Viability Assay
2.4. Visualization of Membrane Blebbing
2.5. Optical Characterization
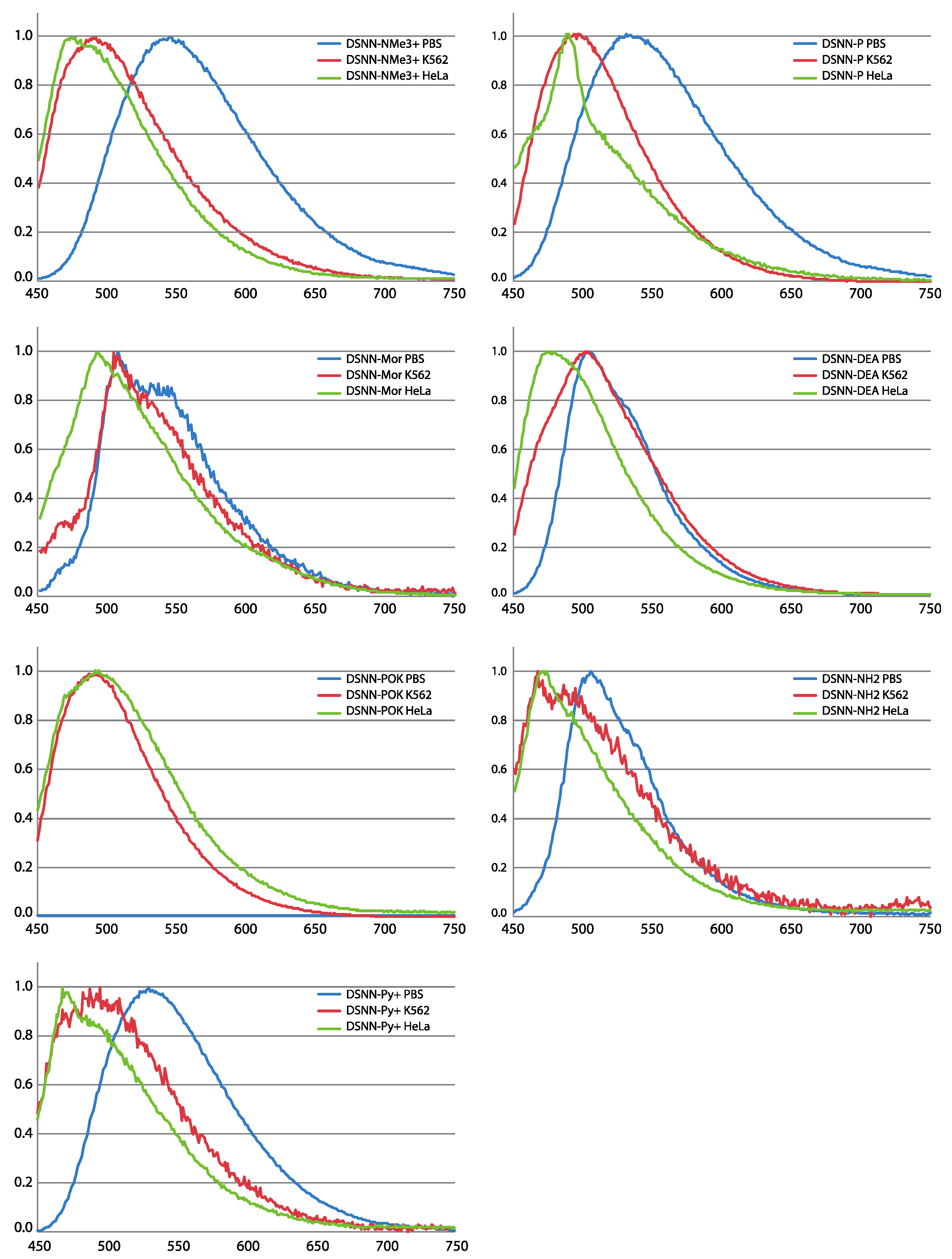
2.5.1. Spectral Properties of SN-COEs
2.5.2. Optical Characterization of SN-COEs within Cellular Membranes
2.6. Fluorescence (FL) and Confocal (CLSM) Microscopy
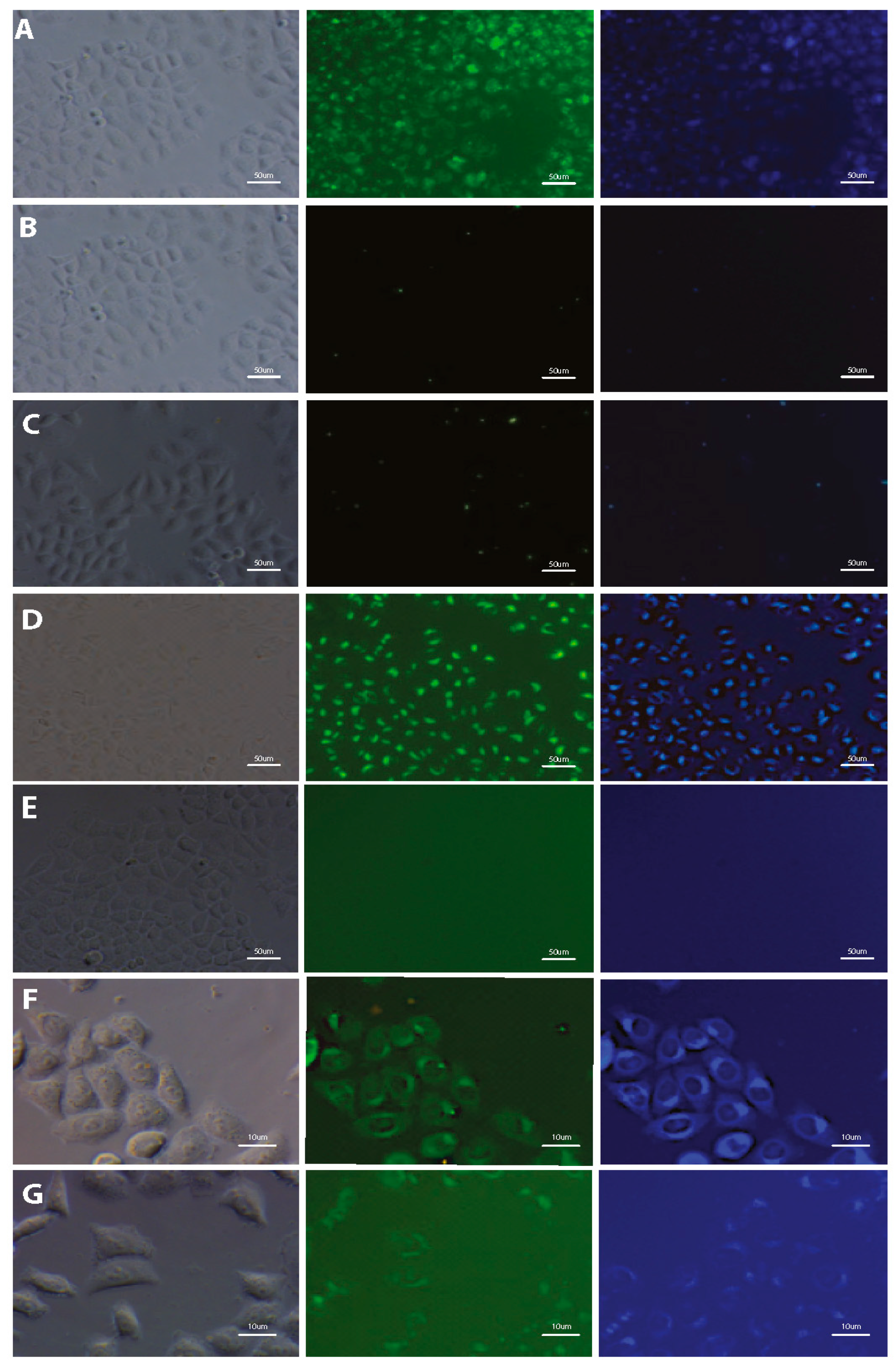
2.6.1. Fluorescence Microscopy Imaging
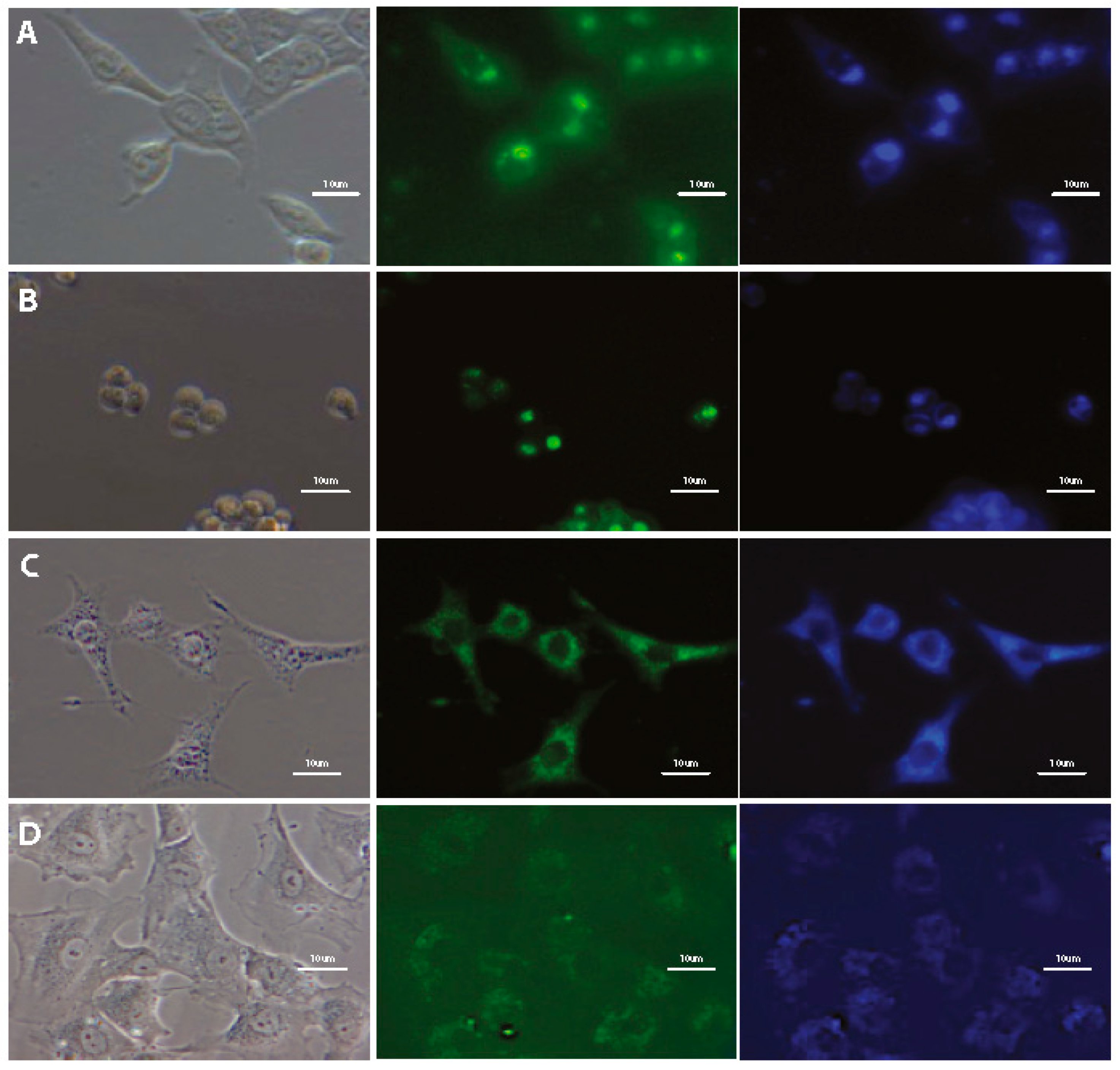
2.6.2. Co-Localization Confocal Microscopy Imaging
2.7. Flow Cytometry Analysis
3. Results and Discussion
3.1. Cytotoxicity Tests
3.2. Evaluation of Spectral Properties of the DSNN-Derivatives
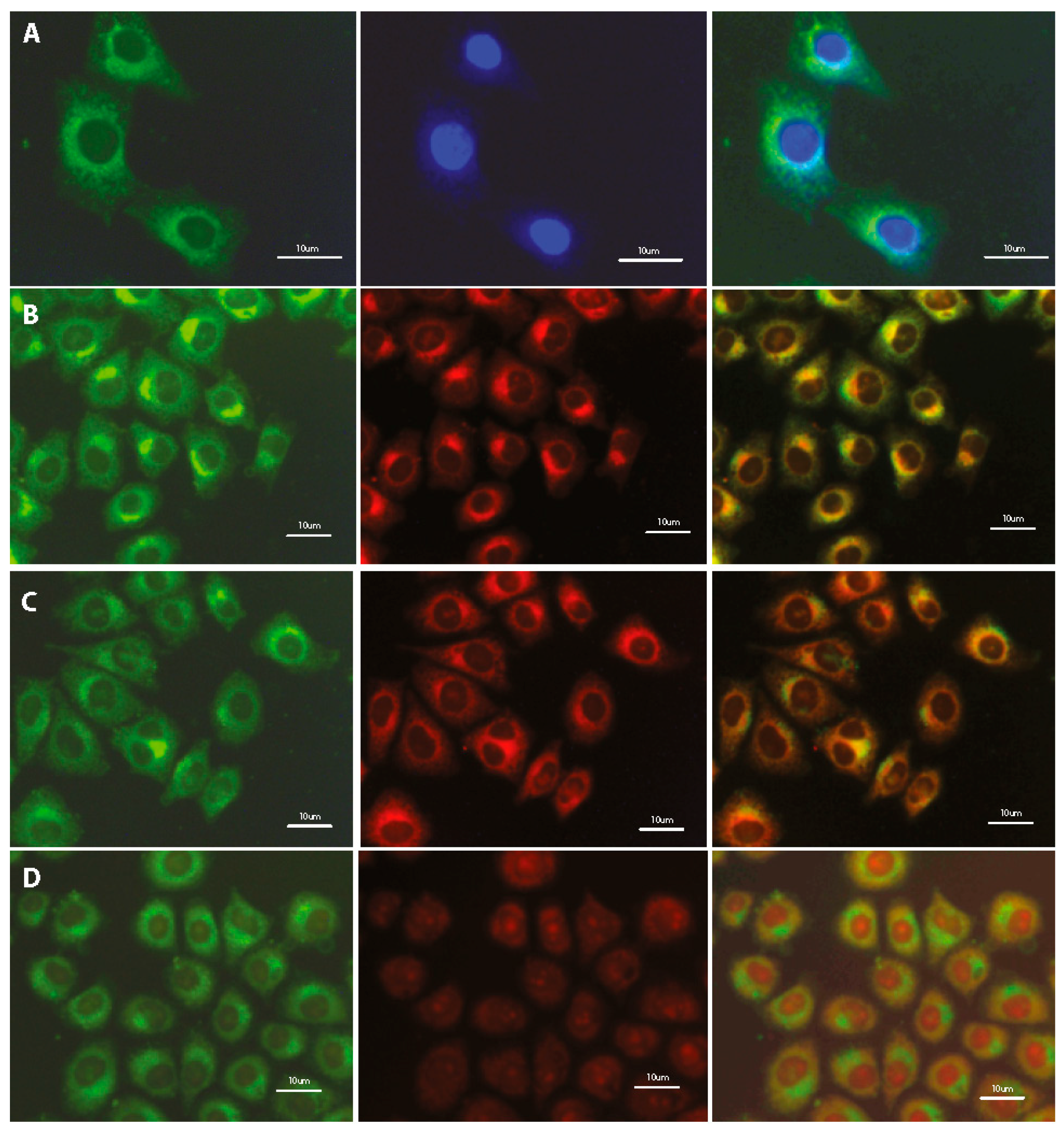
3.3. Imaging of Cells Labeled by DSNN-Derivatives
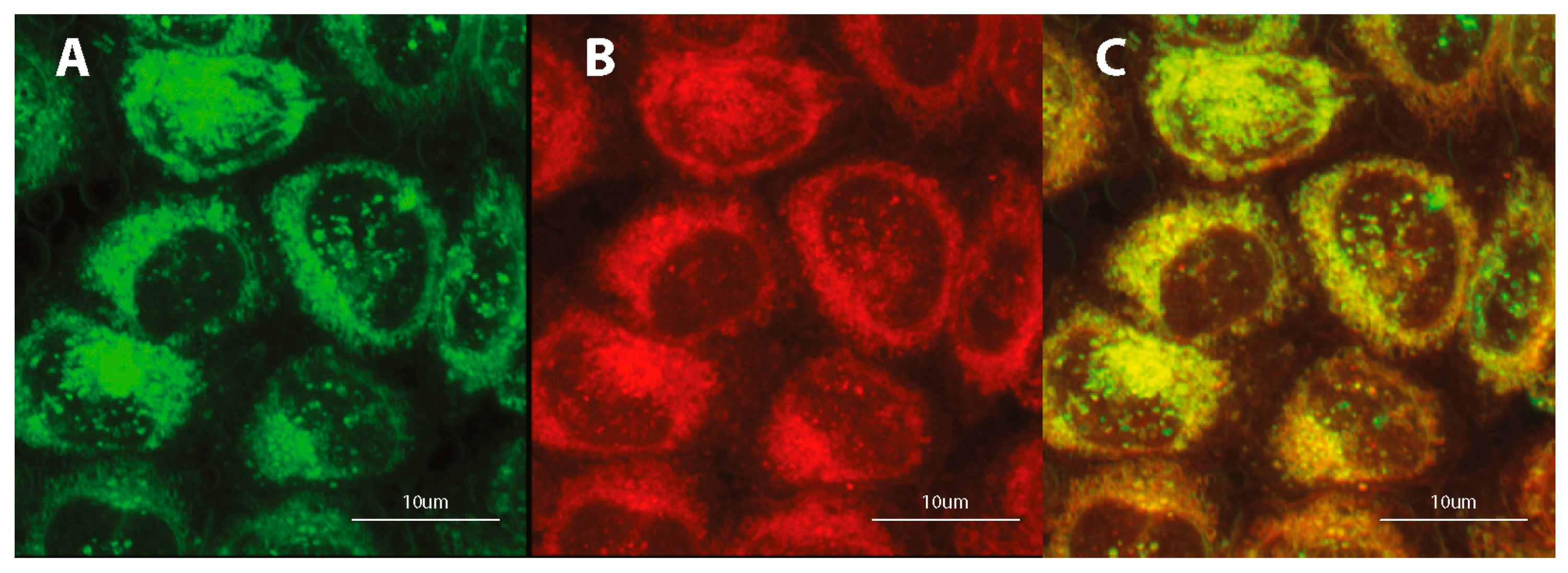
3.4. Subcellular Localization
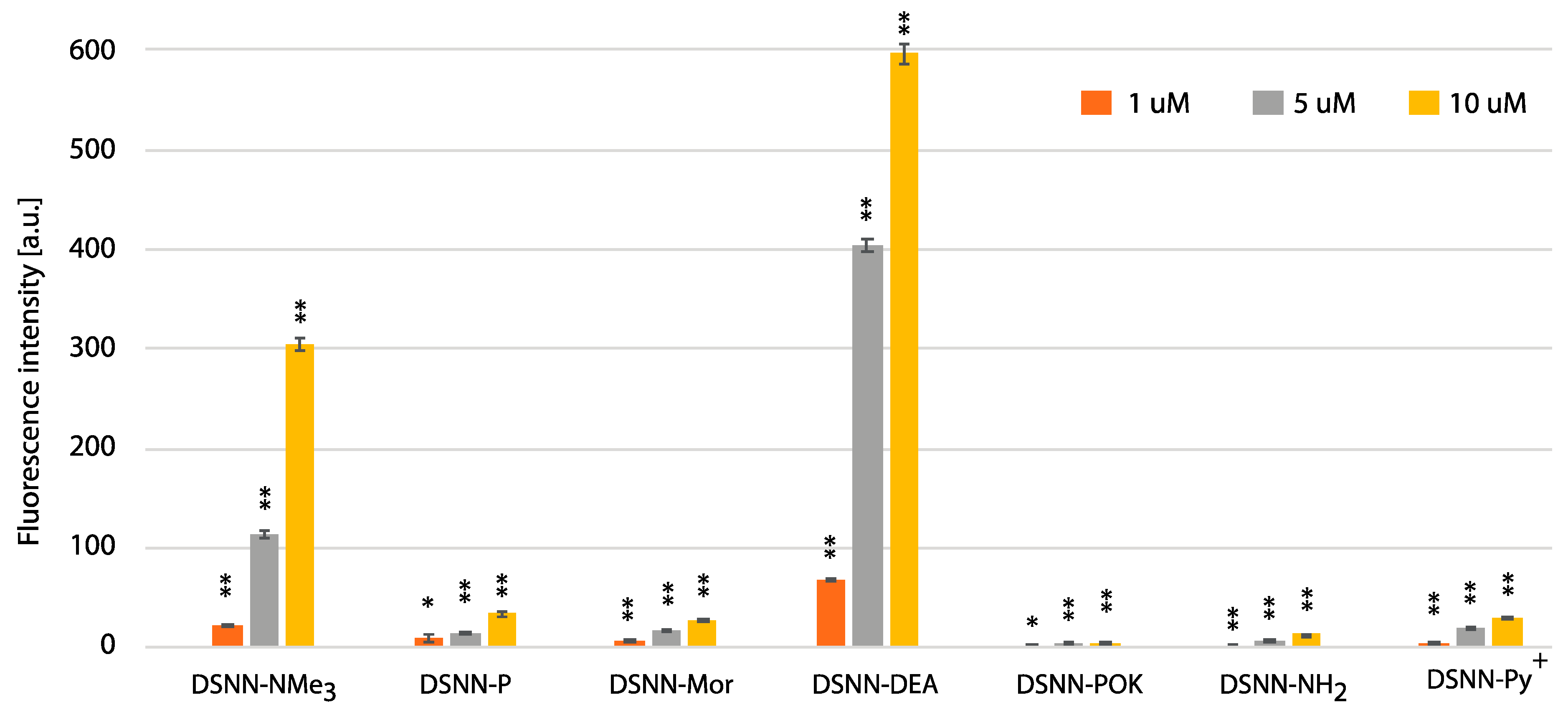
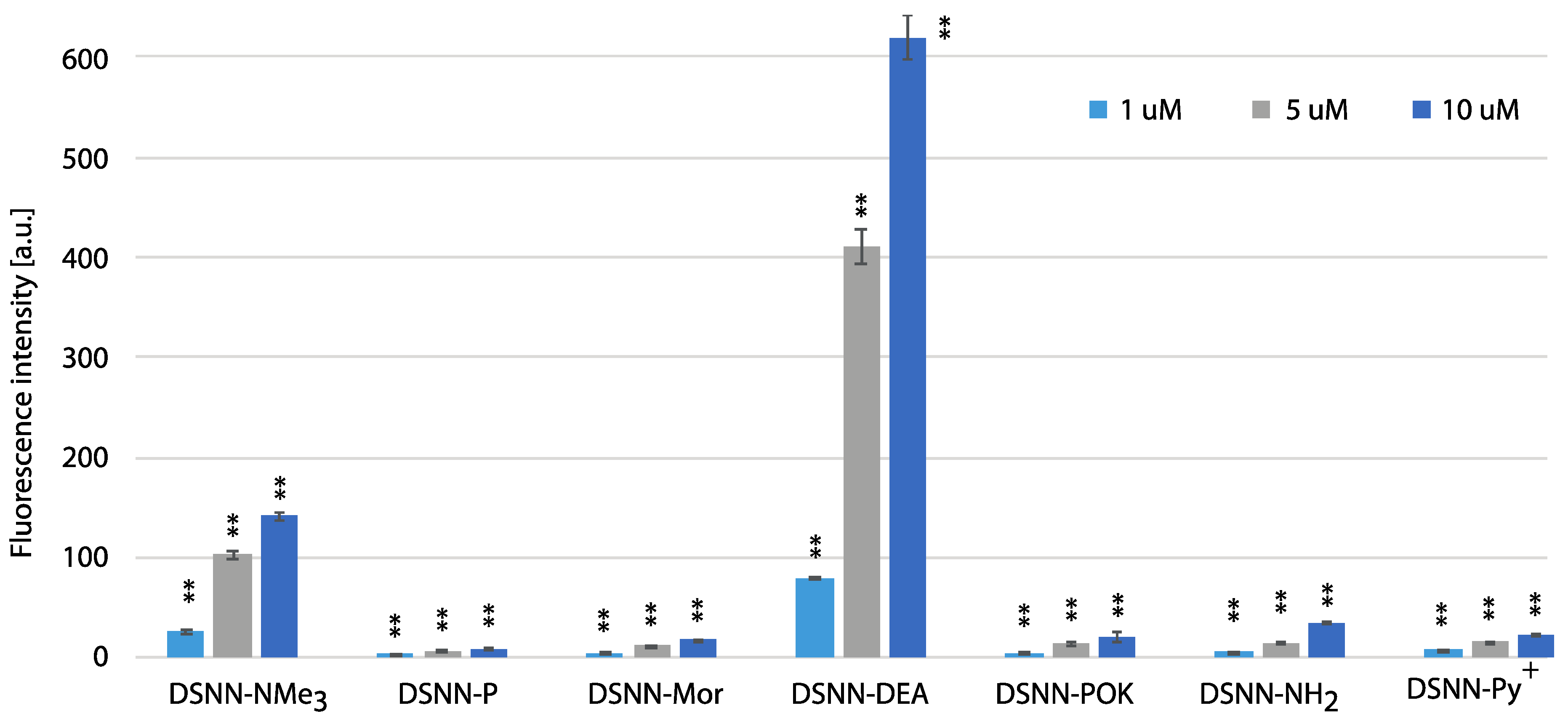
3.5. Quantification of Cellular Uptake by Flow Cytometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Specht, E.A.; Braselmann, E.; Palmer, A.E. A Critical and Comparative Review of Fluorescent Tools for Live-Cell Imaging. Annu. Rev. Physiol. 2017, 79, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, B. Polymer encapsulated conjugated polymer nanoparticles for fluorescence bioimaging. J. Mater. Chem. 2012, 22, 1257–1264. [Google Scholar] [CrossRef]
- Chen, K.; Chen, X. Design and development of molecular imaging probes. Curr. Top. Med. Chem. 2010, 10, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Wiedenmann, J.; Oswald, F.; Nienhaus, G.U. Fluorescent proteins for live cell imaging: Opportunities, limitations, and challenges. IUBMB Life 2009, 61, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Hardman, R. A toxicologic review of quantum dots: Toxicity depends on physicochemical and environmental factors. Environ. Health Perspect. 2006, 114, 165–172. [Google Scholar] [CrossRef]
- Feng, X.L.; Lv, F.T.; Liu, L.B.; Yang, Q.; Wang, S.; Bazan, G.C. A Highly Emissive Conjugated Polyelectrolyte Vector for Gene Delivery and Transfection. Adv. Mater. 2012, 24, 5428–5432. [Google Scholar] [CrossRef]
- Wang, V.B.; Du, J.; Chen, X.F.; Thomas, A.W.; Kirchhofer, N.D.; Garner, L.E.; Maw, M.T.; Poh, W.H.; Hinks, J.; Wuertz, S.; et al. Improving charge collection in Escherichia coli-carbon electrode devices with conjugated oligoelectrolytes. Phys. Chem. Chem. Phys. 2013, 15, 5867–5872. [Google Scholar] [CrossRef]
- Lee, Y.; Yang, I.; Lee, J.E.; Hwang, S.; Lee, J.W.; Um, S.S.; Nguyen, T.L.; Yoo, P.J.; Woo, H.Y.; Park, J.; et al. Enhanced Photocurrent Generation by Forster Resonance Energy Transfer between Phospholipid-Assembled Conjugated Oligoelectrolytes and Nile Red. J. Phys. Chem. C 2013, 117, 3298–3307. [Google Scholar] [CrossRef]
- Hammarstrom, P.; Simon, R.; Nystrom, S.; Konradsson, P.; Aslund, A.; Nilsson, K.P.R. A Fluorescent Pentameric Thiophene Derivative Detects in Vitro-Formed Prefibrillar Protein Aggregates. Biochemistry 2010, 49, 6838–6845. [Google Scholar] [CrossRef]
- Song, W.L.; Jiang, R.C.; Yuan, Y.; Lu, X.M.; Hu, W.B.; Fan, Q.L.; Huang, W. Star-shaped conjugated oligoelectrolyte for bioimaging in living cells. Chin. Sci. Bull. 2013, 58, 2570–2575. [Google Scholar] [CrossRef]
- Thomas, A.W.; Henson, Z.B.; Du, J.; Vandenberg, C.A.; Bazan, G.C. Synthesis, Characterization, and Biological Affinity of a Near-Infrared-Emitting Conjugated Oligoelectrolyte. J. Am. Chem. Soc. 2014, 136, 3736–3739. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.J.; Park, S.; Jeong, J.E.; Hong, Y.; Ku, M.; Kim, B.Y.; Jang, I.H.; Heo, S.C.; Wang, T.; Kim, K.H.; et al. Synthesis and Characterization of Water-Soluble Conjugated Oligoelectrolytes for Near-Infrared Fluorescence Biological Imaging. ACS Appl. Mater. Interfaces 2016, 8, 15937–15947. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yin, H.; Ng, J.C.Y.; Cai, L.P.; Li, J.; Tang, B.Z.; Liu, B. Polyethyleneimine-grafted hyperbranched conjugated polyelectrolytes: Synthesis and imaging of gene delivery. Polym. Chem. 2013, 4, 5297–5304. [Google Scholar] [CrossRef]
- Yan, H.; Rengert, Z.D.; Thomas, A.W.; Rehermann, C.; Hinks, J.; Bazan, G.C. Influence of molecular structure on the antimicrobial function of phenylenevinylene conjugated oligoelectrolytes. Chem. Sci. 2016, 7, 5714–5722. [Google Scholar] [CrossRef]
- Wang, B.; Feng, G.; Seifrid, M.; Wang, M.; Liu, B.; Bazan, G.C. Antibacterial Narrow-Band-Gap Conjugated Oligoelectrolytes with High Photothermal Conversion Efficiency. Angew. Chem. Int. Ed. Engl. 2017, 56, 16063–16066. [Google Scholar] [CrossRef]
- Zhou, C.; Chia, G.W.N.; Ho, J.C.S.; Seviour, T.; Sailov, T.; Liedberg, B.; Kjelleberg, S.; Hinks, J.; Bazan, G.C. Informed Molecular Design of Conjugated Oligoelectrolytes To Increase Cell Affinity and Antimicrobial Activity. Angew. Chem. Int. Ed. Engl. 2018, 57, 8069–8072. [Google Scholar] [CrossRef]
- Yan, H.; Catania, C.; Bazan, G.C. Membrane-intercalating conjugated oligoelectrolytes: Impact on bioelectrochemical systems. Adv. Mater. 2015, 27, 2958–2973. [Google Scholar] [CrossRef]
- Ortony, J.H.; Chatterjee, T.; Garner, L.E.; Chworos, A.; Mikhailovsky, A.; Kramer, E.J.; Bazan, G.C. Self-assembly of an optically active conjugated oligoelectrolyte. J. Am. Chem. Soc. 2011, 133, 8380–8387. [Google Scholar] [CrossRef]
- Ding, D.; Pu, K.Y.; Li, K.; Liu, B. Conjugated oligoelectrolyte-polyhedral oligomeric silsesquioxane loaded pH-responsive nanoparticles for targeted fluorescence imaging of cancer cell nucleus. Chem. Commun. 2011, 47, 9837–9839. [Google Scholar] [CrossRef]
- Woo, H.Y.; Korystov, D.; Mikhailovsky, A.; Nguyen, T.Q.; Bazan, G.C. Two-photon absorption in aqueous micellar solutions. J. Am. Chem. Soc. 2005, 127, 13794–13795. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.Y.; Liu, B.; Kohler, B.; Korystov, D.; Mikhailovsky, A.; Bazan, G.C. Solvent effects on the two-photon absorption of distyrylbenzene chromophores. J. Am. Chem. Soc. 2005, 127, 14721–14729. [Google Scholar] [CrossRef] [PubMed]
- Garner, L.E.; Park, J.; Dyar, S.M.; Chworos, A.; Sumner, J.J.; Bazan, G.C. Modification of the optoelectronic properties of membranes via insertion of amphiphilic phenylenevinylene oligoelectrolytes. J. Am. Chem. Soc. 2010, 132, 10042–10052. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.W.; Garner, L.E.; Nevin, K.P.; Woodard, T.L.; Franks, A.E.; Lovley, D.R.; Sumner, J.J.; Sund, C.J.; Bazan, G.C. A lipid membrane intercalating conjugated oligoelectrolyte enables electrode driven succinate production in Shewanella. Energ. Environ. Sci. 2013, 6, 1761–1765. [Google Scholar] [CrossRef]
- Hou, H.J.; Chen, X.F.; Thomas, A.W.; Catania, C.; Kirchhofer, N.D.; Garner, L.E.; Han, A.; Bazan, G.C. Conjugated Oligoelectrolytes Increase Power Generation in E. coli Microbial Fuel Cells. Adv. Mater. 2013, 25, 1593–1597. [Google Scholar] [CrossRef] [PubMed]
- Gwozdzinska, P.; Pawlowska, R.; Milczarek, J.; Garner, L.E.; Thomas, A.W.; Bazan, G.C.; Chworos, A. Phenylenevinylene conjugated oligoelectrolytes as fluorescent dyes for mammalian cell imaging. Chem. Commun. 2014, 50, 14859–14861. [Google Scholar] [CrossRef] [PubMed]
- Milczarek, J.; Pawlowska, R.; Zurawinski, R.; Lukasik, B.; Garner, L.E.; Chworos, A. Fluorescence and confocal imaging of mammalian cells using conjugated oligoelectrolytes with phenylenevinylene core. J. Photochem. Photobiol. B Biol. 2017, 170, 40–48. [Google Scholar] [CrossRef]
- Czernek, L.; Chworos, A.; Duchler, M. The Uptake of Extracellular Vesicles is Affected by the Differentiation Status of Myeloid Cells. Scand. J. Immunol. 2015, 82, 506–514. [Google Scholar] [CrossRef]
- Pu, K.Y.; Li, K.; Zhang, X.H.; Liu, B. Conjugated Oligoelectrolyte Harnessed Polyhedral Oligomeric Silsesquioxane as Light-Up Hybrid Nanodot for Two-Photon Fluorescence Imaging of Cellular Nucleus. Adv. Mater. 2010, 22, 4186–4189. [Google Scholar] [CrossRef]
- Bang, H.S.; Seo, S.Y.; Choo, D.C.; Kim, T.W.; Lee, S.J.; Seo, J.H.; Kim, Y.K.; Chu, C.; Ha, J. Effect of doped emitting layer on electrical and optical properties in blue organic light-emitting devices. Thin Solid Films 2009, 517, 5314–5317. [Google Scholar] [CrossRef]
- Qi, Q.J.; Wu, X.M.; Hua, Y.L.; Hou, Q.C.; Dong, M.S.; Mao, Z.Y.; Yin, B.; Yin, S.G. Enhancement of performance for blue organic light emitting devices based on double emission layers. Org. Electron. 2010, 11, 503–507. [Google Scholar] [CrossRef]
- Kuboshima, D.; Miyamoto, E.; Hamasaki, K.; Nakai, N.; Inagaki, Y.; Okada, H.; Ichiguchi, T.; Maruo, K. Electrophotographic Photoreceptor and Image Forming Apparatus. Patent WO2006073160, 5 January 2006. [Google Scholar]
- Schinzel, E.; Frischkorn, H.; Roesch, G. 1,2,4-triazolyl (1) Derivatives, Processes for Their Preparation and Their Use as Optical Brightening Agents. Patent US3996213, 7 December 1976. [Google Scholar]
- Sanchez, M.I.; Martinez-Costas, J.; Mascarenas, J.L.; Vazquez, M.E. MitoBlue: A nontoxic and photostable blue-emitting dye that selectively labels functional mitochondria. ACS Chem. Biol. 2014, 9, 2742–2747. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.O.; Carvalho, P.; Correa, J.R.; Guido, B.C.; Medeiros, G.A.; Eberlin, M.N.; Coelho, S.E.; Domingos, J.B.; Neto, B.A.D. Palladium Catalyst with Task-Specific Ionic Liquid Ligands: Intracellular Reactions and Mitochondrial Imaging with Benzothiadiazole Derivatives. J. Organ. Chem. 2019, 84, 5118–5128. [Google Scholar] [CrossRef]
- Lukasik, B.; Milczarek, J.; Pawlowska, R.; Zurawinski, R.; Chworos, A. Facile synthesis of fluorescent distyrylnaphthalene derivatives for bioapplications. New J. Chem. 2017, 41, 6977–6980. [Google Scholar] [CrossRef]
- Chworos, A.; Zurawinski, R.; Lukasik, B.; Pawlowska, R.; Milczarek, J. Pochodne distyrylonaftalenu, sposób ich wytwarzania oraz ich zastosowanie. EP 3072511, 20 June 2018. [Google Scholar]
- Du, J.; Thomas, A.W.; Chen, X.; Garner, L.E.; Vandenberg, C.A.; Bazan, G.C. Increased ion conductance across mammalian membranes modified with conjugated oligoelectrolytes. Chem. Commun. (Camb) 2013, 49, 9624–9626. [Google Scholar] [CrossRef]
- Hinks, J.; Wang, Y.; Poh, W.H.; Donose, B.C.; Thomas, A.W.; Wuertz, S.; Loo, S.C.; Bazan, G.C.; Kjelleberg, S.; Mu, Y.; et al. Modeling cell membrane perturbation by molecules designed for transmembrane electron transfer. Langmuir 2014, 30, 2429–2440. [Google Scholar] [CrossRef]
- Kowalska-Baron, A.; Zurawinski, R.; Lukasik, B.; Chworos, A.; Wysocki, S. Solvent effects on the photophysical properties of distyrylnaphthalene-based conjugated oligoelectrolytes. J. Lumin. 2017, 192, 359–370. [Google Scholar] [CrossRef]
- Kowalska-Baron, A.; Zurawinski, R.; Lukasik, B.; Chworos, A.; Wysocki, S. Theoretical and experimental study on the electronic structure of distyrylnaphthalene-based conjugated oligoelectrolytes. J. Lumin. 2018, 196, 81–89. [Google Scholar] [CrossRef]
- Shim, S.H.; Xia, C.; Zhong, G.; Babcock, H.P.; Vaughan, J.C.; Huang, B.; Wang, X.; Xu, C.; Bi, G.Q.; Zhuang, X. Super-resolution fluorescence imaging of organelles in live cells with photoswitchable membrane probes. Proc. Natl. Acad. Sci. USA 2012, 109, 13978–13983. [Google Scholar] [CrossRef]
- Kwiatek, J.M.; Owen, D.M.; Abu-Siniyeh, A.; Yan, P.; Loew, L.M.; Gaus, K. Characterization of a new series of fluorescent probes for imaging membrane order. PLoS ONE 2013, 8, e52960. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, R.; Sun, Y.; Tian, M.; Zhang, G.; Feng, R.; Li, X.; Yu, X.; He, X. Styrylpyridine salts-based red emissive two-photon turn-on probe for imaging the plasma membrane in living cells and tissues. Analyst 2016, 141, 3228–3232. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, T.; Aravintha Siva, M.; Bajaj, K.; Soppina, V.; Kanvah, S. Imaging mitochondria and plasma membrane in live cells using solvatochromic styrylpyridines. J. Photochem. Photobiol. B, Biol. 2020, 203, 111732. [Google Scholar] [CrossRef] [PubMed]
- Heek, T.; Nikolaus, J.; Schwarzer, R.; Fasting, C.; Welker, P.; Licha, K.; Herrmann, A.; Haag, R. An amphiphilic perylene imido diester for selective cellular imaging. Bioconjug. Chem. 2013, 24, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Collot, M.; Boutant, E.; Lehmann, M.; Klymchenko, A.S. BODIPY with Tuned Amphiphilicity as a Fluorogenic Plasma Membrane Probe. Bioconjug. Chem. 2019, 30, 192–199. [Google Scholar] [CrossRef]

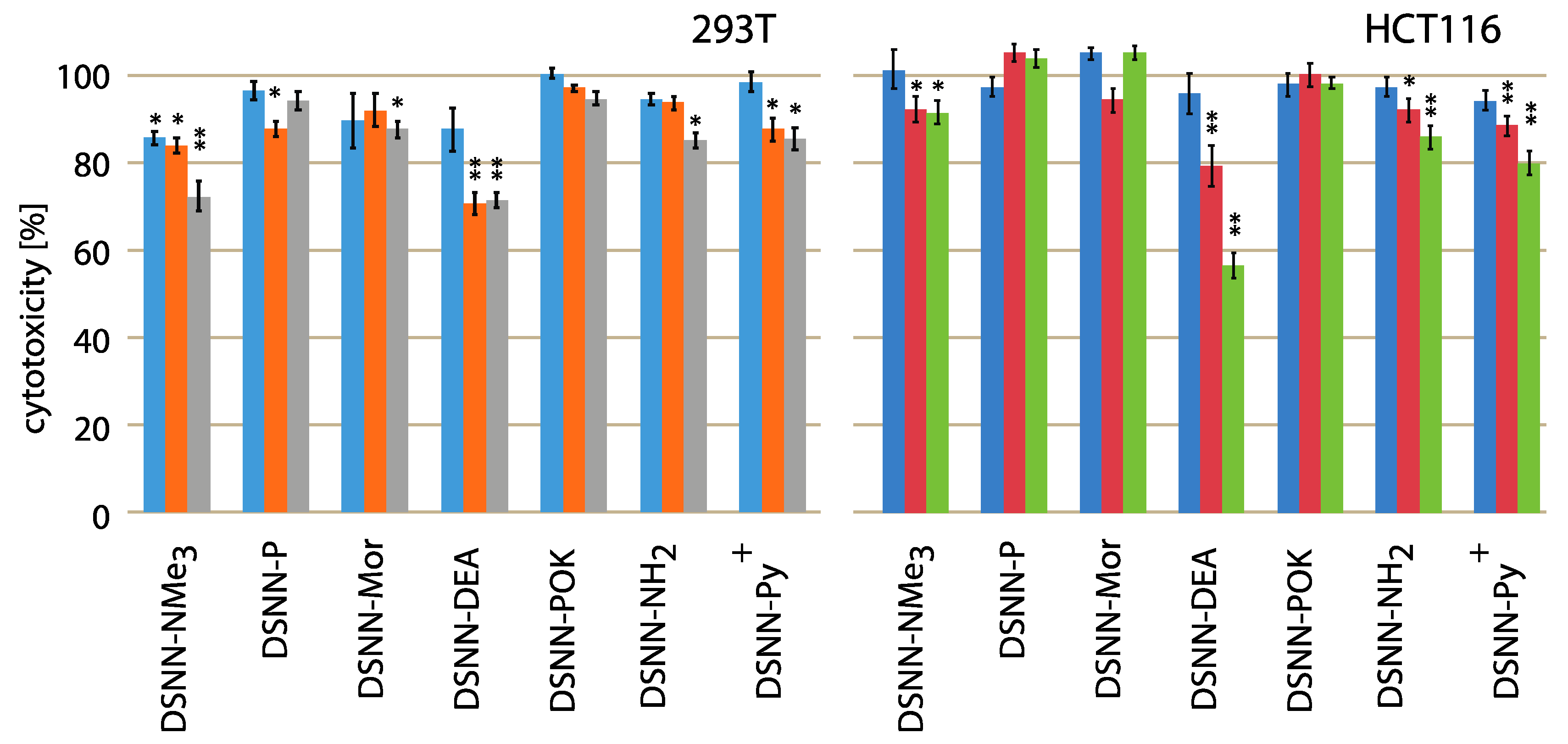
| Compound | Concentration [μM] | Cytotoxicity in Individual Cell Lines [%] | |||||
|---|---|---|---|---|---|---|---|
| MDM | NIH/3T3 | 293T | Fibroblasts | HCT116 | K562 | ||
| DSNN-NMe3+ | 1 | 78.1 ± 0.6 | 96.3 ± 3 | 95.5 ± 2.0 | 56.0 ± 2.8 | 91.9 ± 2.8 | 78.2 ± 2.1 |
| 5 | 48.9 ± 2.8 | 73.0 ± 2.1 | 85.1 ± 3.3 | 54.6 ± 8.5 | 57.5 ± 2.4 | 73.7 ± 1.0 | |
| 10 | 41.2 ± 2.1 | 57.1 ± 2.3 | 77.4 ± 3.5 | 47.2 ± 8.8 | 44.2 ± 1.8 | 64.3 ± 6.2 | |
| DSNN-P | 1 | 111.4 ± 3.0 | 98.2 ± 2.6 | 105.7 ± 3.1 | 83.0 ± 6.9 | 109.0 ± 1.8 | 96.6 ± 5.2 |
| 5 | 105.8 ± 1.7 | 95.5 ± 3.9 | 101.8 ± 3.6 | 90.4 ± 3.2 | 112.7 ± 1.8 | 93.1 ± 1.0 | |
| 10 | 110.5 ± 2.6 | 89.6 ± 2.4 | 103.7 ± 3.6 | 97.6 ± 5.7 | 104.6 ± 2.4 | 89.6 ± 7.9 | |
| DSNN-Mor | 1 | 110.6 ± 1.5 | 96.4 ± 3.1 | 99.7 ± 3.3 | 96.9 ± 5.7 | 95.3 ± 2.0 | 98.7 ± 0.3 |
| 5 | 104.2 ± 2.9 | 99.5 ± 1.4 | 104.8 ± 3.0 | 106.1 ± 4.6 | 116.1 ± 3.5 | 94.4 ± 1.3 | |
| 10 | 115.0 ± 5.3 | 95.0 ± 3.7 | 99.9 ± 3.2 | 99.3 ± 7.0 | 90.6 ± 2.8 | 78.8 ± 0.8 | |
| DSNN-DEA | 1 | 97.8 ± 1.9 | 80.1 ± 1.8 | 98.0 ± 2.7 | 86.4 ± 6.7 | 107.1 ± 1.7 | 101.9 ± 2.4 |
| 5 | 35.3 ± 3.6 | 36.9 ± 2.3 | 81.0 ± 2.8 | 94.6 ± 12.9 | 73.1 ± 1.7 | 54.0 ± 2.3 | |
| 10 | 24.7 ± 3.1 | 39.3 ± 5.4 | 72.0 ± 4.7 | 59.9 ± 5.9 | 20.4 ± 1.8 | 49.2 ± 0.8 | |
| DSNN-POK | 1 | 86.0 ± 2.2 | 99.1 ± 4.6 | 102.5 ± 2.1 | 149.4 ± 4.6 | 102.6 ± 3.0 | 90.8 ± 0.7 |
| 5 | 89.8 ± 5.4 | 100.3 ± 3.8 | 104.4 ± 3.8 | 146.0 ± 9.7 | 101.2 ± 6.4 | 85.0 ± 2.4 | |
| 10 | 81.7 ± 2.8 | 90.0 ± 3.4 | 109.0 ± 3.5 | 111.2 ± 6.4 | 82.6 ± 2.4 | 85.6 ± 0.4 | |
| DSNN-NH2 | 1 | 76.0 ± 1.9 | 94.8 ± 3.5 | 96.9 ± 3.3 | 118.8 ± 4.7 | 95.5 ± 3.1 | 102.8 ± 5.0 |
| 5 | 43.8 ± 0.9 | 70.7 ± 2.8 | 94.9 ± 2.5 | 87.8 ± 5.0 | 72.5 ± 2.2 | 107.8 ± 3.5 | |
| 10 | 47.2 ± 3.2 | 59.8 ± 3.0 | 92.2 ± 2.2 | 80.1 ± 6.2 | 48.0 ± 2.6 | 107.4 ± 0.4 | |
| DSNN-Py+ | 1 | 49.0 ± 0.6 | 80.6 ± 3.0 | 96.8 ± 3.3 | 117.2 ± 6.4 | 81.0 ± 3.4 | 108.6 ± 5.8 |
| 5 | 41.1 ± 3.8 | 68.4 ± 2.0 | 91.1 ± 2.6 | 117.5 ± 9.5 | 51.7 ± 2.7 | 78.7 ± 2.6 | |
| 10 | 24.5 ± 1.5 | 52.6 ± 2.7 | 84.0 ± 2.2 | 91.7 ± 9.9 | 38.4 ± 2.3 | 59.5 ± 2.3 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suwara, J.; Lukasik, B.; Zurawinski, R.; Pawlowska, R.; Chworos, A. Highly Fluorescent Distyrylnaphthalene Derivatives as a Tool for Visualization of Cellular Membranes. Materials 2020, 13, 951. https://doi.org/10.3390/ma13040951
Suwara J, Lukasik B, Zurawinski R, Pawlowska R, Chworos A. Highly Fluorescent Distyrylnaphthalene Derivatives as a Tool for Visualization of Cellular Membranes. Materials. 2020; 13(4):951. https://doi.org/10.3390/ma13040951
Chicago/Turabian StyleSuwara, Justyna, Beata Lukasik, Remigiusz Zurawinski, Roza Pawlowska, and Arkadiusz Chworos. 2020. "Highly Fluorescent Distyrylnaphthalene Derivatives as a Tool for Visualization of Cellular Membranes" Materials 13, no. 4: 951. https://doi.org/10.3390/ma13040951
APA StyleSuwara, J., Lukasik, B., Zurawinski, R., Pawlowska, R., & Chworos, A. (2020). Highly Fluorescent Distyrylnaphthalene Derivatives as a Tool for Visualization of Cellular Membranes. Materials, 13(4), 951. https://doi.org/10.3390/ma13040951






