Abstract
Organoselenium compounds are well-known glutathione peroxidase (GPx) mimetics that possess antioxidants/prooxidant properties and are able to modulate the concentration of reactive oxygen species (ROS), preventing oxidative stress in normal cells or inducing ROS formation in cancer cells leading to apoptosis. The purpose of this study was the synthesis of potent GPx mimics with antioxidant and anticancer activity along with improved bioavailability, as a result of good solubility in protic solvents. As a result of our research, glutathione peroxidase (GPx) mimetics in the form of water-soluble benzeneseleninic acid salts were obtained. The procedure was based on the synthesis of 2-(N-alkylcarboxyamido)benzeneselenenic acids, through the oxidation of benzisoselenazol-3(2H)-ones or analogous arenediselenides with an amido group, which were further converted to corresponding potassium salts by the treatment with potassium tert-butanolate. All derivatives were tested as potential antioxidants and anticancer agents. The areneseleninic acid salts were significantly better peroxide scavengers than analogous acids and the well-known organoselenium antioxidant ebselen. The highest activity was observed for the 2-(N-ethylcarboxyamido)benzeneselenenic acid potassium salt. The strongest cytotoxic effect against breast cancer (MCF-7) and human promyelocytic leukemia (HL-60) cell lines was found for 2-(N-cyclohexylcarboxyamido)benzeneselenenic acid potassium salt and the 2-(N-ethylcarboxyamido)benzeneselenenic acid, respectively. The structure–activity correlations, including the differences in reactivity of benzeneseleninic acids and corresponding salts were evaluated.
1. Introduction
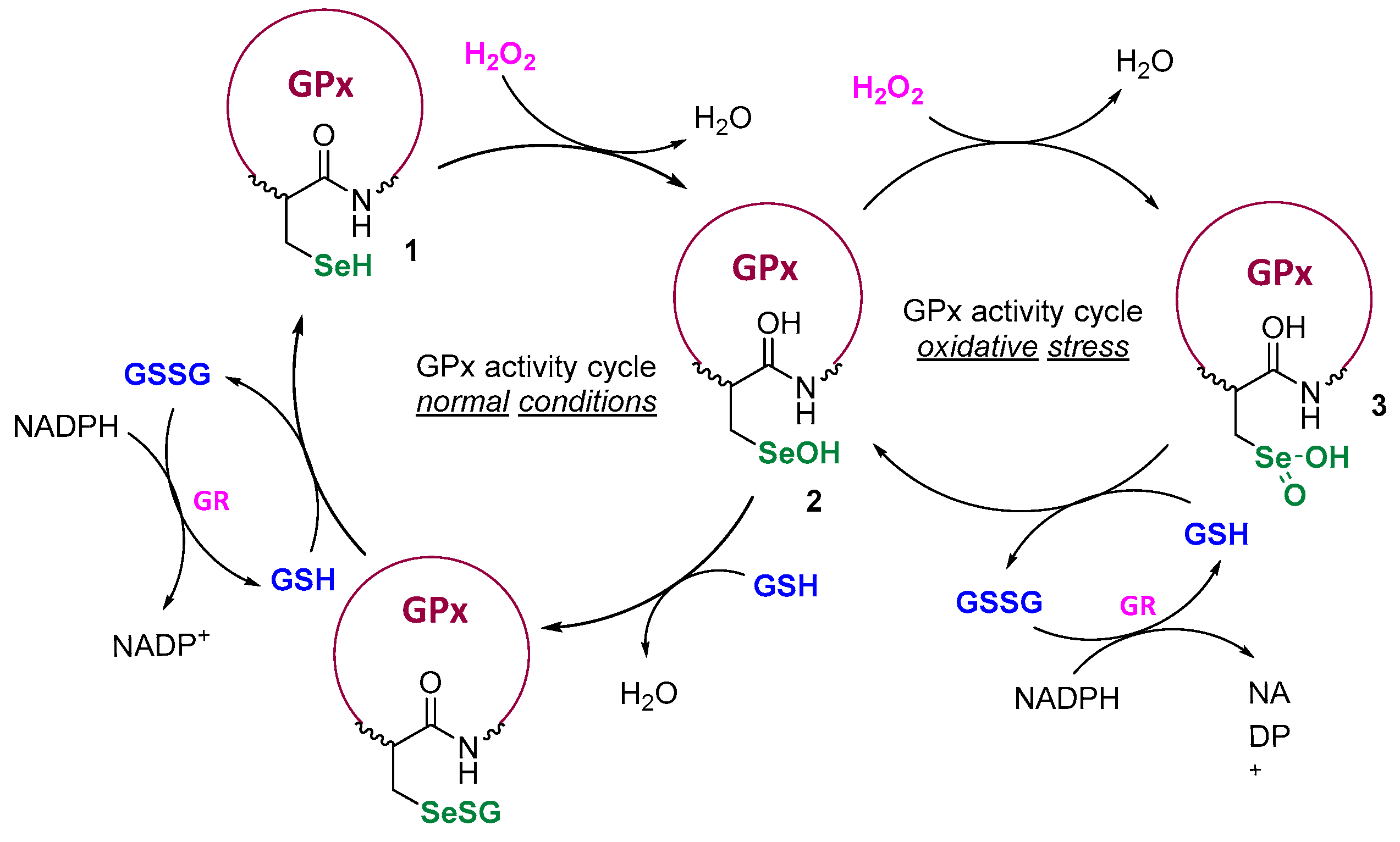
Designing catalysts inspired by enzymes is one of the crucial tactics aimed at influencing cell physiology or reversing a pathological state that triggers a disease to develop. The catalytic systems protecting cells from reactive oxygen/nitrogen species (ROS/RNS) and further oxidative damage, involving selenoenzymes from the glutathione peroxidase (GPx) family, can operate due to the presence of the reactive selenol moiety incorporated in the structure of the amino acid selenocysteine [1,2,3]. In the GPx-catalytic cycle, the initial redox-active -SeH, plays the crucial role at the enzyme’s active site and initiates the cycle through the rapid reaction with H2O2. As it has been presented by Mugesh et al., the regulation of ROS concentrations depends on the levels of peroxide and the thiol co-factor, mostly the glutathione (GSH) [4]. Under physiological conditions, the active selenol 1 is first oxidized to the selenenic acid 2 and next regenerated in the presence of two GHS molecules. In the state of oxidative stress, when the H2O2 level is higher, with a lower amount of thiol, the cycle is extended and further oxidation of the seleninic acid 3 takes place (Scheme 1).
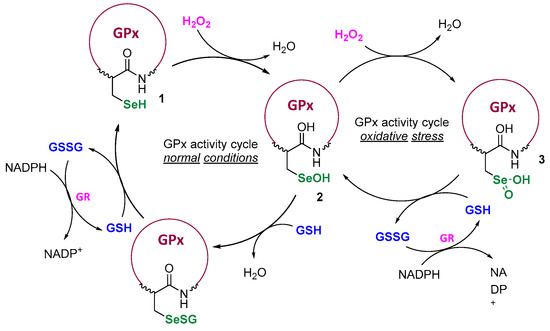
Scheme 1.
GPx activity cycle at physiological conditions and elevated ROS levels.
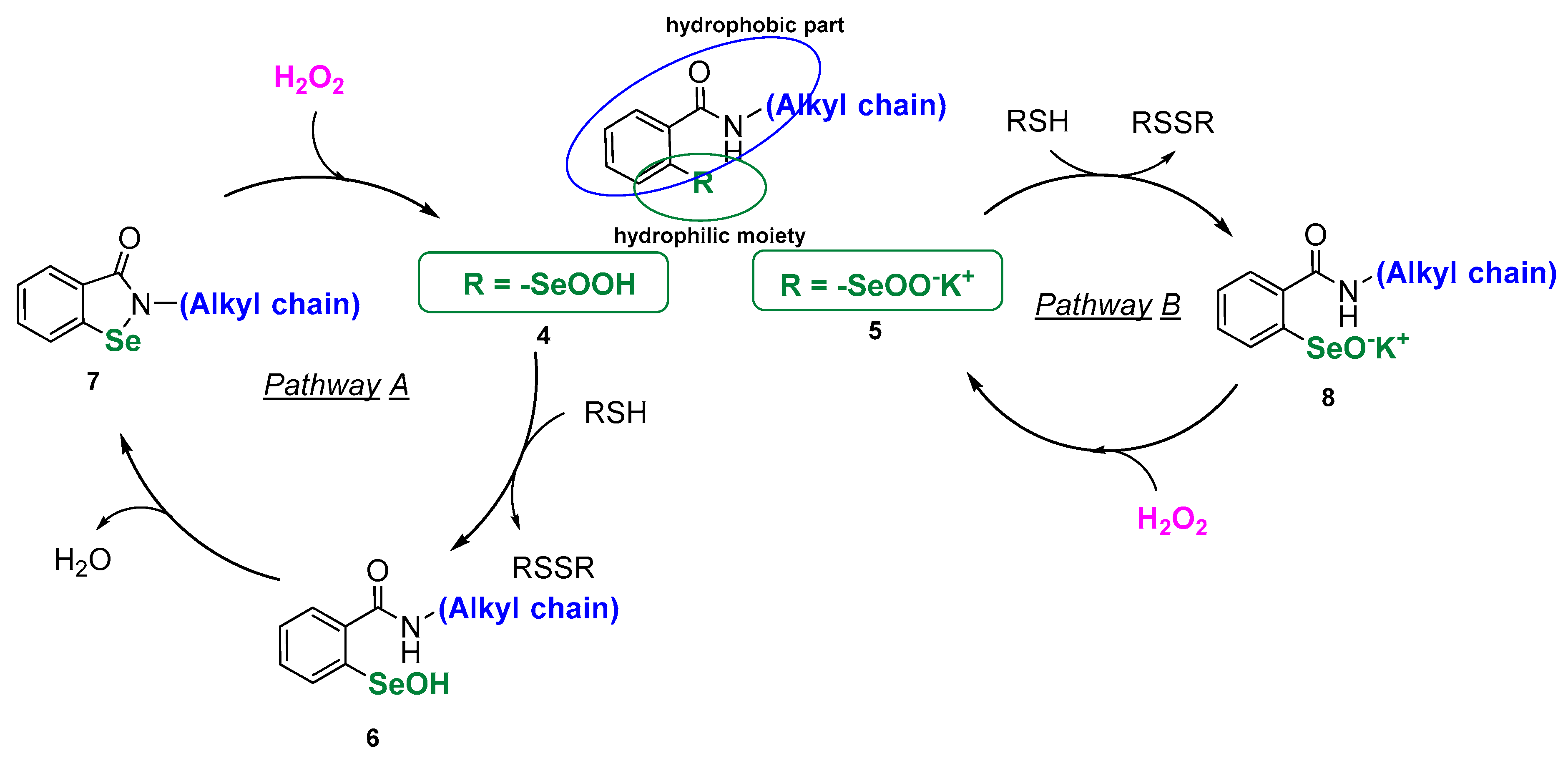
Since it was discovered that small organoselenium compounds can mimic the activity of glutathione peroxidase and reduce peroxides in a similar manner to the above-presented cycle, a lot has been accomplished in the synthesis of redox-active Se-catalysts [5]. Several compounds have been identified and selected as biologically potent, however, low selectivity, leading to higher toxicity, along with solubility problems are still limiting the applicability. When designing new GPx mimics in the form of modified “selenocysteine-like” catalysts, the direct approach to synthetize compounds bearing a free selenol moiety is problematic due to the high instability of the -SeH group and rapid formation of the corresponding diselenide. Thus, an efficient organoselenium antioxidant should possess a masked Se-moiety that could be easily unmasked and transformed to a reactive selenol at the specific place of action. This can be accomplished by synthetizing stable compounds that correspond structurally to a certain isoform of the GPx selenocysteinyl redox center. Herein, we present a series of new peroxide scavengers in the form of seleninic acids 4 and corresponding seleninic salts 5. Both of these Se(IV) compound series 4 and 5 can be qualified as the derivatives of the primal GPx in the form of seleninic acid 3. The structure of the designed molecules is composed of two important features: the hydrophilic -SeOOH/SeOOK group and the hydrophobic N-alkylbenzamide moiety that connects together exhibit amphiphilic properties. As the structure of derivative 4 corresponds to the specific benzseleninic acid intermediated formed in the GPx catalytic cycle of the known drug candidate ebselen, the mechanism of action should include the “ebselen-like” catalytic pathway A. However, we assume that in the case of analogue salt 5, the formation of the Se-N bond will not be achieved, which can accelerate the speed of peroxide reduction (pathway B, Scheme 2).
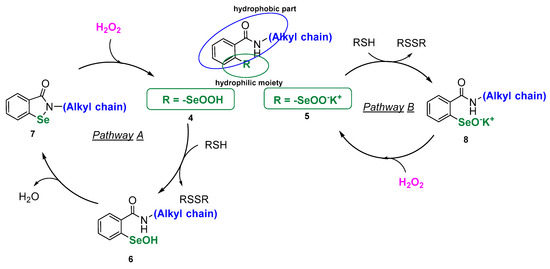
Scheme 2.
Origin of the structure of the designed antioxidants 4 and 5.
Amphiphilic molecules, that can easily penetrate lipid bilayer membranes, may create a delivery system with an incorporated pharmacophore. The Se(IV) molecules can act as “the hidden selenol”, as postulated by Rocha [1], be transported to the specific place of action as stable and soluble RSeOOH/RSeOOK and, at the final stage, be transformed into the bioactive RSeH.
Till now, seleninic acids have found several application routes, including their utilization as ligands [6,7,8,9,10] and reagents in oxidation [11,12,13,14], dehydrogenation [15] and oxidative deoximation reactions [16]. However, their applicability in medicinal chemistry seems to be still an unexplored field with promising perspectives. Only few examples of such areneseleninic acids have been published by Mugesh et al. [4,17,18,19], but analyzed only as intermediates of corresponding benzisoselenazolones, with no activity evaluation. Similar amphiphilic seleninic acids with p-amido function have been presented by Jacobs et al. [20]. The studies revealed that the compounds can easily penetrate cell membranes and possess significantly better antibacterial activity than phenyl seleninic acid and simple surfactants. Nevertheless, the “ebselen-like” catalysts have never been synthetized in the form of seleninic acid salts. The purpose of this study was the synthesis of GPx mimics in the form of RSeOO-M+. The presence of the -SeOO−M+ moiety can improve the solubility in water and change the physicochemical properties of the molecules which, in turn, may strongly influence the metabolism of the presented potential drug candidates.
2. Materials and Methods
2.1. General
Melting points were measured with a Büchi Tottoli SPM-20 heating unit (Büchi Labortechnik AG, Flawil, Switzerland) and were uncorrected. Nuclear magnetic resonance (NMR) spectra were recorded on Bruker Avance III/400 or Bruker Avance III/700 (Karlsruhe, Germany) for 1H and 176.1 MHz or 100.6 MHz for 13C. Chemical shifts were recorded relative to SiMe4 (δ0.00) or solvent resonance (CDCl3 δ7.26, CD3OD δ3.31). Multiplicities were given as: s (singlet), d (doublet), dd (double doublet), ddd (double double doublet), t (triplet), dt (double triplet), and m (multiplet). 77Se NMR spectra were recorded on Bruker Avance III/ 400 or Bruker Avance III/ 700 with diphenyl diselenide as an external standard. NMR spectra were carried out using ACD/NMR Processor Academic Edition. All original NMR spectra are presented in Supplementary Materials. Infrared spectra (IR) were measured on Alpha FT-IR spectrometer from Bruker (Karlsruhe, Germany). Elemental analyses were performed on a Vario MACRO CHN analyzer. Commercially available solvents dimethylformamide (DMF), dichloromethane (DCM), and MeOH (Aldrich, St. Louis, MO, USA) and chemicals were used without further purification. Column chromatography was performed using Merck 40-63D 60Å silica gel (Merck, Darmstadt, Germany).
2.2. Procedures and Analysis Data
2.2.1. Synthesis of 2-(N-alkylcarboxyamido)benzeneselenenic acids 10–15
Method A: To a solution of N-alkylbenzisoselenazol-3(2H)-one (1.00 mmol) in methanol (5 mL), 30% hydrogen peroxide (5.00 mmol) was added and the mixture was stirred at 50 °C for 1 h. Methanol was evaporated, the obtained residue was dissolved in DCM, followed by addition of manganese oxide and anhydrous magnesium sulfate. The mixture was dried for 24 h, filtered and evaporated.
Method B: To a solution of diselenide (1.00 mmol) in methanol (5 mL), 30% hydrogen peroxide (5.00 mmol) was added and the mixture was stirred at 50 °C for 1 h. Methanol was evaporated, the obtained residue was dissolved in DCM, followed by addition of manganese oxide and anhydrous magnesium sulfate. Mixture was dried for 24 h, filtered and evaporated.
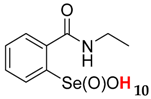
2-(N-ethylcarboxyamido)benzeneselenenic acid 10
Yield: 90%, 84%; mp 135–139 °C; 1H NMR (700 MHz, DMSO) δ = 1.25 (t, J = 7.7 Hz, 3H, CH3), 3.63–3.68 (m, 1H, N-CH2), 3.75–3.81 (m, 1H, N-CH2), 7.75 (dt, J1 = 0.7, J2 = 7.7 Hz, 1H, 1Har), 7.82 (dt, J1 = 1.4, J2 = 7.7 Hz, 1H, 1Har), 7.85 (d, J = 7.7 Hz, 1H, 1Har), 8.16 (d, J = 7.7 Hz, 1H, 1Har); 13C NMR (100.6 MHz, DMSO) δ = 15.83 (CH3), 37.00 (CH2), 127.25 (CHar), 127.82 (CHar), 131.36 (Car), 132.70 (CHar), 134.18 (CHar), 147.64 (Car), 168.31 (C=O); 77Se NMR (76 MHz, DMSO) δ = 1123.91 ppm; IR: 3242, 3074, 2973, 2928, 2872, 2363, 1703, 1686, 1619, 1587, 1567, 1549, 1445, 1380, 1360, 1313, 1291, 1271, 1246, 1145, 1115, 1090, 1056, 1024, 1007 cm−1; Elemental Anal. Calcd for C9H11NO3Se (260.90): C, 41.55; H, 4.26; N, 5.28 Found: C, 41.23; H, 4.18; N, 5.17.
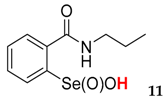
2-(N-propylcarboxyamido)benzeneselenenic acid 11
Yield: 65%, 84%; mp 138–142 °C; 1H NMR (700 MHz, DMSO) δ = 0.91 (t, J = 7.7 Hz, 3H, CH3), 1.63–1.71 (m, 2H, CH2), 3.56–3.61 (m, 1H, N-CH2), 3.66-3.70 (m, 1H, N-CH2), 7.60 (dt, J1 = 0.7, J2 = 7.0 Hz, 1H, 1Har), 7.82 (dt, J1 = 1.4, J2 = 7.7 Hz, 1H, 1Har), 7.85 (d, J = 7.7 Hz, 1H, 1Har), 8.17 (d, J = 7.7 Hz, 1H, 1Har); 13C NMR (100.6 MHz, DMSO) δ = 11.84 (CH3), 23.37 (CH2), 43.66 (CH2), 127.31 (CHar), 127.82 (CHar), 131.24 (Car), 132.71 (CHar), 134.20 (CHar), 147.67 (Car), 168.59 (C=O); 77Se NMR (76 MHz, DMSO) δ = 1126.91 ppm; IR: 3243, 3085, 2953, 2927, 2868, 1667, 1627, 1584, 1456, 1444, 1336, 1291, 1246, 1159, 1113, 1098, 1038, 1021 cm−1; Elemental Anal. Calcd for C10H13NO3Se (289.02): C, 45.84; H, 4.78; N, 5.11 Found: C, 43.53; H, 4.69; N, 5.27.
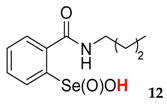
2-(N-butylcarboxyamido)benzeneselenenic acid 12
Yield: 60%, 47%; mp 121–125 °C; 1H NMR (700 MHz, DMSO) δ = 0.89 (t, J = 7.7 Hz, 3H, CH3), 1.34 (m, 2H, CH2), 1.58–1.67 (m, 2H, CH2), 3.58–3.63 (m, 1H, N-CH2), 3.70–3.74 (m, 1H, N-CH2), 7.42 (t, J = 7.0 Hz, 1H, 1Har), 7.82 (t, J = 7.7 Hz, 1H, 1Har), 7.84 (d, J = 7.7 Hz, 1H, 1Har), 8.16 (d, J = 7.7 Hz, 1H, 1Har); 13C NMR (100.6 MHz, DMSO) δ = 14.06 (CH3), 20.06 (CH2), 32.11 (CH2), 41.67 (CH2), 127.33 (CHar), 127.78 (CHar), 131.20 (Car), 132.75 (CHar), 134.23 (CHar), 147.59 (Car), 168.57 (C=O); 77Se NMR (76 MHz, DMSO) δ = 1126.33 ppm; IR: 3325, 3082, 2929,2870, 2851, 1668, 1586, 1586, 1446, 1444, 1357, 1324, 1299, 1248, 1231, 1182, 1150, 1112, 1065, 1022, 1007 cm−1; Elemental Anal. Calcd for C11H15NO3Se (289.02): C, 45.84; H, 5.25; N, 4.86 Found: C, 45.96; H, 5.34; N, 4.99.
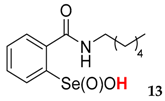
2-(N-hexylcarboxyamido)benzeneselenenic acid 13
Yield: 33%, 36%; mp 84–87 °C; 1H NMR (700 MHz, DMSO) δ = 0.85 (s, 3H, CH3), 1.32–1.36 (m, 6H, 3 × CH2), 1.63–1.65 (m, 2H, CH2), 3.57–3.60 (m, 1H, N-CH2), 3.65–3.72 (m, 1H, N-CH2), 7.76 (t, J = 7.7 Hz, 1H, 1Har), 7.81-7.85 (m, 2H, 2Har), 8.16 (d, J = 7.7 Hz, 1H, 1Har); 13C NMR (100.6 MHz, DMSO) δ = 14.36 (CH3), 22.45 (CH2), 26.48 (CH2), 29.97 (CH2), 31.35 (CH2), 41.93 (CH2), 127.33 (CHar), 127.82 (CHar), 131.21 (Car), 132.76 (CHar), 134.22 (CHar), 147.82 (Car), 168.58 (C=O); 77Se NMR (76 MHz, DMSO) δ = 1122.09 ppm; IR: 3243, 3076, 3058, 0958, 2926, 2856, 1682, 1622, 1585, 1568, 1552, 1460, 1445, 1376, 1329, 1298, 1250, 1215, 1108, 1039, 1026, 1010 cm−1; Elemental Anal. Calcd for C13H19NO3Se (317.05): C, 49.37; H, 6.06; N, 4.43 Found: C, 49.56; H, 6.14; N, 4.59.
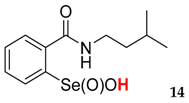
2-(N-(3-methyl)butylcarboxyamido)benzeneselenenic acid 14
Yield: 52%, 72%; mp 119–123 °C; 1H NMR (700 MHz, DMSO) δ = 0.89–0.92 (m, 6H, 2 × CH3), 1.53–1.57 (m, 2H, CH2), 1.62–1.66 (m, 1H, CH), 3.61–3.65 (m, 1H, N-CH2), 3.72–3.77 (m, 1H, N-CH2), 7.76 (t, J = 7.0 Hz, 1H, 1Har), 7.82 (dt, J1 = 0.7, J2 = 7.0 Hz, 1H, 1Har), 7.85 (d, J = 7.0 Hz, 1H, 1Har), 8.16 (d, J = 7.7 Hz, 1H, 1Har); 13C NMR (100.6 MHz, DMSO) δ = 22.78 (CH3), 22.77 (CH3), 25.75 (CH), 38.92 (CH2), 40.30 (CH2), 127.32 (CHar), 127.78 (CHar), 131.21 (Car), 132.75 (CHar), 134.22 (CHar), 147.57 (Car), 168.52 (C=O); 77Se NMR (76 MHz, DMSO) δ = 1126.14 ppm; IR: 3317, 3060, 2956, 2895, 2868, 1666, 1585, 1459, 1445, 1385, 1366, 1328, 1301, 1266, 1248, 1170, 1146, 1128, 1113, 1047, 1032, 1011 cm−1; Elemental Anal. Calcd for C12H17NO3Se (303.04): C, 47.69; H, 5.67; N, 4.63 Found: C, 47.87; H, 5.58; N, 4.79.
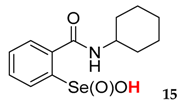
2-(N-cyclohexylcarboxyamido)benzeneselenenic acid 15
Yield: 47%, 66%; mp 108–110 °C; 1H NMR (700 MHz, DMSO) δ = 1.15–1.22 (m, 1H), 1.28–1.40 (m, 2H, CH2), 1.56–1.70 (m, 3H), 1.75–1.83 (m, 2H, CH2), 2.01–2.05 (m, 2H, CH2), 4.07–4.12 (m, 1H, N-CH), 7.75 (dt, J1 = 0.7, J2 = 7.7 Hz, 1H, 1Har), 7.82 (dt, J1 = 1.4, J2 = 7.7 Hz, 1H, 1Har), 7.85 (dd, J1 = 1.4, J2 = 7.0 Hz, 1H, 1Har), 8.13 (d, J = 7.7 Hz, 1H, 1Har); 13C NMR (100.6 MHz, DMSO) δ = 25.44 (CH2), 25.79 (CH2), 25.91 (CH2), 33.71 (CH2), 33.91 (CH2), 54.38 (CH), 127.26 (CHar), 127.55 (CHar), 131.76 (Car), 132.71 (CHar), 134.16 (CHar), 147.42 (Car), 167.92 (C=O); 77Se NMR (76 MHz, DMSO) δ = 1113.88 ppm; IR: 3234, 2927, 2852, 1621, 1588, 1544, 1448, 1329, 1295, 1243, 1147, 1075 cm−1; Elemental Anal. Calcd for C13H17NO3Se (315.04): C, 49.69; H, 5.45; N, 4.46 Found: C, 49.40; H, 5.53; N, 4.32.
2.2.2. Synthesis of 2-(N-alkylcarboxyamido)benzeneselenenic acid potassium salts 16–21
To a solution of benzeneselenic acid (1.00 mmol) in absolute ethanol (5 mL), potassium tert-butanolate (1.00 mmol) in absolute ethanol (2 mL) was added portionswise and stirred at room temperature for 1 h. The solution was evaporated and the residue was washed with diethyl ether (3 × 2 mL). All derivatives were obtained as yellow oil.
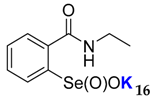
2-(N-ethylcarboxyamido)benzeneselenenic acid potassium salt 16
Yield: 98%; 1H NMR (700 MHz, D2O) δ = 1.12 (t, J = 7.7 Hz, 3H, CH3), 3.30–3.33 (m, 2H, N-CH2), 7.50 (dt, J1 = 1.4, J2 = 7.7 Hz, 1H, 1Har), 7.61 (dd, J1 = 0.7, J2 = 7.7 Hz, 1H, 1Har), 7.65 (dt, J1 = 1.4, J2 = 7.7 Hz, 1H, 1Har), 7.99 (dd, J1 = 0.7, J2 = 7.7 Hz, 1H, 1Har); 13C NMR (100.6 MHz, D2O) δ = 13.53 (CH3), 35.13 (CH2), 124.47 (CHar), 127.36 (CHar), 130.96 (CHar), 132.34 (CHar), 133.14 (Car), 150.98 (Car), 169.27 (C=O); 77Se NMR (76 MHz, D2O) δ = 1142.60 ppm; IR: 3192, 3052, 2971, 2932, 1628, 1587, 1543, 1444, 1376, 1359, 1314, 1146, 1051 cm−1; Elemental Anal. Calcd for C9H10KNO3Se (298.85): C, 36.25; H, 3.38; N, 4.70 Found: C, 36.58; H, 3.29; N, 4.85.
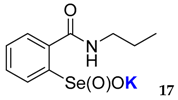
2-(N-propylcarboxyamido)benzeneselenenic acid potassium salt 17
Yield: 97%; 1H NMR (700 MHz, D2O) δ = 0.87 (t, J=7.0 Hz, 3H, CH3), 1.53–1.57 (m, 2H, CH2), 3.28 (t, J = 7.0 Hz, 2H, N-CH2), 7.52 (dt, J1 = 1.4, J2 = 7.7 Hz, 1H, 1Har), 7.63 (dd, J1 = 1.4, J2 = 7.7 Hz, 1H, 1Har), 7.67 (dt, J1 = 1.4, J2 = 7.7 Hz, 1H, 1Har), 8.00 (dd, J1 = 1.4, J2 = 7.7 Hz, 1H, 1Har); 13C NMR (100.6 MHz, D2O) δ = 10.73 (CH3), 21.89 (CH2), 41.83 (CH2), 124.47 (CHar), 127.35 (CHar), 130.94 (CHar), 132.32 (CHar), 133.23 (Car), 150.94 (Car), 169.53 (C=O); 77Se NMR (76 MHz, D2O) δ = 1142.45 ppm; IR: 3196, 3050, 2952, 2867, 1630, 1590, 1560, 1458, 1443, 1374, 1358, 1339, 1320, 1286, 1244, 1148 cm−1; Elemental Anal. Calcd for C10H12KNO3Se (312.96): C, 38.46; H, 3.87; N, 4.49 Found: C, 38.72; H, 3.93; N, 4.61.
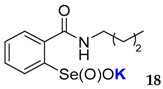
2-(N-butylcarboxyamido)benzeneselenenic acid potassium salt 18
Yield: 96%; 1H NMR (700 MHz, D2O) δ = 0.83 (t, J=7.7 Hz, 3H, CH3), 1.28–1.33 (m, 2H, CH2), 1.49–1.55 (m, 2H, CH2), 3.31 (t, J = 7.7 Hz, 2H, N-CH2), 7.51 (t, J = 7.7 Hz, 1H, 1Har), 7.61 (d, J = 7.7 Hz, 1H, 1Har), 7.66 (dt, J1 = 0.7, J2 = 7.0 Hz, 1H, 1Har), 8.00 (d, J = 7.7 Hz, 1H, 1Har); 13C NMR (100.6 MHz, D2O) δ = 13.02 (CH3), 19.54 (CH2), 30.49 (CH2), 39.78 (CH2), 124.45 (CHar), 127.33 (CHar), 130.93 (CHar), 132.29 (CHar), 133.24 (Car), 150.93 (Car), 169.46 (C=O); 77Se NMR (76 MHz, D2O) δ = 1142.95 ppm; IR: 3216, 3061, 2957, 2930, 2870, 1628, 1588, 1547, 1460, 1440, 1318, 1258, 1148, 1125 cm−1; Elemental Anal. Calcd for C10H12KNO3Se (326.98): C, 40.49; H, 4.32; N, 4.29 Found: C, 40.23; H, 4.41; N, 4.44.
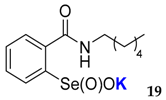
2-(N-hexylcarboxyamido)benzeneselenenic acid potassium salt 19
Yield: 89%; 1H NMR (700 MHz, D2O) δ = 0.76 (t, J = 7.0 Hz, 3H, CH3), 1.20–1.22 (m, 4H, 2 × CH2), 1.51–1.55 (m, 2H, CH2), 3.30 (t, J = 7.0 Hz, 2H, N-CH2), 7.50 (dt, J1 = 0.7, J2=7.7 Hz, 1H, 1Har), 7.59 (dd, J1 = 1.4, J2 = 7.0 Hz, 1H, 1Har), 7.65 (dt, J1 = 1.4, J2 = 7.0 Hz, 1H, 1Har), 7.99 (d, J = 7.7 Hz, 1H, 1Har); 13C NMR (100.6 MHz, D2O) δ=13.33 (CH3), 21.95 (CH2), 25.85 (CH2), 28.27 (CH2), 30.73 (CH2), 40.06 (CH2), 124.48 (CHar), 127.30 (CHar), 130.95 (CHar), 132.31 (CHar), 133.25 (Car), 150.98 (Car), 169.40 (C=O); 77Se NMR (76 MHz, D2O) δ = 1142.17 ppm; IR: 3218, 3058, 2955, 2926, 2856, 1626, 1588, 1543, 1459, 1436, 1400, 1376, 1314, 1252, 1120, 1012 cm−1; Elemental Anal. Calcd for C13H18KNO3Se (355.01): C, 44.06; H, 5.12; N, 3.95 Found: C, 44.38; H, 5.03; N, 4.19.
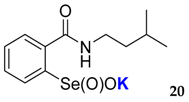
2-(N-(3-methyl)butylcarboxyamido)benzeneselenenic acid potassium salt 20
Yield: 91%; 1H NMR (700 MHz, D2O) δ = 0.85 (d, J = 7.0 Hz, 6H, 2 × CH3), 1.43–1.46 (m, 2H, CH2), 1.58–1.62 (m, 1H, CH), 3.34 (t, J = 7.0 Hz, 2H, N-CH2), 7.51 (dt, J1 = 0.7, J2 = 7.7 Hz, 1H, 1Har), 7.60 (dd, J1 = 1.4, J2 = 7.7 Hz, 1H, 1Har), 7.66 (dt, J1 = 1.4, J2 = 7.7 Hz, 1H, 1Har), 8.00 (d, J = 7.0 Hz, 1H, 1Har); 13C NMR (100.6 MHz, D2O) δ = 21.71 (2 × CH3), 25.28 (CH), 37.24 (CH2), 38.43 (CH2), 124.49 (CHar), 127.34 (CHar), 130.95 (CHar), 132.32 (CHar), 133.28 (Car), 150.98 (Car), 169.40 (C=O); 77Se NMR (76 MHz, D2O) δ = 1142.29 ppm; IR: 3189, 3057, 2954, 2929, 2869, 1627, 1588, 1545, 1461, 1365, 1315, 1260, 1227 cm−1; Elemental Anal. Calcd for C12H16KNO3Se (340.99): C, 42.35; H, 4.74; N, 4.12 Found: C, 42.13; H, 4.68; N, 4.27.
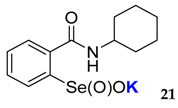
2-(N-cyclohexylcarboxyamido)benzeneselenenic acid potassium salt 21
Yield: 89%; 1H NMR (700 MHz, D2O) δ = 1.08 (m, 1H, CH), 1.22-1.31 (m, 4H, 2 × CH2), 1.53–1.54 (m, 1H, CH), 1.64–1.68 (m, 2H, CH2), 1.85–1.86 (m, 2H, CH2), 3.70–3.73 (m, 1H, CH), 7.49 (dt, J1 = 0.7, J2 = 7.7 Hz, 1H, 1Har), 7.58 (dd, J1 = 0.7, J2=7.0 Hz, 1H, 1Har), 7.65 (dt, J1 = 0.7, J2 = 7.7 Hz, 1H, 1Har), 7.98 (d, J = 7.7 Hz, 1H, 1Har); 13C NMR (176.08 MHz, D2O) δ = 24.16 (2 × CH2), 24.56 (CH2), 31.54 (2 × CH2), 49.40 (CH), 123.95 (CHar), 126.97 (CHar), 130.46 (CHar), 131.79 (CHar), 133.03 (Car), 150.35 (Car), 168.16 (C=O); 77Se NMR (76 MHz, D2O) δ = 1142.44 ppm; IR: 3183, 3057, 2927, 2853, 1628, 1587, 1540, 1449, 1369, 1336, 1300, 1257, 1072, 1053 cm−1; Elemental Anal. Calcd for C13H16KNO3Se (340.99): C, 44.32; H, 4.58; N, 3.98 Found: C, 44.55; H, 4.64; N, 3.82.
2.3. Antioxidant Activity Assay
Compounds 10–21 (0.1, 0,01 or 0,0075equiv.) and dithiothreitol DTTred (0.15 mmol) were dissolved in 1.1 mL of CD3OD or D2O. Next, 30% H2O2 (0.15 mmol) was added and 1H NMR spectra were directly measured in specific time intervals. Following the changes in the integration on the 1H NMR spectra, the decay of the substrate was evaluated.
2.4. 3-(4,5-Dimethyldiazol-2-yl)-2,5 Diphenyl Tetrazolium Bromide (MTT) Viability Assay
The MTT test was conducted by Mosmann methodology [21].
3. Results and Discussion
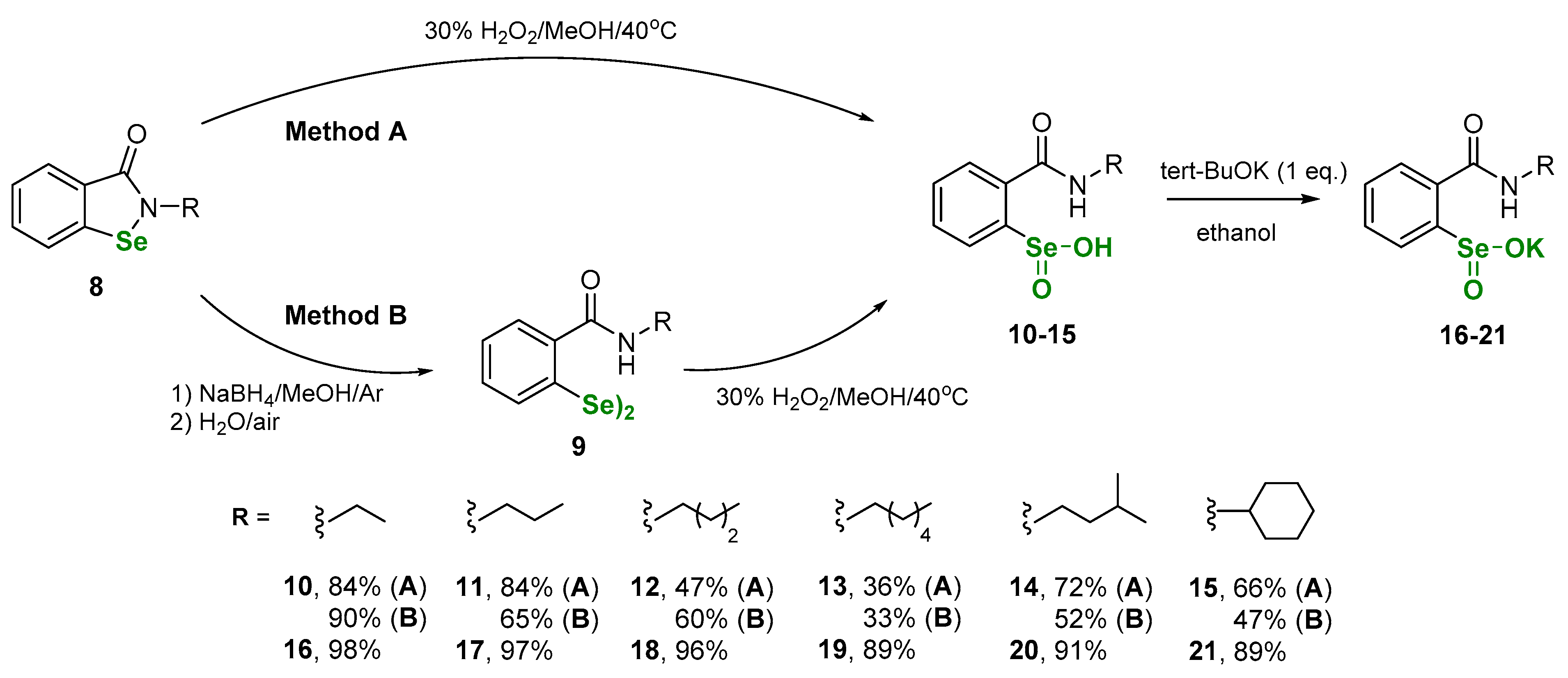
The first step of this study involved the synthesis of N-alkyl benzeneselenenic acids with o-amido function. The compounds were obtained by two different methods based on the oxidation of N-alkylbenzisoselenazol-3(2H)-ones 8 - method A, or corresponding diselenides 9 - method B. Derivatives 8 and 9 were synthetized according to our previously published procedures [22,23,24,25]. The overall yields of both methods A and B were comparable. Next, benzeneseleninic acids 10-15 were transformed to the corresponding benzeneseleninic salts 16–21. The reaction was conducted using potassium tert-butanolate in anhydrous ethanol (Scheme 3).
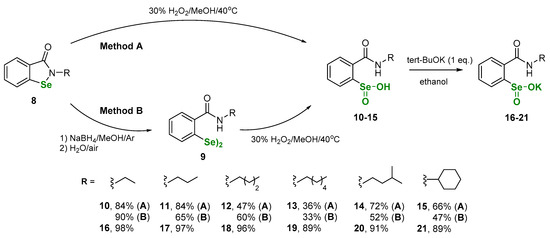
Scheme 3.
Sythesis of benzeneseleninic acids 10–15 and corresponding potassium salts 16–21.
The structures of both, benzeneseleninic acids and their potassium salts were fully characterized by 1H, 13C and 77Se NMR. The spectra were obtained in deuterated dimethyl sulfoxide (for compounds 10–15) and water (16–21). According to the best of our knowledge, NMR spectra of benzeneseleninic acids salts have never been published before.
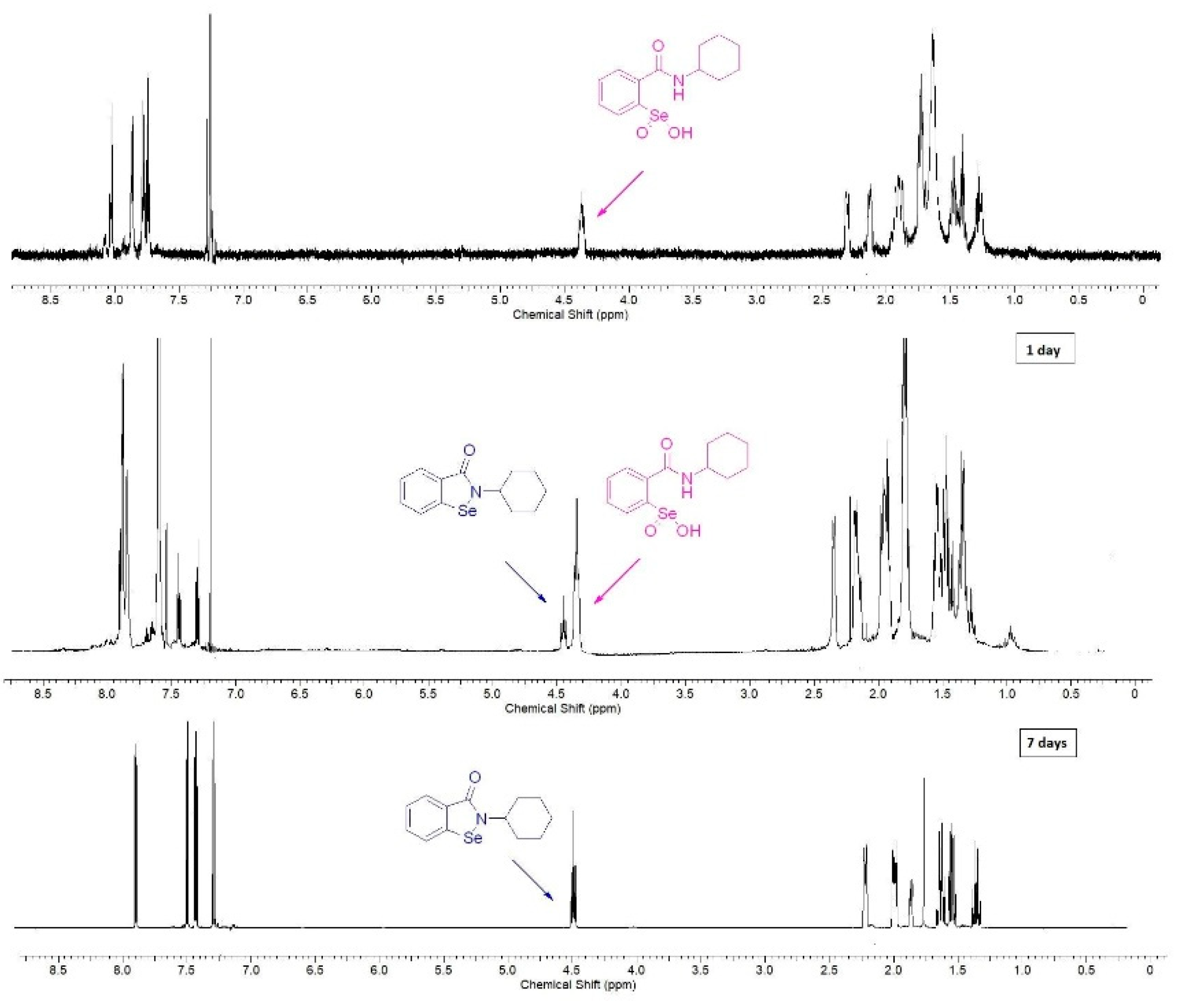
We observed that the stability of derivatives 10–15 in solution depends on the used solvent. Compound 15, dissolved in chloroform, was partially decomposed in 24 h and fully converted into the corresponding benzisoselenazolone in 7 days (Figure 1).
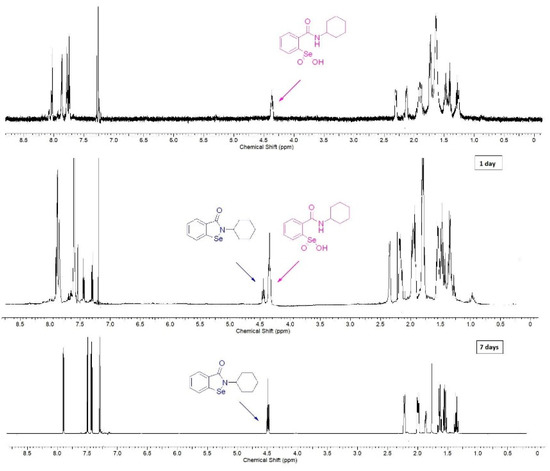
Figure 1.
Decomposition of benzeneseleninic acid 15 to the corresponding benzisoselenazolone.
On the contrary, in DMSO, the decomposition was significantly slower and the sample could be stored for one week with no side-product formation. As it has been presented in Scheme 2, the transformation of benzeneseleninic acid 5 to ebselen 7 takes place through the formation of the selenenic acid 6. This could indicate that the rate of the reduction of R-SeOOH to R-SeOH can be influenced by the type of a solvent.
All derivatives were tested as antioxidants using the NMR activity assay proposed by Iwaoka and co-workers [26]. The efficiency of the peroxide scavenging potential was measured by the rate of dithiothreitol oxidation (DTTred to DTTox) in the presence of the selenocatalyst and equimolar amount of H2O2. Compounds 10–15 were applied in 0.1 molar equivalent and dissolved in deuterated methanol. Results are presented in Table 1.

Table 1.
Results of the antioxidant activity measurement.
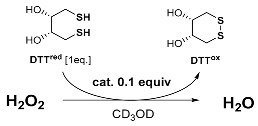
All benzeneseleninic acids 10–15 were more reactive than ebselen (N-phenylbenzisoselenazol-3(2H)-one) 7. The activity was higher for compounds 14 and 15, possessing more bulky substituents. The best result was obtained for N-cyclohexylbenzisoselenazol-3(2H)-one 15, for which only 2% of the substrate was observed after 60 minutes of reaction time. When the -SeOOH group was exchanged for -SeOOK, the activity increased drastically. The reaction was completed in 3 minutes. Consequently, all benzeneseleninic acid salts 16–21 were evaluated by the same procedure but using 0.01 equivalent of the Se-catalyst (Table 2).

Table 2.
Results of the antioxidant activity measurement – 0.01 eq. of Se-catalyst.
In these conditions, almost no conversion was observed for ebselen. The N-ethyl derivative 16 was the most active one. Other results corresponded to the ones obtained for areneseleninic acids, with higher antioxidant potential observed for more sterically hindered compounds 20 and 21. The differences between antioxidant properties was initially evaluated by t-test. Due to various variance of tested groups, nonparametric Mann-Whitney test was additionally performed to confirm it. Both tests showed that the antioxidant properties of areneseleninic acid salts are significantly better than the well-known organoselenium antioxidant ebselen.
As salts 16–21 are soluble in water, they were additionally tested using the procedure proposed by Santi et. al. [27], in which deuterated water was used as a solvent. The reaction was very fast; thus, all compounds were used in only 0.0075 molar equivalents. The amount of a substrate was measured e for each catalyst after 2 min of reaction time (Table 3).

Table 3.
Results of the antioxidant activity measurement performed in D2O – 0.0075 eq. of Se-catalyst.
The best activity was observed for N-butyl 18 and N-ethyl derivative 16, as in the test performed in methanol (Table 2). The amount of DTTred was 15% and 19% respectively, whereas when no catalyst was present still 93% of substrate remained in the reaction mixture. The rate of the process was also significantly improved when N-cyclohexylbenzeneseleninic acid salt 21 was applied. The test was also performed using benzeneseleninic acid potassium salt PhSeOOK. The obtained result indicates that the presence of an electron withdrawing group such as the o-amido function can improve the antioxidant properties.
Finally, the cytotoxic activity of acids 10–15 and salts 16–21 against breast cancer MCF-7 and human promyelocytic leukemia HL-60 cell lines was assessed using the cell viability assay (MTT) [21]. As a reference compound, we have used carboplatin. The results are shown in Table 4.

Table 4.
Cytotoxic activity of acids 1–14 and corresponding salts 16–20.
The highest cytotoxic activity against MCF-7 cells was found for N-cyclohexyl potassium salt 21 with IC50 16.6 ± 1.1 µM. For most compounds, with the exception of acid 12, the conversion of -SeOOH to -SeOOK improved cytotoxicity. In HL-60 cell line the lowest IC50 value of 11.7 ± 1.0 µM was observed for N-ethyl benzeneseleninic acid 10. In general, with one exception (derivative 21), acids with -SeOOH group were more cytotoxic than the corresponding salts, in contrary to the IC50 values obtained for MCF-7 cell line.
4. Conclusions
This article presents the synthesis of the first water-soluble organoselenium antioxidants. A series of N-alkylcarboxyamidobenzeneseleninic acids and N-alkylcarboxyamidobenzeneseleninic acid potassium salts were obtained and evaluated as potential antioxidants and anticancer agents. All benzeneseleninic acid salts exhibited significant peroxide scavenging properties, both in methanol and water. The best obtained antioxidant, 2-(N-ethylcarboxyamido)benzeneselenenic acid potassium salt, used in only 0.01 equivalent, significantly increased the rate of H2O2 reduction, in comparison to the corresponding acid, and the well-known antioxidants, e.g., ebselen and the phenylseleninic acid potassium salt PhSeOOK. It indicates the necessity of adding the N-alkyl-o-amido function to the structure of the designed catalysts. The compounds can be applied in submicromolar amount what can decrease toxic side effects. The highest cytotoxic activity was observed for 2-(N-cyclohexyl-carboxyamido)benzeneselenenic acid potassium salt 21 in MCF-7 cells (IC50 16.6 ± 1.1 µM) and for 2-(N-ethylcarboxyamido)benzeneselenenic acid 15 in HL-60 cells (IC50 11.7 ± 1.0 µM). Generally, the compounds with -SeOOK moiety were more cytotoxic for breast cancer cells, whereas compounds with -SeOOH group were more potent against leukemia cells.
Supplementary Materials
The following are available online at https://www.mdpi.com/1996-1944/13/3/661/s1, Figure S1. (a) 1H NMR, (b) 13C NMR, and (c) 77Se NMR spectra of 2-(N-ethylcarboxyamido)benzeneselenenic acid 10. Figure S2. (a) 1H NMR, (b) 13C NMR, and (c) 77Se NMR spectra of 2-(N-propylcarboxyamido)benzeneselenenic acid 11. Figure S3. (a) 1H NMR, (b) 13C NMR, and (c) 77Se NMR spectra of 2-(N-butylcarboxyamido)benzeneselenenic acid 12. Figure S4. (a) 1H NMR, (b) 13C NMR, and (c) 77Se NMR spectra of 2-(N-hexylcarboxyamido)benzeneselenenic acid 13. Figure S5. (a) 1H NMR, (b) 13C NMR, and (c) 77Se NMR spectra of 2-(N-(3-methyl)butylcarboxyamido)benzeneselenenic acid 14. Figure S6. (a) 1H NMR, (b) 13C NMR, and (c) 77Se NMR spectra of 2-(N-cyclohexylcarboxyamido)benzeneselenenic acid 15. Figure S7. (a) 1H NMR, (b) 13C NMR, and (c) 77Se NMR spectra of 2-(N-ethylcarboxyamido)benzeneselenenic acid potassium salt 16. Figure S8. (a) 1H NMR, (b) 13C NMR, and (c) 77Se NMR spectra of 2-(N-propylcarboxyamido)benzeneselenenic acid potassium salt 17. Figure S9. (a) 1H NMR, (b) 13C NMR, and (c) 77Se NMR spectra of 2-(N-butylcarboxyamido)benzeneselenenic acid potassium salt 18. Figure S10. (a) 1H NMR, (b) 13C NMR, and (c) 77Se NMR spectra of 22-(N-hexylcarboxyamido)benzeneselenenic acid potassium salt 19. Figure S11. (a) 1H NMR, (b) 13C NMR, and (c) 77Se NMR spectra of 2-(N-(3-methyl)butylcarboxyamido)benzeneselenenic acid potassium salt 20. Figure S12. (a) 1H NMR, (b) 13C NMR, and (c) 77Se NMR spectra of 2-(N-cyclohexylcarboxyamido)benzeneselenenic acid potassium salt 21.
Author Contributions
Conceptualization, J.Ś.; Data curation, M.O., A.L. and A.J.P.; Formal analysis, M.O., A.L., A.D.-P. and A.J.P.; Investigation, M.O., A.L., A.J.P., A.D.-P. and A.J.; Writing—original draft, A.J.P. and J.S.; Writing—review & editing, A.J. and J.Ś. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Centre, Poland, grant number 2019/03/X/ST4/00121.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barbosa, N.V.; Nogueira, C.W.; Nogara, P.A.; de Bem, A.F.; Aschnerc, M.; Rocha, J.B.T. Organoselenium compounds as mimics of selenoproteins and thiol modifier agents. Metallomics 2017, 9, 1703–1734. [Google Scholar] [CrossRef] [PubMed]
- Orian, L.; Toppo, S. Organochalcogen peroxidase mimetics as potential drugs: a long story of a promise still unfulfilled. Free Rad. Biol. Med. 2014, 66, 65–74. [Google Scholar]
- Weekley, C.M.; Harris, H.H. Which form is that? The importance of selenium speciation and metabolism in the prevention and treatment of disease. Chem. Soc. Rev. 2013, 42, 8870–8894. [Google Scholar] [CrossRef] [PubMed]
- Ungati, H.; Govindaraj, V.; Narayanan, M.; Mugesh, G. Probing the formation of a Seleninic Acid in Living Cells by a Fluorescence Switching of a Glutathione Peroxidase Mimetic. Angew. Chem. Int. Ed. 2019, 58, 8156–8160. [Google Scholar] [CrossRef]
- Pacuła, A.J.; Mangiavacchi, F.; Sancineto, L.; Lenardao, E.J.; Ścianowski, J.; Santi, C. An Update on “Selenium Containing Compounds from Poison to Drug Candidates: A Review on the GPx-like Activity”. Curr. Chem. Biol. 2015, 9, 97–112. [Google Scholar] [CrossRef]
- Candrini, G.; Malavasi, W.; Preti, C.; Tosi, G.; Zannini, P. Substituted benzeneseleninic acids as bidentate ligands. Synthesis and spectroscopic studies of manganese(H) and iron complexes. Spetrochim. Acta. 1983, 39, 635–639. [Google Scholar] [CrossRef]
- Graziosi, G.; Preti, C.; Tosi, G.; Zannini, P. Osmium (111) Halide Complexes with para- and meta Substituted Benzeneseleninato Ligands. Aust. J. Chem. 1985, 38, 1675–1684. [Google Scholar] [CrossRef]
- Yao, N.-T.; Zhang, R.-F.; Zhang, S.-L.; Li, Q.-L.; Ma, C.-L. A novel octa-nuclear 32-membered zirconocene macrocycle based on the aromatic selenite. Dalton Trans. 2017, 46, 524–528. [Google Scholar] [CrossRef]
- Ugandhar, U.; Baskar, V. Monoorganoantimony(V) Phosphonates and PhosphoSelininates. Dalton Trans. 2016, 45, 6269–6274. [Google Scholar] [CrossRef]
- Yan, M.; Zhang, R.-F.; Zhang, S.-L.; Ru, J.; Du, J.-Y.; Ma, C.-L. Novel cobalt (II) coordination complexes based on 3,4,5-trifluorobenzeneseleninic acid and different N-donor ligands. Polyhedron 2018, 146, 172–179. [Google Scholar] [CrossRef]
- Syper, L.; Młochowski, J. Benzeneperoxyseleninic Acids - Synthesis and Properties. Tetrahedron 1987, 43, 207–213. [Google Scholar] [CrossRef]
- Hori, T.; Sharpless, K.B. Synthetic Applications of Arylselenenic and Arylseleninic Acids. Conversion of Olefins to Allylic Alcohols and Epoxides. J. Org. Chem. 1978, 43, 1689–1694. [Google Scholar] [CrossRef]
- Taylor, T.R.; Flood, L.A. Polystyrene-Bound Phenylseleninic Acid: Catalytic Oxidations of Olefins, Ketones, and Aromatic Systems. J. Org. Chem. 1983, 48, 5160–5164. [Google Scholar] [CrossRef]
- Back, G.T.; Collins, S.; Kerr, R.G. Oxidation of Hydrazines with Benzeneseleninic Acid and Anhydride. J. Org. Chem. 1981, 46, 1564–1570. [Google Scholar] [CrossRef]
- Teskey, Ch.J.; Adler, P.; Gonzalves, C.; Maulide, N. Chemoselective α,β-Dehydrogenation of Saturated Amides. Angew.Chem. Int. Ed. 2019, 58, 447–451. [Google Scholar] [CrossRef]
- Jing, X.; Yuan, D.; Yua, L. Green and Practical Oxidative Deoximation of Oximes to Ketones or Aldehydes with Hydrogen Peroxide/Air by Organoselenium Catalysis. Adv. Synth. Catal. 2017, 359, 1194–1201. [Google Scholar] [CrossRef]
- Sarma, B.K.; Mugesh, G. Antioxidant Activity of the Anti-Inflmmatory Compound Ebselen: A Reversible Cyclization Pathway via Selenenic and Seleninic Acid Intermediates. Chem. Eur. J. 2008, 14, 10603–10614. [Google Scholar] [CrossRef]
- Bhabak, K.P.; Mugesh, G. Amide-Based Glutathione Peroxidase Mimics: Effect of Secondary and Tertiary Amide Substituents on Antioxidant Activity. Chem. Asian J. 2009, 4, 974–983. [Google Scholar] [CrossRef]
- Bhabak, K.P.; Vernekar, A.A.; Jakka, S.R.; Roy, G.; Mugesh, G. Mechanistic investigations on the efficient catalytic decomposition of peroxynitrite by ebselen analogues. Org. Biomol. Chem. 2011, 9, 5193–5200. [Google Scholar] [CrossRef]
- Du, P.; Viswanathan, U.M.; Xu, Z.; Ebrahimnejad, H.; Hanf, B.; Burkholz, T.; Schneider, M.; Bernhardt, I.; Kirsch, G.; Jacob, C. Synthesis of amphiphilic seleninic acid derivatives with considerable activity against cellular membranes and certain pathogenic microbes. J. Haz. Mat. 2014, 269, 74–82. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Pacuła, A.J.; Ścianowski, J.; Aleksandrzak, K.B. Highly efficient synthesis and antioxidant capacity of N-substituted benzisoselenazol-3(2H)-ones. RSC Advances 2014, 4, 48959–48962. [Google Scholar] [CrossRef]
- Pacuła, A.J.; Kaczor, K.B.; Wojtowicz, A.; Antosiewicz, J.; Janecka, A.; Długosz, A.; Janecki, T.; ´Scianowski, J. New glutathione peroxidase mimetics—Insights into antioxidant and cytotoxic activity. Bioorg. Med. Chem. 2017, 25, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Pacuła, A.J.; Kaczor, K.B.; Antosiewicz, J.; Janecka, A.; Długosz, A.; Janecki, T.; Wojtczak, A.; Ścianowski, J. New Chiral Ebselen Analogues with Antioxidant and Cytotoxic Potential. Molecules 2017, 22, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Pacuła, A.J.; Obieziurska, M.; Ścianowski, J.; Kaczor, K.B.; Antosiewicz, J. Synthesis of biologically active diaryl diselenides under water control. Arkivoc 2018, iii, 144–155. [Google Scholar]
- Kumakura, F.; Mishra, B.; Priyadarsini, K.I.; Iwaoka, M. A Water-Soluble Cyclic Selenide with Enhanced Glutathione Peroxidase-Like Catalytic Activities. Eur. J. Org. Chem. 2010, 3, 440–444. [Google Scholar] [CrossRef]
- Tidei, C.; Piroddi, M.; Galli, F.; Santi, C. Oxidation of thiols promoted by PhSeZnCl. Tetrahedron Lett. 2012, 53, 232–234. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).