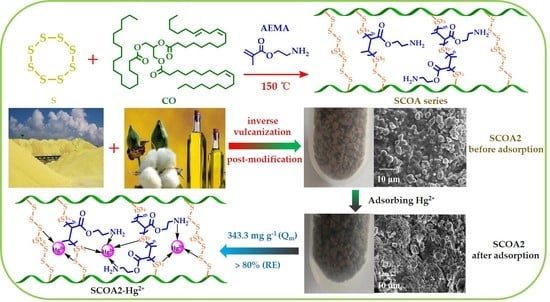
Preparation and Modification of Biomass-Based Functional Rubbers for Removing Mercury(II) from Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
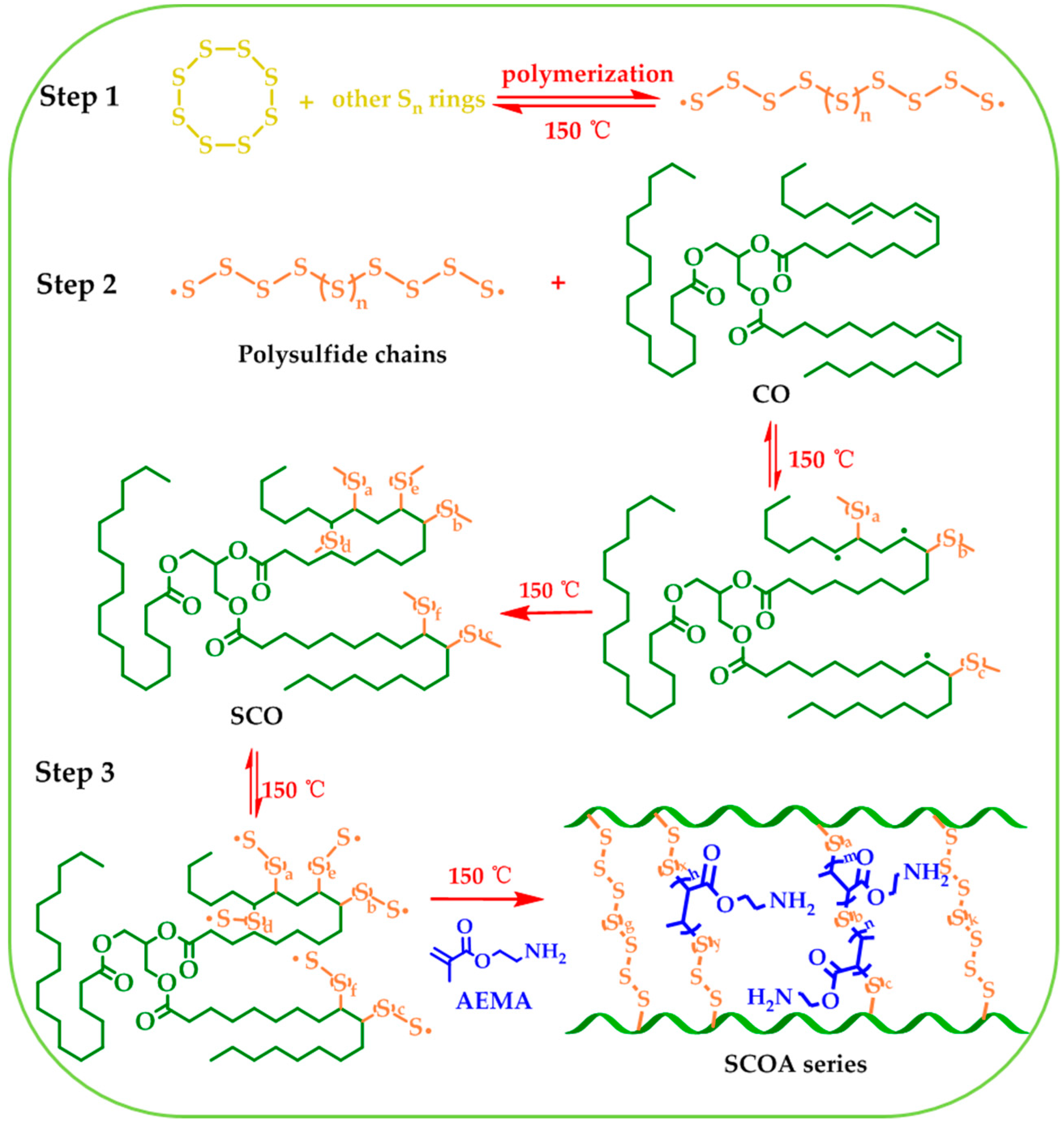
2.2. Preparation of Polysulfide-Co-Cottonseed Oil (SCO)
2.3. Preparation of Post-Modified SCO
2.4. Characterization
2.5. Batch Mercury Adsorption Experiments
2.6. The Calculation of Adsorption Capacities of the Related Control Samples
2.7. Adsorption Kinetic Study
2.8. Adsorption Isotherm Study
2.9. Reusability Test of Adsorbents
3. Results and Discussion
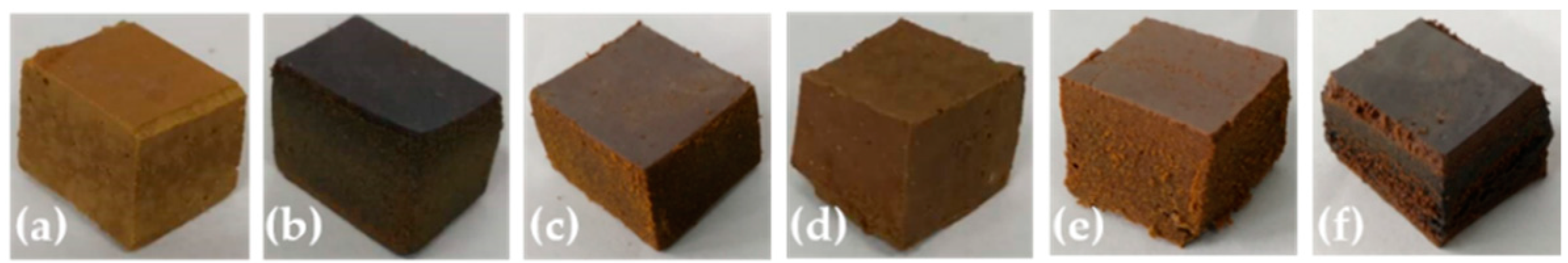
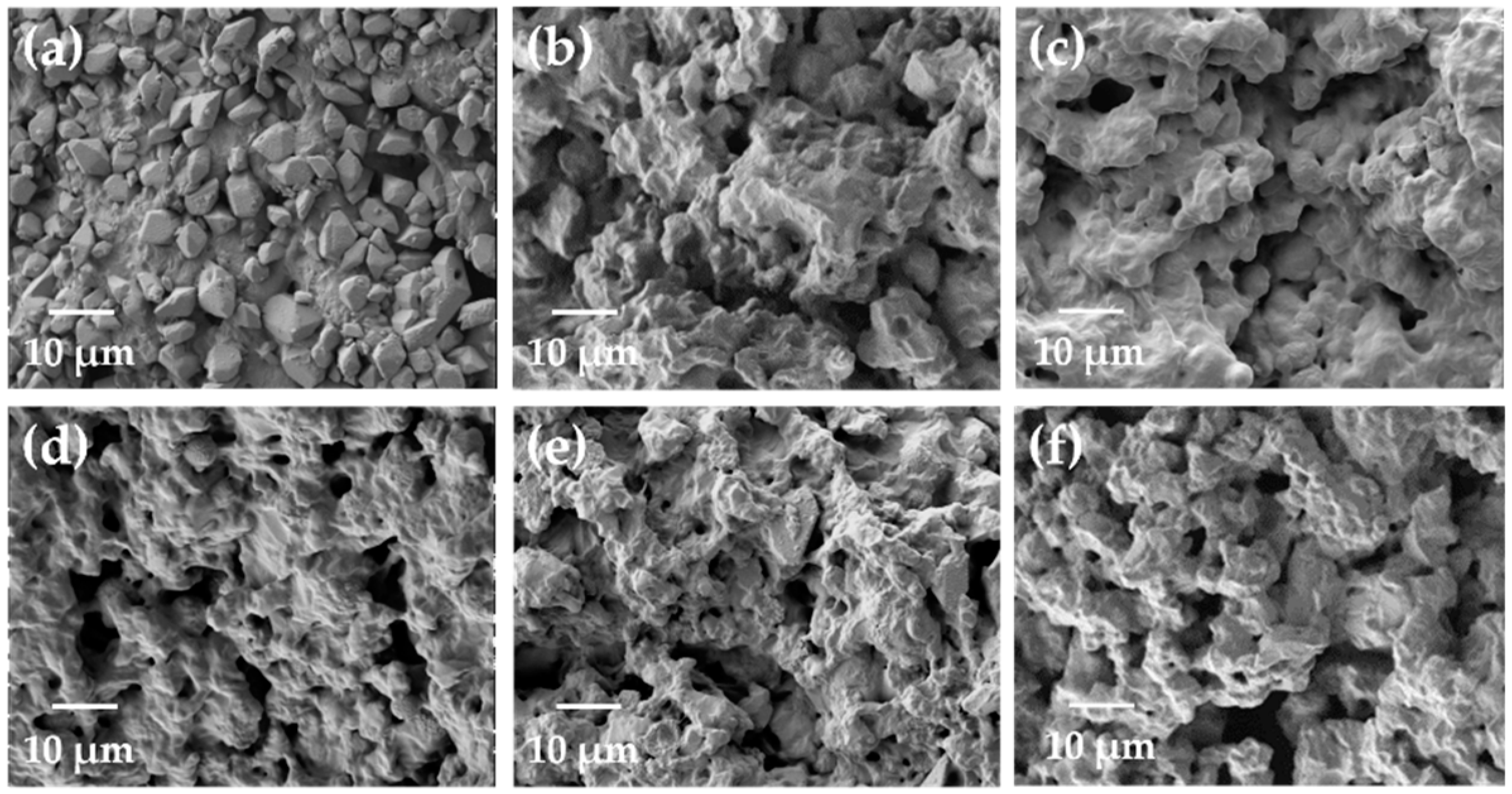
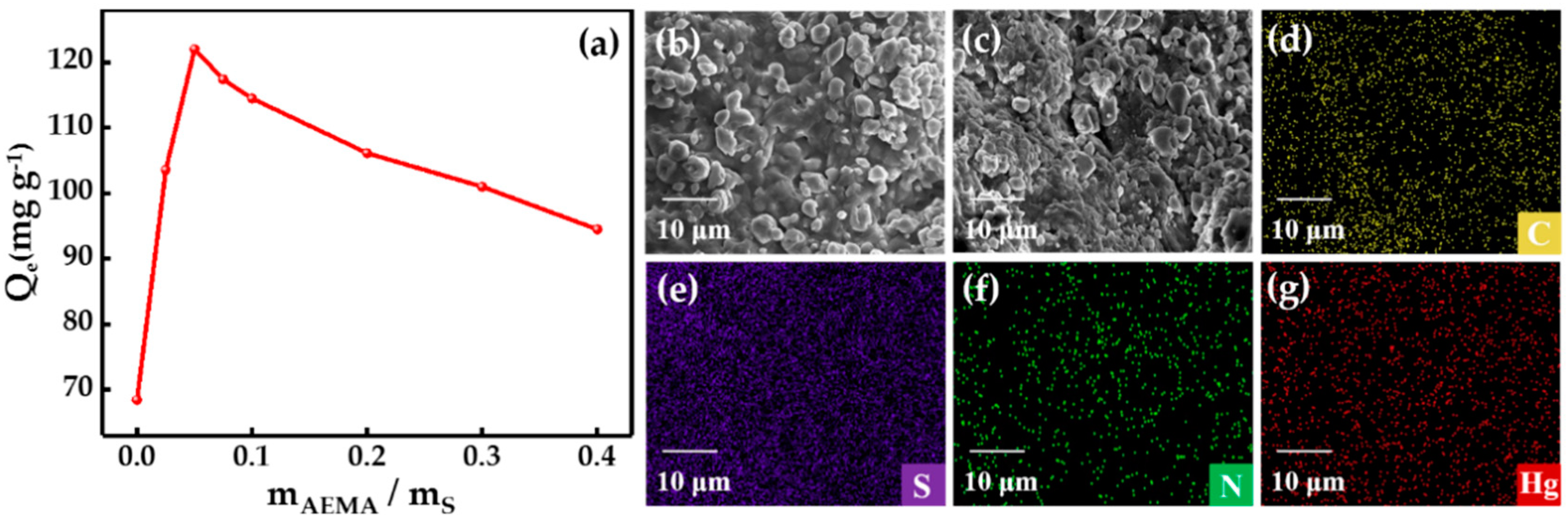
3.1. Synthesis and Characterization
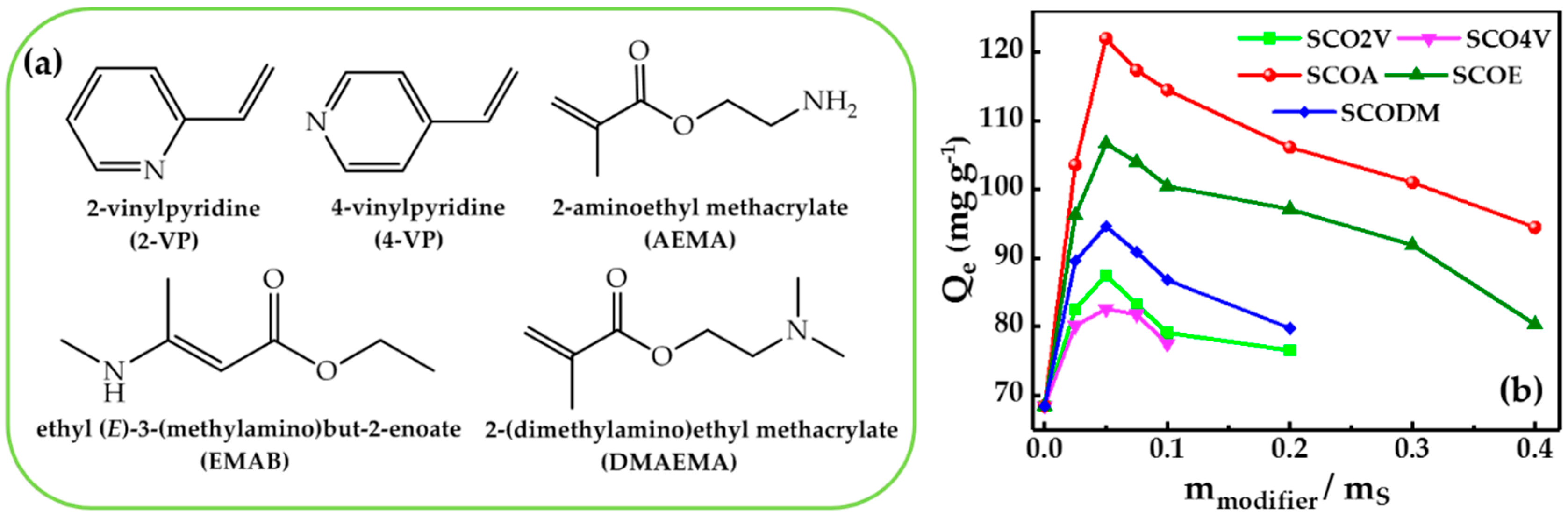
3.2. Effect of Types of Nitrogen-Containing Groups in Modifiers on Adsorption Capacities of Samples
3.3. Effect of Dosage of AEMA on Adsorption Capacities of SCOA Serial Samples
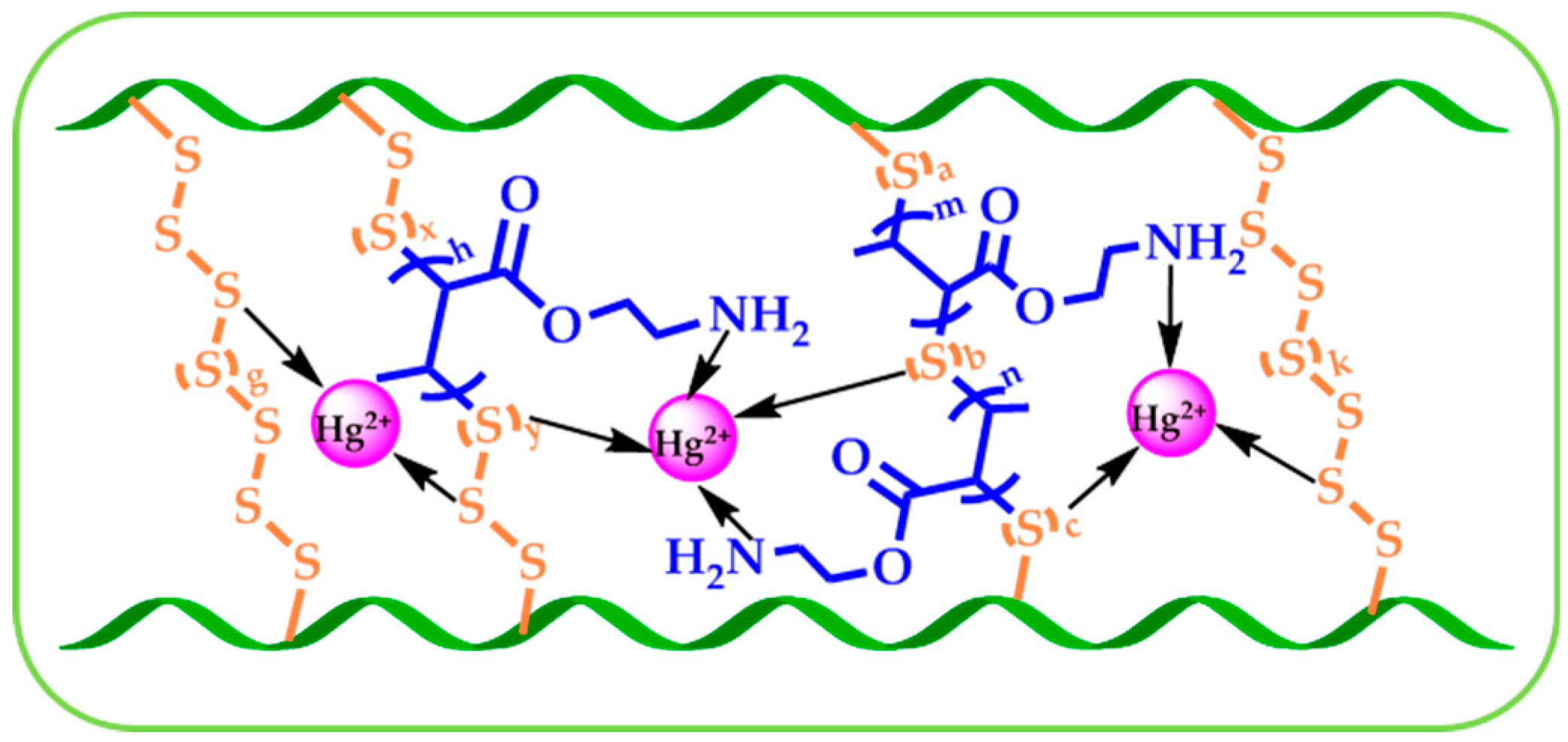
3.4. Adsorption Mechanism
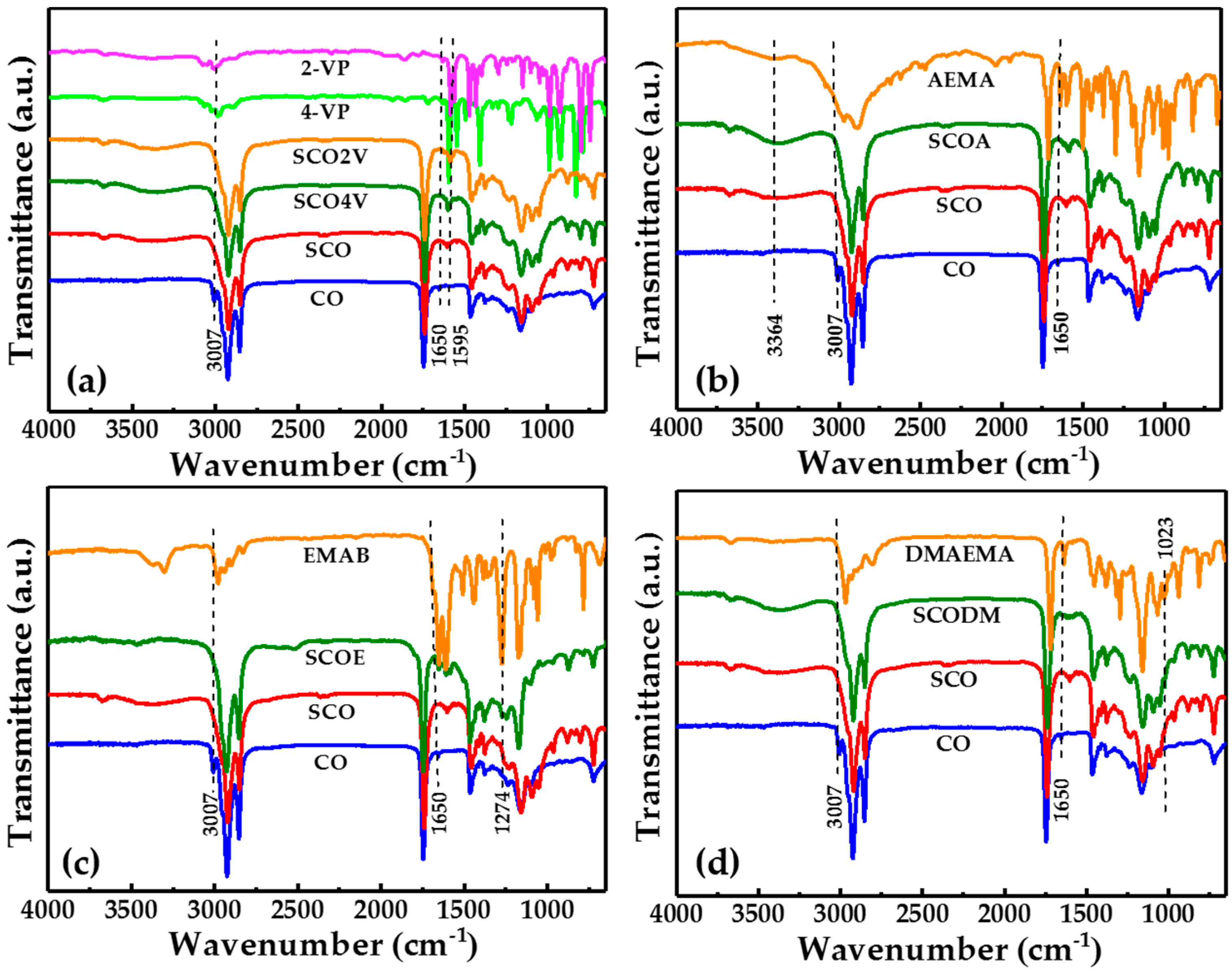
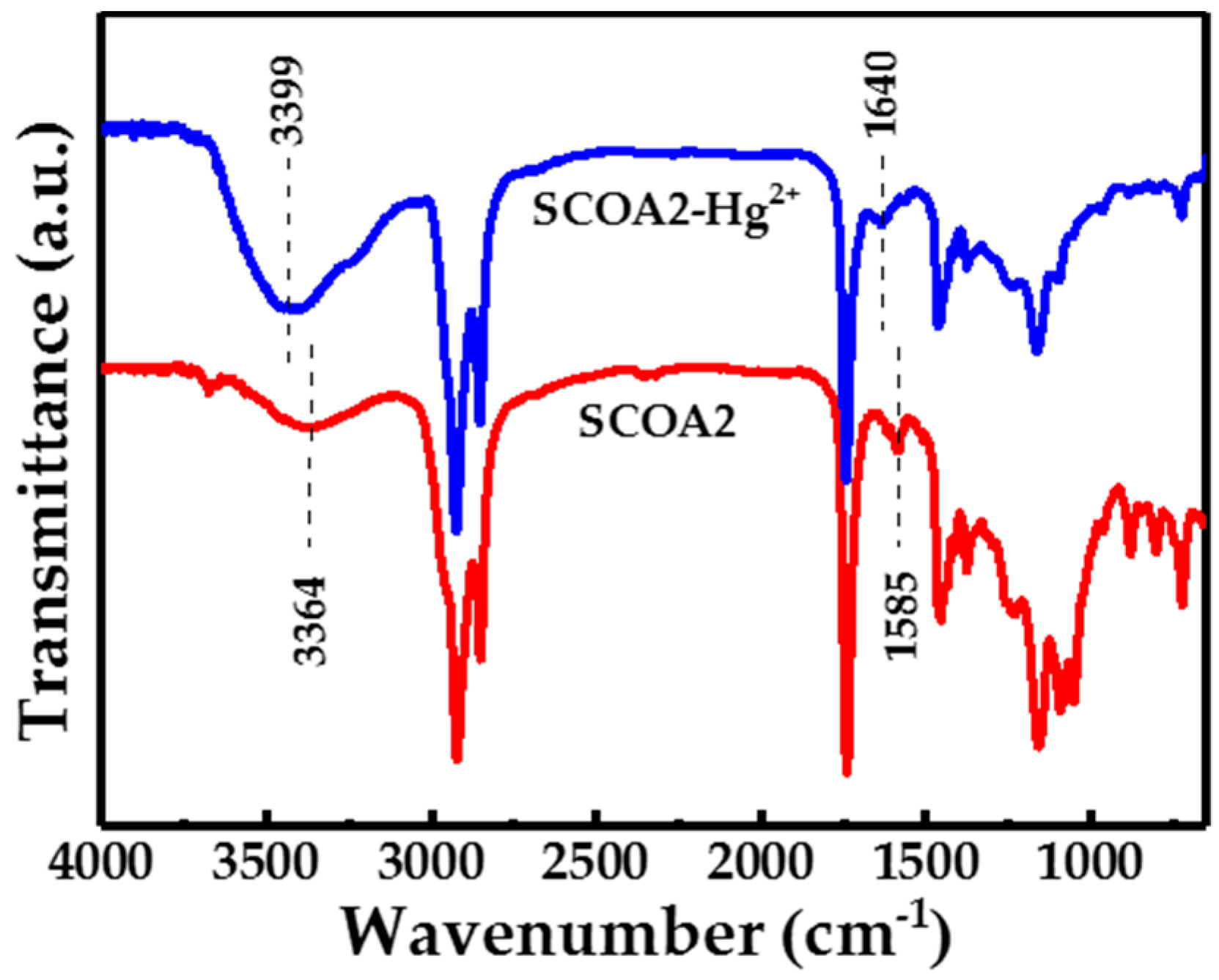
3.4.1. FT-IR Analysis
3.4.2. XPS Analysis
3.5. Adsorption Kinetics
3.6. Adsorption Isotherm
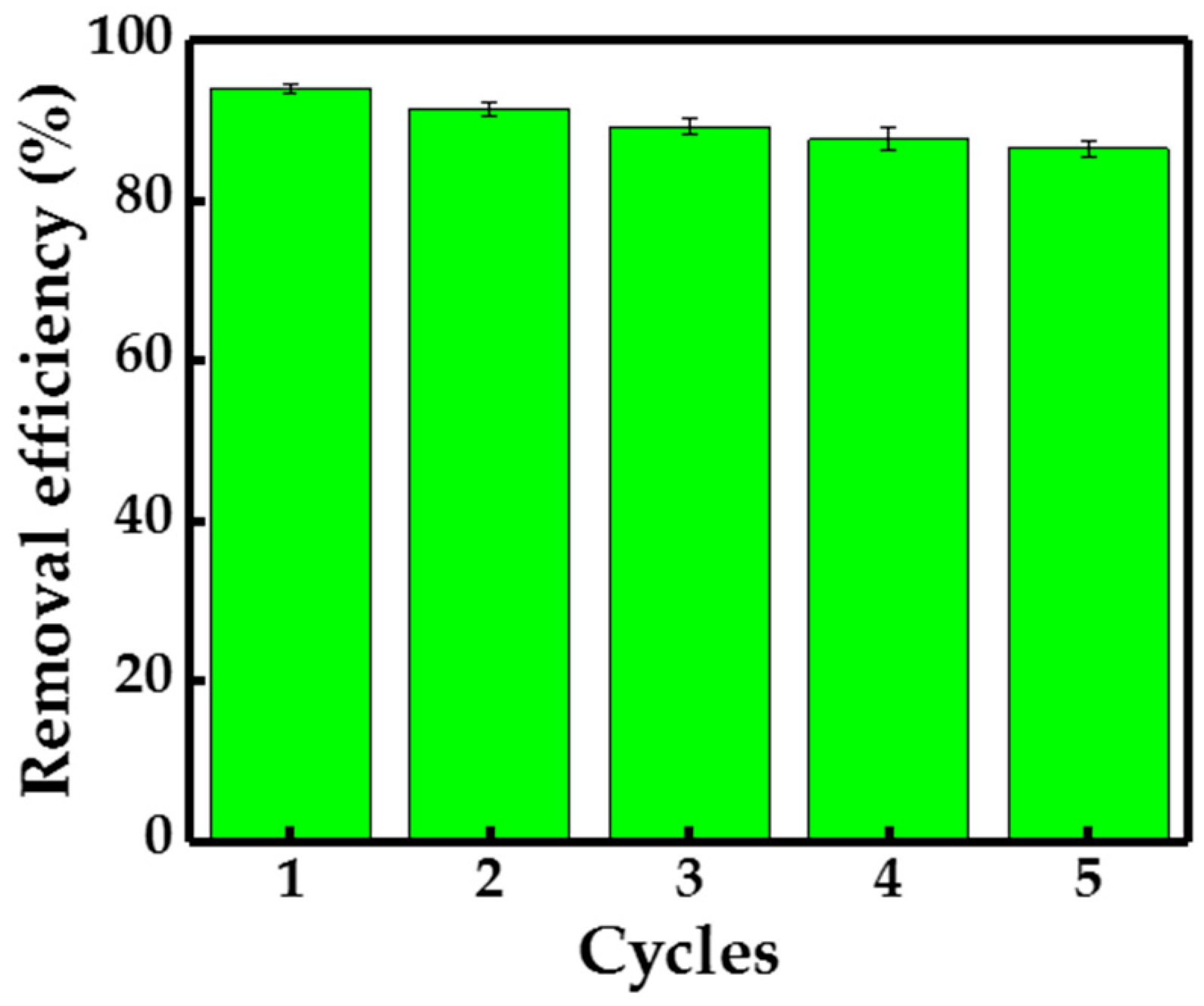
3.7. Reusability of Adsorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sheldon, R.A. Green chemistry, catalysis and valorization of waste biomass. J. Mol. Catal. A Chem. 2016, 422, 3–12. [Google Scholar] [CrossRef]
- Rastogi, T.; Leder, C.; Kümmerer, K. A sustainable chemistry solution to the presence of pharmaceuticals and chemicals in the aquatic environment—The example of re-designing β-blocker Atenolol. RSC Adv. 2015, 5, 27–32. [Google Scholar] [CrossRef]
- Mekonnen, T.; Mussone, P.; Bressler, D. Valorization of rendering industry wastes and co-products for industrial chemicals, materials and energy: Review. Crit. Rev. Biotechnol. 2016, 36, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Glass, R.S.; Char, K.; Pyun, J. From waste to valuable plastics-discovery of new paradigms from well-studied systems with elemental sulfur. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 157–161. [Google Scholar] [CrossRef]
- Feng, Y.; Liang, H.; Yang, Z.; Yuan, T.; Luo, Y.; Li, P.; Yang, Z.; Zhang, C. A solvent-free and scalable method to prepare soybean-oil-based polyols by thiol–ene photo-click reaction and biobased polyurethanes therefrom. ACS Sustain. Chem. Eng. 2017, 5, 7365–7373. [Google Scholar] [CrossRef]
- Poonyakan, A.; Rachakornkij, M.; Wecharatana, M.; Smittakorn, W. Potential use of plastic wastes for low thermal conductivity concrete. Materials 2018, 11, 1938. [Google Scholar] [CrossRef]
- Griebel, J.J.; Glass, R.S.; Char, K.; Pyun, J. Polymerizations with elemental sulfur: A novel route to high sulfur content polymers for sustainability, energy and defense. Prog. Polym. Sci. 2016, 58, 90–125. [Google Scholar] [CrossRef]
- Chung, W.J.; Griebel, J.J.; Kim, E.T.; Yoon, H.; Simmonds, A.G.; Ji, H.J.; Dirlam, P.T.; Glass, R.S.; Wie, J.J.; Nguyen, N.A.; et al. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 2013, 5, 518–524. [Google Scholar] [CrossRef]
- Worthington, M.J.H.; Kucera, R.L.; Chalker, J.M. Green chemistry and polymers made from sulfur. Green Chem. 2017, 19, 2748–2761. [Google Scholar] [CrossRef]
- Sun, Z.; Xiao, M.; Wang, S.; Han, D.; Song, S.; Chen, G.; Meng, Y. Sulfur-rich polymeric materials with semi-interpenetrating network structure as a novel lithium–sulfur cathode. J. Mater. Chem. A 2014, 2, 9280–9286. [Google Scholar] [CrossRef]
- Lin, H.K.; Liu, Y.L. Sulfur radical transfer and coupling reaction to benzoxazine groups: A new reaction route for preparation of polymeric materials using elemental sulfur as a feedstock. Macromol. Rapid Commun. 2018, 39, 1700832. [Google Scholar] [CrossRef]
- Gutarowska, B.; Kotynia, R.; Bielinski, D.; Anyszka, R.; Wreczycki, J.; Piotrowska, M.; Kozirog, A.; Berlowska, J.; Dziugan, P. New sulfur organic polymer-concrete composites containing waste materials: Mechanical characteristics and resistance to biocorrosion. Materials 2019, 12, 2602. [Google Scholar] [CrossRef]
- Griebel, J.J.; Namnabat, S.; Kim, E.T.; Himmelhuber, R.; Moronta, D.H.; Chung, W.J.; Simmonds, A.G.; Kim, K.J.; van der Laan, J.; Nguyen, N.A.; et al. New infrared transmitting material via inverse vulcanization of elemental sulfur to prepare high refractive index polymers. Adv. Mater. 2014, 26, 3014–3018. [Google Scholar] [CrossRef] [PubMed]
- Dirlam, P.T.; Simmonds, A.G.; Kleine, T.S.; Nguyen, N.A.; Anderson, L.E.; Klever, A.O.; Florian, A.; Costanzo, P.J.; Theato, P.; Mackay, M.E.; et al. Inverse vulcanization of elemental sulfur with 1,4-diphenylbutadiyne for cathode materials in Li–S batteries. RSC Adv. 2015, 5, 24718–24722. [Google Scholar] [CrossRef]
- Hasell, T.; Parker, D.J.; Jones, H.A.; McAllister, T.; Howdle, S.M. Porous inverse vulcanised polymers for mercury capture. Chem. Commun. 2016, 52, 5383–5386. [Google Scholar] [CrossRef]
- Deng, Z.; Hoefling, A.; Théato, P.; Lienkamp, K. Surface properties and antimicrobial activity of poly(sulfur-co-1,3-diisopropenylbenzene) copolymers. Macromol. Chem. Phys. 2018, 219, 1700497. [Google Scholar] [CrossRef]
- Worthington, M.J.H.; Kucera, R.L.; Albuquerque, I.S.; Gibson, C.T.; Sibley, A.; Slattery, A.D.; Campbell, J.A.; Alboaiji, S.F.K.; Muller, K.A.; Young, J.; et al. Laying waste to mercury: Inexpensive sorbents made from sulfur and recycled cooking oils. Chemistry 2017, 23, 16219–16230. [Google Scholar] [CrossRef]
- Hoefling, A.; Lee, Y.J.; Theato, P. Sulfur-based polymer composites from vegetable oils and elemental sulfur: A sustainable active material for Li-S batteries. Macromol. Chem. Phys. 2017, 218, 1600303. [Google Scholar] [CrossRef]
- Lin, H.K.; Lai, Y.S.; Liu, Y.L. Cross-linkable and self-foaming polysulfide materials for repairable and mercury capture applications. ACS Sustain. Chem. Eng. 2019, 7, 4515–4522. [Google Scholar] [CrossRef]
- Xu, G.; Zhao, Y.; Hou, L.; Cao, J.; Tao, M.; Zhang, W. A recyclable phosphinic acid functionalized polyacrylonitrile fiber for selective and efficient removal of Hg2+. Chem. Eng. J. 2017, 325, 533–543. [Google Scholar] [CrossRef]
- Mackey, T.K.; Contreras, J.T.; Liang, B.A. The Minamata Convention on mercury: Attempting to address the global controversy of dental amalgam use and mercury waste disposal. Sci. Total Environ. 2014, 472, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Esfandiyari, T.; Nasirizadeh, N.; Dehghani, M.; Ehrampoosh, M.H. Graphene oxide based carbon composite as adsorbent for Hg removal: Preparation, characterization, kinetics and isotherm studies. Chin. J. Chem. Eng. 2017, 25, 1170–1175. [Google Scholar] [CrossRef]
- Qu, Z.; Fang, L.; Chen, D.; Xu, H.; Yan, N. Effective and regenerable Ag/graphene adsorbent for Hg(II) removal from aqueous solution. Fuel 2017, 203, 128–134. [Google Scholar] [CrossRef]
- Monier, M. Adsorption of Hg2+, Cu2+ and Zn2+ ions from aqueous solution using formaldehyde cross-linked modified chitosan-thioglyceraldehyde Schiff’s base. Int. J. Biol. Macromol. 2012, 50, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Monier, M.; Abdel-Latif, D.A. Preparation of cross-linked magnetic chitosan-phenylthiourea resin for adsorption of Hg(II), Cd(II) and Zn(II) ions from aqueous solutions. J. Hazard. Mater. 2012, 209, 240–249. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Deliyanni, E.A. Mercury(II) removal with modified magnetic chitosan adsorbents. Molecules 2013, 18, 6193–6214. [Google Scholar] [CrossRef]
- Liang, W.; Li, M.; Zhang, Z.; Jiang, Y.; Awasthi, M.K.; Jiang, S.; Li, R. Decontamination of Hg(II) from aqueous solution using polyamine-co-thiourea inarched chitosan gel derivatives. Int. J. Biol. Macromol. 2018, 113, 106–115. [Google Scholar] [CrossRef]
- Zeng, H.; Wang, L.; Zhang, D.; Yan, P.; Nie, J.; Sharma, V.K.; Wang, C. Highly efficient and selective removal of mercury ions using hyperbranched polyethylenimine functionalized carboxymethyl chitosan composite adsorbent. Chem. Eng. J. 2019, 358, 253–263. [Google Scholar] [CrossRef]
- Crockett, M.P.; Evans, A.M.; Worthington, M.J.; Albuquerque, I.S.; Slattery, A.D.; Gibson, C.T.; Campbell, J.A.; Lewis, D.A.; Bernardes, G.J.; Chalker, J.M. Sulfur-limonene polysulfide: A material synthesized entirely from industrial by-products and its use in removing toxic metals from water and soil. Angew. Chem. Int. Ed. Engl. 2016, 55, 1714–1718. [Google Scholar] [CrossRef]
- Qu, Z.; Yan, L.; Li, L.; Xu, J.; Liu, M.; Li, Z.; Yan, N. Ultraeffective ZnS nanocrystals sorbent for mercury(II) removal based on size-dependent cation exchange. ACS Appl. Mater. Interfaces 2014, 6, 18026–18032. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, J.; Hu, Y.; Zhao, H.; Zhou, J.; Liu, Y.; Lou, Z.; Xu, X. Functional nanomaterials: Study on aqueous Hg(II) adsorption by magnetic Fe3O4@SiO2-SH nanoparticles. J. Taiwan Inst. Chem. Eng. 2016, 60, 394–402. [Google Scholar] [CrossRef]
- Navarro, R.R.; Sumi, K.; Fujii, N.; Matsumura, M. Mercury removal from wastewater using porous cellulose carrier modified with polyethyleneimine. Water Res. 1996, 30, 2488–2494. [Google Scholar] [CrossRef]
- Wang, J.; Deng, B.; Chen, H.; Wang, X.; Zheng, J. Removal of Aqueous Hg(II) by Polyaniline: Sorption Characteristics and Mechanisms. Environ. Sci. Technol. 2009, 43, 5223–5228. [Google Scholar] [CrossRef] [PubMed]
- Kagaya, S.; Miyazaki, H.; Ito, M.; Tohda, K.; Kanbara, T. Selective removal of mercury(II) from wastewater using polythioamides. J. Hazard. Mater. 2010, 175, 1113–1115. [Google Scholar] [CrossRef]
- Westerman, C.R.; Jenkins, C.L. Dynamic sulfur bonds initiate polymerization of vinyl and allyl ethers at mild temperatures. Macromolecules 2018, 51, 7233–7238. [Google Scholar] [CrossRef]
- Kleine, T.S.; Nguyen, N.A.; Anderson, L.E.; Namnabat, S.; LaVilla, E.A.; Showghi, S.A.; Dirlam, P.T.; Arrington, C.B.; Manchester, M.S.; Schwiegerling, J.; et al. High refractive index copolymers with improved thermomechanical properties via the inverse vulcanization of sulfur and 1,3,5-triisopropenylbenzene. ACS Macro. Lett. 2016, 5, 1152–1156. [Google Scholar] [CrossRef]
- Griebel, J.J.; Li, G.; Glass, R.S.; Char, K.; Pyun, J. Kilogram scale inverse vulcanization of elemental sulfur to prepare high capacity polymer electrodes for Li-S batteries. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 173–177. [Google Scholar] [CrossRef]
- Lin, Y.C.; Wang, H.P.; Gohar, F.; Ullah, M.H.; Zhang, X.; Xie, D.F.; Fang, H.; Huang, J.; Yang, J.X. Preparation and copper ions adsorption properties of thiosemicarbazide chitosan from squid pens. Int. J. Biol. Macromol. 2017, 95, 476–483. [Google Scholar] [CrossRef]
- Ahmad, M.; Manzoor, K.; Venkatachalam, P.; Ikram, S. Kinetic and thermodynamic evaluation of adsorption of Cu(II) by thiosemicarbazide chitosan. Int. J. Biol. Macromol. 2016, 92, 910–919. [Google Scholar] [CrossRef]
- Molinelli, A.; O’Mahony, J.; Nolan, K.; Smyth, M.R.; Jakusch, M.; Mizaikoff, B. Analyzing the mechanisms of selectivity in biomimetic self-assemblies via IR and NMR spectroscopy of prepolymerization solutions and molecular dynamics simulations. Anal. Chem. 2005, 77, 5196–5204. [Google Scholar] [CrossRef]
- Biniak, S.; Szymański, G.; Siedlewski, J.; Świątkowski, A. The characterization of activated carbons with oxygen and nitrogen surface groups. Carbon 1997, 35, 1799–1810. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Lu, M.; Tao, P.Y.; Zhang, Y.J.; Gong, X.T.; Yang, Z.; Zhang, G.Q.; Li, H.L. Hierarchically porous and heteroatom doped carbon derived from tobacco rods for supercapacitors. J. Power Sources 2016, 307, 391–400. [Google Scholar] [CrossRef]
- Yuan, W.; Xu, W.; Zhang, Z.; Wang, X.; Zhang, Q.; Bai, J.; Wang, J. Rapid Cr(VI) reduction and immobili- zation in contaminated soil by mechanochemical treatment with calcium polysulfide. Chemosphere 2019, 227, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liang, W.; Li, M.; Jiang, S.; Huang, H.; Zhang, Z.; Wang, J.J.; Awasthi, M.K. Removal of Cd(II) and Cr(VI) ions by highly cross-linked Thiocarbohydrazide-chitosan gel. Int. J. Biol. Macromol. 2017, 104, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Z.; Li, R.; Wang, J.J.; Ali, A. Removal of Pb(II) and Cd(II) ions from aqueous solution by thiosemicarbazide modified chitosan. Int. J. Biol. Macromol. 2016, 86, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.; Bajpai, J.; Bajpai, A.K. Chitosan-alginate nanoparticles (CANPs) as potential nanosorbent for removal of Hg (II) ions. Environmental Nanotechnology. Monit. Manag. 2016, 6, 32–44. [Google Scholar] [CrossRef]
- Xu, G.; Wang, L.; Xie, Y.; Tao, M.; Zhang, W. Highly selective and efficient adsorption of Hg2+ by a recyclable aminophosphonic acid functionalized polyacrylonitrile fiber. J. Hazard. Mater. 2018, 344, 679–688. [Google Scholar] [CrossRef]
- Ge, H.; Huang, H.; Xu, M.; Chen, Q. Cellulose/poly(ethylene imine) composites as efficient and reusable adsorbents for heavy metal ions. Cellulose 2016, 23, 2527–2537. [Google Scholar] [CrossRef]



| Samples | C (%) | H (%) | O (%) | N (%) | S (%) |
|---|---|---|---|---|---|
| SCO | 38.88 | 5.721 | 1.983 | 0.006 | 53.41 |
| SCOA2 | 38.86 | 5.742 | 2.519 | 0.309 | 52.57 |
| SCOE2 | 39.06 | 5.728 | 2.081 | 0.241 | 52.89 |
| SCODM2 | 38.42 | 5.689 | 2.553 | 0.188 | 53.15 |
| SCO2V2 | 38.93 | 5.741 | 1.642 | 0.147 | 53.54 |
| SCO4V2 | 38.88 | 5.692 | 1.424 | 0.114 | 53.89 |
| Samples | SBET (m2 g−1) | Pore Volume (cm3 g−1) | Pore Diameter (nm) | Adsorption Capacities (mg g−1) |
|---|---|---|---|---|
| SCO | 591.4 | 0.236 | 0.523 | 68.45 |
| SCOA2 | 893.6 | 0.196 | 0.524 | 121.98 |
| SCOE2 | 892.6 | 0.183 | 0.528 | 106.66 |
| SCODM2 | 896.1 | 0.188 | 0.519 | 94.58 |
| SCO2V2 | 890.3 | 0.241 | 0.527 | 87.45 |
| SCO4V2 | 894.6 | 0.229 | 0.517 | 82.56 |
| Samples | mAEMA/mS | C (%) | H (%) | O (%) | N (%) | S (%) |
|---|---|---|---|---|---|---|
| SCO | 0 | 38.88 | 5.721 | 1.983 | 0.006 | 53.41 |
| SCOA1 | 0.025 | 39.03 | 5.722 | 1.656 | 0.262 | 53.33 |
| SCOA2 | 0.05 | 38.86 | 5.742 | 2.119 | 0.309 | 52.97 |
| SCOA3 | 0.075 | 38.78 | 5.737 | 3.318 | 0.395 | 51.77 |
| SCOA4 | 0.1 | 38.93 | 5.735 | 2.996 | 0.549 | 51.79 |
| SCOA5 | 0.2 | 39.01 | 5.802 | 3.585 | 0.713 | 50.89 |
| SCOA6 | 0.3 | 39.14 | 5.795 | 5.001 | 0.984 | 49.08 |
| SCOA7 | 0.4 | 39.26 | 5.827 | 4.748 | 1.215 | 48.95 |
| Samples | SBET (m2 g−1) | Pore Volume (cm3 g−1) | Pore Diameter (nm) | Adsorption Capacities (mg g−1) |
|---|---|---|---|---|
| SCO | 591.356 | 0.236 | 0.523 | 68.45 |
| SCOA1 | 883.561 | 0.228 | 0.529 | 103.56 |
| SCOA2 | 893.605 | 0.196 | 0.524 | 121.98 |
| SCOA3 | 884.873 | 0.203 | 0.526 | 117.36 |
| SCOA4 | 894.586 | 0.189 | 0.518 | 114.45 |
| SCOA5 | 889.112 | 0.193 | 0.527 | 106.11 |
| SCOA6 | 887.563 | 0.209 | 0.521 | 100.99 |
| SCOA7 | 895.229 | 0.211 | 0.525 | 94.47 |
| Adsorbents | pH | te (min) | T (°C) | Qm (mg g−1) | Isotherm Model | Kinetic Model | References |
|---|---|---|---|---|---|---|---|
| CSTG | 5.0 | 60 | 30 | 98.0 | Langmuir | pseudo-second-order | [24] |
| Fe3O4 @SiO2-SH | 3-8 | 1440 | 25 | 132.0 | Langmuir | pseudo-second-order | [31] |
| Chit-PTU | 5.0 | 60 | 30 | 135.0 | Langmuir | pseudo-second-order | [25] |
| CSm | 5.0 | 200 | 25 | 152.0 | Langmuir | pseudo-second-order | [26] |
| TC-EDA-CS | ≥4.0 | 1440 | 25 | 217.1 | Langmuir | pseudo-second-order | [27] |
| Cell-PEI | 7.0 | 500 | 25 | 288.0 | Langmuir | pseudo-second-order | [32] |
| SCOA2 | 6.5 | 600 | 30 | 343.3 | Langmuir-Freundlich | pseudo-second-order | this work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Yasin, A.; Zhang, Y.; Zan, X.; Liu, Y.; Zhang, L. Preparation and Modification of Biomass-Based Functional Rubbers for Removing Mercury(II) from Aqueous Solution. Materials 2020, 13, 632. https://doi.org/10.3390/ma13030632
Chen Y, Yasin A, Zhang Y, Zan X, Liu Y, Zhang L. Preparation and Modification of Biomass-Based Functional Rubbers for Removing Mercury(II) from Aqueous Solution. Materials. 2020; 13(3):632. https://doi.org/10.3390/ma13030632
Chicago/Turabian StyleChen, Yurong, Akram Yasin, Yagang Zhang, Xingjie Zan, Yanxia Liu, and Letao Zhang. 2020. "Preparation and Modification of Biomass-Based Functional Rubbers for Removing Mercury(II) from Aqueous Solution" Materials 13, no. 3: 632. https://doi.org/10.3390/ma13030632
APA StyleChen, Y., Yasin, A., Zhang, Y., Zan, X., Liu, Y., & Zhang, L. (2020). Preparation and Modification of Biomass-Based Functional Rubbers for Removing Mercury(II) from Aqueous Solution. Materials, 13(3), 632. https://doi.org/10.3390/ma13030632