The Impact of Isothermal Treatment on the Microstructural Evolution and the Precipitation Behavior in High Strength Linepipe Steel
Abstract
1. Introduction
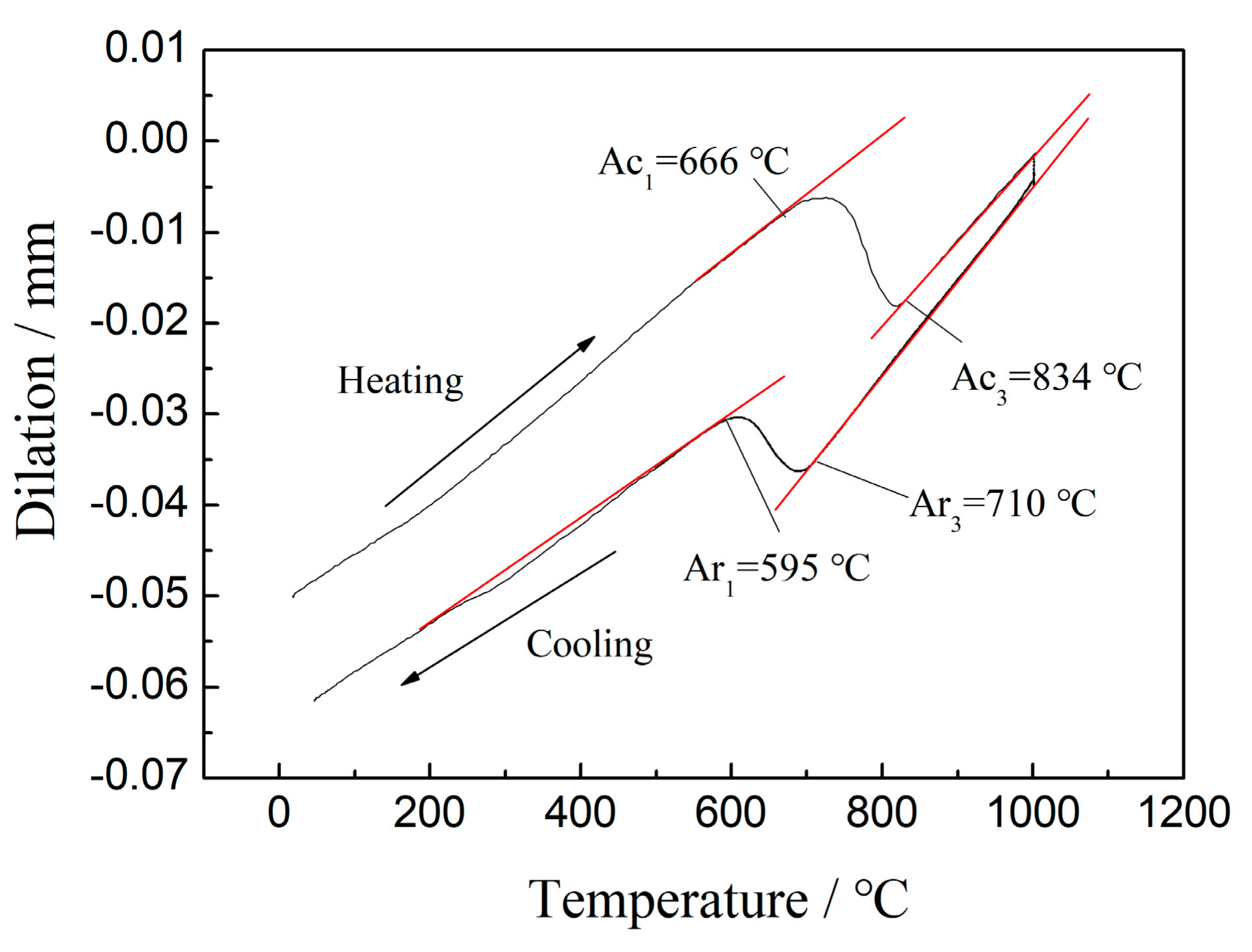
2. Materials and Methods
3. Results
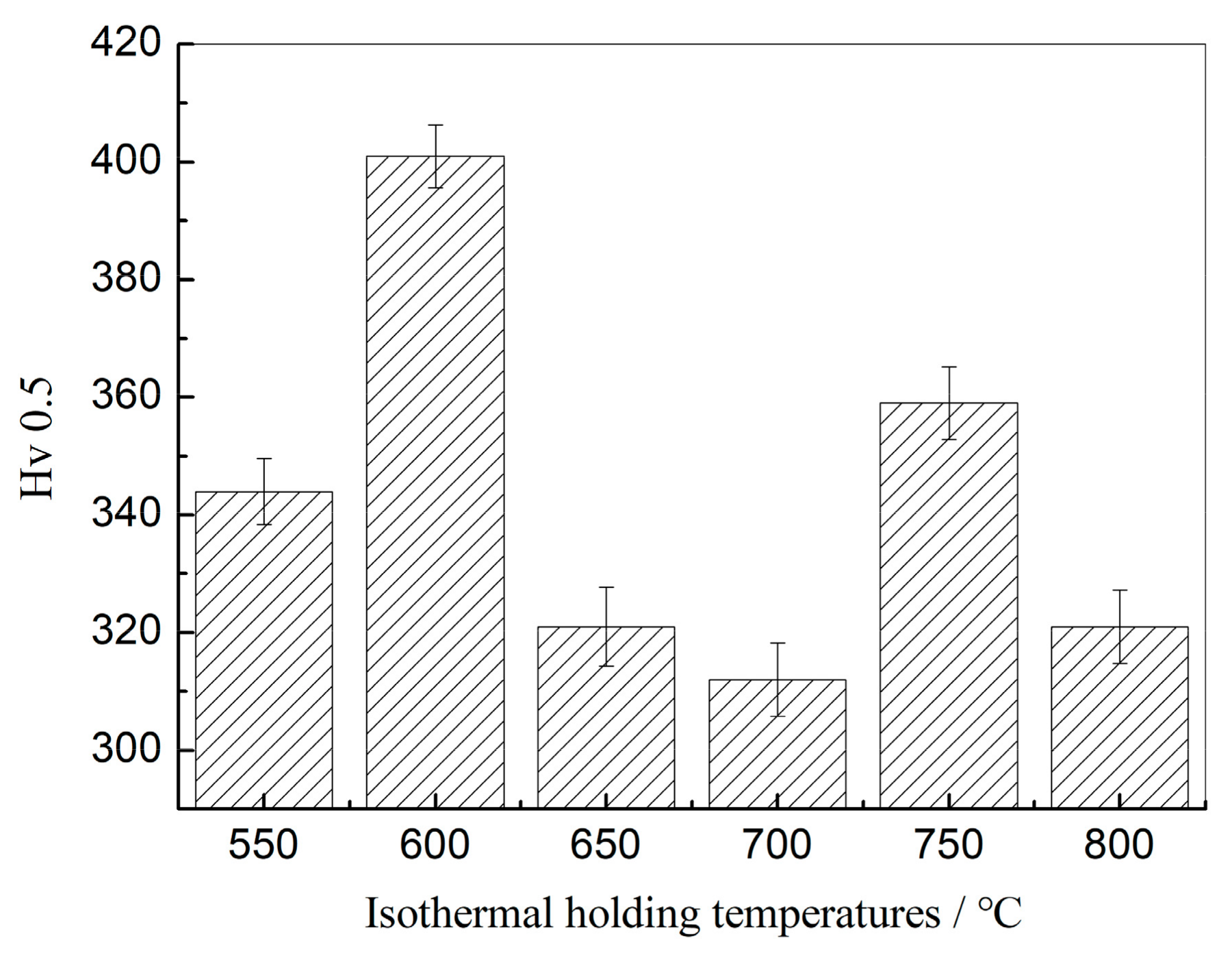
3.1. Vickers Hardness
3.2. Optical Microscopy
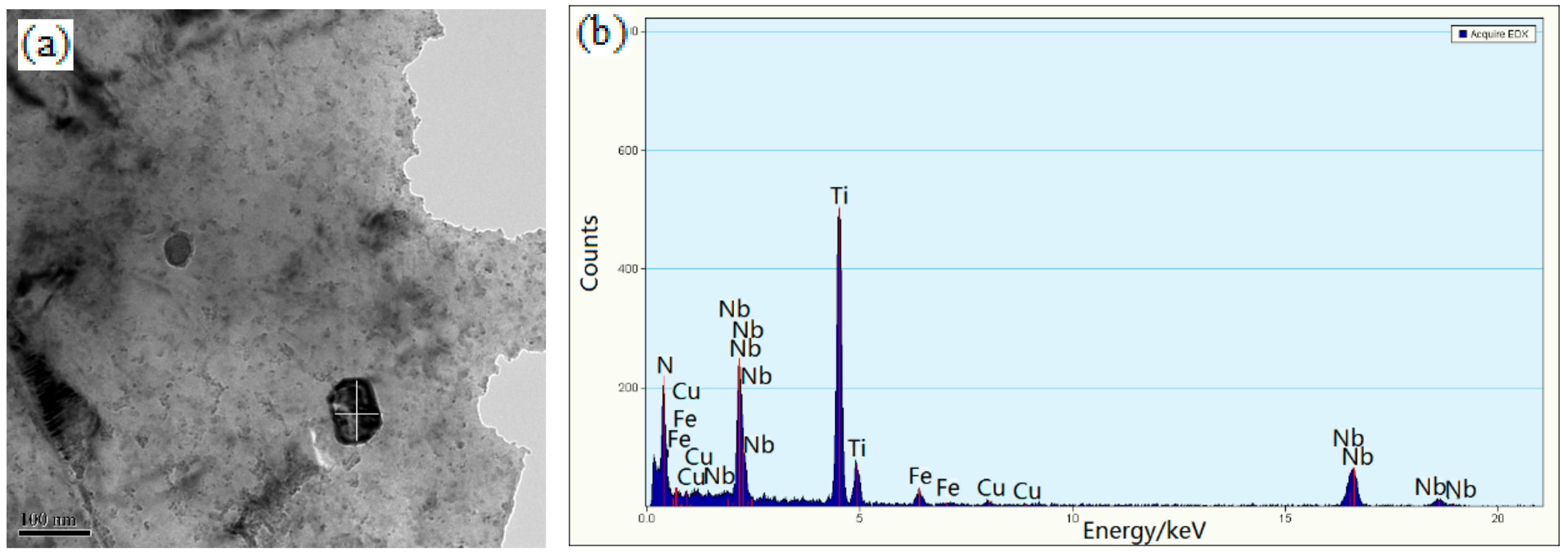
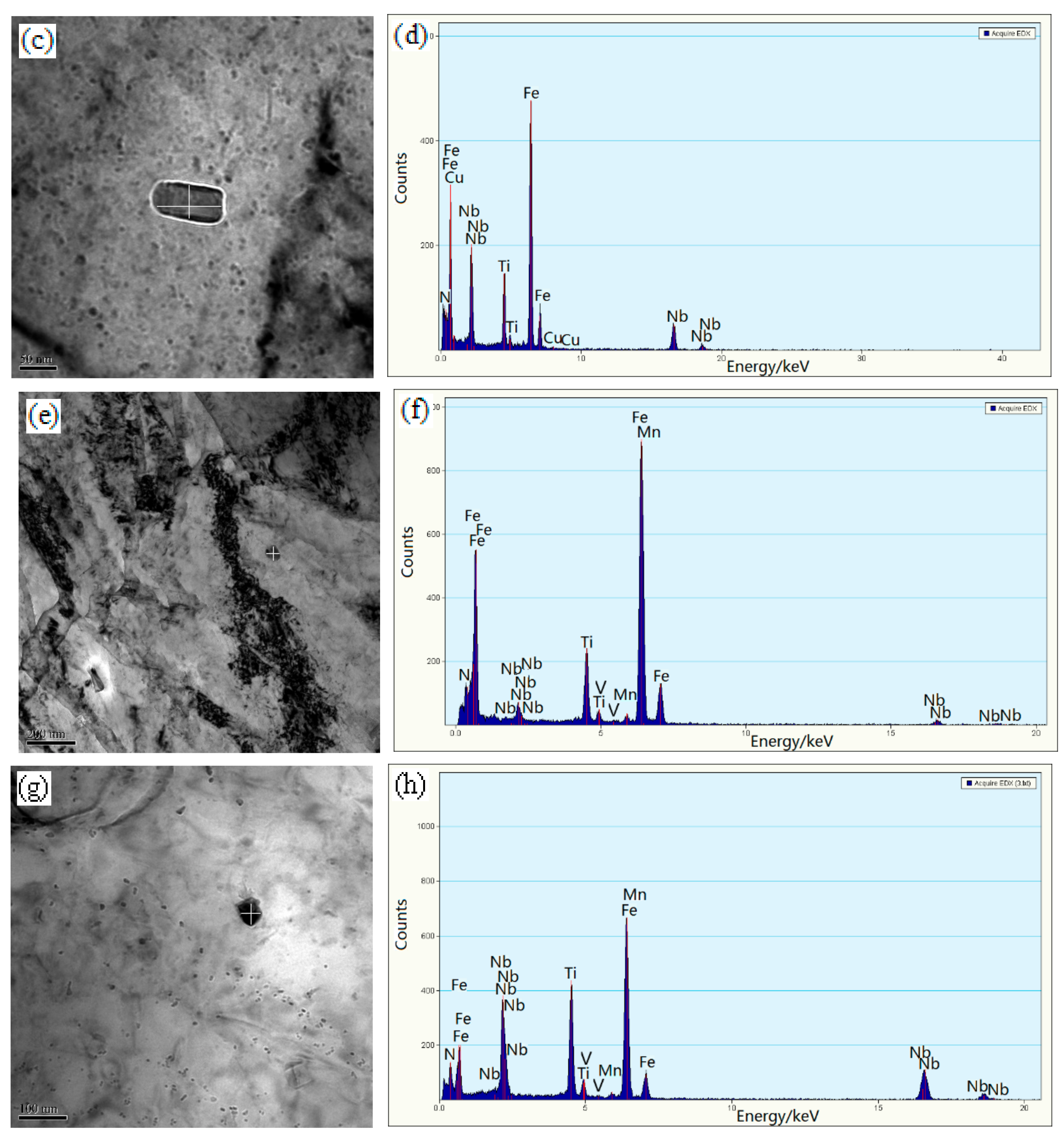
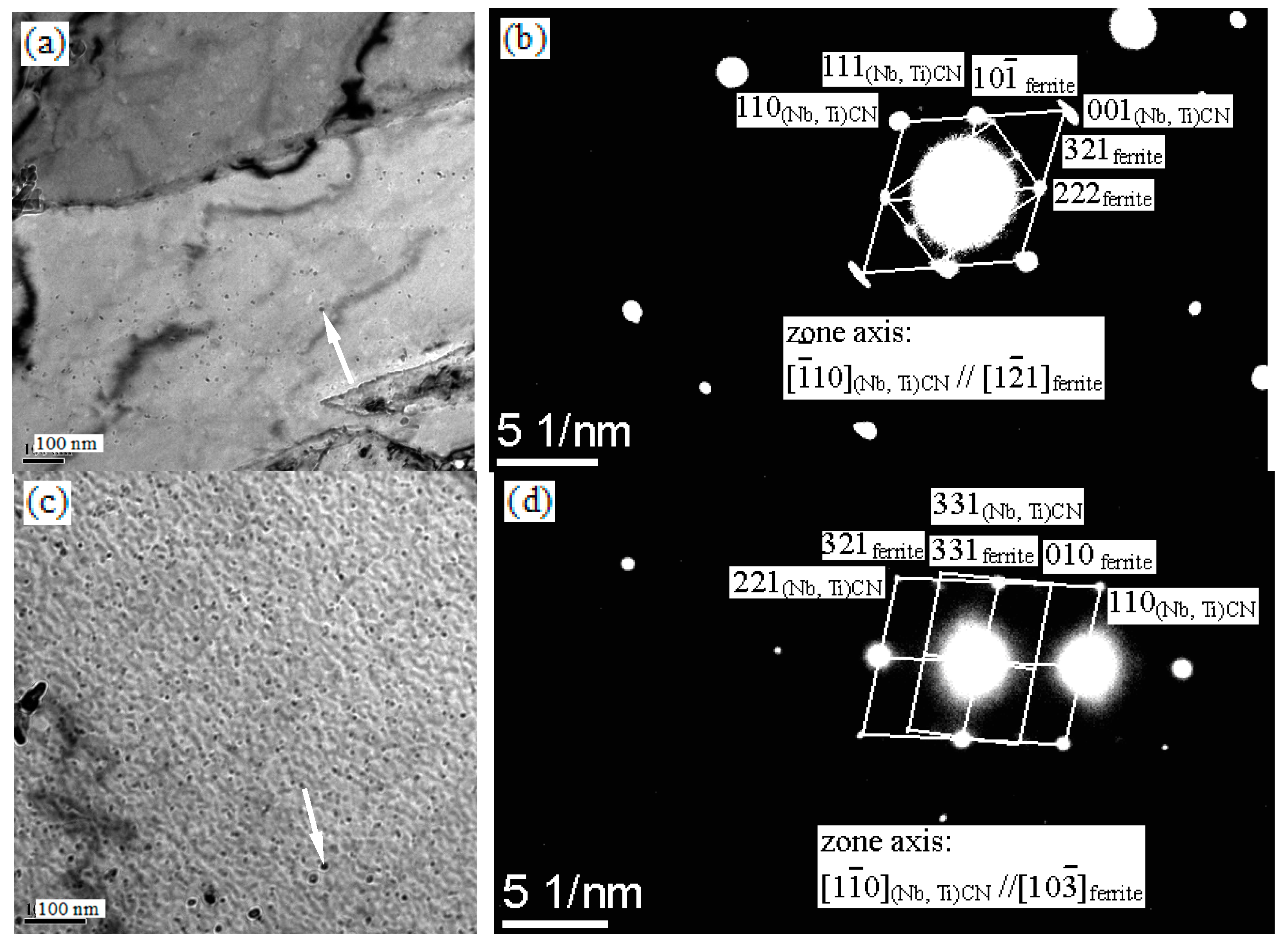
3.3. Electron Microscopy
4. Discussion
4.1. Microstructural Evolution
4.2. The Precipitation Behavior
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Villalobos, J.; Del-Pozo, A.; Campillo, B.; Mayen, J.; Serna, S. Microalloyed steels through history until 2018: review of chemical composition, processing and hydrogen service. Metals 2018, 8, 351. [Google Scholar] [CrossRef]
- Di Schino, A.; Di Nunzio, P.E. Effect of Nb microalloying on the heat affected zone microstructure of girth welded joints. Mater. Lett. 2017, 186, 86–89. [Google Scholar] [CrossRef]
- Shajan, N.; Arora, K.S.; Asati, B.; Sharma, V.; Shome, M. Effects of Post-Weld Heat Treatment on the Microstructure and Toughness of Flash Butt Welded High-Strength Low-Alloy Steel. Metall. Mater. Trans. A 2018, 49, 1276–1286. [Google Scholar] [CrossRef]
- Farzampour, A. Evaluating Shear links for Use in Seismic Structural Fuses. Ph.D. Thesis, Virginia Tech, Blacksburg, VA, USA, 28 January 2019. [Google Scholar]
- Karmakar, A.; Sahu, P.; Neogy, S.; Chakrabarti, D.; Mitra, R.; Mukherjee, S.; Kundu, S. Effect of Cooling Rate and Chemical Composition on Microstructure and Properties of Naturally Cooled Vanadium-Microalloyed Steels. Metall. Mater. Trans. A 2017, 48, 1581–1595. [Google Scholar] [CrossRef]
- McCann, F.; Ridolfi, G.; Karjadi, E.; Demmink, H.; Boyd, H. Numerical modelling of hot polymer-coated steel pipeline joints in bending. Ocean Eng. 2018, 160, 354–367. [Google Scholar] [CrossRef]
- Chatzidouros, E.V.; Traidia, A.; Devarapalli, R.S.; Pantelis, D.I.; Steriotis, T.A.; Jouiad, M. Effect of hydrogen on fracture toughness properties of a pipeline steel under simulated sour service conditions. Int. J. Hydrogen Energy. 2018, 43, 5747–5759. [Google Scholar] [CrossRef]
- Han, S.Y.; Shin, S.Y.; Lee, S.; Kim, N.J.; Bae, J.H.; Kim, K. Denting the oil pipelines by a rigid cylindrical indenter with conical nose by the numerical and experimental analyses. Thin Wall Struct. 2018, 124, 312–322. [Google Scholar]
- Shao, Y.; Liu, C.; Yan, Z.; Li, H.; Liu, Y. Formation mechanism and control methods of acicular ferrite in HSLA steels: A review. J. Mater. Sci. Technol. 2018, 34, 737–744. [Google Scholar] [CrossRef]
- Niu, Y.; Jia, S.; Liu, Q.; Tong, S.; Li, B.; Ren, Y.; Wang, B. Influence of Effective Grain Size on Low Temperature Toughness of High-Strength Pipeline Steel. Materials 2019, 12, 3672. [Google Scholar] [CrossRef]
- Takahashi, J.; Kawakami, K.; Kobayashi, Y. Origin of hydrogen trapping site in vanadium carbide precipitation strengthening steel. Acta Mater. 2018, 153, 193–204. [Google Scholar] [CrossRef]
- Dong, J.; Zhou, X.; Liu, Y.; Li, C.; Liu, C.; Guo, Q. Carbide precipitation in Nb-V-Ti microalloyed ultra-high strength steel during tempering. Mat. Sci. Eng. A-Struct. 2017, 683, 215–226. [Google Scholar] [CrossRef]
- Liu, S.; Challa, V.S.A.; Natarajan, V.V.; Misra, R.D.K.; Sidorenko, D.M.; Mulholland, M.D.; Hartmann, J.E. Significant influence of carbon and niobium on the precipitation behavior and microstructural evolution and their consequent impact on mechanical properties in microalloyed steels. Mater. Sci. Eng. A-Struct. 2017, 683, 70–82. [Google Scholar] [CrossRef]
- Gong, P.; Palmiere, E.J.; Rainforth, W.M. Thermomechanical processing route to achieve ultrafine grains in low carbon microalloyed steels. Acta Mater. 2016, 119, 43–54. [Google Scholar] [CrossRef]
- Xiao, X.; Shi, G.; Zhang, S.; Wang, Q. Effect of Cooling Path on Microstructure Features and Tensile Properties in a Low Carbon Mo-V-Ti-N Steel. Metals 2018, 8, 677. [Google Scholar] [CrossRef]
- Hardy, M. Metallurgical Effects of Niobium and Molybdenum on Heat-Affected Zone Toughness in Low-Carbon Steel. Appl. Sci. 2019, 9, 1847. [Google Scholar]
- Mohrbacher, H. Property optimization in as-quenched martensitic steel by molybdenum and niobium alloying. Metals 2018, 8, 234. [Google Scholar] [CrossRef]
- Hannula, J.; Porter, D.; Kaijalainen, A.; Somani, M.; Kömi, J. Mechanical Properties of Direct-Quenched Ultra-High-Strength Steel Alloyed with Molybdenum and Niobium. Metals 2019, 9, 350. [Google Scholar] [CrossRef]
- Ebrahimi, G.R.; Momeni, A.; Kazemi, S.; Alinejad, H. Flow curves, dynamic recrystallization and precipitation in a medium carbon low alloy steel. Vacuum 2017, 142, 135–145. [Google Scholar] [CrossRef]
- Abbasi, E.; Rainforth, W.M. Effect of Nb-Mo additions on precipitation behaviour in V microalloyed TRIP-assisted steels. Mater. Sci. Technol. 2016, 32, 1721–1729. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Liang, B.; Du, L.; Di, H. Study on Isothermal Precipitation Behavior of Nano-Scale (Nb, Ti) C in Ferrite/Bainite in 780 MPa Grade Ultra-High Strength Steel. Steel Res. Int. 2013, 4, 402–409. [Google Scholar] [CrossRef]
- Girault, E.; Jacques, P.; Harlet, P.; Mols, K.; van Humbeeck, J.; Aernoudt, E.; Delannay, F. Metallographic methods for revealing the multiphase microstructure of TRIP-assisted steels. Mater. Charact. 1998, 40, 111–118. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, H.T.; Wang, Z.D.; Misra, R.D.K.; Wang, G.D. Microstructural Evolution and the Precipitation Behavior in X90 Linepipe Steel During Isothermal Processing. J. Mater. Eng. Perform. 2018, 27, 1494–1504. [Google Scholar] [CrossRef]
- Ioannidou, C.; Arechabaleta, Z.; Navarro-López, A.; Rijkenberg, A.; Dalgliesh, R.M.; Kölling, S.; Offerman, S.E. Interaction of precipitation with austenite-to-ferrite phase transformation in vanadium micro-alloyed steels. Acta Mater. 2019, 181, 10–24. [Google Scholar] [CrossRef]
- Miyamoto, G.; Hori, R.; Poorganji, B.; Furuhara, T. Interphase Precipitation of VC and Resultant Hardening in V-added Medium Carbon Steels. ISIJ Int. 2011, 51, 1733–1739. [Google Scholar] [CrossRef]
- Yen, H.W.; Chen, C.Y.; Wang, T.Y.; Huang, C.Y.; Yang, J.R. Orientation relationship transition of nanometre sized interphase precipitated TiC carbides in Ti bearing steel. Mater. Sci. Technol. 2010, 26, 421–430. [Google Scholar] [CrossRef]
- Nafisi, S.; Amirkhiz, B.S.; Fazeli, F.; Arafin, M.; Glodowski, R.; Collins, L. Effect of Vanadium Addition on the Strength of API X100 Linepipe Steel. ISIJ Int. 2016, 56, 154–160. [Google Scholar] [CrossRef]
- Kostryzhev, A.G.; Al Shahrani, A.; Zhu, C.; Cairney, J.M.; Ringer, S.P.; Killmore, C.R.; Pereloma, E.V. Effect of niobium clustering and precipitation on strength of an NbTi-microalloyed ferritic steel. Mater. Sci. Eng. A-Struct. 2014, 607, 226–235. [Google Scholar] [CrossRef]
- Grajcar, A.; Kwaśny, W.; Zalecki, W. Microstructure–property relationships in TRIP aided medium-C bainitic steel with lamellar retained austenite. Mater. Sci. Technol. 2015, 31, 781–794. [Google Scholar] [CrossRef]
- Kim, J.D.Y.J.; Jung, J.G.; Kim, D.H.; Lee, Y.K. The kinetics of Nb(C,N) precipitation during the isothermal austenite to ferrite transformation in a low-carbon Nb-microalloyed steel. Acta Mater. 2013, 61, 7437–7443. [Google Scholar] [CrossRef]
- Jang, J.H.; Heo, Y.U.; Lee, C.H.; Bhadeshia, H.K.D.H.; Suh, D.W. Interphase precipitation in Ti-Nb and Ti-Nb-Mo bearing steel. Mater. Sci. Technol. 2013, 29, 309–313. [Google Scholar] [CrossRef]


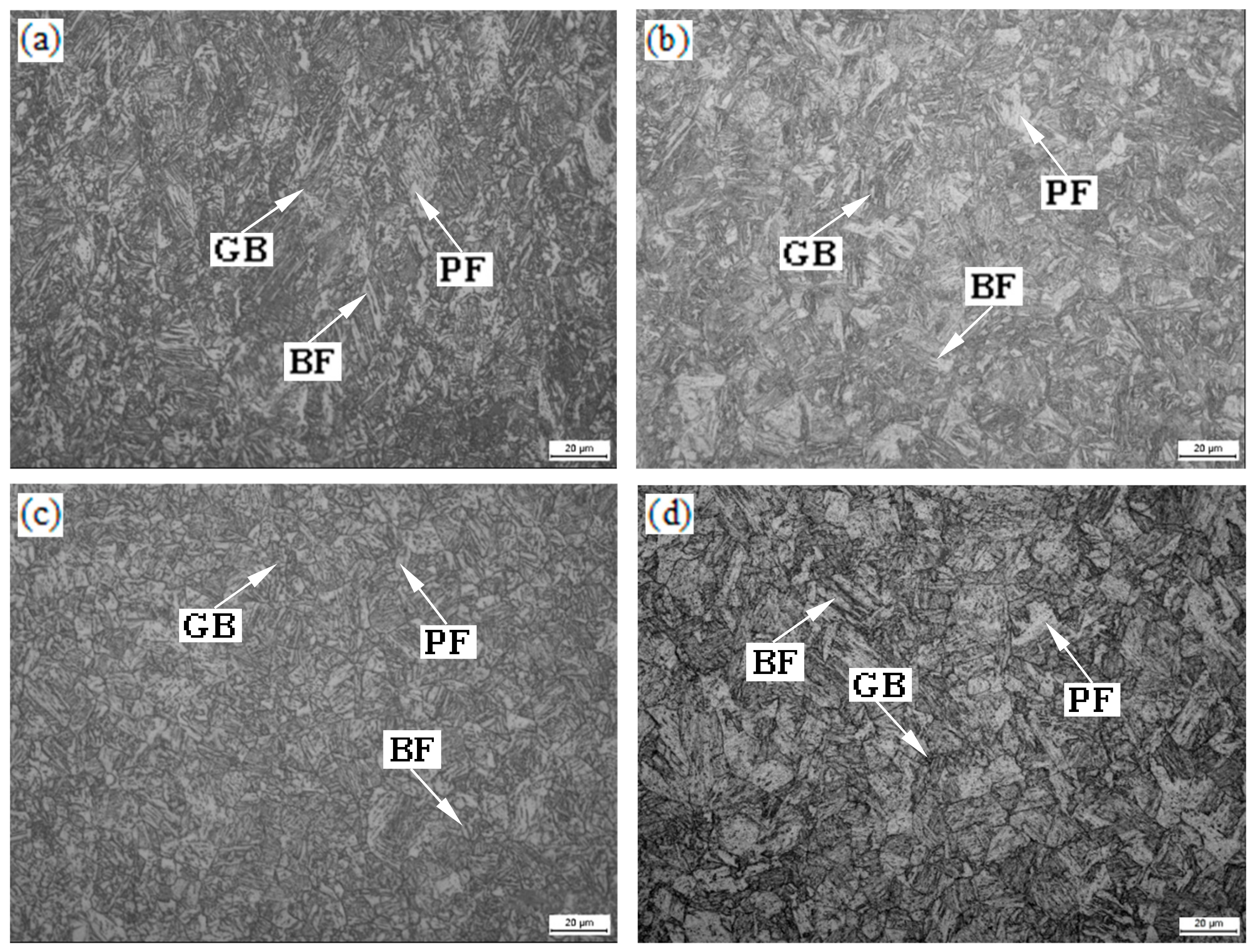
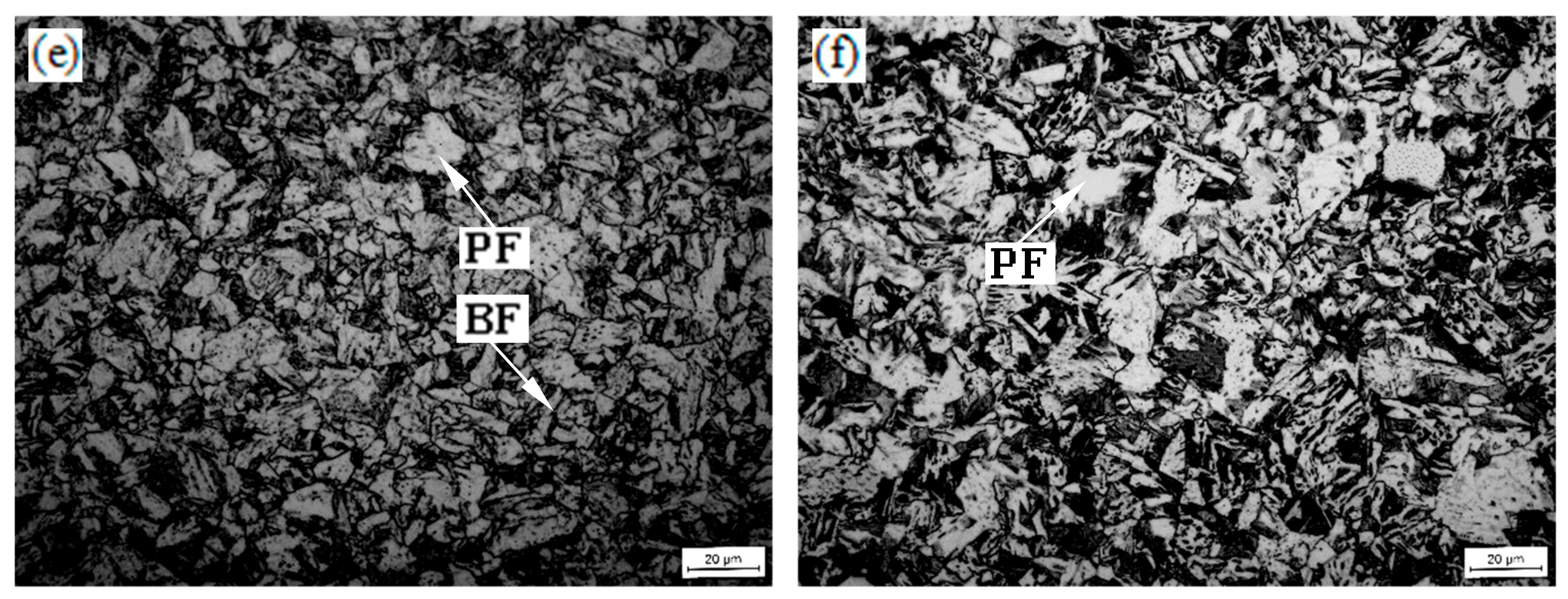
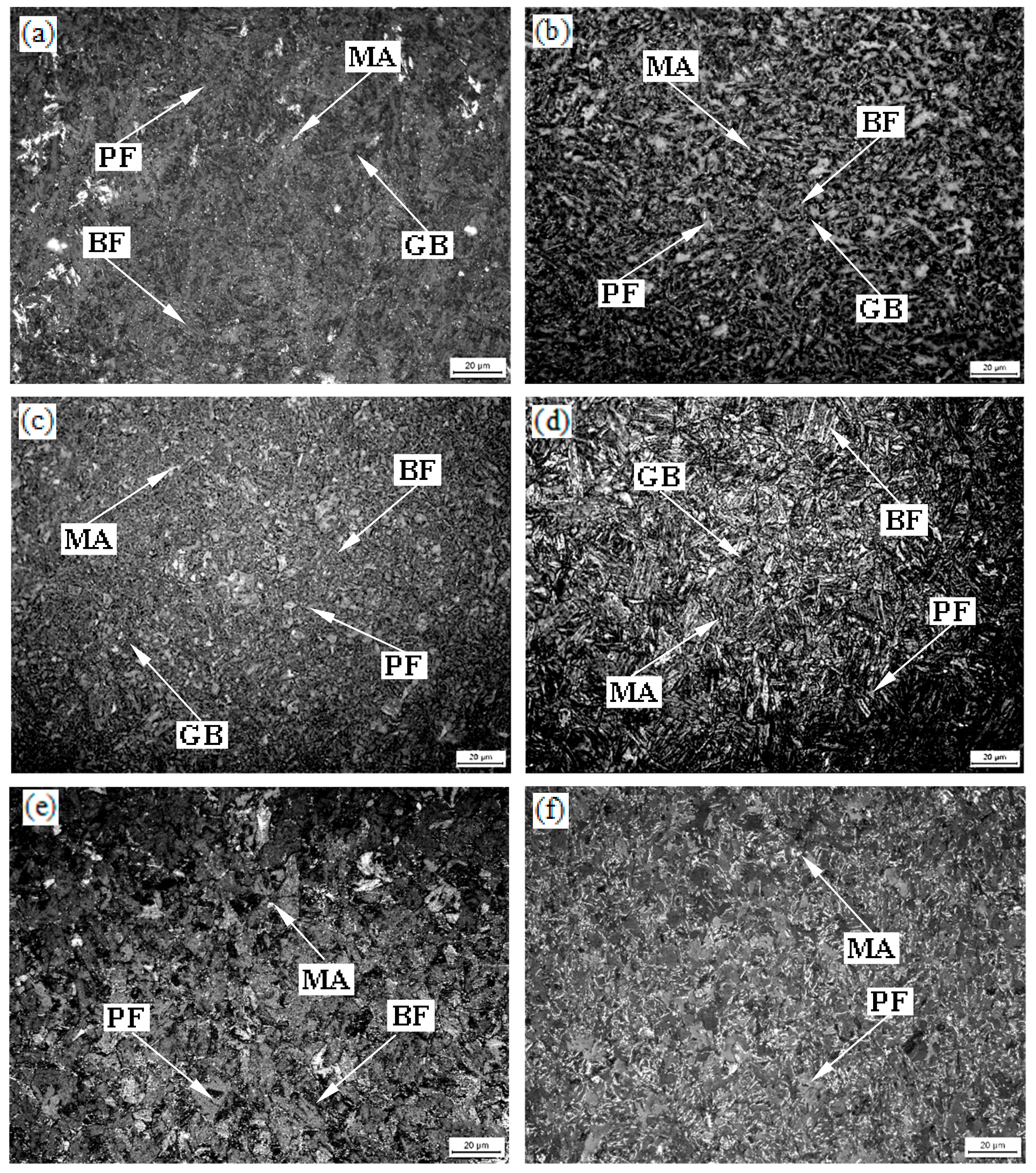
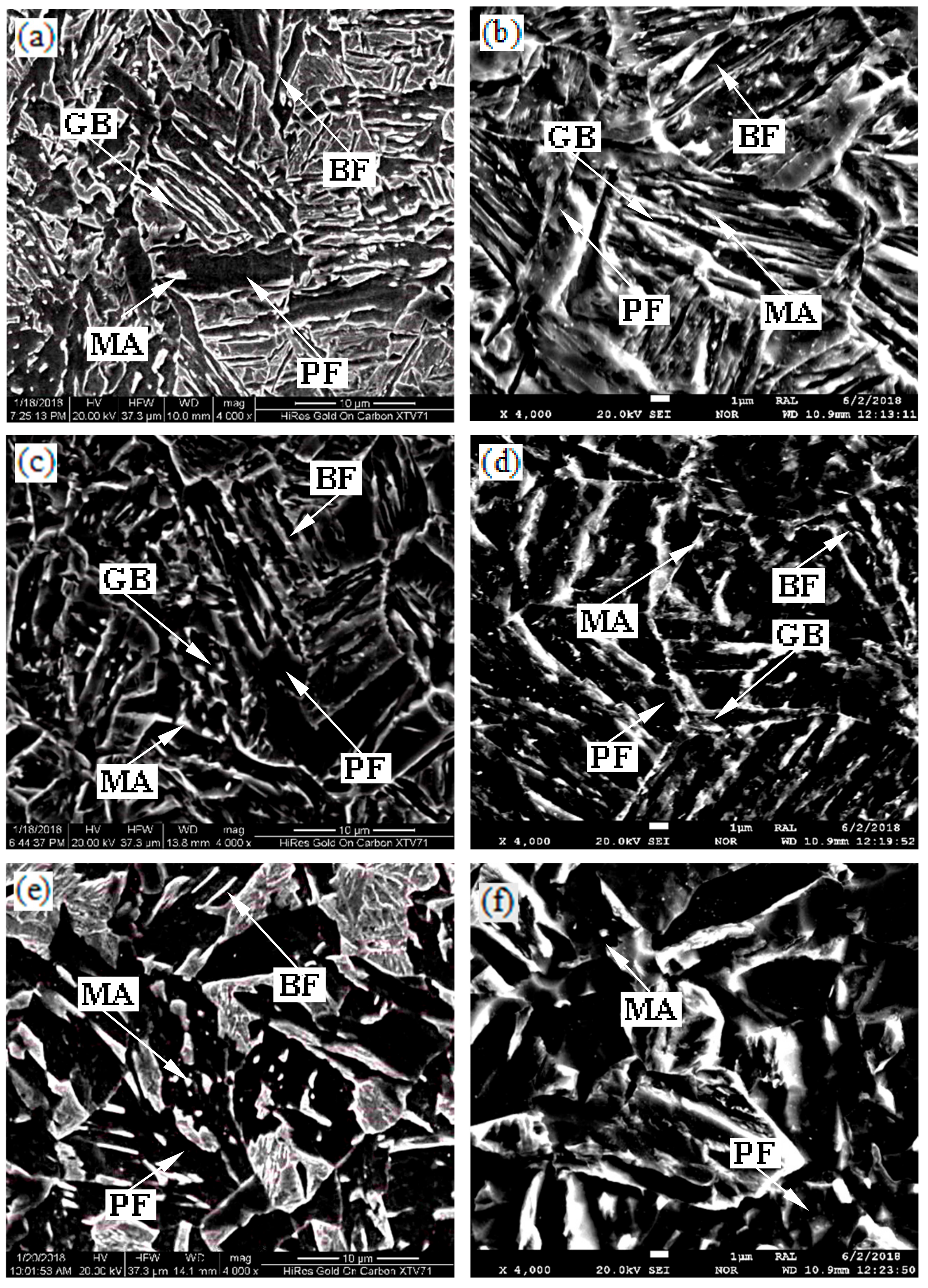
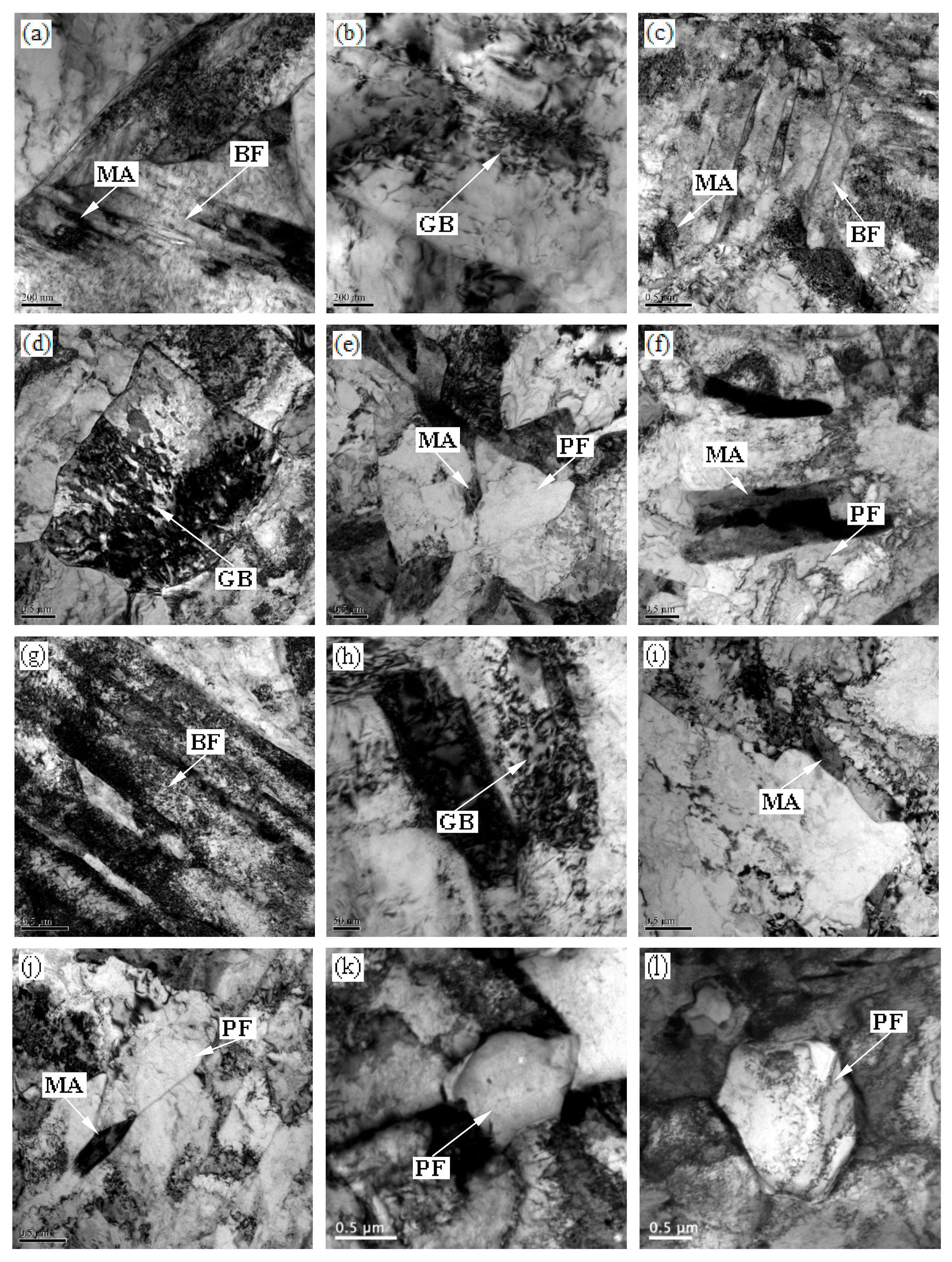
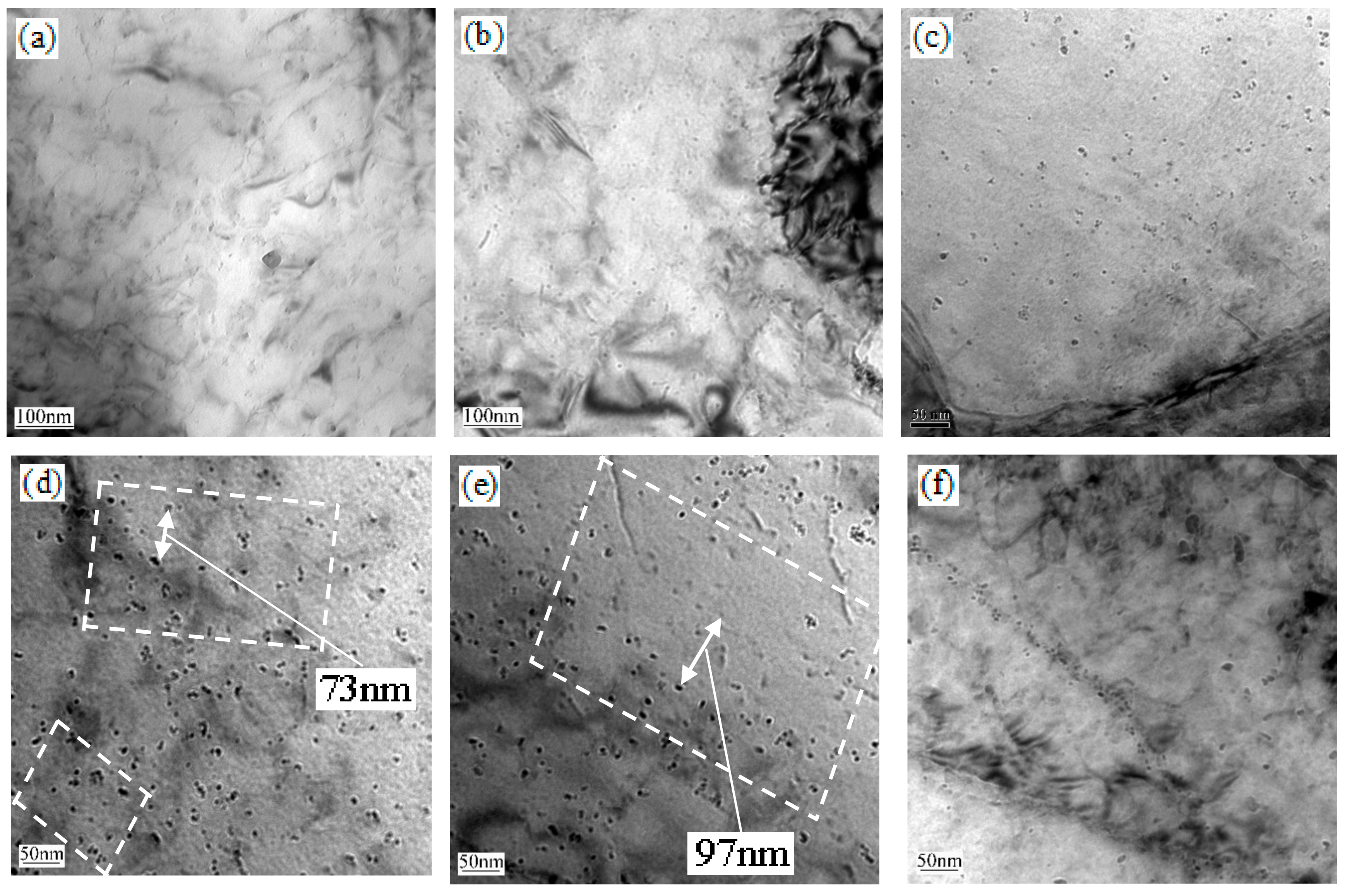
| Specimens (°C) | GB (%) | BF (%) | PF (%) | MA Islands (%) | Average Grain Size of PF (μm) |
|---|---|---|---|---|---|
| 550 | 36.76 ± 3.9 | 63.86 ± 3.1 | 1.39 ± 1.6 | 7.03 ± 0.6 | |
| 600 | 37.75 ± 4.2 | 60.84 ± 4.5 | 1.65 ± 0.4 | 7.19 ± 0.15 | |
| 650 | 20.37 ± 3.7 | 77.64 ± 2.8 | 1.03 ± 0.5 | 7.24 ± 0.54 | |
| 700 | 28.37 ± 3.2 | 70.57 ± 2.9 | 1.27 ± 0.6 | 7.52 ± 0.40 | |
| 750 | - | 98.64 ± 4.5 | 1.27 ± 1.2 | 8.90 ± 0.94 | |
| 800 | - | 92.46 ± 3.7 | 7.54 ± 1.0 | 10.26 ± 0.13 | |
| Specimens (°C) | GB (Hv 0.5) | BF (Hv 0.5) | PF (Hv 0.5) | MA Islands (GPa) |
|---|---|---|---|---|
| 550 | 216.2 ± 12.8 | 227.1 ± 12.1 | 175.9 ± 17.0 | 4.027 ± 0.68 |
| 600 | 337.2 ± 27.0 | 346.7 ± 20.1 | 266.3 ± 7.3 | 7.817 ± 0.75 |
| 650 | 221.0 ± 14.8 | 299.6 ± 38.8 | 189.6 ± 9.7 | 6.334 ± 0.40 |
| 700 | 229.1 ± 16.6 | 207.9 ± 6.6 | 209.6 ± 6.6 | 4.349 ± 0.57 |
| 750 | 178.0 ± 6.0 | 183.7 ± 14.3 | 155.6 ± 14.0 | 3.950 ± 0.42 |
| 800 | 300.5 ± 28.5 | 195.3 ± 25.2 | 138.5 ± 5.8 | 3.756 ± 0.72 |
| Specimens (°C) | Volume Fraction (%) | Average Size (nm) | Strength Increase (MPa) |
|---|---|---|---|
| 550 | - | - | - |
| 600 | 0.698 × 10−3 | 3.14 | 101.38 |
| 650 | 0. 613 × 10−3 | 4.47 | 78.08 |
| 700 | 0. 635 × 10−3 | 4.74 | 75.54 |
| 750 | 0. 623 × 10−3 | 4.74 | 75.80 |
| 800 | 0. 332 × 10−3 | 4.83 | 54.70 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Wang, H.; Xu, X.; Wang, Z.; Misra, R.D.K.; Wang, G. The Impact of Isothermal Treatment on the Microstructural Evolution and the Precipitation Behavior in High Strength Linepipe Steel. Materials 2020, 13, 634. https://doi.org/10.3390/ma13030634
Tian Y, Wang H, Xu X, Wang Z, Misra RDK, Wang G. The Impact of Isothermal Treatment on the Microstructural Evolution and the Precipitation Behavior in High Strength Linepipe Steel. Materials. 2020; 13(3):634. https://doi.org/10.3390/ma13030634
Chicago/Turabian StyleTian, Yong, Hongtao Wang, Xiaoning Xu, Zhaodong Wang, R.D.K. Misra, and Guodong Wang. 2020. "The Impact of Isothermal Treatment on the Microstructural Evolution and the Precipitation Behavior in High Strength Linepipe Steel" Materials 13, no. 3: 634. https://doi.org/10.3390/ma13030634
APA StyleTian, Y., Wang, H., Xu, X., Wang, Z., Misra, R. D. K., & Wang, G. (2020). The Impact of Isothermal Treatment on the Microstructural Evolution and the Precipitation Behavior in High Strength Linepipe Steel. Materials, 13(3), 634. https://doi.org/10.3390/ma13030634







