PDC Glass/Ceramic Coatings Applied to Differently Pretreated AISI441 Stainless Steel Substrates
Abstract
1. Introduction
2. Materials and Methods
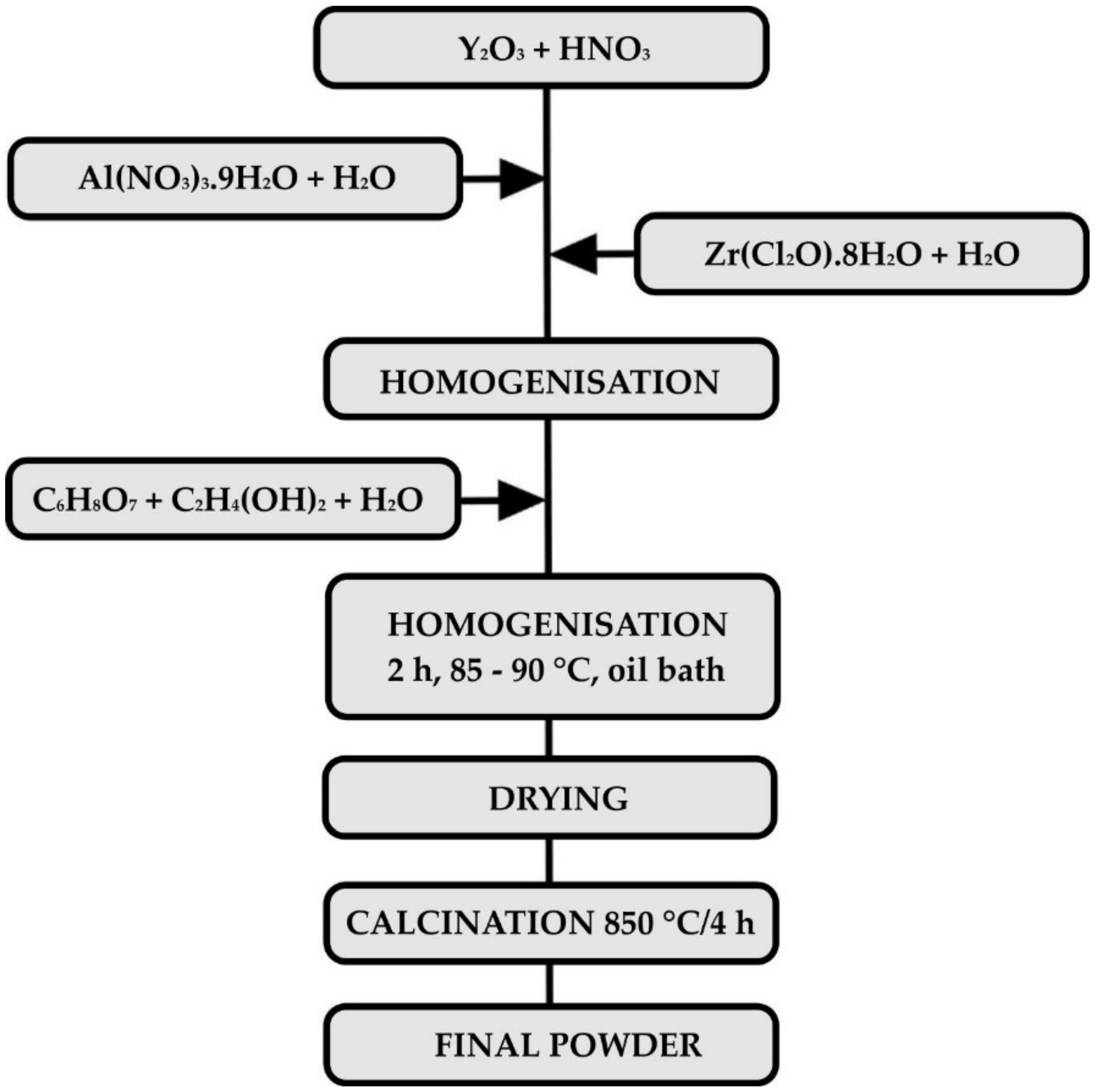
2.1. Preparation of the Precursor Powder
2.2. Pretreatment of the Stainless Steel
2.3. Preparation of the Coatings
2.4. Characterisation Methods
2.5. Oxidation Tests
3. Results and Discussion
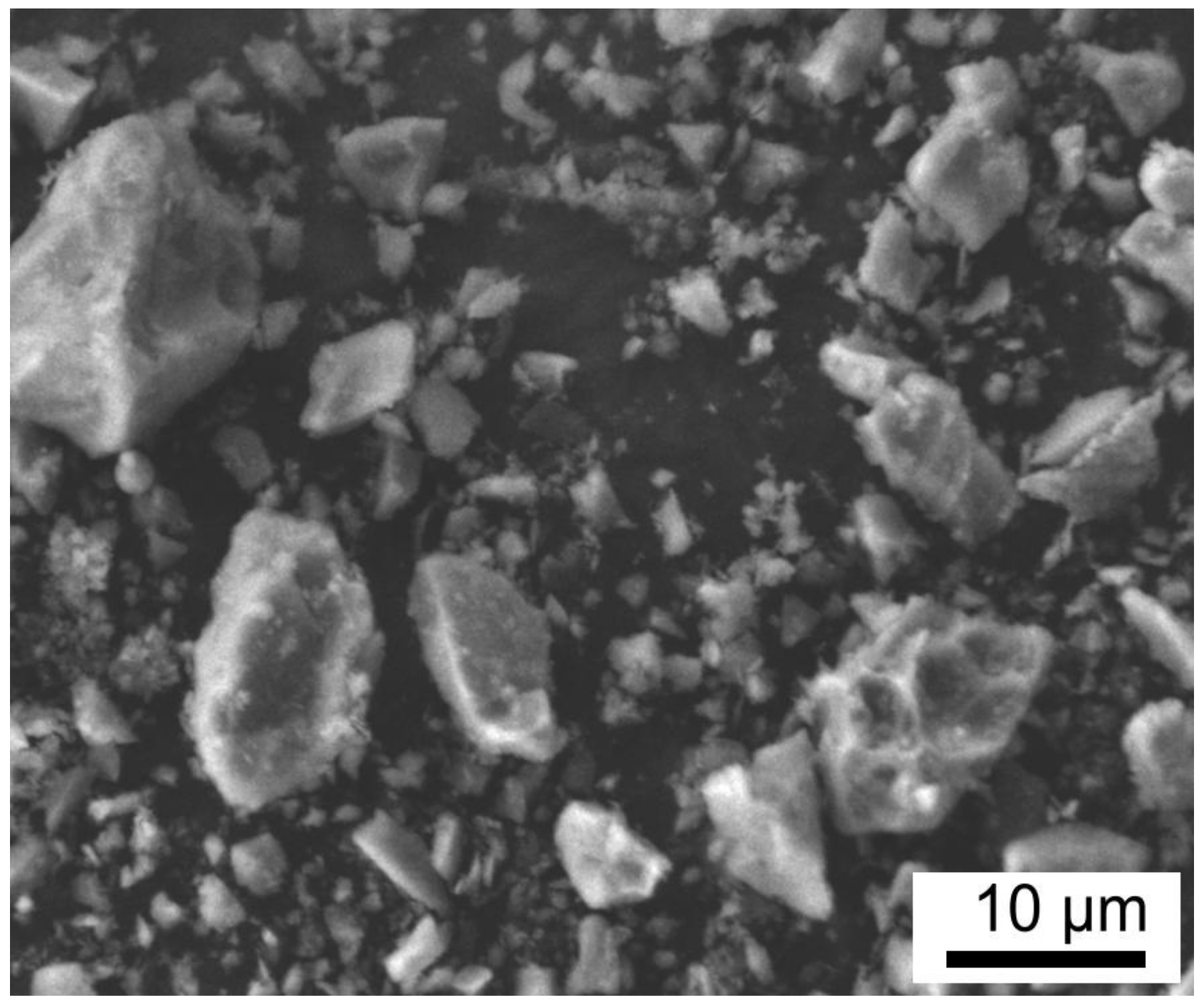
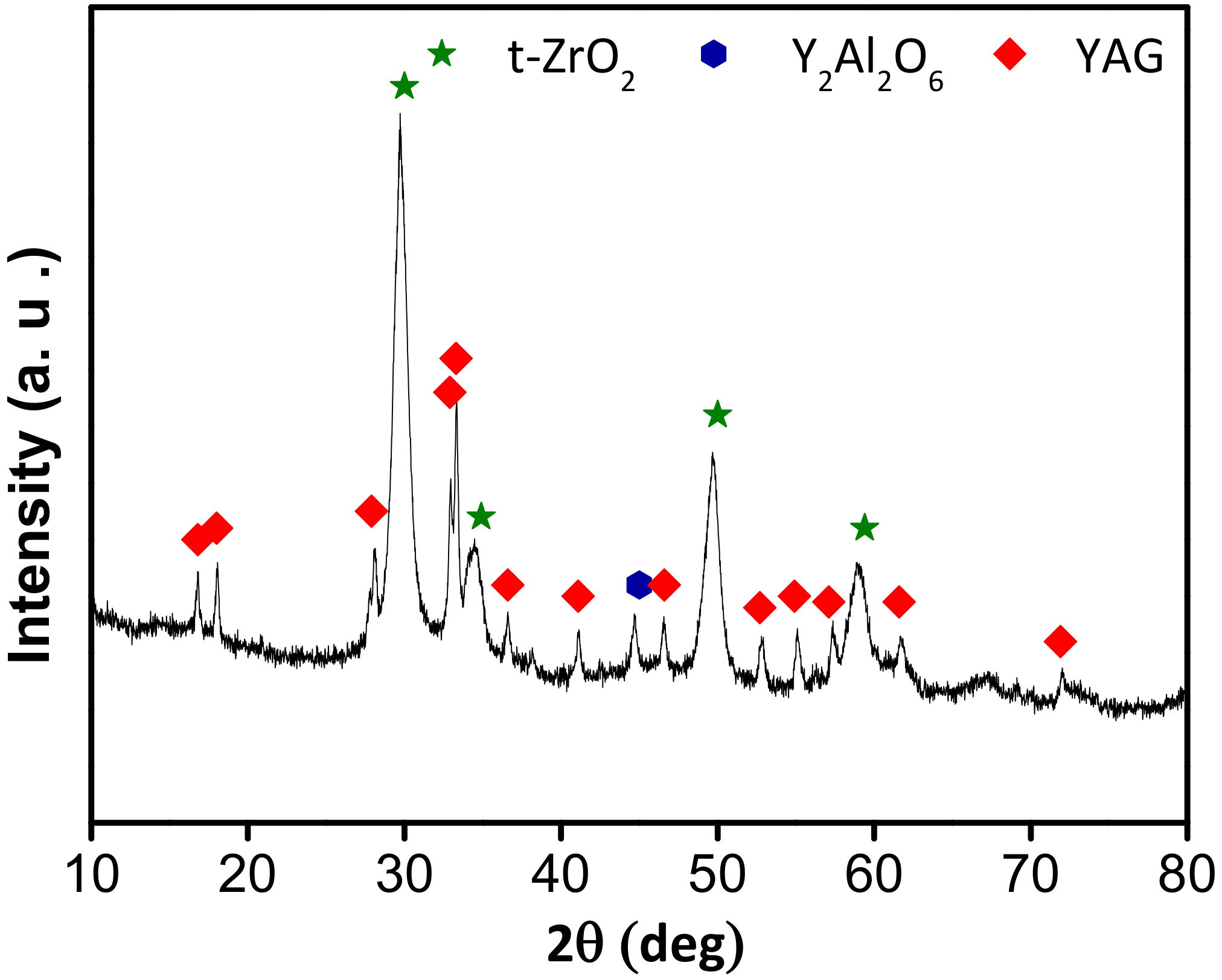
3.1. Characterisation of the AYZ Filler
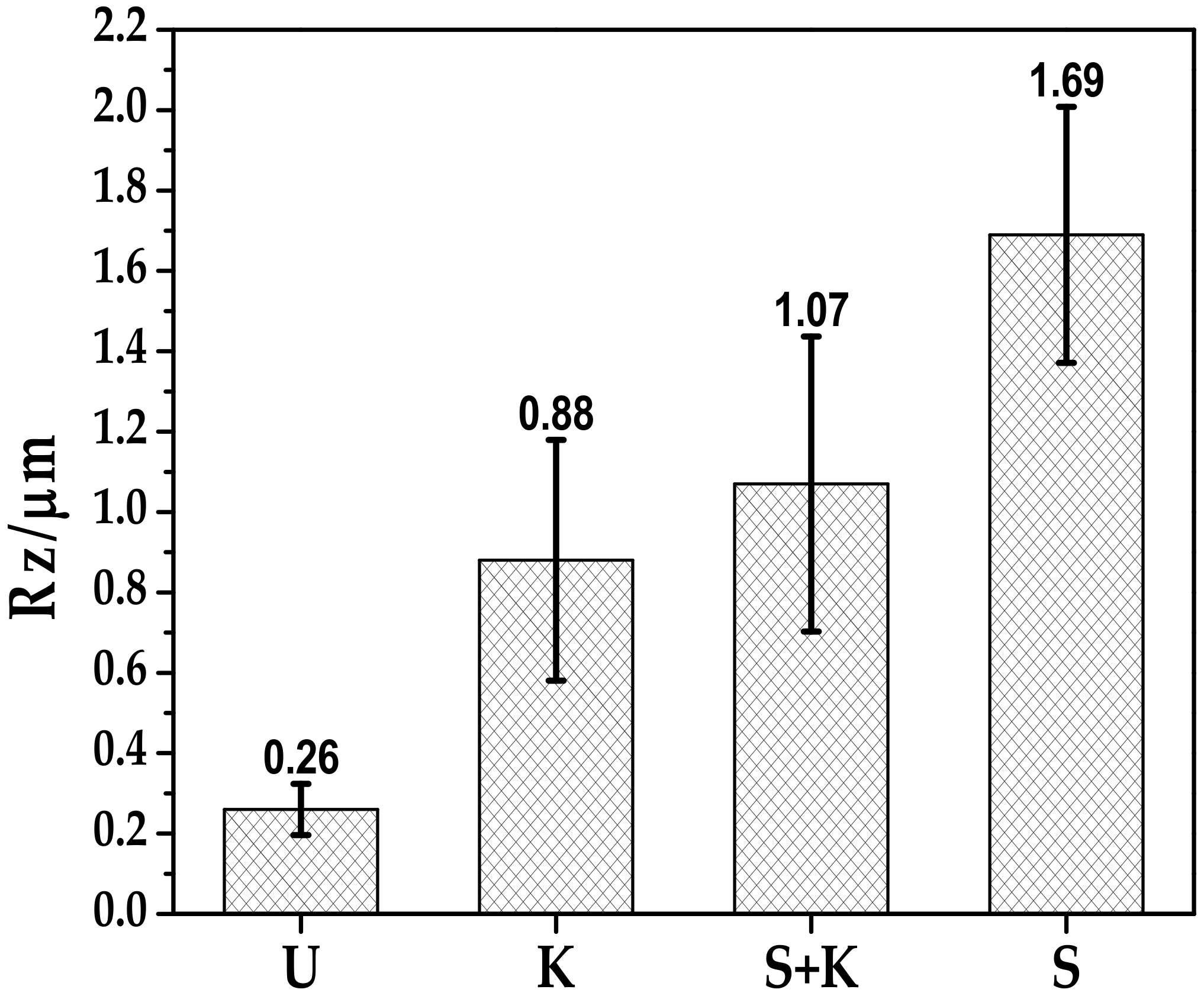
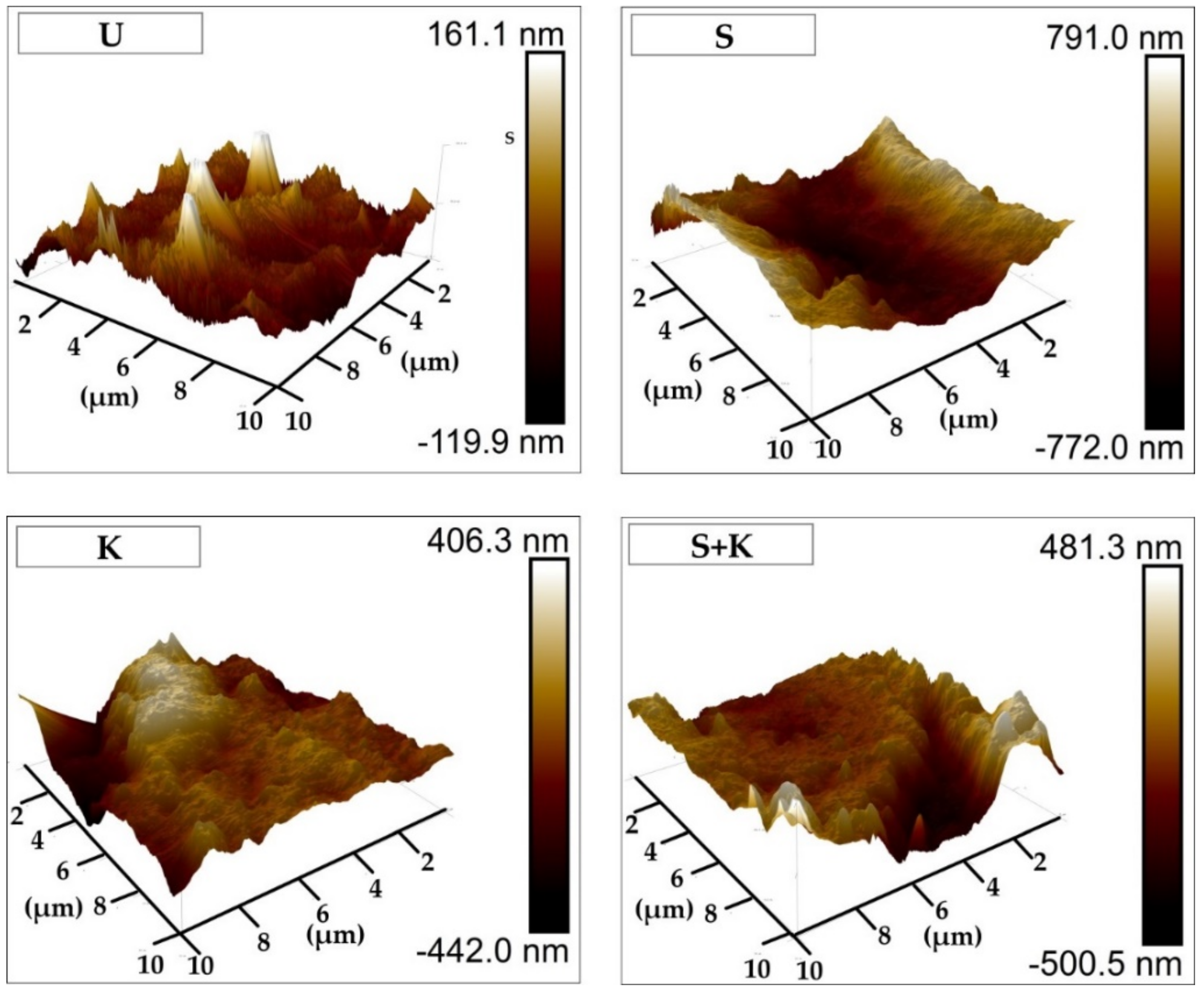
3.2. Treated Steel Surfaces
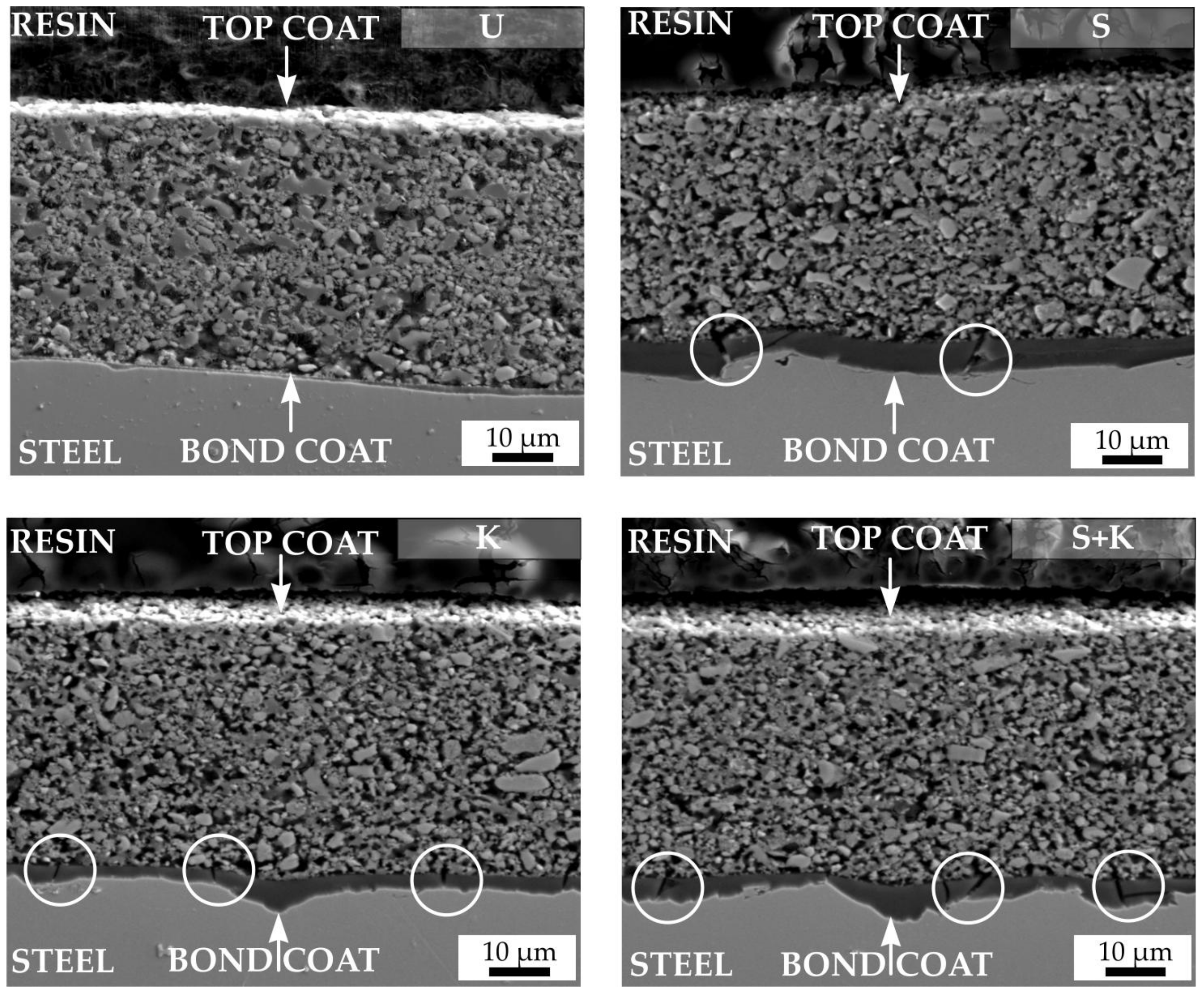
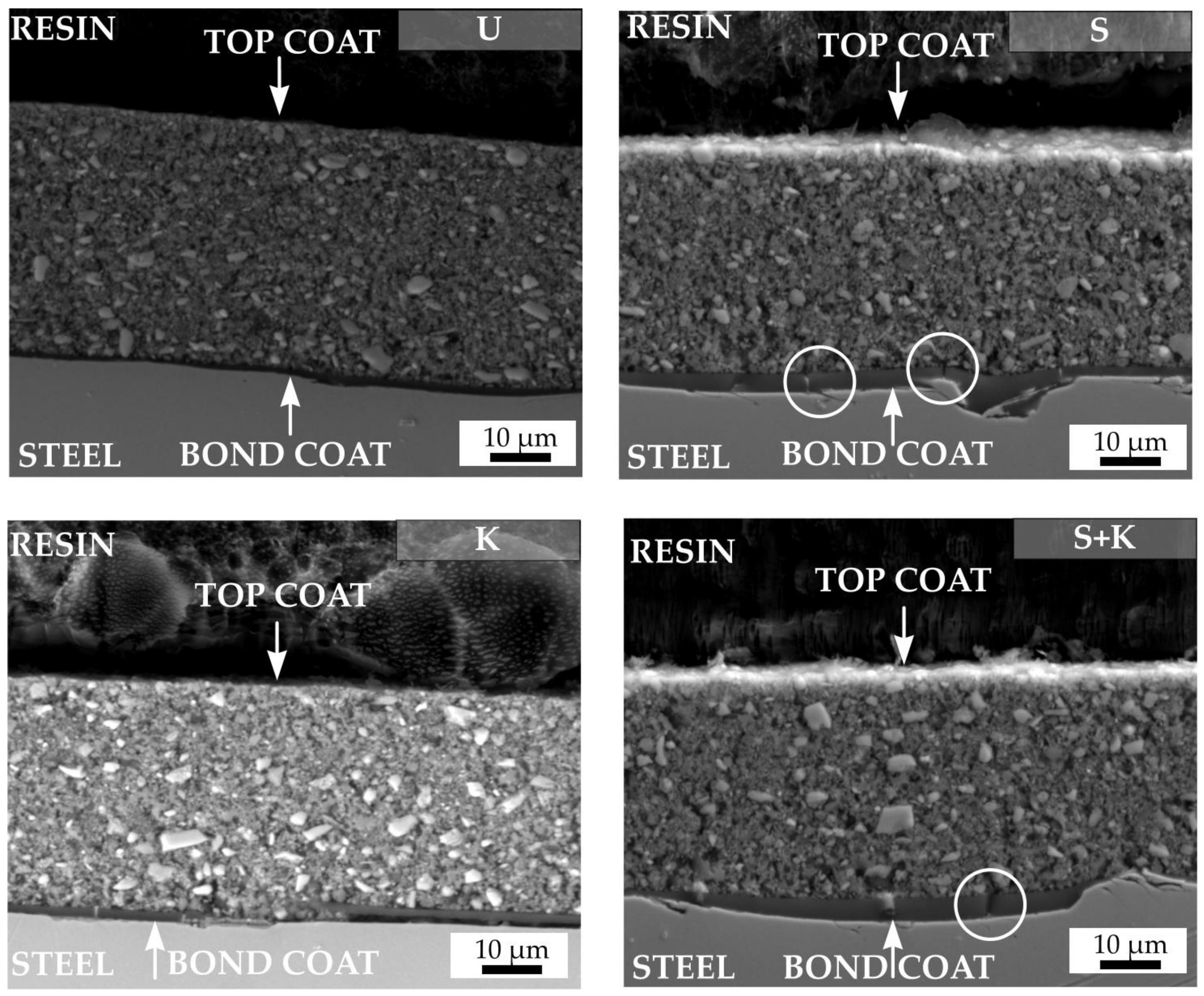
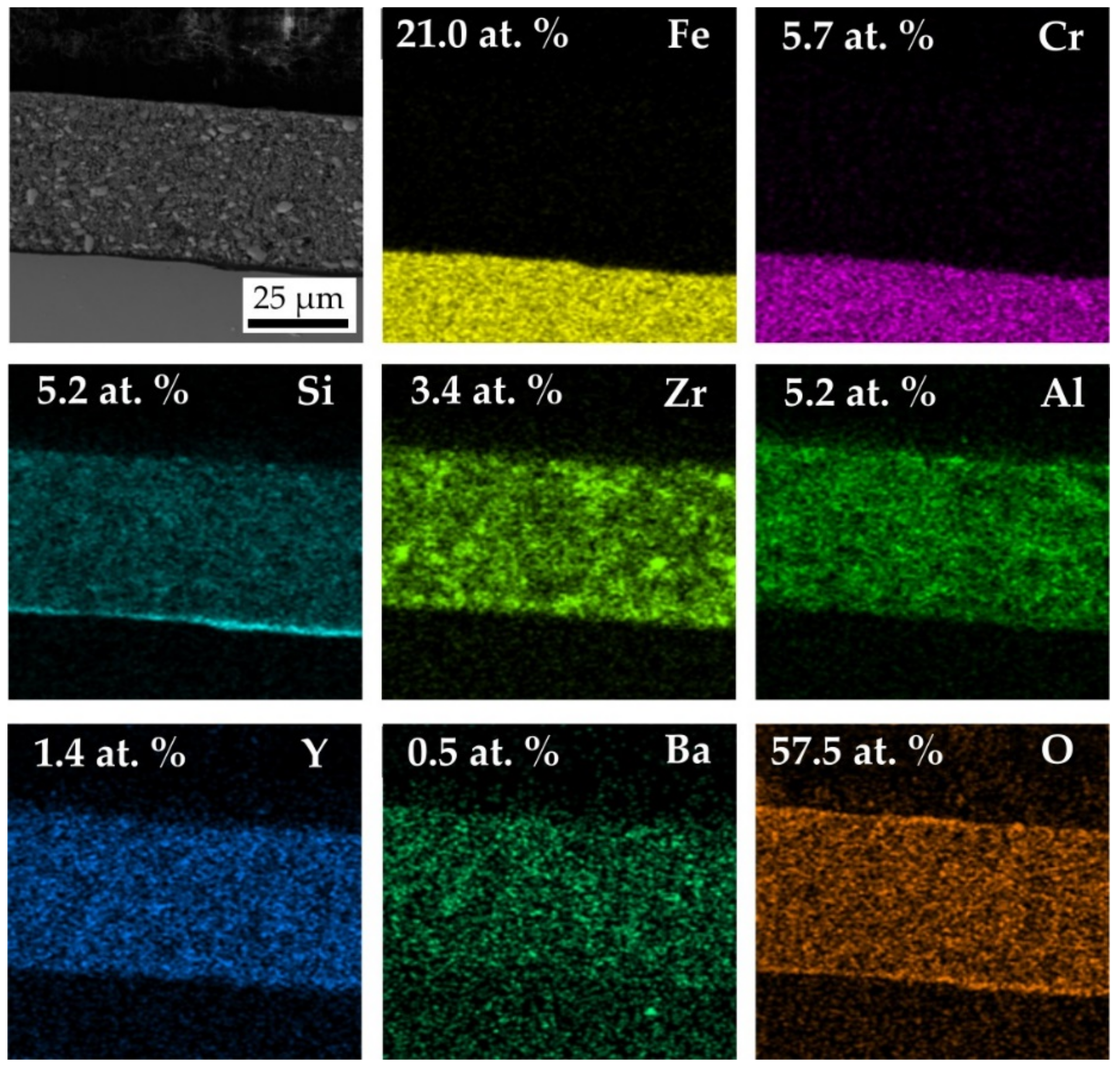
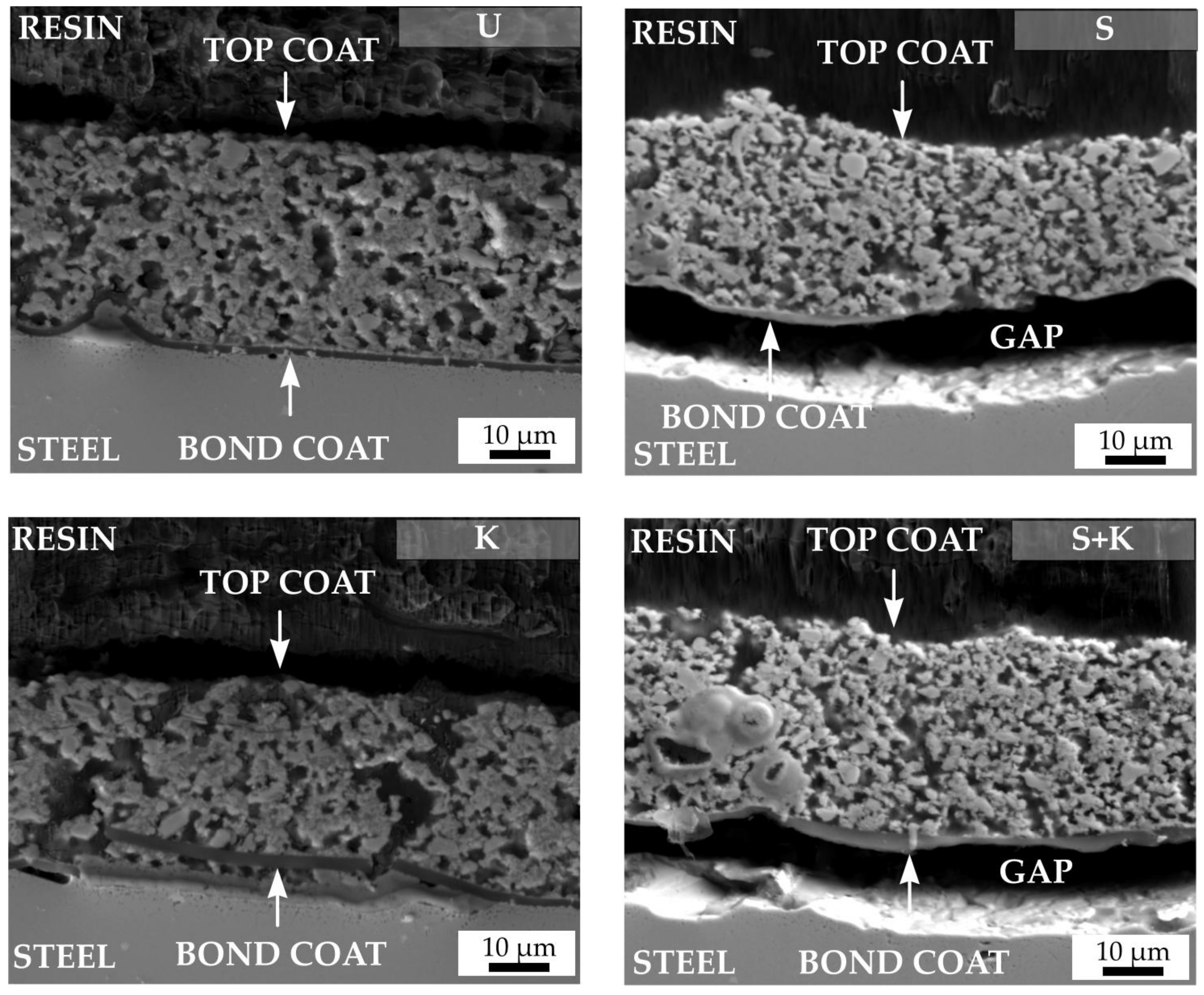
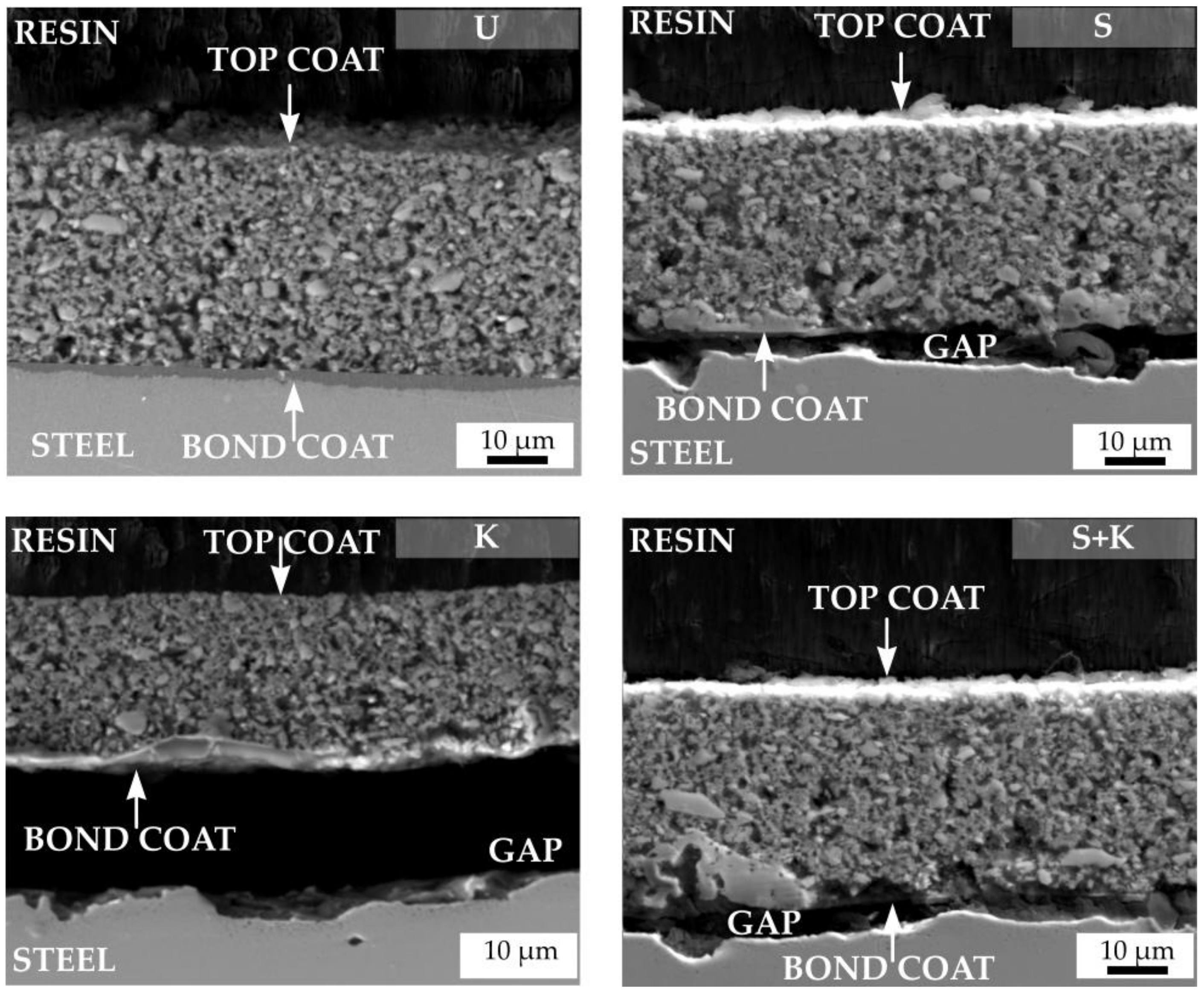
3.3. Characterisation of the Coatings
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, K.; Günthner, M.; Motz, G.; Bordia, R.K. High performance environmental barrier coatings, Part II: Active filler loaded SiOC system for superalloys. J. Eur. Ceram. Soc. 2011, 31, 3011–3020. [Google Scholar] [CrossRef]
- Greil, P. Polymer Derived Engineering Ceramics. Adv. Eng. Mater. 2000, 2, 339–348. [Google Scholar] [CrossRef]
- Torrey, J.D.; Bordia, R.K. Processing of polymer-derived ceramic composite coatings on steel. J. Am. Ceram. Soc. 2008, 91, 41–45. [Google Scholar] [CrossRef]
- Colombo, P.; Paulson, T.E.; Pantano, C.G. Synthesis of Silicon Carbide Thin Films with Polycarbosilane (PCS). J. Am. Ceram. Soc. 2005, 40, 2333–2340. [Google Scholar] [CrossRef]
- Lukacs, A. Polysilazane precursors to advanced ceramics. Am. Ceram. Soc. Bull. 2007, 86, 9301–9306. [Google Scholar]
- Seifert, M.; Travitzky, N.; Krenkel, W.; Motz, G. Multiphase ceramic composites derived by reaction of Nb and SiCN precursor. J. Eur. Ceram. Soc. 2014, 34, 1913–1921. [Google Scholar] [CrossRef]
- Günthner, M.; Pscherer, M.; Kaufmann, C.; Motz, G. High emissivity coatings based on polysilazanes for flexible Cu(In,Ga)Se2 thin-film solar cells. Sol. Energy Mater. Sol. Cells 2014, 123, 97–103. [Google Scholar] [CrossRef]
- Kroke, E.; Li, Y.; Konetschny, C.; Lecomte, E.; Fasel, C.; Riedel, R. Silazane derived ceramics and related materials. Mater. Sci. Eng. R Rep. 2000, 26, 97–199. [Google Scholar] [CrossRef]
- Lenormand, P.; Caravaca, D.; Laberty-Robert, C.; Ansart, F. Thick films of YSZ electrolytes by dip-coating process. J. Eur. Ceram. Soc. 2005, 25, 2643–2646. [Google Scholar] [CrossRef]
- Kappa, M.; Kebianyor, A.; Scheffler, M. A two-component preceramic polymer system for structured coatings on metals. Thin Solid Film. 2010, 519, 301–305. [Google Scholar] [CrossRef]
- Morlier, A.; Cros, S.; Garandet, J.P.; Alberola, N. Thin gas-barrier silica layers from perhydropolysilazane obtained through low temperature curings: A comparative study. Thin Solid Film. 2012, 524, 62–66. [Google Scholar] [CrossRef]
- Barroso, G.S.; Krenkel, W.; Motz, G. Low thermal conductivity coating system for application up to 1000 °C by simple PDC processing with active and passive fillers. J. Eur. Ceram. Soc. 2015, 35, 3339–3348. [Google Scholar] [CrossRef]
- Parchovianský, M.; Barroso, G.; Petríková, I.; Motz, G.; Galusková, D.; Galusek, D. Polymer derived glass ceramic layers for corrosion protection of metals. Ceram. Trans. 2016, 256, 187–200. [Google Scholar] [CrossRef]
- Goerke, O.; Feike, E.; Heine, T.; Trampert, A.; Schubert, H. Ceramic coatings processed by spraying of siloxane precursors (polymer-spraying). J. Eur. Ceram. Soc. 2004, 24, 2141–2147. [Google Scholar] [CrossRef]
- Günthner, M.; Wang, K.; Bordia, R.K.; Motz, G. Conversion behaviour and resulting mechanical properties of polysilazane-based coatings. J. Eur. Ceram. Soc. 2012, 32, 1883–1892. [Google Scholar] [CrossRef]
- Greil, P. Active Filler Controlled Pyrolysis of Preceramic Polymers. J. Am. Ceram. Soc. 1995, 78, 835–848. [Google Scholar] [CrossRef]
- Greil, P. Near Net Shape Manufacturing of Ceramic Components. Cfi Ceram. Forum Int. 1998, 75, 15–21. [Google Scholar] [CrossRef]
- Petríková, I.; Parchovianský, M.; Lenz-Leite, M.; Švančárek, P.; Motz, G.; Galusek, D. Passive Filler loaded Polysilazane-Derived Glass/Ceramic coating system applied to AISI 441 stainless steel, Part 1: Processing and Characterisation. Int. J. Appl. Ceram. Technol. 2019. [Google Scholar] [CrossRef]
- Bernardo, E.; Colombo, P.; Hampshire, S. SiAlON-based ceramics from filled preceramic polymers. J. Am. Ceram. Soc. 2006, 89, 3839–3842. [Google Scholar] [CrossRef]
- Labrousse, M.; Nanot, M.; Boch, P.; Chassagneux, E. Ex-polymer SiC coatings with Al2O3 particulates as filler materials. Ceram. Int. 1993, 19, 259–267. [Google Scholar] [CrossRef]
- Scheffler, M.; Dernovsek, O.; Schwarze, D.; Science, M.; Science, M. Polymer / filler derived NbC composite ceramics. J. Mater. Sci. 2003, 8, 4925–4931. [Google Scholar] [CrossRef]
- Schütz, A.; Günthner, M.; Motz, G.; Greißl, O.; Glatzel, U. Characterisation of novel precursor-derived ceramic coatings with glass filler particles on steel substrates. Surf. Coat. Technol. 2012, 207, 319–327. [Google Scholar] [CrossRef]
- Günthner, M.; Kraus, T.; Krenkel, W.; Motz, G.; Dierdorf, A.; Decker, D. Particle-Filled PHPS silazane-based coatings on steel. Int. J. Appl. Ceram. Technol. 2009, 6, 373–380. [Google Scholar] [CrossRef]
- Günthner, M.; Schütz, A.; Glatzel, U.; Wang, K.; Bordia, R.K.; Greißl, O.; Krenkel, W.; Motz, G. High performance environmental barrier coatings, Part I: Passive filler loaded SiCN system for steel. J. Eur. Ceram. Soc. 2011, 31, 3003–3010. [Google Scholar] [CrossRef]
- Parchovianský, M.; Petíková, I.; Barroso, G.S.; Galuskova, D.; Motz, G.; Galusek, D. Corrosion and oxidation behavior of polymer derived ceramic coatings with passive glass fillers on AISI 441. Ceram. Silik. 2018, 62, 146–157. [Google Scholar] [CrossRef]
- Pechini, M.P. Method of Preparing Lead and Alkaline Earth Titanates and Niobates and Coating Method Using the Same to Form a Capacitor. US Patent No. 3330697, 11 July 1967. [Google Scholar]
- Günthner, M.; Kraus, T.; Dierdorf, A.; Decker, D.; Krenkel, W.; Motz, G. Advanced coatings on the basis of Si(C)N precursors for protection of steel against oxidation. J. Eur. Ceram. Soc. 2009, 29, 2061–2068. [Google Scholar] [CrossRef]
- Bauer, F.; Decker, U.; Dierdorf, A.; Ernst, H.; Heller, R.; Liebe, H.; Mehnert, R. Preparation of moisture curable polysilazane coatings: Part I. Elucidation of low temperature curing kinetics by FT-IR spectroscopy. Prog. Org. Coat. 2005, 53, 183–190. [Google Scholar] [CrossRef]
- Bahloul, D.; Pereira, M.; Goursat, P.; Yive, N.S.C.K.; Corriu, R.J.; Mixte, R.I. Preparation of silicon carbonitrides from organosilicon polymer. I. Thermal decomposition of the cross-linked polysilazane. J. Am. Ceram. Soc. 1993, 75, 1156–1162. [Google Scholar] [CrossRef]
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal barrier coatings for gas-turbine engine applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef]
- Soliman, H.M.; Waheed, A.F. Effect of differential thermal expansion coefficient on stresses generated in coating. J. Mater. Sci. Technol. 1999, 15, 457–462. [Google Scholar]


| Symbol | Pretreatment of Stainless Steel |
|---|---|
| U | 3-step ultrasonic vibration cleaning in acetone, ethanol and deionised water (10 min each) |
| S | Sandblasting with glass beads (70–110 μm), ultrasonic cleaning in deionised water |
| K | Chemical etching—Kroll’s reagent (deionised water, HNO3, HF), 20 s |
| S+K | Sandblasting with glass beads + etching with Kroll’s reagent |
| Passive Fillers | d50 (µm) | ρ (g/cm3) | CTE (10−6/K) |
|---|---|---|---|
| 8YSZ | 0.5 | 6.1 | 11.5 |
| AYZ | 1–10 | 4.6 | 8.6 |
| G018-281 | 0.5–5 | 2.7 | 12.1 |
| Sample Name | Durazane 1800 | 8YSZ | AYZ | G018-281 | CTE (10−6/K) |
|---|---|---|---|---|---|
| C2c | 30 | 35 | - | 35 | 10.1 |
| D2-PP | 25 | 20 | 20 | 35 | 9.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parchovianský, M.; Parchovianská, I.; Švančárek, P.; Motz, G.; Galusek, D. PDC Glass/Ceramic Coatings Applied to Differently Pretreated AISI441 Stainless Steel Substrates. Materials 2020, 13, 629. https://doi.org/10.3390/ma13030629
Parchovianský M, Parchovianská I, Švančárek P, Motz G, Galusek D. PDC Glass/Ceramic Coatings Applied to Differently Pretreated AISI441 Stainless Steel Substrates. Materials. 2020; 13(3):629. https://doi.org/10.3390/ma13030629
Chicago/Turabian StyleParchovianský, Milan, Ivana Parchovianská, Peter Švančárek, Günter Motz, and Dušan Galusek. 2020. "PDC Glass/Ceramic Coatings Applied to Differently Pretreated AISI441 Stainless Steel Substrates" Materials 13, no. 3: 629. https://doi.org/10.3390/ma13030629
APA StyleParchovianský, M., Parchovianská, I., Švančárek, P., Motz, G., & Galusek, D. (2020). PDC Glass/Ceramic Coatings Applied to Differently Pretreated AISI441 Stainless Steel Substrates. Materials, 13(3), 629. https://doi.org/10.3390/ma13030629






