Flexible and Electroactive Ionogel Graphene Composite Actuator
Abstract
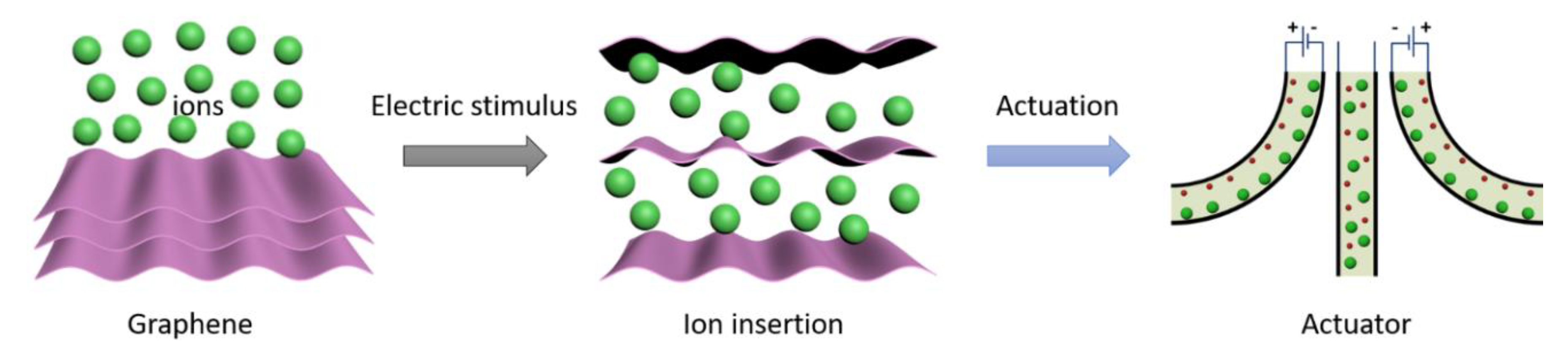
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Ionogel Electrolyte
2.3. Fabrication of Ionogel Graphene Composite Actuator
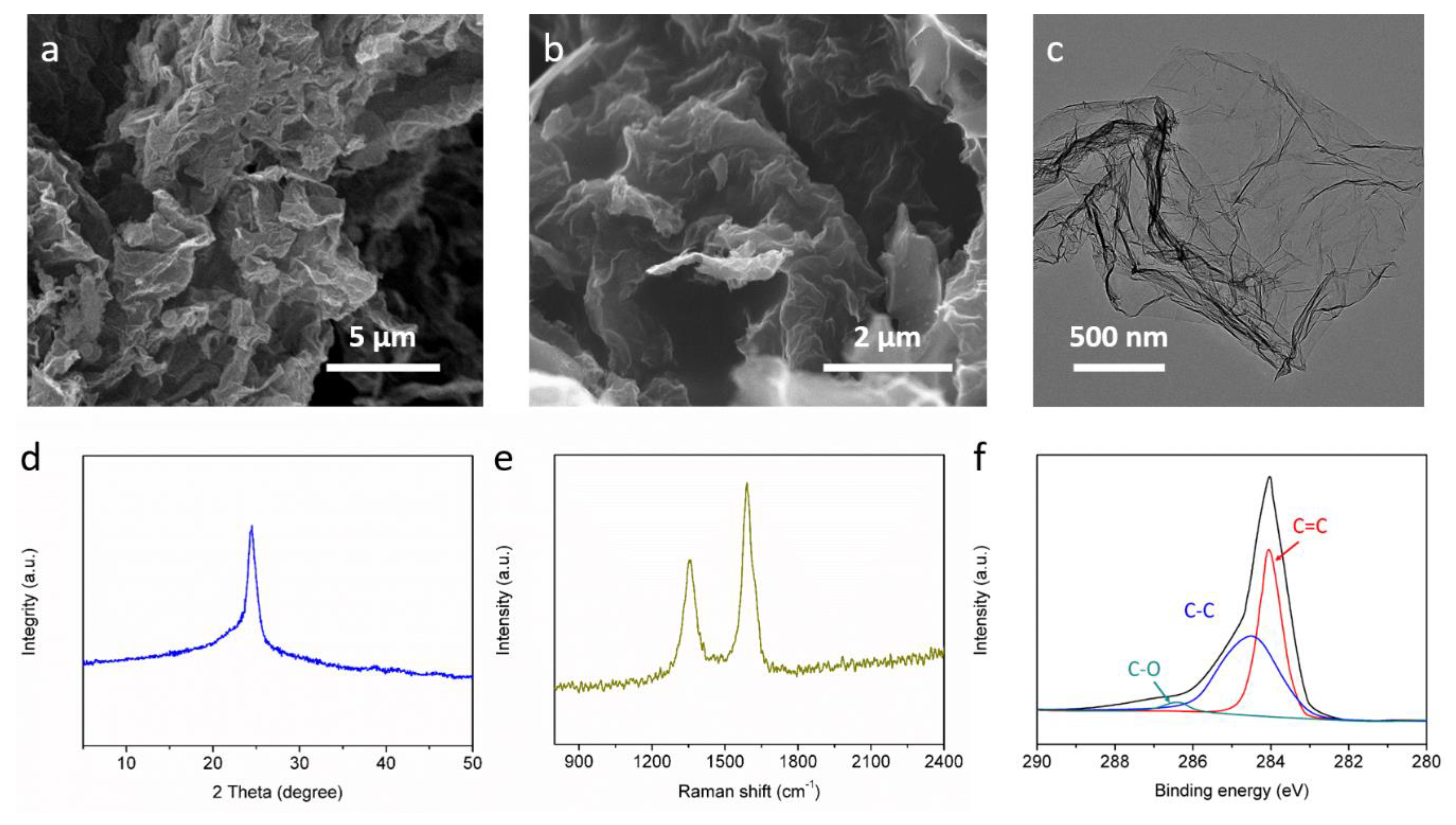
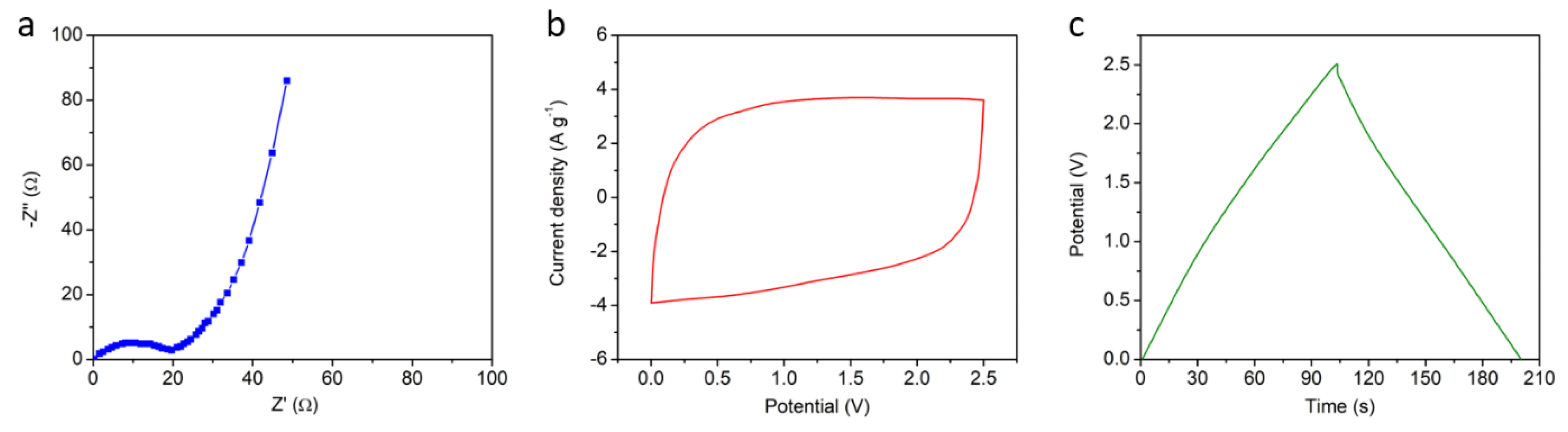
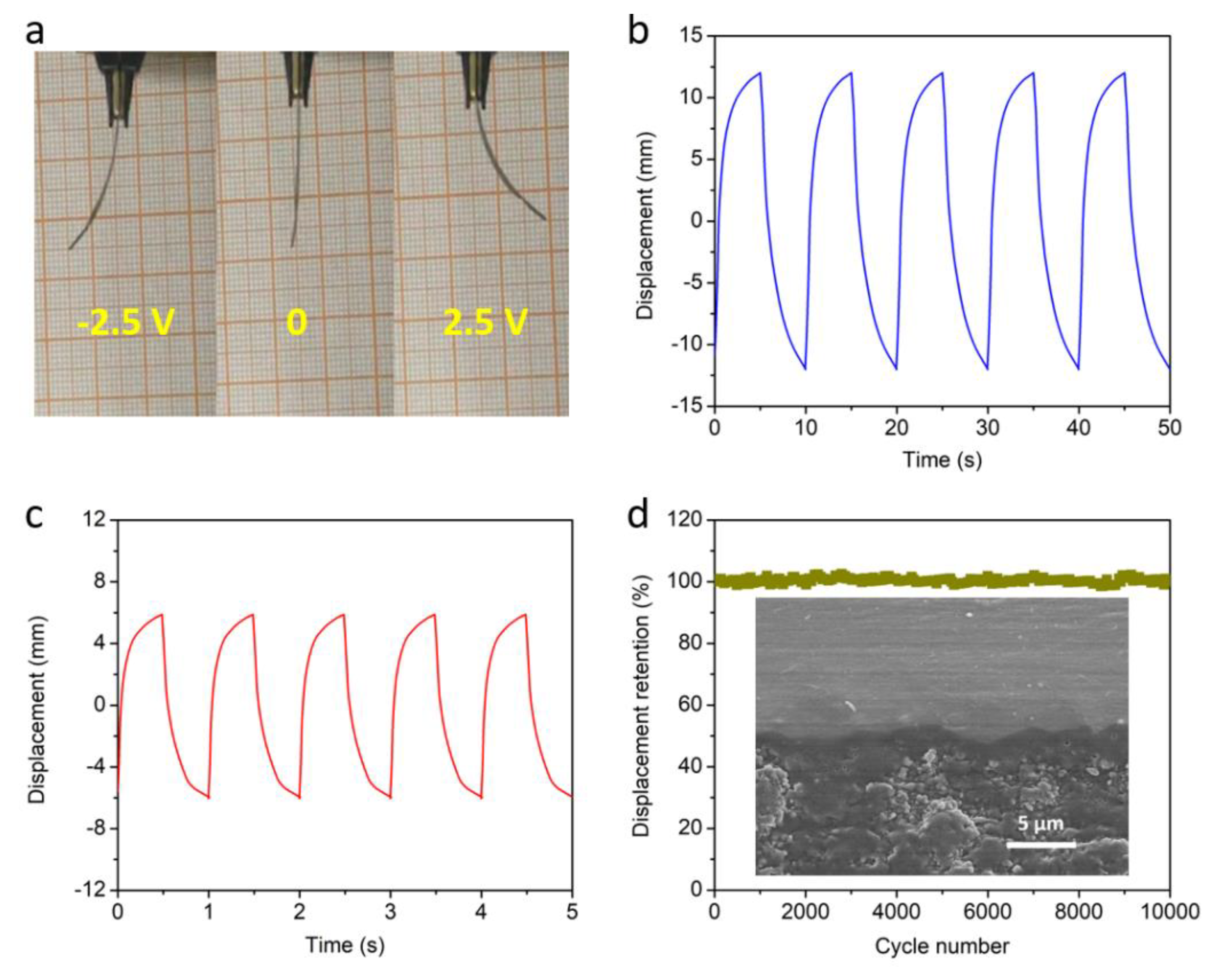
2.4. Characterizations
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gong, J.; Lin, H.; Dunlop, J.W.C.; Yuan, J. Hierarchically arranged helical fiber actuators derived from commercial cloth. Adv. Mater. 2017, 29, 1605103. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Yang, Y.; Wang, J.; Fu, R.; Zhao, X.; Zhao, L.; Ming, Y.; Hu, Y.; Lin, H.; Tao, X.; et al. High-performance graphdiyne-based electrochemical actuators. Nat. Commun. 2018, 9, 752. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Tarakanova, A.; Hsu, C.C.; Yu, M.; Zheng, S.; Yu, L.; Liu, J.; He, Y.; Dunstan, D.J.; Buehler, M.J. Spider dragline silk as torsional actuator driven by humidity. Sci. Adv. 2019, 5, eaau9183. [Google Scholar] [CrossRef] [PubMed]
- Umrao, S.; Tabassian, R.; Kim, J.; Nguyen, V.H.; Zhou, Q.; Nam, S.; Oh, I.-K. MXene artificial muscles based on ionically cross-linked Ti3C2Tx electrode for kinetic soft robotics. Sci. Rob. 2019, 4, eaaw7797. [Google Scholar] [CrossRef]
- Kong, L.; Chen, W. Carbon nanotube and graphene-based bioinspired electrochemical actuators. Adv. Mater. 2014, 26, 1025–1043. [Google Scholar] [CrossRef]
- Shahinpoory, M.; Bar-Cohenz, Y.; Simpsonx, J.O.; Smithx, J. Ionic polymer-metal composites (IPMCs) as biomimetic sensors, actuators and artificial muscles—A review. Smart Mater. Struct. 1998, 7, 15–30. [Google Scholar] [CrossRef]
- Lee, J.A.; Li, N.; Haines, C.S.; Kim, K.J.; Lepro, X.; Ovalle-Robles, R.; Kim, S.J.; Baughman, R.H. Electrochemically powered, energy-conserving carbon nanotube artificial muscles. Adv. Mater. 2017, 29, 1700870. [Google Scholar] [CrossRef]
- Shao, L.-H.; Biener, J.; Jin, H.-J.; Biener, M.M.; Baumann, T.F.; Weissmüller, J. Electrically tunable nanoporous carbon hybrid actuators. Adv. Funct. Mater. 2012, 22, 3029–3034. [Google Scholar] [CrossRef]
- Fukushima, T.; Asaka, K.; Kosaka, A.; Aida, T. Fully plastic actuator through layer-by-layer casting with ionic-liquid-based bucky gel. Angew. Chem. Int. Ed. Engl. 2005, 44, 2410–2413. [Google Scholar] [CrossRef]
- Kim, O.; Kim, S.J.; Park, M.J. Low-voltage-driven soft actuators. Chem. Commun. 2018, 54, 4895–4904. [Google Scholar] [CrossRef]
- Lu, C.; Chen, X. In situ synthesized PEO/NBR composite ionogels for high-performance all-solid-state supercapacitors. Chem. Commun. 2019, 55, 8470–8473. [Google Scholar] [CrossRef]
- Huang, X.; Zeng, Z.; Fan, Z.; Liu, J.; Zhang, H. Graphene-based electrodes. Adv. Mater. 2012, 24, 5979–6004. [Google Scholar] [CrossRef]
- Lu, C.; Yang, Y.; Chen, X. Ultrathin conductive graphitic carbon nitride assembly through van der Waals epitaxy toward high energy-density flexible supercapacitors. Nano Lett. 2019, 19, 4103–4111. [Google Scholar] [CrossRef]
- Groenewolt, M.; Antonietti, M. Synthesis of g-C3N4 nanoparticles in mesoporous silica host matrices. Adv. Mater. 2005, 17, 1789–1792. [Google Scholar] [CrossRef]
- Lu, C.; Chen, X. Sea-island nanostructured polyvinylidene fluoride/zeolitic imidazolate framework-8 polyelectrolyte for high-performance all-solid-state supercapacitors. J. Power Sources 2020, 448, 227587. [Google Scholar] [CrossRef]
- Li, W.H.; Ding, K.; Tian, H.R.; Yao, M.S.; Nath, B.; Deng, W.H.; Wang, Y.; Xu, G. Conductive metal–organic framework nanowire array electrodes for high-performance solid-state supercapacitors. Adv. Funct. Mater. 2017, 27, 1702067. [Google Scholar] [CrossRef]
- Kaasik, F.; Must, I.; Baranova, I.; Põldsalu, I.; Lust, E.; Johanson, U.; Punning, A.; Aabloo, A. Scalable fabrication of ionic and capacitive laminate actuators for soft robotics. Sens. Actuators B 2017, 246, 154–163. [Google Scholar] [CrossRef]
- Palmre, V.; Lust, E.; Jänes, A.; Koel, M.; Peikolainen, A.-L.; Torop, J.; Johanson, U.; Aabloo, A. Electroactive polymer actuators with carbon aerogel electrodes. J. Mater. Chem. 2011, 21, 2577–2583. [Google Scholar] [CrossRef]
- Lu, C.; Wang, D.; Zhao, J.; Han, S.; Chen, W. A continuous carbon nitride polyhedron assembly for high-performance flexible supercapacitors. Adv. Funct. Mater. 2017, 27, 1606219. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Liang, J.; Chen, Y. Hydrous RuO2-decorated mxene coordinating with silver nanowire inks enabling fully printed micro-supercapacitors with extraordinary volumetric performance. Adv. Energy Mater. 2019, 9, 1803987. [Google Scholar] [CrossRef]
- Lu, C.; Chen, X. An interfacial polymerization strategy towards high-performance flexible supercapacitors. J. Mater. Chem. A 2019, 7, 20158–20161. [Google Scholar] [CrossRef]
- Le Bideau, J.; Viau, L.; Vioux, A. Ionogels, ionic liquid based hybrid materials. Chem. Soc. Rev. 2011, 40, 907–925. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Liu, J.; Hu, Y.; Zhang, Y.; Randriamahazaka, H.; Chen, W. Highly stable air working bimorph actuator based on a graphene nanosheet/carbon nanotube hybrid electrode. Adv. Mater. 2012, 24, 4317–4321. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.P.; Yang, M.C.; Yang, C.H.; Zhong, D.X.; Hsu, M.C.; Chen, Y. Newton output blocking force under low-voltage stimulation for carbon nanotube-electroactive polymer composite artificial muscles. ACS Appl. Mater. Interfaces 2017, 9, 5550–5555. [Google Scholar] [CrossRef]
- Wu, G.; Hu, Y.; Liu, Y.; Zhao, J.; Chen, X.; Whoehling, V.; Plesse, C.; Nguyen, G.T.; Vidal, F.; Chen, W. Graphitic carbon nitride nanosheet electrode-based high-performance ionic actuator. Nat. Commun. 2015, 6, 7258. [Google Scholar] [CrossRef]
- Jung, K.; Nam, J.; Choi, H. Investigations on actuation characteristics of IPMC artificial muscle actuator. Sens. Actuators A Phys. 2003, 107, 183–192. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.; Chen, X. Flexible and Electroactive Ionogel Graphene Composite Actuator. Materials 2020, 13, 656. https://doi.org/10.3390/ma13030656
Lu C, Chen X. Flexible and Electroactive Ionogel Graphene Composite Actuator. Materials. 2020; 13(3):656. https://doi.org/10.3390/ma13030656
Chicago/Turabian StyleLu, Chao, and Xi Chen. 2020. "Flexible and Electroactive Ionogel Graphene Composite Actuator" Materials 13, no. 3: 656. https://doi.org/10.3390/ma13030656
APA StyleLu, C., & Chen, X. (2020). Flexible and Electroactive Ionogel Graphene Composite Actuator. Materials, 13(3), 656. https://doi.org/10.3390/ma13030656




