Chitosan-TiO2: A Versatile Hybrid Composite
Abstract
1. Introduction
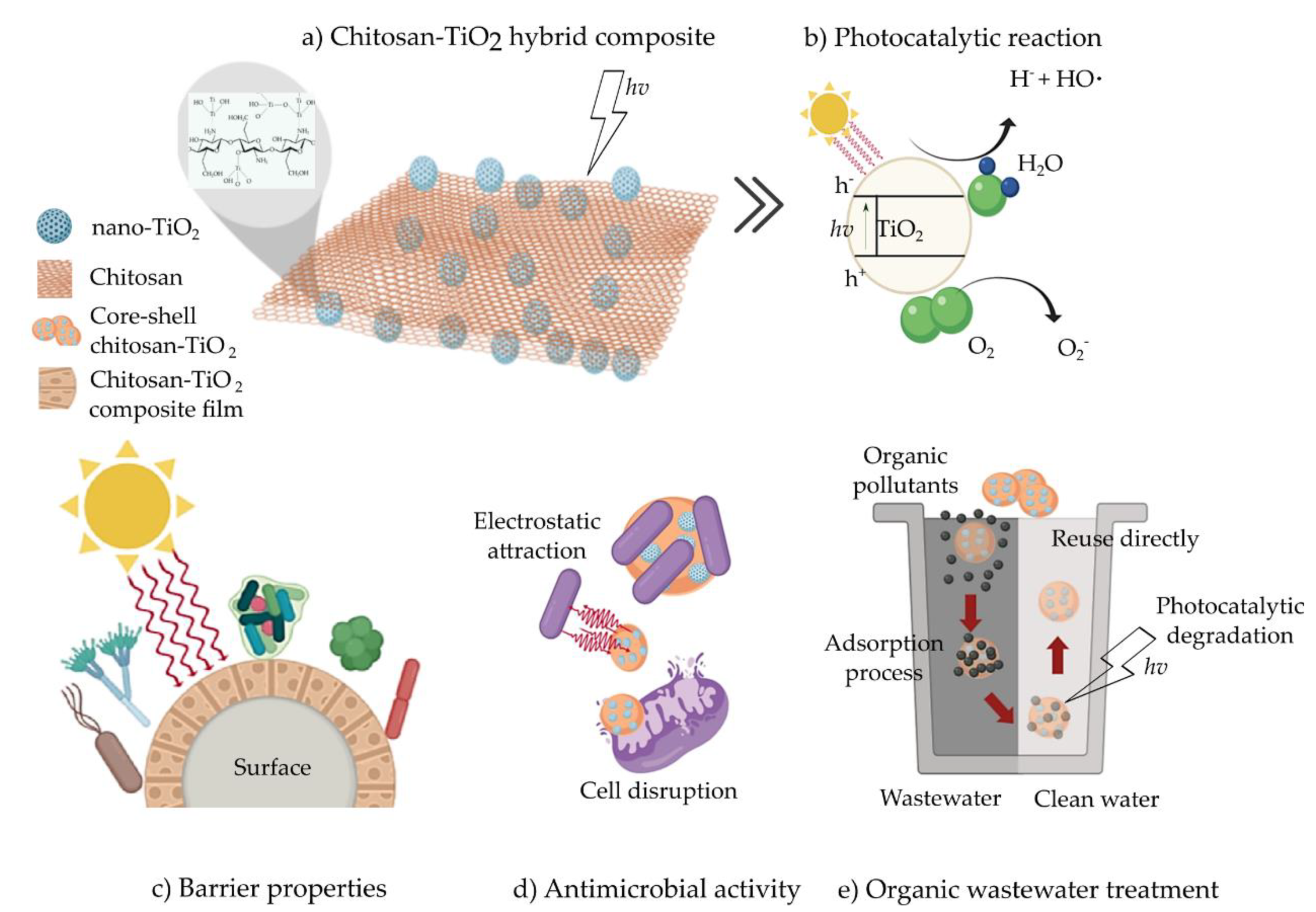
2. Hybrid Composites
3. Chitosan–TiO2 (CS–TiO2) Composite
4. Applications of CS–TiO2 Composite
4.1. Antimicrobial Activity
4.2. Environmental Applications
4.3. Biomedical Applications
4.4. Food Preservation Applications
4.5. Other Applications
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Müller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Sanz, Y.E.; Lagaron, J.M.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Bölz, U.; et al. Review on the processing and properties of polymer nanocomposites and nanocoatings and their applications in the packaging, automotive and solar energy fields. Nanomaterials 2017, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Chen, B.Y.; Den, W. Chitosan as a natural polymer for heterogeneous catalysts support: A short review on its applications. Appl. Sci. 2015, 5, 1272–1283. [Google Scholar] [CrossRef]
- Bui, V.K.H.; Park, D.; Lee, Y.C. Chitosan combined with ZnO, TiO2 and Ag nanoparticles for antimicrobial wound healing applications: A mini review of the research trends. Polymers 2017, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.S.; Das, U.; Kumar, A.; Bissoyi, A.; Singh, A.K. Chitosan/TiO2 composite membrane improves proliferation and survival of L929 fibroblast cells: Application in wound dressing and skin regeneration. Int. J. Biol. Macromol. 2017, 98, 329–340. [Google Scholar] [CrossRef]
- Kaewklin, P.; Siripatrawan, U.; Suwanagul, A.; Lee, Y.S. Active packaging from chitosan-titanium dioxide nanocomposite film for prolonging storage life of tomato fruit. Biol. Macromol. 2018, 112, 523–529. [Google Scholar] [CrossRef]
- Xiao, G.; Zhang, X.; Zhang, W.; Zhang, S.; Su, H.; Tan, T. Visible-light-mediated synergistic photocatalytic antimicrobial effects and mechanism of Ag-nanoparticles@chitosan–TiO2 organic–inorganic composites for water disinfection. Appl. Catal. B 2015, 170, 255–262. [Google Scholar] [CrossRef]
- Dedloff, M.R.; Effler, C.S.; Holban, A.M.; Gestal, M.C. Use of biopolymers in mucosally-administered vaccinations for respiratory disease. Materials 2019, 12, 2445. [Google Scholar] [CrossRef]
- Zainal, Z.; Hui, L.K.; Hussein, M.Z.; Abdullah, A.H.; Hamadneh, I.R. Characterization of TiO2–Chitosan/Glass photocatalyst for the removal of a monoazo dye via photodegradation–adsorption process. J. Hazard. Mater. 2009, 164, 138–145. [Google Scholar] [CrossRef]
- Li, B.; Yang, Z.; Yang, Y.; Wen, Q.; Wang, X.; Liu, B.; Wang, Y.; Sun, G. Synthesis, characterization, and antibacterial activity of chitosan/TiO2 nanocomposite against Xanthomonas oryzae pv. oryzae. Carbohydr. Polym. 2016, 152, 825–831. [Google Scholar] [CrossRef]
- Raut, A.V.; Yadav, H.M.; Gnanamani, A.; Pushpavanam, S.; Pawar, S.H. Synthesis and characterization of chitosan-TiO2:Cu nanocomposite and their antimicrobial activity with visible light. Colloids Surf. B 2016, 148, 566–575. [Google Scholar] [CrossRef]
- El-Wakil, N.A.; Hassan, E.A.; Abou-Zeid, R.E.; Dufrense, A. Development of wheat gluten/nanocellulose/titanium dioxide nanocomposites for active food packaging. Carbohydr. Polym. 2015, 124, 337–346. [Google Scholar] [CrossRef]
- Catauro, M.; Tranquillo, E.; Dal Poggetto, G.; Pasquali, M.; Dell’Era, A.; Vecchio Ciprioti, S. Influence of the heat treatment on the particle size and on the crystalline phase of TiO2 synthetized by the Sol-gel method. Materials 2018, 11, 2364. [Google Scholar] [CrossRef]
- Ali, H.M.; Babar, H.; Shah, T.Z.; Sajid, M.U.; Qasim, M.A.; Javed, S. Preparation techniques of TiO2 nanofluids and challenges: A review. Appl. Sci. 2018, 8, 587. [Google Scholar]
- Anaya-Esparza, L.M.; Montalvo-González, E.; González-Silva, N.; Méndez-Robles, M.D.; Romero-Toledo, R.; Yahia, E.M.; Pérez-Larios, A. Synthesis and characterization of TiO2-ZnO-MgO mixed oxide and their antibacterial activity. Materials 2019, 12, 698. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Visurraga, J.; Meléndrez, M.F.; García, A.; Paulraj, M.; Cárdenas, G. Semitransparent chitosan-TiO2 nanotubes composite film for food package applications. J. Appl. Polym. Sci. 2009, 116, 3503–3515. [Google Scholar]
- Saravanan, R.; Aviles, J.; Gracia, F.; Mosquera, E.; Gupta, V.K. Crystallinity and lowering band gap induced visible light photocatalytic activity of TiO2/CS (Chitosan) nanocomposites. Int. J. Biol. Macromol. 2018, 109, 1239–1245. [Google Scholar] [CrossRef]
- Razzaz, A.; Ghorban, S.; Hosayni, L.; Irani, M.; Aliabadi, M. Chitosan nanofibers functionalized by TiO2 nanoparticles for the removal of heavy metal ions. J. Taiwan Inst. Chem. Eng. 2015, 58, 333–343. [Google Scholar] [CrossRef]
- Arjunan, N.; Kumari, H.L.J.; Singaravelu, C.M.; Kandasamy, R.; Kandasamu, J. Physicochemical investigations of biogenic chitosan-silver nanocomposite as antimicrobial and anticancer agent. Int. J. Biol. Macromol. 2016, 92, 77–87. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Guibal, E. Arsenic(V) sorption using chitosan/Cu(OH)2 and chitosan/CuO composite sorbents. Carbohydr. Polym. 2015, 134, 190–204. [Google Scholar] [CrossRef]
- Liu, Y.; Kim, H.I. Characterization and antibacterial properties of genipin-crosslinked chitosan/poly(ethylene glycol)/ZnO/Ag nanocomposites. Carbohydr. Polym. 2012, 89, 111–116. [Google Scholar] [CrossRef]
- Li, L.H.; Deng, J.C.; Deng, H.R.; Liu, Z.L.; Li, X.L. Preparation, characterization and antimicrobial activities of chitosan/Ag/ZnO blend films. Chem. Eng. J. 2010, 160, 378–382. [Google Scholar] [CrossRef]
- Miyazaki, T.; Ishikawa, K.; Shirosaki, Y.; Ohtsuki, C. Organic–Inorganic composites designed for biomedical applications. Biol. Pharm. Bull. 2013, 36, 1670–1675. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.A.M.; Panhius, M.I.H. Reinforced materials based on chitosan, TiO2 and Ag composites. Polymers 2012, 4, 590–599. [Google Scholar] [CrossRef]
- Ahmadi, R.; Tanomand, A.; Kazeminava, F.; Kamounah, F.S.; Ayaseh, A.; Yousefi, M.; Katourani, A.; Yousefi, B.; Kafil, H.S. Fabrication and characterization of a titanium dioxide (TiO2) nanoparticles reinforced bio-nanocomposite containing Miswak (Salvadora persica L.) extract – the antimicrobial, thermo-physical and barrier properties. Int. J. Nanomed. 2019, 14, 3439–3454. [Google Scholar] [CrossRef] [PubMed]
- Fathi, N.; Almasi, H.; Pirouzifard, M.K. Sesame protein isolate based bionanocomposite films incorporated with TiO2 nanoparticles: Study on morphological, physical and photocatalytic properties. Polym. Test. 2019, 77, 105919. [Google Scholar] [CrossRef]
- Kochkina, N.E.; Butikova, O.A. Effect of fibrous TiO2 filler on the structural, mechanical, barrier and optical characteristics of biodegradable maize starch/PVA composite films. Biol. Macromol. 2019, 139, 431–439. [Google Scholar] [CrossRef] [PubMed]
- González-Calderon, J.A.; Vallejo-Montesinos, J.; Martínez-Martínez, H.N.; Cerecero-Enríquez, R.; López-Zamora, L. Effect of chemical modification of titanium dioxide particles via silanization under properties of chitosan/potato starch film. Rev. Mex. Ing. Química 2019, 18, 913–927. [Google Scholar]
- Li, W.; Zheng, K.; Chen, H.; Feng, S.; Wang, W.; Qin, C. Influence of nano titanium dioxide and clove oil on chitosan–starch film characteristics. Polymers 2019, 11, 1418. [Google Scholar] [CrossRef]
- Hamad, H.; Bailón-García, E.; Morales-Torres, S.; Carrasco-Marín, F.; Pérez-Cardenas, A.; Maldonado-Hódar, F.J. Physicochemical properties of new Cellulose-TiO2 composites for the removal of water pollutants: Developing specific interactions and performances by cellulose functionalization. J. Environ. Chem. Eng. 2018, 6, 5032–5041. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Ye, S.; Song, X. Construction of Bi2WO6–TiO2/starch nanocomposite films for visible-light catalytic degradation of ethylene. Food Hydrocoll. 2018, 88, 92–100. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Kim, S.S.; Lee, J.; Lee, J. Effect of TiO2 on highly elastic, stretchable UV protective nanocomposite films formed by using a combination of k-Carrageenan, xanthan gum and gellan gum. Biol. Macromol. 2018, 123, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Gjipalaj, J.; Alessandri, I. Easy recovery, mechanical stability, enhanced adsorption capacity and recyclability of alginate-TiO2 macrobead photocatalysts for water treatment. J. Environ. Chem. Eng. 2017, 5, 1763–1770. [Google Scholar] [CrossRef]
- Oleyaei, S.A.; Zahedi, Y.; Ghanbarzadeh, B.; Maoyedi, A.A. Modification of physicochemical and thermal properties of starchfilms by incorporation of TiO2 nanoparticles. Int. J. Biol. Macromol. 2016, 89, 256–264. [Google Scholar] [CrossRef]
- Zheng, P.; Du, Y.; Chang, P.R.; Ma, X. Amylose–halloysite–TiO2 composites: Preparation, characterization and photodegradation. Appl. Surf. Sci. 2015, 329, 256–261. [Google Scholar] [CrossRef]
- Sreekumar, P.A.; Al-Hartchi, M.A.; De, S.K. Reinforcement of starch/polyvinyl alcohol blend using nano-titanium dioxide. J. Compos. Mater. 2012, 46, 3181–3187. [Google Scholar] [CrossRef]
- Rani, A.A.D.; Ramachandran, R.; Chennazhi, K.P.; Tamura, H.; Nair, S.V.; Jayakumar, R. Fabrication of alginate/nanoTiO2 needle composite scaffolds for tissue engineering applications. Carbohydr. Polym. 2011, 83, 858–864. [Google Scholar] [CrossRef]
- Buzarovska, A.; Grozdanov, A. Biodegradable Poly(L-lactic acid)/TiO2 nanocomposites: Thermal properties and degradation. J. Appl. Polym. Sci. 2012, 123, 2187–2193. [Google Scholar] [CrossRef]
- Zhou, J.J.; Wang, S.Y.; Gunasekaran, S. Preparation and characterization of whey protein film incorporated with TiO2 nanoparticles. J. Food Sci. 2009, 74, N50–N56. [Google Scholar] [CrossRef]
- Zhang, X.; Su, H.; Zhao, Y.; Tan, T. Antimicrobial activities of hydrophilic polyurethane/titanium dioxide complex film under visible light irradiation. J. Photochem. Photobiol. A 2008, 199, 123–129. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Kaewklin, P. Fabrication and characterization of chitosan-titanium dioxide nanocomposite film as ethylene scavenging and antimicrobial active food packaging. Food Hydrocoll. 2018, 84, 125–134. [Google Scholar] [CrossRef]
- Xiao, G.; Zhang, X.; Zhao, Y.; Su, H.; Tan, T. The behavior of active bactericidal and antifungal coating undervisible light irradiation. Appl. Surf. Sci. 2014, 292, 756–763. [Google Scholar] [CrossRef]
- Qian, T.; Su, H.; Tan, T. The bactericidal and mildew-proof activity of a TiO2–chitosan composite. J. Photochem. Photobiol. A 2011, 218, 130–136. [Google Scholar] [CrossRef]
- Raddaha, N.S.J.; Seuss, S.; Boccaccini, A. Study of the electrophoretic deposition of chitosan/halloysite nanotubes/titanium dioxide composite coatings using Taguchi experimental design approach. Key Eng. Mater. 2015, 654, 230–239. [Google Scholar] [CrossRef]
- Lin, B.; Luo, Y.; Teng, Z.; Zhang, B.; Zhou, B.; Wang, Q. Development of silver/titanium dioxide/chitosan adipate nanocomposite as an antibacterial coating for fruit storage. LWT Food Sci. Technol. 2015, 63, 1206–1213. [Google Scholar] [CrossRef]
- Nithya, A.; Jothivenkatachalam, K. Visible light assisted TiO2-chitosan composite for removal of reactive dye. J. Environ. Nanotechnol. 2014, 3, 20–26. [Google Scholar]
- Kavitha, K.; Prabhu, M.; Rajendran, V.; Manivasankan, P.; Prabu, P.; Jayakumar, T. Optimization of nano-titania and titania–chitosan nanocomposite to enhance biocompatibility. Curr. Nanosci. 2013, 9, 308–317. [Google Scholar] [CrossRef]
- Jayakumar, R.; Ramachandran, R.; Divyarani, V.V.; Chennazhi, K.P.; Tamura, K.P.; Nair, S.V. Fabrication of chitin–chitosan/nano TiO2-composite scaffolds for tissue engineering applications. Int. J. Biol. Macromol. 2011, 48, 336–344. [Google Scholar] [CrossRef]
- Habiba, U.; Joo, T.C.; Siddique, T.A.; Salleh, A.; Ang, B.C.; Afifi, A.M. Effect of Degree of Deacetylation of chitosan on adsorption capacity and reusability of Chitosan/Polyvinylalcohol/TiO2 Nano Composite. Int. J. Biol. Macromol. 2017, 104, 1133–1142. [Google Scholar] [CrossRef]
- Archana, D.; Dutta, J.; Dutta, P.K. Evaluation of chitosan nano dressing for wound healing: Characterization, in vitro and in vivo studies. Int. J. Biol. Macromol. 2013, 57, 193–203. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, W.; Liu, X.; Zhang, W. Synthesis of titanium cross-linked chitosan composite for efficient adsorption and detoxification of hexavalent chromium from water. J. Mater. Chem. A 2015, 3, 331–340. [Google Scholar] [CrossRef]
- Wiacek, A.E.; Godzecka, A.; Malgortzata, J. Physicochemical characteristics of chitosan–TiO2 biomaterial. 1. Stability and swelling properties. Ind. Eng. Chem. Res. 2018, 57, 1859–1870. [Google Scholar] [CrossRef]
- Vallejo-Montesinos, J.; Gámez-Cordero, J.; Zarraga, R.; Pérez-Pérez, M.C.; González-Calderon, J.A. Influence of the surface modification of titanium dioxide nanoparticles TiO2 under efficiency of silver nanodots deposition and its effect under the properties of starch–chitosan (SC) films. Polym. Bull. 2020, 77, 107–133. [Google Scholar] [CrossRef]
- Cano, L.; Pollet, E.; Avérous, L.; Tercjak, A. Effect of TiO2 nanoparticles on the properties of thermoplastic chitosan-based nano-biocomposites obtained by mechanical kneading. Compos. Part A Appl. Sci. Manfacturing 2016, 93, 33–40. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr. Polym. 2017, 169, 101–107. [Google Scholar] [CrossRef]
- Qu, L.; Chen, G.; Dong, S.; Huo, Y.; Yin, Z.; Li, S.; Chen, Y. Improved mechanical and antimicrobial properties of zein/chitosan films by adding highly dispersed nano-TiO2. Ind. Crop. Prod. 2019, 130, 450–458. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Shi, H.; Wang, X.; Qi, K.; Zhou, X.; Xin, J.H. Carboxymethyl chitosan coating to block photocatalytic activity of TiO2 nanoparticles. Text. Res. J. 2010, 80, 2214–2220. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Yong, H.; Qin, Y.; Liu, J.; Liu, J. Development of multifunctional food packaging films based on chitosan, TiO2 nanoparticles and anthocyanin-rich black plum peel extract. Food Hydrocoll. 2019, 94, 84–92. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Zeng, J.; Zhang, F.; Zhou, K.; Bowen, C.R.; Zhang, D. Aligned macroporous TiO2/chitosan/reduced graphene oxide (rGO)composites for photocatalytic applications. Appl. Surf. Sci. 2017, 424, 170–176. [Google Scholar] [CrossRef]
- Peng, C.C.; Yang, M.H.; Chiu, W.T.; Chiu, C.H.; Yang, C.S.; Chen, Y.W.; Chen, K.C.; Peng, R.Y. Composite nano-titanium oxide–chitosan artificial skin exhibits strong wound-healing effect—An approach with anti-inflammatory and bactericidal kinetics. Macromol. Biosci. 2008, 8, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Lian, Z.; Zhang, Y.; Zhao, Y. Nano-TiO2 particles and high hydrostatic pressure treatment for improving functionality of polyvinyl alcohol and chitosan composite films and nano-TiO2 migration from film matrix in food simulants. Innov. Food Sci. Emerg. Technol. 2015, 33, 145–153. [Google Scholar] [CrossRef]
- Tang, Y.; Hu, X.; Zhang, X.; Guo, D.; Zhang, J.; Kong, F. Chitosan/titanium dioxide nanocomposite coatings: Rheological behavior and surface application to cellulosic paper. Carbohydr. Polym. 2016, 151, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Al-Sagheer, F.A.; Merchant, S. Visco-elastic properties of chitosan–titania nano-composites. Carbohydr. Polym. 2011, 85, 356–362. [Google Scholar] [CrossRef]
- Norranattrakul, P.; Siralertmukul, K.; Nuisin, R. Fabrication of chitosan/titanium dioxide composites film for the photocatalytic degradation of dye. J. Met. Mater. Min. 2013, 23, 9–22. [Google Scholar]
- Kamal, T.; Yasir, A.; Bahadar, K.S.; Saeed, C.M.T.; Abdullah, A. Dye adsorption and bactericidal properties of TiO2/Chitosan coating layer. Carbohydr. Polym. 2016, 148, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Alvarado, E.; Peña-Juárez, M.; Pérez-Pérz, C.; Pérez, E.; González-Calderon, A. Improvement in the dispersion of TiO2 particles inside Chitosan-Methyl cellulose. J. Mex. Chem. Soc. 2019, 63, 154–168. [Google Scholar] [CrossRef]
- Brasselet, C.; Pierre, G.; Dubessay, P.; Dols-Lafargue, M.; Coulon, J.; Maupeu, J.; Vallet-Courbin, A.; de Baynast, H.; Doco, T.; Michaud, P.; et al. Modification of chitosan for the generation of functional derivatives. Appl. Sci. 2019, 9, 1321. [Google Scholar] [CrossRef]
- Alex, M.J.; Periasamy, P.; Mohan, K.; Sekar, S.; Kandiah, K.; Prabha, S.; Venkatachalam, R. In situ synthesised TiO2-chitosan-chondroitin 4–sulphate nanocomposites for bone implant applications. IET Nanobiotechnol. 2016, 10, 107–113. [Google Scholar] [CrossRef]
- Kavitha, K.; Sutha, S.; Prabhu, M.; Rajendran, V.; Jayakumar, T. In situ synthesized novel biocompatible titania–chitosan nanocomposites with high surface area and antibacterial activity. Carbohydr. Polym. 2013, 93, 731–739. [Google Scholar] [CrossRef]
- Alagumuthu, G.; Kumar, A. Synthesis and characterization of chitosan/TiO2 nanocomposites using liquid phase deposition technique. Int. J. Nanosci. Nanotechnol. 2013, 4, 105–111. [Google Scholar]
- Huang, K.S.; Grumezescu, A.M.; Chang, C.Y.; Yang, C.H.; Wang, C.Y. Immobilization and stabilization of TiO2 nanoparticles in alkaline solidificated chitosan spheres without cross-linking agent. Int. J. Latest Res. Sci. Technol. 2014, 3, 174–178. [Google Scholar]
- Habiba, U.; Islam, M.d.S.; Siddique, T.A.; Afifi, A.M.; Ang, B.C. Adsorption and photocatalytic degradation of anionic dyes onChitosan/PVA/Na–Titanate/TiO2composites synthesized by solution casting method. Carbohydr. Polym. 2016, 149, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, B.; Xia, K.; Li, Y.; Han, J.; Zhao, C. Enhanced antibacterial activity of silver doped titanium dioxide-chitosan composites under visible light. Materials 2018, 11, 1403. [Google Scholar] [CrossRef]
- Chen, A.; Zang, G.; Chen, G.; Hu, X.; Yan, M.; Guan, S.; Shang, C.; Lu, L.; Zou, Z.; Xie, G. Novel thiourea-modified magnetic ion-imprinted chitosan/TiO2 composite for simultaneous removal of cadmium and 2,4-dichlorophenol. Chem. Eng. J. 2012, 191, 85–94. [Google Scholar] [CrossRef]
- Haldorai, Y.; Shim, J.J. Novel chitosan-TiO2 nanohybrid: Preparation, characterization, antibacterial, and photocatalytic properties. Polym. Compos. 2014, 1, 327–333. [Google Scholar] [CrossRef]
- Farzana, M.H.; Meenakshi, S. Photocatalytic aptitude of titanium dioxide impregnated chitosan beads for the reduction of Cr(VI). Int. J. Biol. Macromol. 2014, 72, 1265–1271. [Google Scholar] [CrossRef]
- Mohandas, A.; Deepthi, S.; Biswas, R.; Jayakumar, R. Chitosan based metallic nanocomposite scaffolds as antimicrobial wound dressings. Bioact. Mater. 2017, 3, 267–277. [Google Scholar] [CrossRef]
- Zhu, H.; Jiang, R.; Xiao, L.; Chang, Y.; Guan, Y.; Li, X.; Zeng, G. Photocatalytic decolorization and degradation of Congo Red on innovative crosslinked chitosan/nano-CdS composite catalyst under visible light irradiation. J. Hazard. Mater. 2009, 169, 933–940. [Google Scholar] [CrossRef]
- Kim, T.Y.; Lee, Y.H.; Park, K.H.; Kim, S.J.; Cho, S.Y. A study of photocatalysis of TiO2 coated onto chitosan based and activated carbon. Res. Chem. Int. 2005, 31, 343–358. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, X.; Su, H.; Tan, T. Antibacterial and anti-mildew behavior of chitosan/nano-TiO2 composite emulsion. Korean J. Chem. Eng. 2008, 25, 1434–1438. [Google Scholar] [CrossRef]
- Xu, W.; Xie, W.; Huang, X.; Chen, X.; Huang, N.; Wang, X.; Liu, J. The graphene oxide and chitosan biopolymer loads TiO2 for antibacterial and preservative research. Food Chem. 2017, 221, 267–277. [Google Scholar] [CrossRef]
- Li, Y.S.; Han, Y.; Qin, J.T.; Song, Z.Y.; Cai, H.H.; Du, J.F.; Sun, S.F.; Liu, Y. Photosensitive antibacterial and cytotoxicity performances of a TiO2/carboxymethyl chitosan/poly(vinyl alcohol) nanocomposite hydrogel by in situ radiation construction. J. Appl. Polym. Sci. 2016, 133, 44150. [Google Scholar] [CrossRef]
- Longo, V.M.; Picon, F.C.; Zamperini, C.; Albuquerque, A.R.; Sambrano, J.R.; Vergani, C.E.; Machado, A.L.; Andrés, J.; Hernandes, A.C.; Varela, J.A.; et al. Experimental and theoretical approach of nanocrystalline TiO2 with antifungal activity. Chem. Phys. Lett. 2013, 577, 114–120. [Google Scholar] [CrossRef]
- Yazadani, M.; Bhatnagar, A.; Vahala, R. Synthesis, characterization and exploitation of nano-TiO2/feldspar-embedded chitosan beads towards UV-assisted adsorptive abatement of aqueous arsenic (As). Chem. Eng. J. 2017, 316, 370–382. [Google Scholar] [CrossRef]
- Zhu, H.; Jiang, R.; Xiao, L.; Liu, L.; Cao, C.; Zeng, G. CdS nanocrystals/TiO2/crosslinked chitosan composite: Facile preparation, characterization and adsorption-photocatalytic properties. Appl. Surf. Sci. 2013, 273, 661–669. [Google Scholar] [CrossRef]
- Miller, S.M.; Spaulding, M.L.; Zimmerman, J.B. Optimization of capacity and kinetics for a novel bio-based arsenic sorbent, TiO2-impregnated chitosan bead. Water Res. 2011, 45, 5745–5754. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Su, H.; Tan, T. Synthesis of ion-imprinted chitosan-TiO2 adsorbent and its multi-functional performances. Biochem. Eng. J. 2008, 38, 212–218. [Google Scholar] [CrossRef]
- Pincus, L.N.; Melnikov, F.; Yamani, J.S.; Zimmerman, J.B. Multifunctional photoactive and selective adsorbent for arsenite and arsenate: Evaluation of nano titanium dioxide-enabled chitosan cross-linked with copper. J. Hazard. Mater. 2018, 358, 145–154. [Google Scholar] [CrossRef]
- Dhanya, A.; Aparana, K. Synthesis and evaluation of TiO2/chitosan based hydrogel for the adsorptional photocatalytic degradation of Azo and Anthraquinone dye under UV light irradiation. Procedia Technol. 2016, 24, 611–618. [Google Scholar] [CrossRef]
- Xiao, G.; Zhao, Y.; Li, L.; Pratt, J.O.; Su, H.; Tan, T. Facile synthesis of dispersed Ag nanoparticles on chitosan-TiO2 composites as recyclable nanocatalysts for 4-nitrophenol reduction. Nanotechnology 2018, 29, 1–9. [Google Scholar] [CrossRef]
- Wu, S.; Kan, J.; Dai, X.; Shen, X.; Zhang, K.; Zhu, M. Ternary carboxymethyl chitosan-hemicellulose-nanosized TiO2 composite as effective adsorbent for removal of heavy metal contaminants from water. Fibers Polym. 2017, 18, 22–32. [Google Scholar] [CrossRef]
- Xie, Z.; Fei, J.; Huang, M. Differential pulse anodic stripping voltammetric determination of Cadmium(II) at a glassy carbon electrode modified with a nano-TiO2/chitosan composite film. Aust. J. Chem. 2008, 61, 1000–1005. [Google Scholar] [CrossRef]
- Zhou, J.; Hao, B.; Wang, L.; Ma, J. Preparation and characterization of nano-TiO2/chitosan/poly(N-isopropylacrylamide) composite hydrogel and its application for removal of ionic dyes. Sep. Purif. Technol. 2016, 176, 193–199. [Google Scholar] [CrossRef]
- Alizadeh, B.; Delnavaz, M.; Shakeri, A. Removal of Cd(II) and phenol using novel cross-linked magnetic EDTA/chitosan/TiO2 nanocomposite. Carbohydr. Polym. 2017, 181, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Samsudin, E.M.; Mun, L.K.; Julkapli, N.M.; Hamid, S.B.A. Room temperature synthesis of TiO2 supported chitosan photocatalyst: Study on physicochemical and adsorption photo-decolorization properties. Mater. Res. Bull. 2017, 86, 22–29. [Google Scholar] [CrossRef]
- Liu, L.F.; Zhang, P.H.; Yang, F.L. Adsorptive removal of 2,4-DCP from water by fresh or regenerated chitosan/ACF/TiO2 membrane. Sep. Purif. Technol. 2010, 70, 354–361. [Google Scholar] [CrossRef]
- Miller, S.M.; Zimmerman, J.B. Novel, bio-based, photoactive arsenic sorbent: TiO2-impregnated chitosan bead. Water Res. 2010, 44, 5722–5729. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jiang, R.; Fu, Y.; Guan, Y.; Yao, J.; Xiao, L.; Zeng, G. Effective photocatalytic decolorization of methyl orange utilizing TiO2/ZnO/chitosan nanocomposite films under simulated solar irradiation. Desalination 2012, 286, 41–48. [Google Scholar] [CrossRef]
- Huang, K.J.; Li, J.; Wu, Y.Y.; Liu, Y.M. Amperometric immunobiosensor for α-fetoprotein using Au nanoparticles/chitosan/TiO2–graphene composite based platform. Biolectrochemistry 2013, 90, 18–23. [Google Scholar] [CrossRef]
- Al-Mokaram, A.M.A.A.A.; Yahya, R.; Abdi, M.M.; Mahmud, H.N.M.E. The Development of non-enzymatic glucose biosensors based on electrochemically prepared polypyrrole–chitosan–titanium dioxide nanocomposite films. Nanomaterials 2017, 7, 129. [Google Scholar] [CrossRef]
- Zhang, M.; Yuan, R.; Chai, Y.; Li, W.; Zhong, H.; Wang, C. Glucose biosensor based on titanium dioxide-multiwall carbon nanotubes-chitosan composite and functionalized gold nanoparticles. Bioprocess. Biosyst. Eng. 2011, 34, 1143–1150. [Google Scholar] [CrossRef]
- Woo, C.H.; Choi, Y.C.; Choi, J.S.; Lee, H.Y.; Cho, Y.W. A bilayer composite composed of TiO2-incorporated electrospun chitosan membrane and human extracellular matrix sheet as a wound dressing. J. Biomater. Sci. 2015, 26, 841–854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, L.; Liu, C.; Feng, X.; Wei, L.; Shao, L. Self-assembly chitosan/gelatin composite coating on icariin-modified TiO2 nanotubes for the regulation of osteoblast bioactivity. Mater. Des. 2016, 92, 471–479. [Google Scholar] [CrossRef]
- Safari, M.; Ghiaci, M.; Jafari-Asl, M.; Ensafi, A.A. Nanohybrid organic-inorganic chitosan/dopamine/TiO2 composites with controlled drug-delivery properties. Appl. Surf. Sci. 2015, 342, 26–33. [Google Scholar] [CrossRef]
- Malhotra, B.D.; Kaushik, A. Metal oxide–chitosan based nanocomposite for cholesterol biosensor. Thin Solid Films 2009, 518, 614–620. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, M.; Xia, L. Effects of chitosan/TiO2 composite coating on keeping-fresh of stauntonvine. Adv. Mater. Res. 2012, 530, 68–73. [Google Scholar] [CrossRef]
- Tian, F.; Chen, W.; Wu, C.E.; Kou, X.; Fan, G.; Li, T.; Wu, Z. Preservation of ginkgo (Ginkgo biloba L.) seeds by coating with chitosan/nano-TiO2 and chitosan/nano-SiO2 films. Int. J. Biol. Macromol. 2018, 126, 917–925. [Google Scholar] [CrossRef] [PubMed]
- García, C.V.; Shin, G.H.; Kim, J.T. Metal oxide-based nanocomposites in food packaging: Applications, migration, and regulations. Trends Food Sci. Technol. 2018, 82, 21–31. [Google Scholar] [CrossRef]
- Hsieh, S.H.; Zhang, F.R.; Li, H.S. Anti-ultraviolet and physical properties of woolen fabrics cured with citric acid and TiO2/chitosan. J. Appl. Polym. Sci. 2006, 100, 4311–4319. [Google Scholar] [CrossRef]
- Balaji, J.; Sethuraman, M.G. Chitosan-doped-hybrid/TiO2 nanocomposite based sol-gel coating for the corrosion resistance of aluminum metal in 3.5% NaCl medium. Int. J. Biol. Macromol. 2017, 104, 1730–1739. [Google Scholar]
- Ledwing, P.; Kot, M.; Moskalewics, T.; Dubiel, B. Electrophoretic deposition of nc-TiO2/chitosan composite coatings on X2CrNiMo17-12-2 stainless steel. Arch. Metall. Mater. 2017, 62, 405–410. [Google Scholar] [CrossRef]
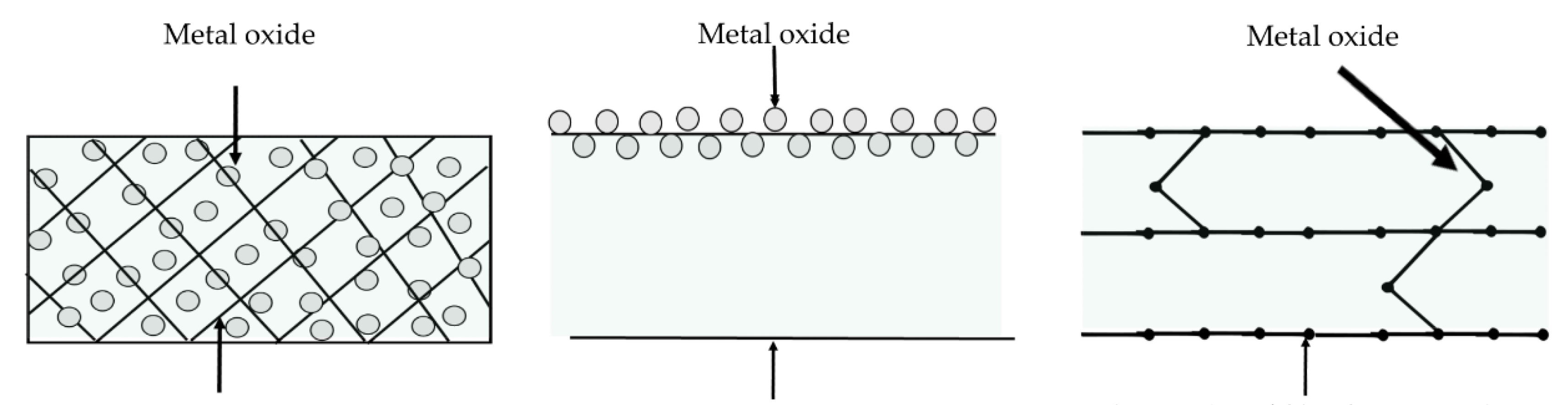
| Material | Application | Ref. |
|---|---|---|
| Corn starch | Bioplastic with potential use as packaging material | [23] |
| Carboxymethyl cellulose containing miswak extract | Nanocomposite with potential use as food packaging | [24] |
| Sesame protein extract | Films for food active packaging applications and photo-decolorization purposes | [25] |
| Corn starch/PVA | Potential application in food and non-food industries as UV shielding packaging materials | [26] |
| Chitosan/potato-starch | Films with potential use as food packaging | [27] |
| Chitosan/starch | Films with potential use as food packaging | [28] |
| Cellulose | Removal of water pollutants | [29] |
| Bi2WO6-TiO2/corn starch | Films with ethylene scavenging activity, potential use for fruits and vegetables preservation | [30] |
| KC/X/G | Potential application in food and non-food industries as UV shielding packaging materials | [31] |
| Alginate | Removal of water pollutants | [32] |
| Potato starch | Films with potential use as food packaging | [33] |
| Amylose-halloysite composite | Composite with potential environmental applications as wastewater treatment | [34] |
| Wheat/cellulose | Films with antibacterial properties | [11] |
| Corn starch/PVA | Nanocomposite with potential use as packaging material | [35] |
| Alginate | Medical applications as tissue regenerator | [36] |
| Poly(L-lactic acid) | Potential use as drug delivery system | [37] |
| Whey protein | Biopolymer with potential use as packaging material | [38] |
| Hydrophilic polyurethane | Films with antibacterial properties | [39] |
| Parameter | Characterization Technique | Results | Ref. |
|---|---|---|---|
| Structural properties | SEM | Good dispersion of TiO2 nanoparticles into the CS film. | [40] |
| EDX | TiO2 is uniformly distributed on the surface of the composite. | [41] | |
| AFM | Composite exhibited rough and porous surface. | [41] | |
| XRD | Characteristics peaks (2θ) for TiO2 (25.4°) and CS (20.4°) were reported on CS–TiO2 composite. | [5] | |
| UV-Vis | Composite exhibited a strong absorption range at 300–500 nm. | [42] | |
| Zeta–potential | CS–Ag–TiO2 coating exhibited good stability (z–potential of 33 mV) after 60 days of storage. | [43,44] | |
| Textural properties | Ads–Des isotherm | Composite is classified as a macroporous material (isotherm Type II). | [45] |
| SSA | Composite’s SSA is dependent of the CS:TiO2 ratio. A major presence of CS promotes a decrease in SSA. | [46] | |
| Pore volume | Decrease in pore volume in CS from 0.25 cm3 g−1 to 0.15 cm3 g−1 in CS–TiO2 composite. | [44] | |
| Pore size | TiO2 decreases the pore size of the composite. | [47] | |
| Thermal properties | DSC | The presence of TiO2 enhances the thermal stability of CS. | [48,49,50,51,52,53] |
| TGA | Composite film exhibited lesser degradation than a CS–film. | [4] | |
| Optical properties | Color | CS film–forming solution turned whiter when TiO2 was added, affecting the color and transparency of the composite film. | [15] |
| Light transmittance | Presence of TiO2 reduces the optical transmittance of the composite film. | [54,55,56] | |
| Mechanical properties | Thickness | TiO2 promotes an increase in the composite film thickness. | [57] |
| Young’s modulus | TiO2 improves the flexibility of the composite (increase of 11.8–fold in Young’s modulus). | [23] | |
| Tensile strength | TiO2 improves the elongation at break in 70%. | [54] | |
| Toughness | TiO2 enhances the toughness of composite (a six–fold increase). | [23] | |
| Viscosity | The viscosity of film-forming solution of CS–TiO2 is influenced by TiO2 content. | [58] | |
| Density | Composite–film exhibited low density (0.33 mg mm−3). | [59] | |
| Vapor barrier properties | Water vapor barrier transmission rate | Incorporation of TiO2 on CS promotes a decrease in water vapor permeability. | [40] |
| Oxygen barrier transmission rate | Presence of TiO2 significantly reduced the oxygen permeability. However, its effectiveness is TiO2 concentration-dependent. | [60] | |
| Water solubility | TiO2 did not influence in the water solubility behavior CS–TiO2 of composite. | [57] | |
| Biodegradability | Swelling study | TiO2 controls the swelling capacity of CS film. | [45] |
| Biodegradation rate | CS–TiO2 composite exhibited moderate biodegradability (<3%) and favorable time–dependent biodegradability (0.0521 mg mL−1 h−1 on day zero; 0.6 ng mL−1 h−1 post–covering). | [59] |
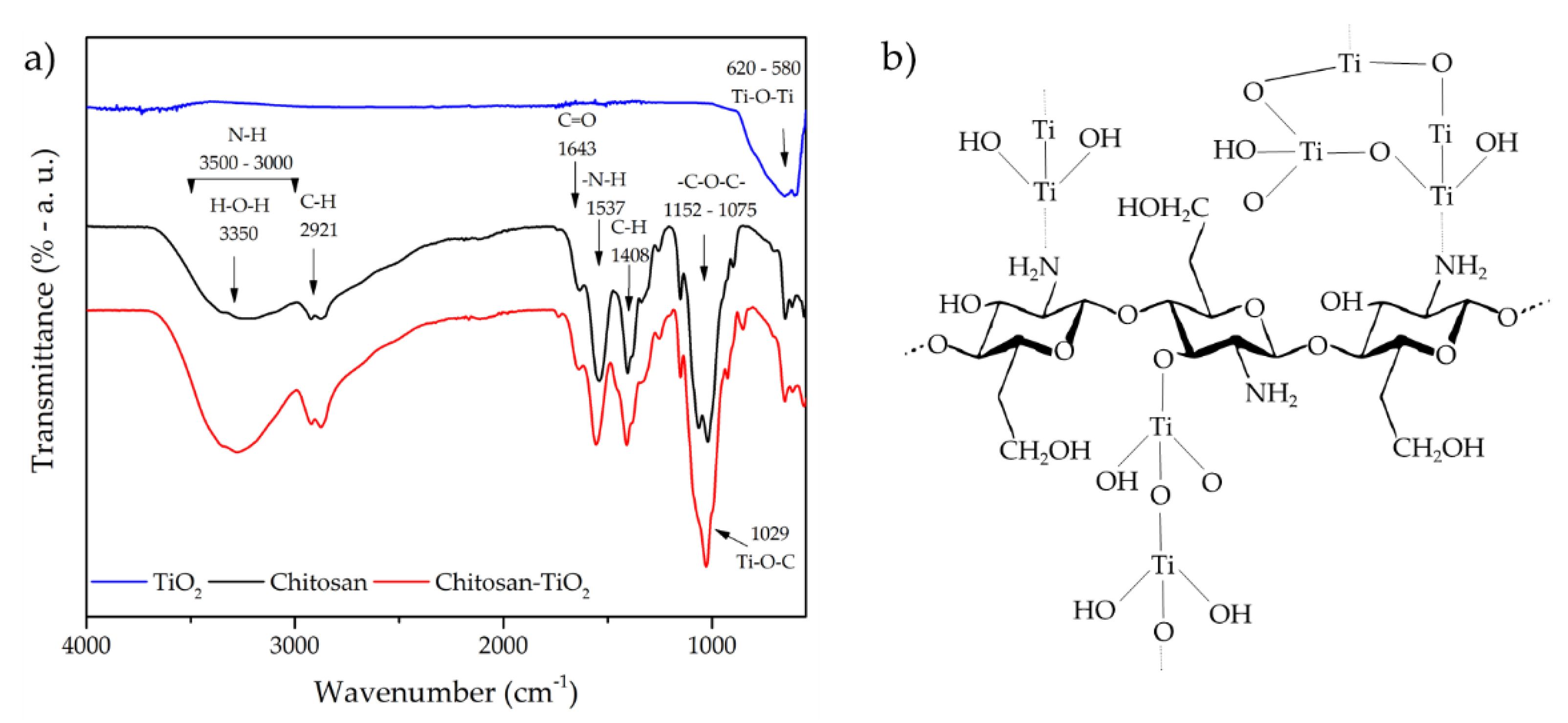
| Band Position | Assignment | Ref. |
|---|---|---|
| 3450 | OH bond of chitosan; it could exist an electrostatic interaction of N–H–O–Ti | [15,16] |
| 3350 | Combined peaks of the NH2 and OH group stretching vibrations | [69] |
| 3300 | Strong interaction between NH2 and OH with TiO2 | [40,69] |
| 2934 | Asymmetrical stretching vibration of the C–H in CH2 and CH3 groups | [55,70] |
| 2923–2872 | C–H asymmetric and symmetric vibrations, TiO2–OH functional group | [8] |
| 1735–1733 | O–C–NH2 indicated the presence of titanates in the composite | [71] |
| 1637–1715 | N–H scissoring from the primary amine, it could exist an interaction of Ti4+ with –NH2 | [50,68,72,73] |
| 1577–1589 | Angular deformation of N–H bonds | [51,74] |
| 1538 | Secondary amide (amide II), CH2 bending | [60] |
| 1528–1534 | C–N and C–N–H bending mode | [5,68] |
| 1421 | C–N axial deformation (amine group); C–O stretching (amide I) | [45,70] |
| 1370–1420 | C–O–C stretching bands, N = O vibrations, –NH deformation, CH3 group | [15,70,73,74] |
| 1287 | Ti–OH and Ti–O bonds | [15,70] |
| 1029–1152 | C–N bending vibrations and asymmetrical stretching vibrations of C–O–C glycosidic bonds, Ti–O–C bending mode, Ti–OH bond | [10,15,16,17,70,74,75] |
| 600–900 | Ti–O–Ti bond, asymmetric stretching mode of Ti–O, immobilization of TiO2 onto the CS matrix | [5,15,16,70,74] |
| 385–600 | Ti–O–C, it could exist an interaction of Ti Lewis site with -NH2 groups of chitosan chain | [42,50,69] |
| Microorganism | Material | Composition | Results | Ref. |
|---|---|---|---|---|
| Staphylococus aureus, Escherichia coli, Salmonella Typhimurium, Pseudomonas aeruginosa, Aspergillus, Penicillium | CS–TiO2 film | CS (2% w/v), nano–TiO2 (1% w/w) | The film exhibited antimicrobial activity against Gram–positive and Gram–negative bacteria, and fungi. | [40] |
| Sthapylococcus aureus, Pseudomona aureginosa | CS–TiO2:Ag composite | CS (1.5% w/v), TiO2 (0.1% w/v), Ag (1 mol L−1) | CS–TiO2:Ag exhibited major antibacterial activity than CS–TiO2 composite against E. coli, S. aureus and P. aureginosa. | [72] |
| Sthapylococcus aureus | CS–TiO2 membrane | CS (1% w/v), TiO2 (0.25% w/v) | Enhanced antibacterial activity against S. aureus by CS–TiO2 compared to CS films. | [4] |
| Escherichia coli | CS–Ag–TiO2 coating | CS (1% w/v), AgNO3 solution (1% w/v), 0.5% TiO2 NPs | CS–Ag–TiO2 exhibited higher antimicrobial activity (MIC of 0.38 µg mL−1) than the individual components (CS, Ag, TiO2) against E. coli (MIC > 4 µg mL−1). | [44] |
| Escherichia coli, Sthapylococcus aureus, Candida albicans | Ag–CS–TiO2 composite | CS–TiO2 (0.15 g) mixed in 40 mL of AgNO3 (Ag+ 200 mg L−1) | Composite exhibited favorable antimicrobial activity against E. coli, S. aureus and C. albicans, without significant losses of its activity even after five consecutive cycles. | [6] |
| Escherichia coli, Sthapylococcus aureus, Salmonella enterica ser. Typhimurium | CS–TiO2 film | CS (2% w/v), TiO2 (0.1% w/v) NPs | Films were effective in reducing the microbial concentration in liquid culture for S. aureus, E. coli and Salmonella enterica ser. Typhimurium, but effectiveness was dependent on the strain and TiO2 content. | [15] |
| Escherichia coli, Sthapylococcus aureus, Candida albicans, Aspergillus niger | CS–TiO2 film | CS (2.5% w/v), TiO2 NPs (2.5% w/v) | CS–TiO2 films exhibited photocatalytic antimicrobial activity against E. coli, S. aureus, C. albicans, and A. niger. | [54] |
| Salmonella choleraesuis | TiO2 on CS beads and activated carbon | CS (2% w/v), nano–TiO2 (5% w/v) | TiO2 coated on activated carbon and chitosan beads served as a strong anti-bacterial agent against S. choleraesuis subsp. | [78] |
| Escherichia coli, Staphylococcus aureus | CS–TiO2:Cu composite | CS (1% w/v), nano–TiO2:Cu (0.2 mg mL−1) | CS–TiO2: Cu exhibited antimicrobial activity against E. coli and S. aureus, it was enhanced in 200% in presence of UV-light. | [10] |
| Escherichia coli | TiO2/CS/CMM coating layer | CS (1,3,5% w/t), TiO2 (2%), CMM | TiO2/CS exhibited significant reduction (93%) in viable cells E. coli in viable cells after 24 h. | [64] |
| Escherichia coli, Aspergillus niger, Candida albicans | CS/TiO2 emulsion deposited on gauzes | CS (0.1% w/v), TiO2 (0.05 g), gauze 25 cm2 | Gauze treated with chitosan/nano–TiO2 composite emulsion showed antibacterial activities against E. coli, A. niger and C. albicans. | [79] |
| Escherichia coli, Candida albicans, Aspergillus niger | CS–Fe–TiO2 coatings | Chitosan (0.1% w/v), Fe–TiO2 (0.05 g) | Antimicrobial activity against E. coli, C. albicans and A. niger under visible light irradiation. | [41] |
| Aspergillus niger, Bacillus subtilis | CS–GO–TiO2 coating | CS (1% w/v), GO:TiO2 ratio 1:2 | Self-assembled film of GO–CS with nano–TiO2 exhibited high antibacterial activity against biofilm forming A. niger and B. subtilis. | [80] |
| Escherichia coli, Staphylococcus aureus, Aspergillus niger | CS–TiO2 incorporated in cotton fibers | CS (1% w/v), TiO2 (0.5 g), cotton fiber 250 cm2 | More than 99% of E. coli, S. aureus and A. niger of viable cells were inactivated. | [42] |
| Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae | CS–TiO2 composite | CS:TiO2 (2:1) | Moderate antibacterial activity against S. aureus, but no for E. coli and K. pneumoniae. | [68] |
| Escherichia coli | CS–TiO2 composite | CS (1% w/v), TiO2 (1% w/v) | CS–TiO2 exhibited a complete inactivation of E. coli (100% of reduction) in comparison with a CS alone (7.5%) after 24 h of exposure. | [74] |
| Escherichia coli, Salmonella enteritidis, Staphylococcus aureus | Zein/CS/TiO2 film | CS (0.12 g), Zein (2 g), TiO2 (0.25% w/w) | Antimicrobial activity of Zein/CS was improved by the incorporation of TiO2. | [55] |
| Xanthomonas oryzae pv. Oryzae | CS–TiO2 composite | CS (1% w/v), TiO2 (0.50% w/w) | CS–TiO2 exhibited enhanced antibacterial activity compared with the individual components. | [9] |
| Escherichia coli, Staphylococcus aureus | TiO2–CS–PVA composite | TiO2 (0.1 g), CS (0.5 g), PVA solution (10%), mass ratio 1:5:20 | Composite exhibited enhanced antibacterial activity against E. coli and S. aureus compared with the individual components. | [81] |
| Pollutant | Material | * Composition | Relevant Results | Ref. |
|---|---|---|---|---|
| Methyl orange | CS–TiO2 composite | Different weight ratios of TiO2 and CS were evaluated (75:25, 50:50, 25:75) being the composite 1% of the total volume of polluted water. | TiO2–CS composite (75:25 w/w) showed great degradation efficiency against methyl orange dye and the recycling outcome material shows effective stability. | [16] |
| Thymol violet | TiO2/CS/CMM coating layer | CS (1,3,5% w/t), TiO2 (2%), CMM | TiO2/CS/CMM composites could adsorb thymol violet for its removal from water. | [64] |
| Methyl orange/Congo red | CS/PVA/Na–Titanate/TiO2 composites | CS–PVA ratio 60:40, 80:20, 90:10, TiO2 (1% w/v) | 100% of methyl orange removal was obtained under UV irradiation, and 99% for Congo red removal. | [48] |
| Cu(II) and Pb(II) heavy metal ions | CS–TiO2 composite | CS (7% w/v), TiO2 (1% w/v) | CS–TiO2 exhibited higher potential for metal ions sorption compared with CS. | [17] |
| Methylene blue | CS–TiO2 nanohybrid | CS (1% w/v), TiO2 (1% w/v) | Exhibited high photocatalytic activity degradation of methylene blue dye under UV–light illumination even after five cycles of reuse. | [74] |
| Cr(VI) | CS–TiO2 beads | CS (1% w/v), TiO2 (1% w/v) | CS–TiO2 composite exhibited high reduction of Cr(VI) in water in comparison with CS. | [75] |
| Methyl orange | CdS/TiO2/CS coating | CS (2% w/v), TiO2 (0.3% w/w), CdCl2 (0.912% w/v) | CdS/TiO2/CSC exhibited enhanced photocatalytic activity under simulated solar light irradiation and represents a suitable and promising photocatalyst for effective decolorization treatment of dye–containing effluents. | [84] |
| Methyl orange | TiO2/ZnO/CS composite | CS (2% w/v), TiO2 (0.2 g), ZnO (1.17 g of zinc acetate) | Exhibited high photocatalytic activity for methyl orange degradation (97%) under simulated solar radiation. | [77] |
| Methyl orange | CS–TiO2 composite | CS (1 g in 36 mL of acetic acid), TiO2 (0.025 g) | Enhanced photocatalytic selectivity for methyl orange compared with CS, and could be reused up to 10 cycles without desorption and regeneration while preserving 60% of its photocatalytic efficiency. | [6] |
| Cr(VI) | CS–TiO2 composite | CS (3.22% w/v), TiO2 solution | The CS–TiO2 composite was quite effective for adsorption and detoxification of Cr(VI) in water with a maximum adsorption capacity of 171 mg g−1 for Cr(VI). | [50] |
| As | CS–TiO2 composite | CS (1 g), TiO2 (0.42g g−1 CS) | Exhibited good Arsenic removal from water, but the effectiveness is TiO2 concentration dependent. | [85] |
| Congo red | CS–TiO2 glass photocatalyst | CS (0.83% w/v), TiO2 (0.83% w/v) | The study suggests a new method that has the advantages of photodegradation and adsorption in the abatement of various wastewater pollutants. | [8] |
| Methyl orange | CS–TiO2 composite | CS (0.5 g 25 mL−1), TiO2 (0.2 g 25 mL−1) | Exhibited degradation of methyl orange and adsorption on Ni2+ ions. | [86] |
| Cd, 2,4-DCP | MICT composite | CS (2.5 g 500 mL−1), TiO2 (1 g TiO2), Fe3O4 (1.25 g) | The composite is effective for cadmium adsorption (256 mg g−1) and 2,4–dichlorophenol degradation (98%), and could be used up to five cycles with preserving 69% of its adsorption properties. | [73] |
| Methyl orange | TiO2–CS–rGO | CS (2% w/v), 4 mL of TiO2 suspension (50 mg mL−1), rGo (1% w/w) | Hybrid composite showed photocatalytic degradation (97%) of methyl orange. | [58] |
| Methylene blue | CS–TiO2 composite | CS (1% w/v), TiO2 (1 g in 20 mL of CS solution) | Composite is effective for methylene blue (100%) degradation under UV–light, but the effectiveness was catalyst concentration–dependent. | [45] |
| As(III), As(V) | CS–TiO2:Cu composite | CS (1% w/v), TiO2 (0.6% w/w), Cu (0.7% w/w) | The composite showed good photo–oxidation and selective removal for As(III) and As(V). | [87] |
| Methyl orange | CS–TiO2 composite | CS (1% w/v), TiO2 (2% w/w) | CS is an excellent support for TiO2 immobilization, which exhibited a complete photodegradation of methyl orange and alizarin red S after 3 h of treatment. | [88] |
| 4-NPh | CS–TiO2:Ag composite | CS (1% w/v), TiO2 (0.4% w/v), Ag+ (200 mg L−1) | Incorporation of Ag+ ions into the surface of CS–TiO2 composite improved the catalytic activity in the reduction of 4–NPh to 4–APh (100% in 120 min) and preserve the catalytic activity in five continuous cycles. | [89] |
| Ni, Cd, Cu, Hg, Mn and Cr heavy metal ions | CS–HC–TiO2 composite | CS (1.5 g in 56 mL), TiO2 (0.04 g), HC (6 g) | Composite is effective for heavy metal ions (Ni, Cd, Cu, Hg, Mn and Cr) adsorption from aqueous solution. | [90] |
| As(III), As(V) | TiO2/feldspar(FP)-embedded in CS beads | CS (1% w/v), TiO2 (0.5 g/1g CS), FP (0.5 g−1 g−1 CS) | The composite exhibited good adsorption properties for arsenite and aresenate removal from aqueous solution. | [83] |
| Cd(II) | Cd sensor | CS–TiO2 composite film onto a glassy carbone electrode | The modified electrode exhibited a detection limit of 2 × 10−10 mol L−1 Cd for 180 s accumulation. | [91] |
| Acid fuchsin | TiO2/CS/PNIPAAm) composite hydrogel | CS (0.15 g dissolved in 10 mL of 1% acetic acid solution), TiO2 (0.5 g), PNIPAAm (0.5 g) | Composite hydrogel exhibited high efficiency photocatalytic degradation for acid fuchsin, and the removal reached 90% after 160 min. | [92] |
| Cd(II) | EDTA/CS/TiO2 composite | CS (0.2 g), TiO2 (0.1 g) | Composite exhibited high adsorption properties from Cd (II) removal and high phenol degradation efficiency. | [93] |
| Methyl orange | CS–TiO2 composite | CS (2% w/v), TiO2 (NI) | A complete degradation of methyl orange under UV exposition during 90 min was obtained. | [94] |
| 2-4-DCP | CS/ACF/TiO2 membrane | CS (1% w/v), TiO2 (NI), ACF (NI) | The membrane exhibited high efficiency on 2–4–DCP removal from aqueous solution. | [95] |
| Methyl orange | CS/PVA/TiO2 composite | CS (7 wt. %.), PVA (8 wt. %.), TiO2 (1 wt. %.) | Deacetylation degree of chitosan have an impact on methyl orange removal. | [48] |
| Application | Material | Composition | Relevant Results | Ref. |
|---|---|---|---|---|
| Wound healing | CS–TiO2 membrane | CS (1% w/v), TiO2 (0.25% w/w) | Membranes allow proliferation, survival, and decreased oxidative stress and apoptosis of L929 cells. | [4] |
| Immuno-biosensors | Au/CS/TiO2–graphene composite | CS (1 mg mL−1), TiO2-Gr (1 mg), Au (NI) | Biosensor exhibited good bioactivity, sensitivity (0.1–300 ng mL−1) and selectivity for α-fetoprotein detection. Possible applications on the detection of other antigens or biocompounds. | [98] |
| Wound healing | CS–TiO2 with collagen artificial skin (NTCAS) | CS (2%), TiO2 (0.40%) | In an animal model, NTCAS had better outcomes with regard to integrated wound healing than a commercial product. | [59] |
| Glucose biosensor | Ppy–CS–TiO2 nanocomposite film | CS (50 mg mL−1), TiO2 (NI), Ppy (NI) | Biosensor showed good sensitivity over linear range of 1–14 mM with detection limit of 614 µM for glucose (R2 = 0.989). | [99] |
| Glucose biosensor | TiO2–CN–CS composite functionalized with nano–Au | 2 mg TiO2-CN was dispersed in 2 mL CS solution (1 mg mL−1), Au (NI) | Biosensor showed good response performance to glucose with a linear range of 6 µM to 1.2 mM with a detection limit of 0.1 µM glucose. | [100] |
| Tissue engineering applications | chitin–CS/nano–TiO2 composite scaffolds | CS (2% w/v), TiO2 (2% w/w) | No cytotoxic effects on MG-63, L929, and hMSCs cell lines were observed. | [47] |
| Wound healing | CS–Pectin–TiO2 composite | CS:Pectin (1:1), TiO2 (0.001% w/w) | The wounds treated with CS-Pectin-TiO2 dressing material healed faster than CS-treated and gauze. | [49] |
| Wound healing | TiO2–CS–ECM | CS (8.6% w/v), TiO2 (1% w/w), ECM sheets | TiO2-CS-ECM exhibited wound healing acceleration effects. | [101] |
| Tissue engineering applications | CS–TiO2 composite | CS (1% w/v), TiO2 (2:1) | No cytotoxic effect of the composite on a gastric carcinoma human cell line. The preparation method has a remarkable effect on composite biocompatibility. | [46] |
| Regulation of osteoblast bioactivity | CS–gelatin composite coating on ICA–modified TiO2 nanotubes | CS (10 mg mL−1), gelatin (NI), TiO2 (0.5 mg mL−1), ICA (NI) | Composite promote osteoblast proliferation and up-regulation on the expression of bone-related genes (osteopontin, type I collagen, and osteoprotegerin) while down-regulating RANKL mRNA expression. | [102] |
| Drug delivery system | CS/DOP/TiO2 composite | CS (1% w/v), DOP (0.01–0.04% w/v), TiO2 (30% mass ratio) | Incorporation of TiO2 on CS/DOP composite considerably reduces the drug release (16 h) in comparison with CS/DOP system (10 min). | [103] |
| Bone regeneration | TiO2–CS–H4S composite | TiO2-CS-CH4S molar ration 2:1:0.125 | The composite exhibited high bioactivity and biocompatibility with human MG–63 cell line. | [67] |
| Medical dressing | TiO2–CS–PVA | TiO2 (0.1 g), CS (0.5 g), PVA solution (10%), mass ratio 1:5:20 | Composite did not show toxicity against L929 cell line. | [9] |
| Food | Material | Composition | Relevant Results | Ref. |
|---|---|---|---|---|
| Cherry tomatoes | CS–TiO2 film | CS (1% w/v), TiO2 (1% w/v) of TiO2 | Cherry tomatoes packaged with CS–TiO2 nanocomposite film had lower quality changes (firmness, weight loss, color, TSS, lycopene and AA content, and concentration of ethylene and CO2) and delayed the ripening process. | [5] |
| Cantaloupe rind | CS–Ag–TiO2 coating | CS (1% w/v), AgNO3 solution (1% w/v), TiO2 (0.5% w/v) | The CS–Ag–TiO2 exhibited good adherence on cantaloupe rind surface. | [44] |
| Red grape | CS–TiO2 film | CS (2.5% w/v), TiO2 (2.5% w/v) | The CS–TiO2 film prevented microbial infection and increased the shelf life of red grape to 36 days at 37 °C. | [54] |
| Strawberries and Mangoes | CS–GO–TiO2 coating | CS (1% w/v), GO:TiO2 ratio 1:2 | Coated fruits exhibited less than 5% weight loss and maintained color attributes compared to uncoated fruits. PPO activity was diminished in coated fruits. | [80] |
| Stauntonive fruit | CS–TiO2 coating | CS (1% w/v), TiO2 (0.03% w/w) | The fruit treated with the composite coating exhibited a reduction on the CO2 transmission coefficient compared to the CS–treated, without significant changes in quality parameters during 45 days of storage. | [105] |
| Gingko biloba seeds | CS–TiO2 coating films | CS (1% w/v), TiO2 (0.02% w/v) | The composite preserved the quality parameters (firmness and antioxidant activity) of gingko seeds and prevented the mildew apparition. | [106] |
| Application | Material | Composition | Relevant Results | Ref. |
|---|---|---|---|---|
| Textile | CS–TiO2 coated onto cotton | CS (10 g in 50 wt. % NaOH solution), 1 mL of TiO2 (3.28 mmol) | The treated cotton (CS–TiO2) showed excellent protection against UV radiation in comparison to a cotton–TiO2 system. | [56] |
| Textile | TiO2–CS and CA | TiO2:CS:CA relation 1:3:2 wt. % | The treated wool fabrics cured with CA and TiO2–CS showed good protection against UV–radiation. | [108] |
| Metal corrosion resistance | CS–TiO2 composite | CS:TiO2 molar ratio 1:1 | CS–TiO2 composite improved the corrosion resistance of aluminum metal compared with chitosan. | [109] |
| Metal corrosion resistance | CS–TiO2 coating | CS (0.5 g L−1), TiO2 (3 g L−1) | Hybrid coating improved the corrosion resistance of X2CrNiMo17–12–2 stainless steel. | [110] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anaya-Esparza, L.M.; Ruvalcaba-Gómez, J.M.; Maytorena-Verdugo, C.I.; González-Silva, N.; Romero-Toledo, R.; Aguilera-Aguirre, S.; Pérez-Larios, A.; Montalvo-González, E. Chitosan-TiO2: A Versatile Hybrid Composite. Materials 2020, 13, 811. https://doi.org/10.3390/ma13040811
Anaya-Esparza LM, Ruvalcaba-Gómez JM, Maytorena-Verdugo CI, González-Silva N, Romero-Toledo R, Aguilera-Aguirre S, Pérez-Larios A, Montalvo-González E. Chitosan-TiO2: A Versatile Hybrid Composite. Materials. 2020; 13(4):811. https://doi.org/10.3390/ma13040811
Chicago/Turabian StyleAnaya-Esparza, Luis Miguel, José Martín Ruvalcaba-Gómez, Claudia Ivette Maytorena-Verdugo, Napoleón González-Silva, Rafael Romero-Toledo, Selene Aguilera-Aguirre, Alejandro Pérez-Larios, and Efigenia Montalvo-González. 2020. "Chitosan-TiO2: A Versatile Hybrid Composite" Materials 13, no. 4: 811. https://doi.org/10.3390/ma13040811
APA StyleAnaya-Esparza, L. M., Ruvalcaba-Gómez, J. M., Maytorena-Verdugo, C. I., González-Silva, N., Romero-Toledo, R., Aguilera-Aguirre, S., Pérez-Larios, A., & Montalvo-González, E. (2020). Chitosan-TiO2: A Versatile Hybrid Composite. Materials, 13(4), 811. https://doi.org/10.3390/ma13040811







