Influence of Glass Additions on Illitic Clay Ceramics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Feedstock
2.3. Milling and Mixing by Disintegration
- D50—average particle size before disintegration, mm;
- d50—average particle size after disintegration, mm.
2.4. Characterization of the Sintering Process and Phase Transformation
2.5. Characterization of Physico-Mechanical Properties
3. Results and Discussion
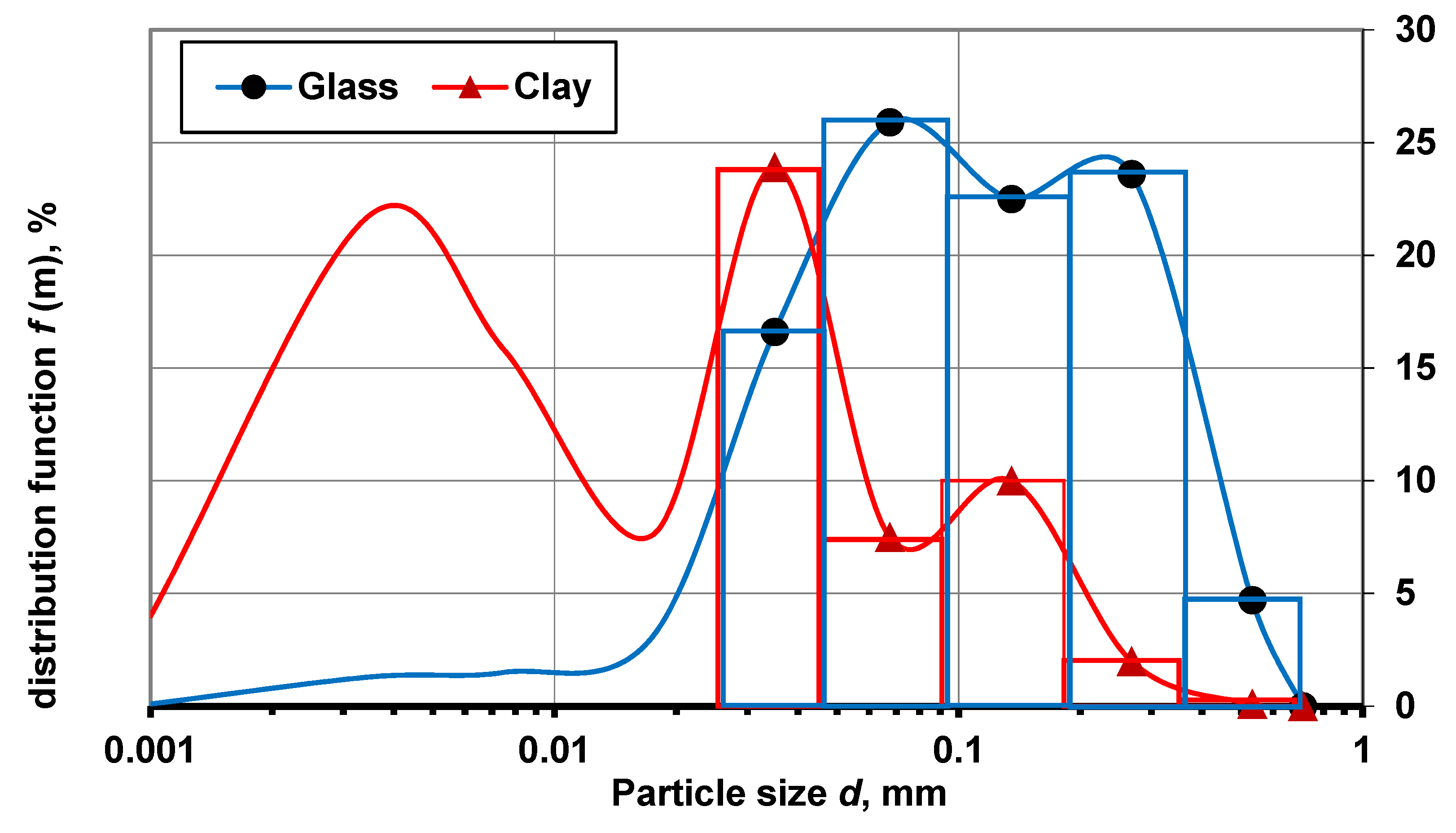
3.1. Efficiency of Clay and Glass Disintegration
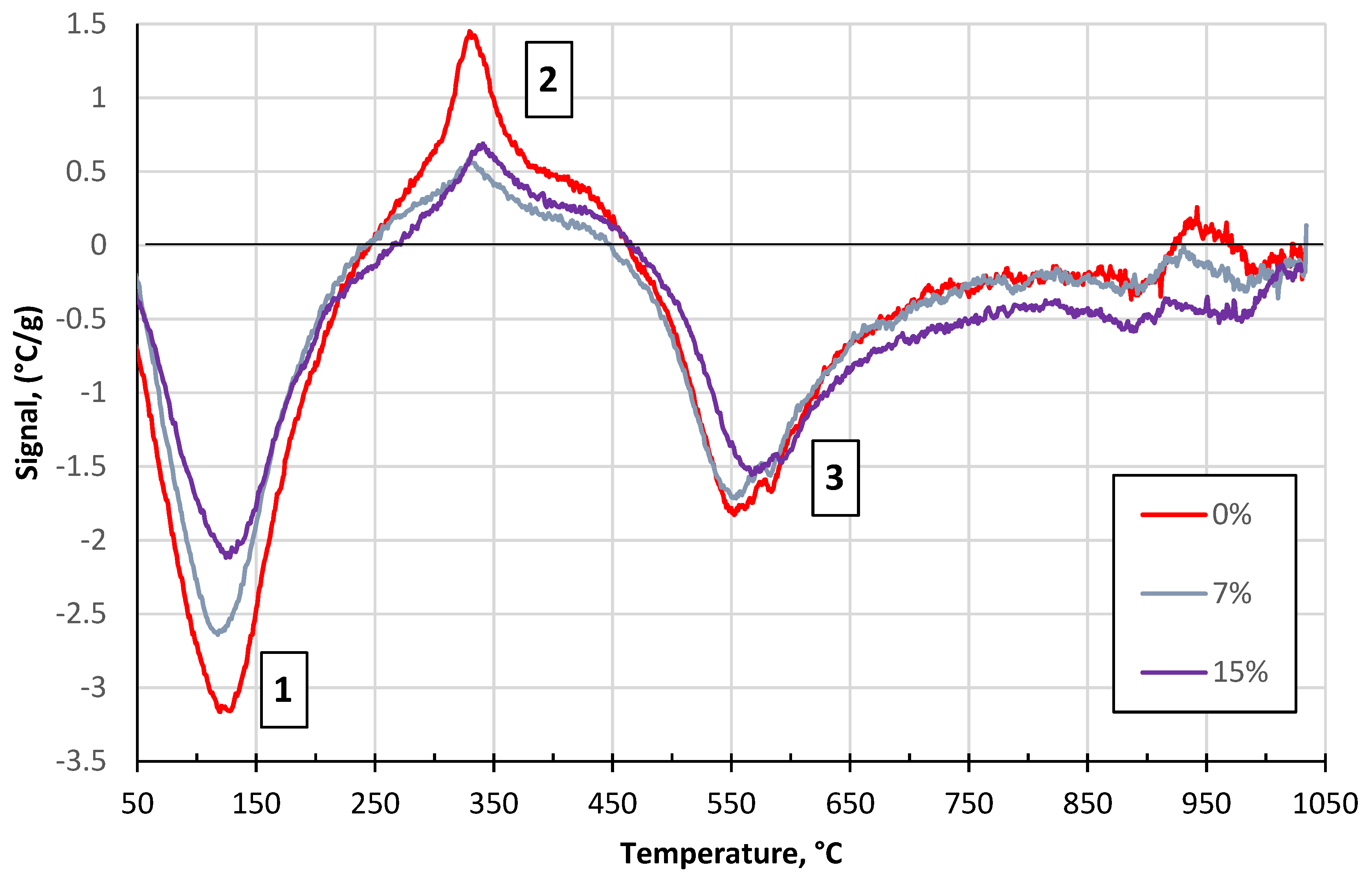
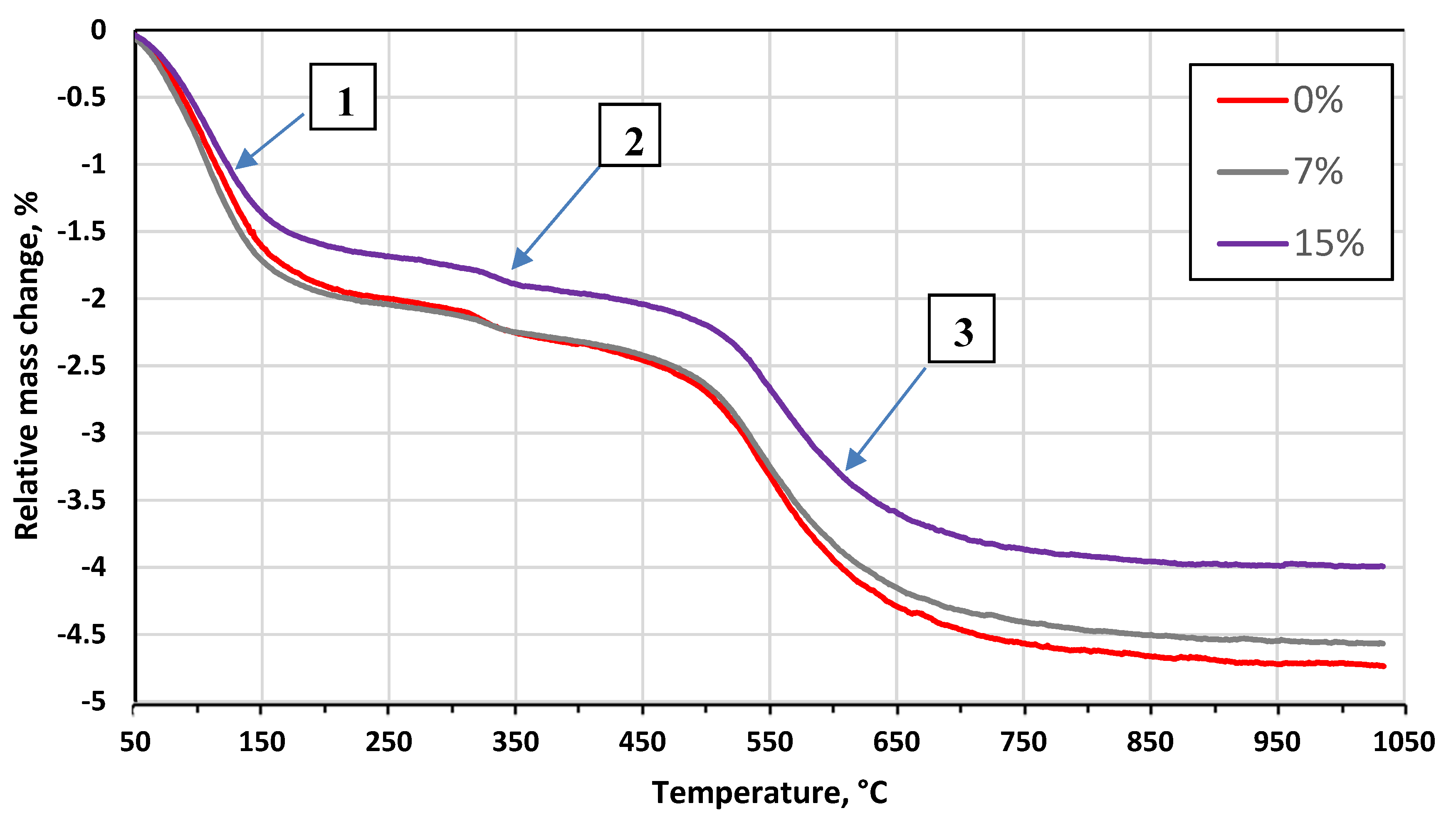
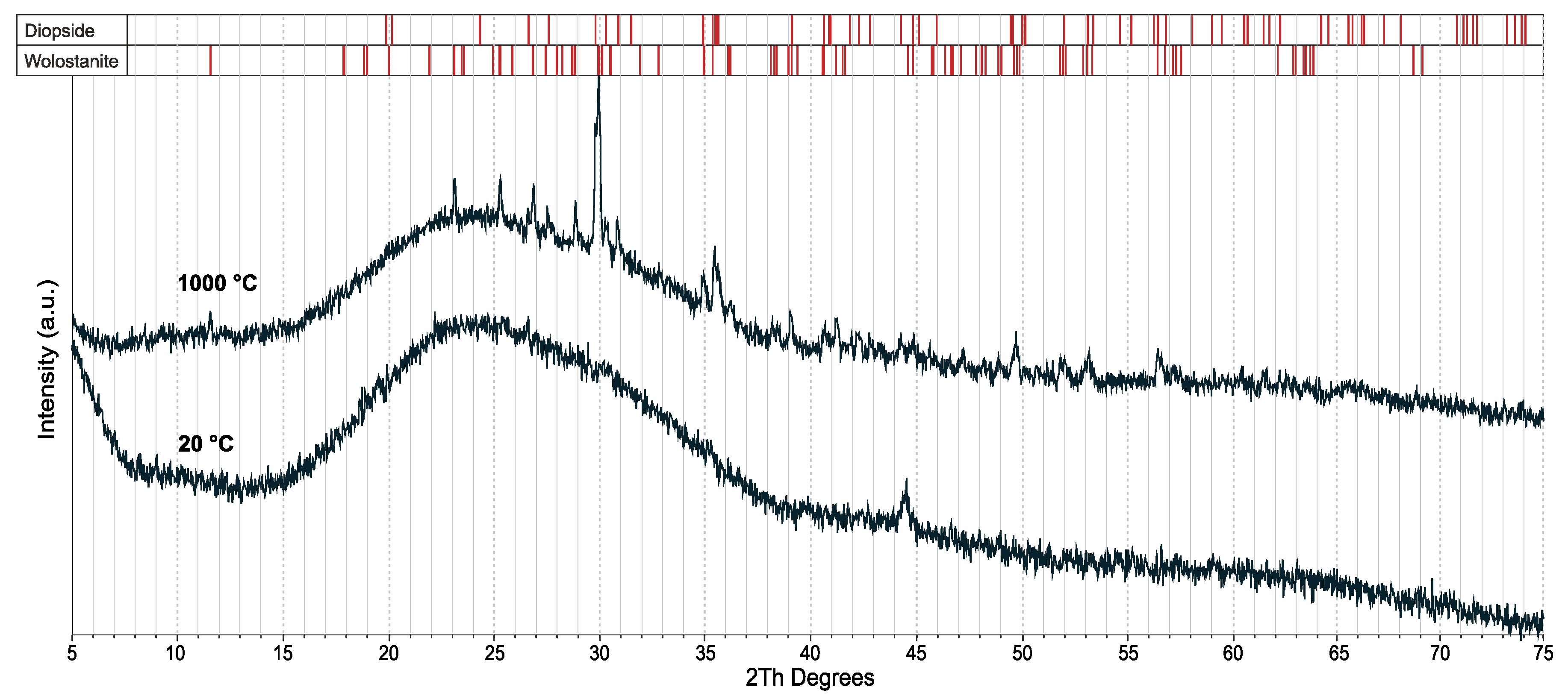
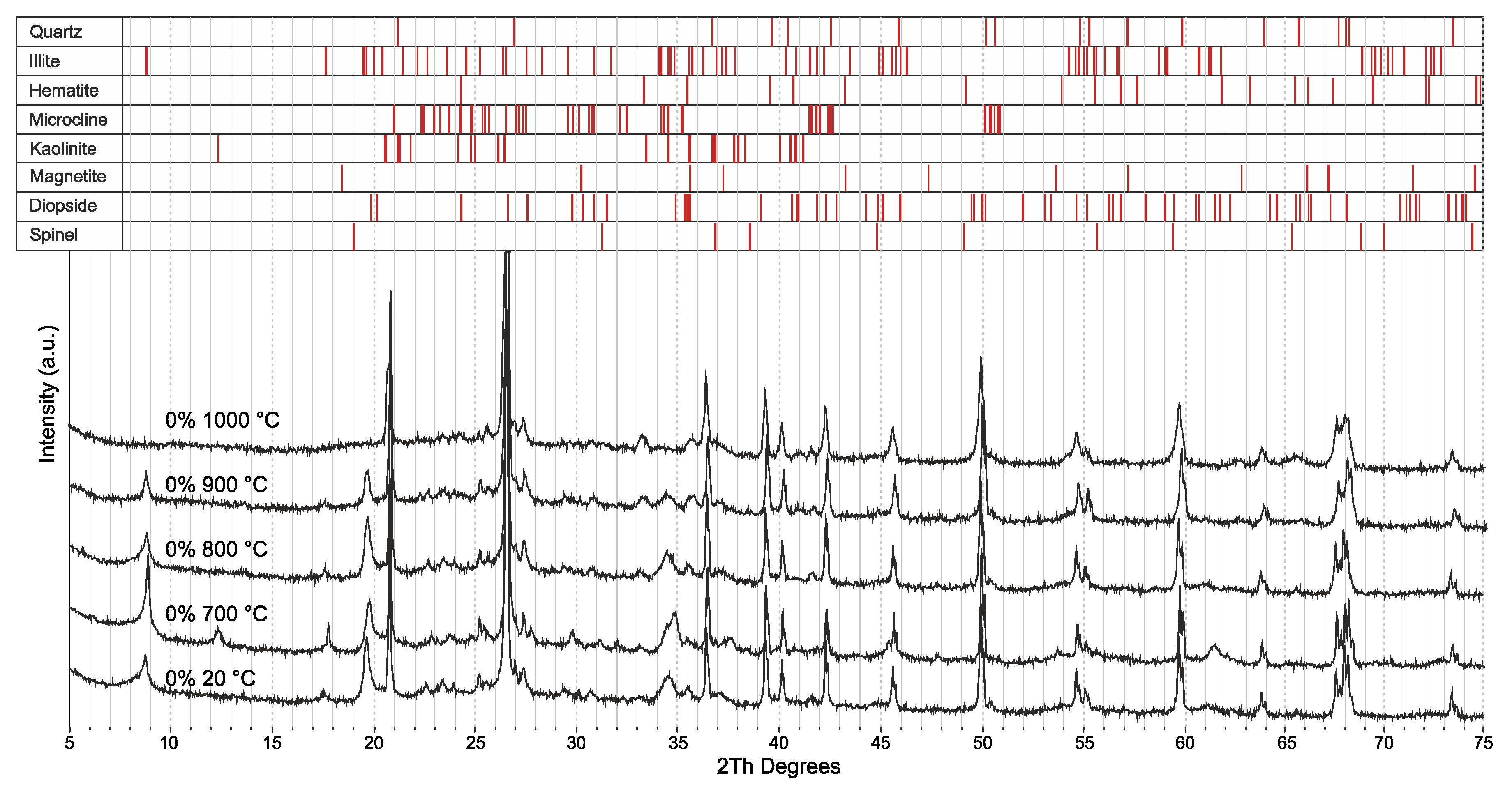
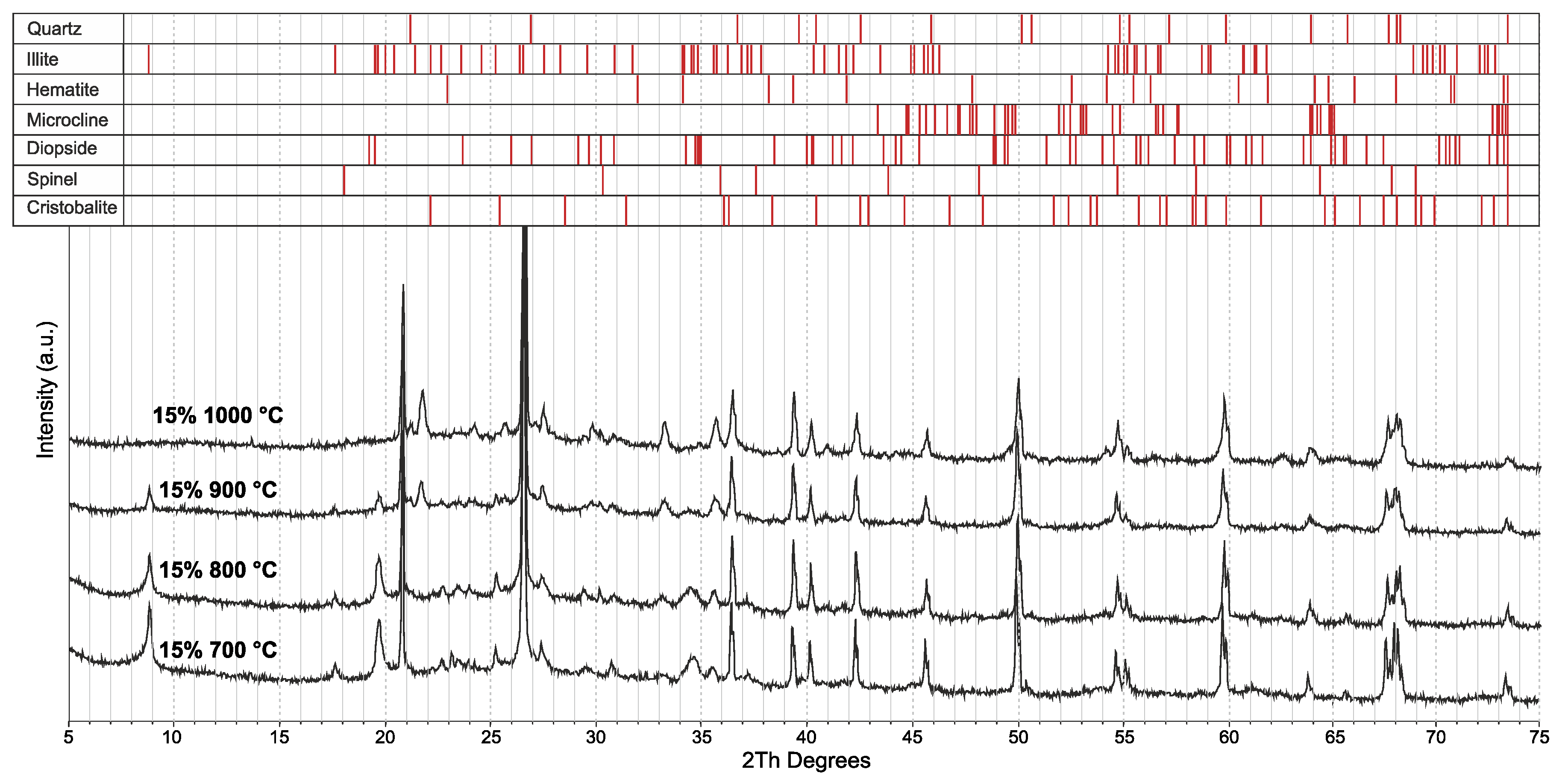
3.2. Effect of the Glass Concentration on the Sintering Processes
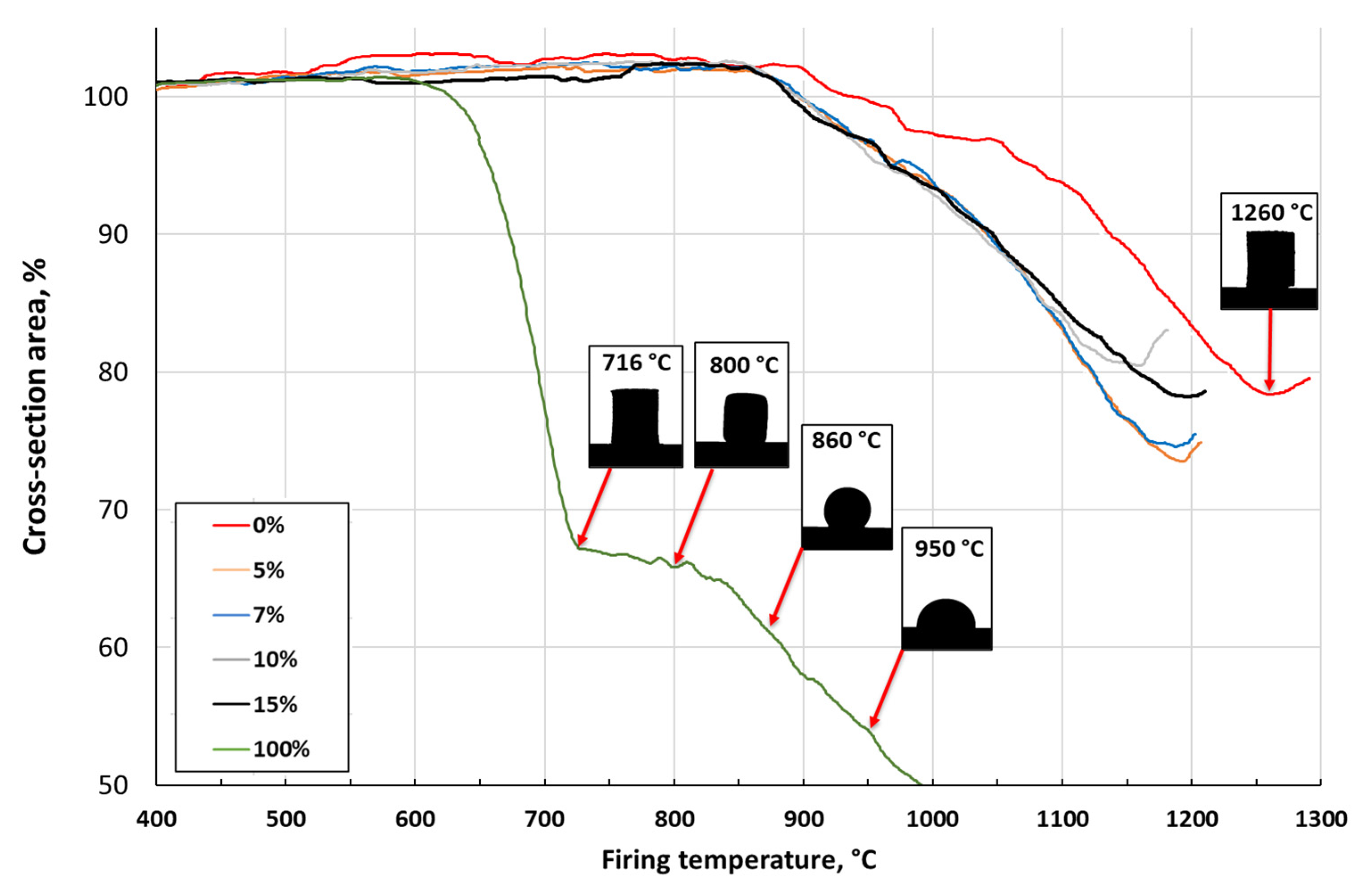
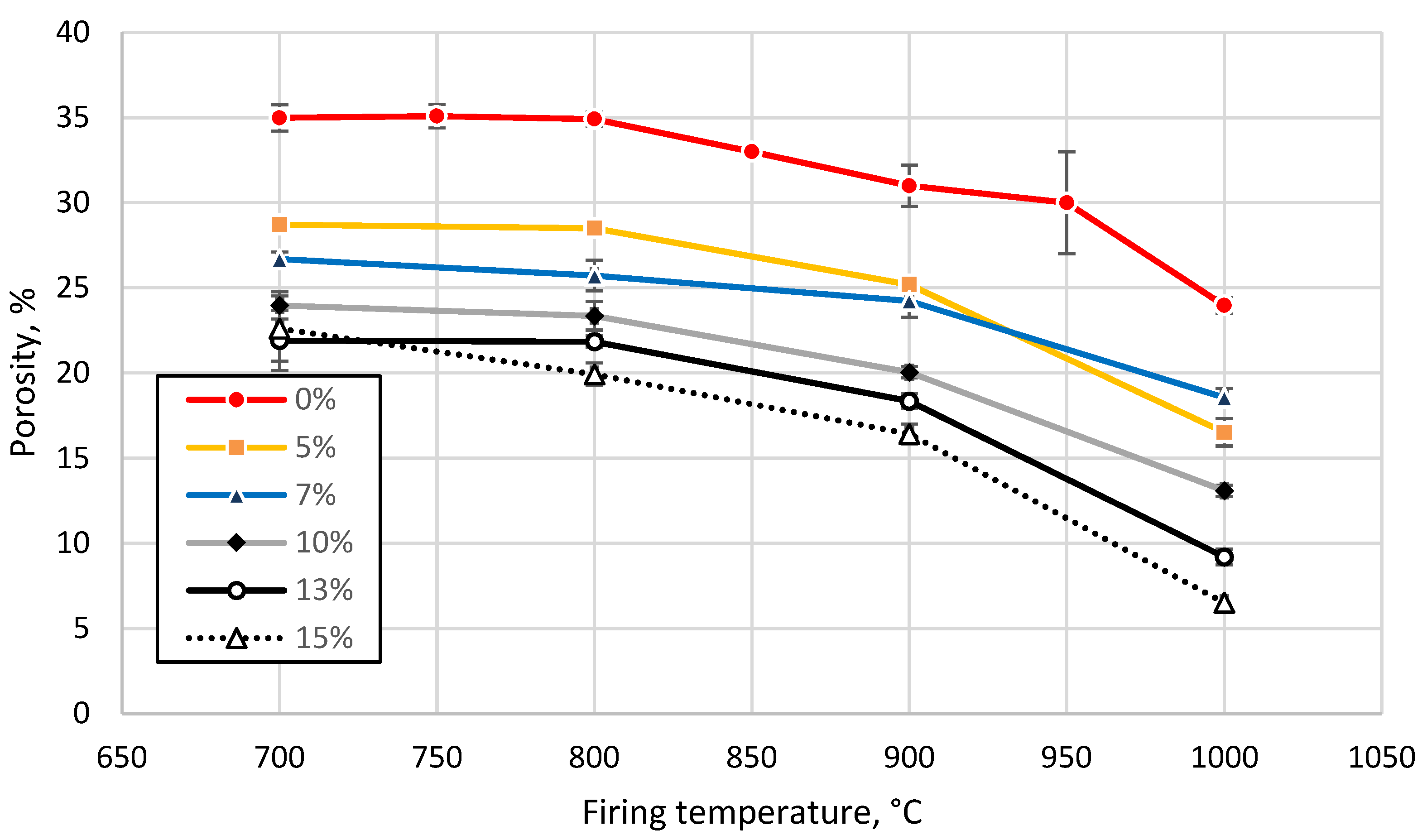
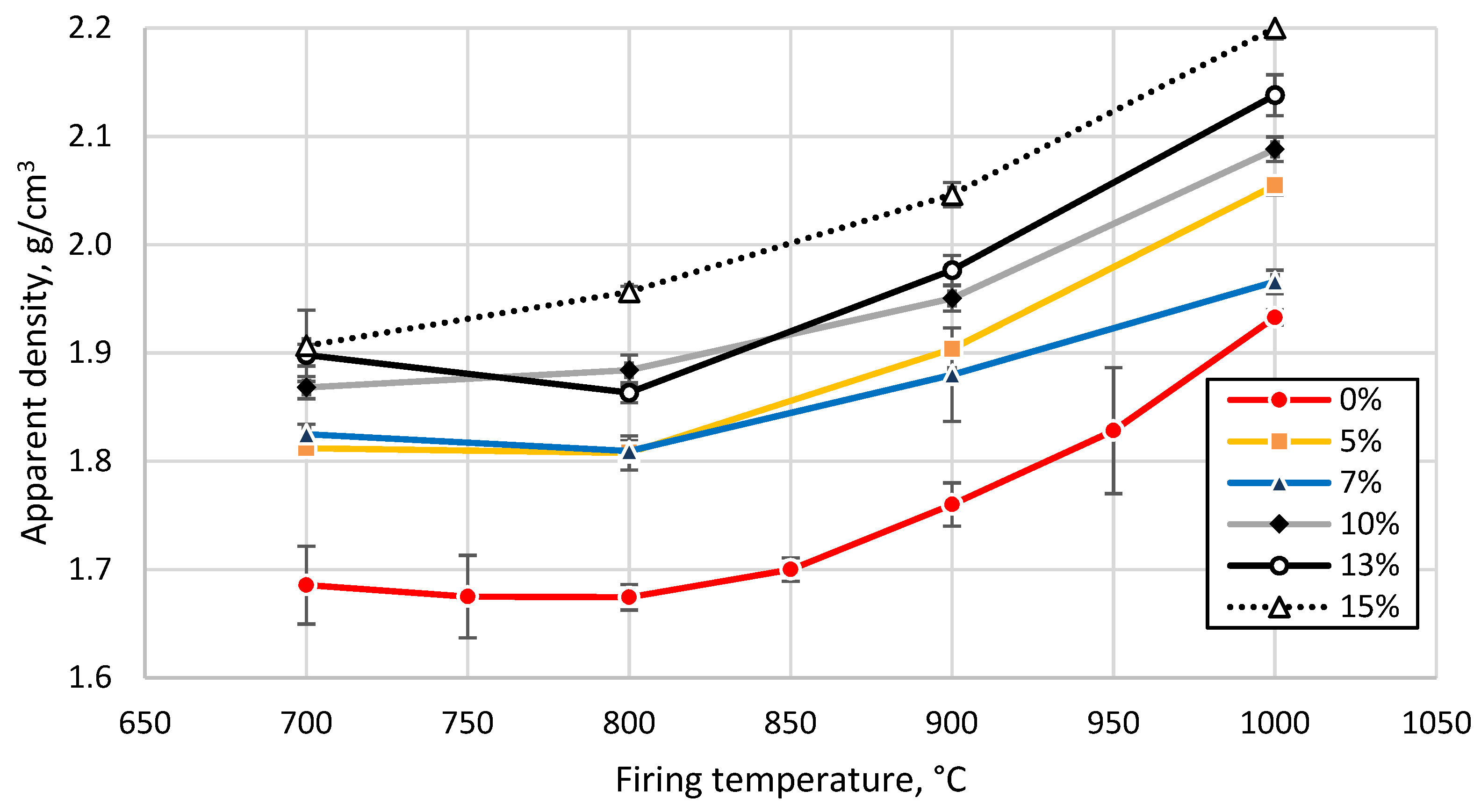
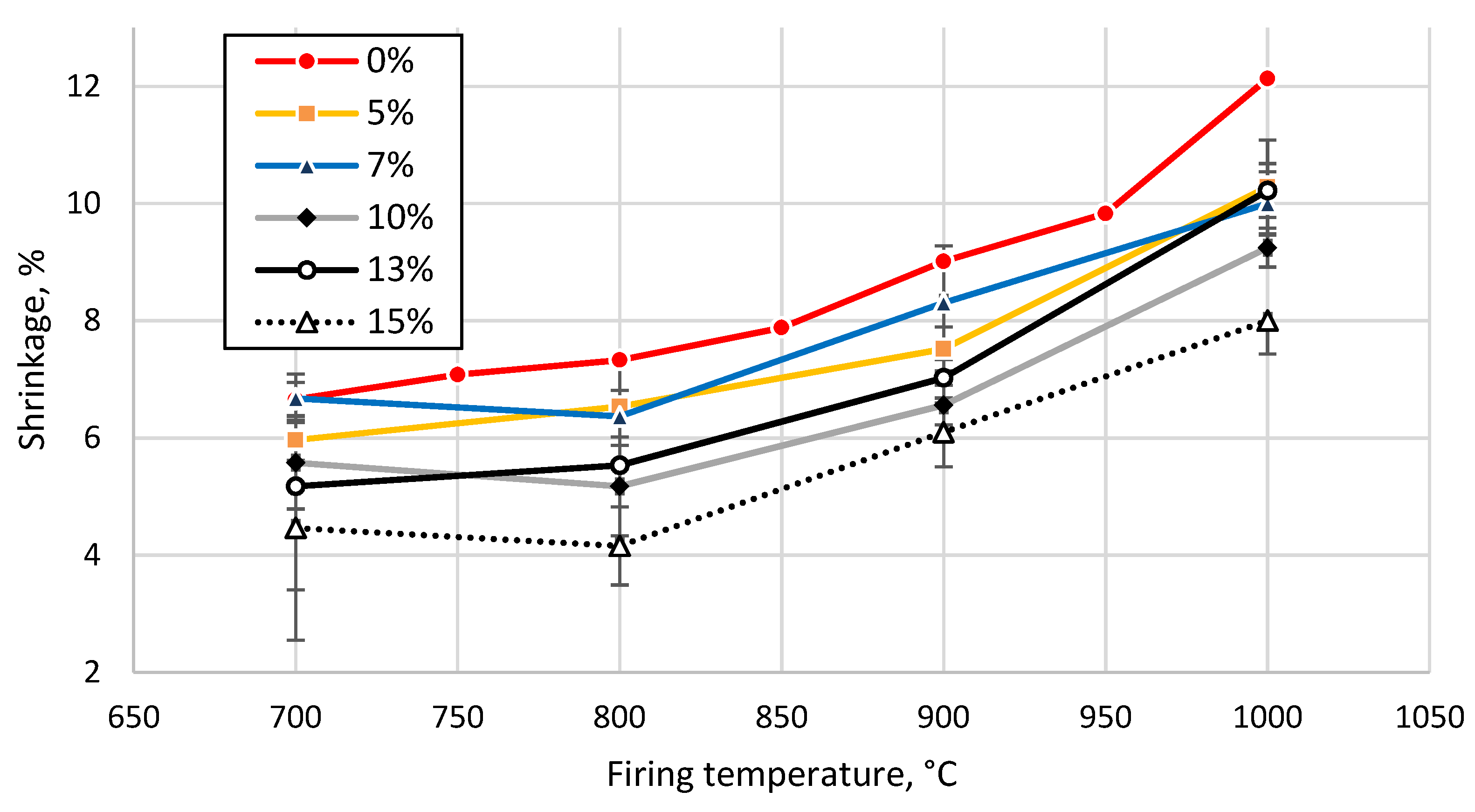
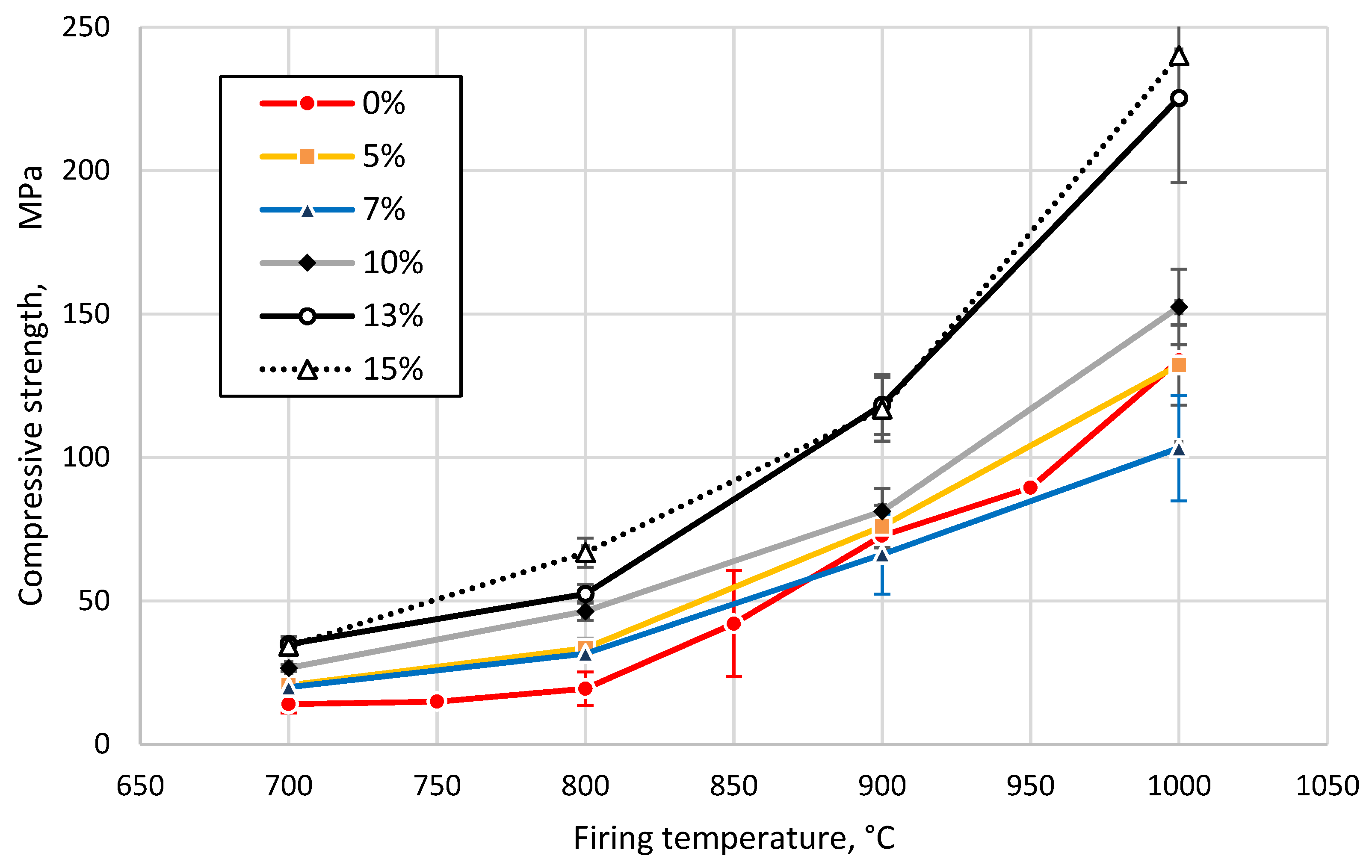
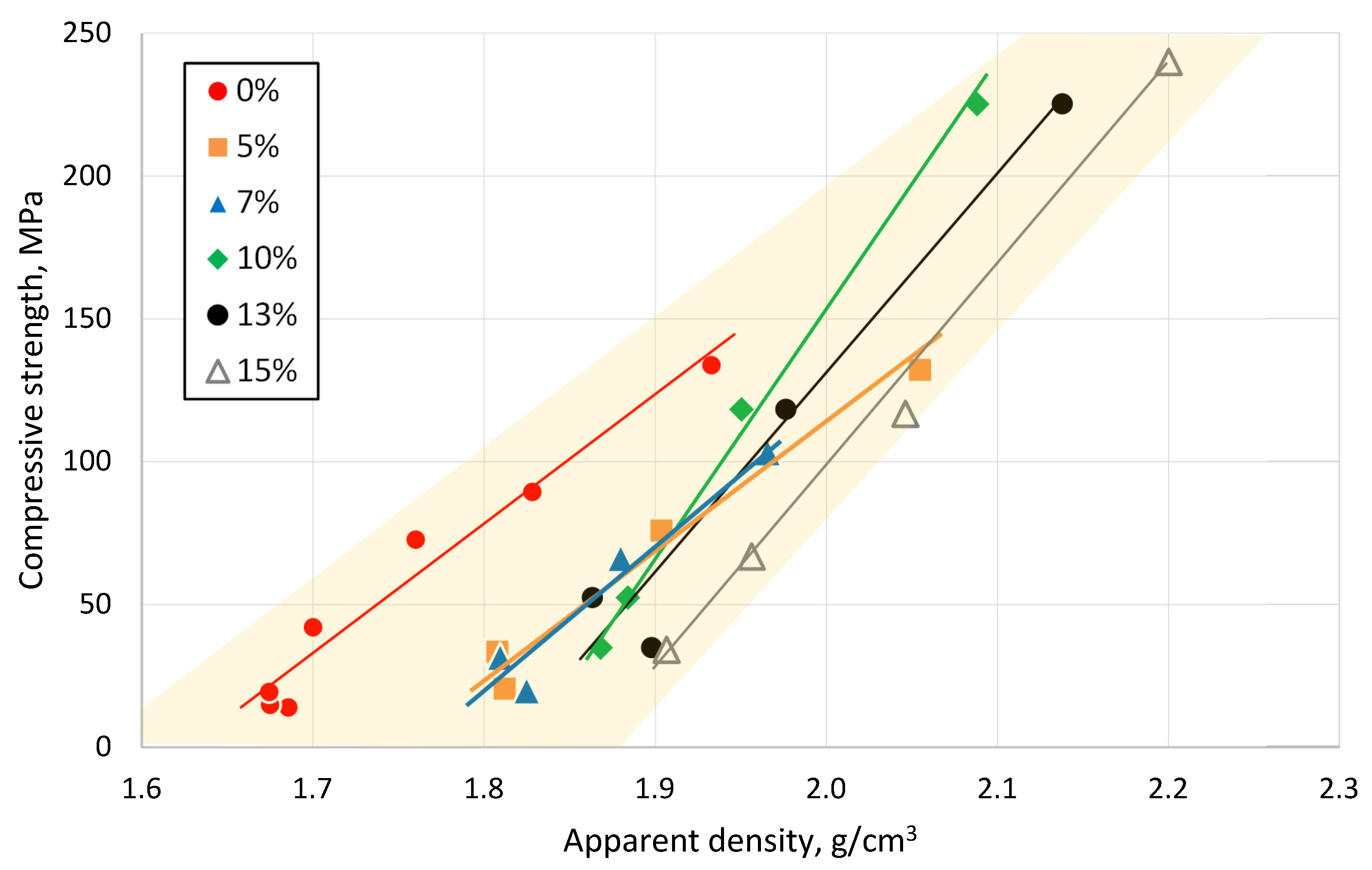
3.3. Effect of Sintering Temperature and Glass Concentration on the Physico-Mechanical Properties of the Clay Ceramics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eurostat Municipal waste landfilled, incinerated, recycled and composted in the EU-27, 1995 to 2015. Available online: http://ec.europa.eu/eurostat/statistics-explained/index.php/File:Municipal_waste_landfilled,_incinerated,_recycled_and_composted_in_the_EU-27,_1995_to_2015_update.png (accessed on 19 October 2017).
- Eurostats Treatment of waste by waste category, hazardousness and waste management operations. Available online: https://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=env_wastrt&lang=en (accessed on 11 November 2019).
- Taveri, G.; Tousek, J.; Bernardo, E.; Toniolo, N.; Boccaccini, A.R.; Dlouhy, I. Proving the role of boron in the structure of fly-ash/borosilicate glass based geopolymers. Mater. Lett. 2017, 200, 105–108. [Google Scholar] [CrossRef]
- Hardy, F. The physical significance of the shrinkage coefficient of clays and soils. J. Agric. Sci. 1923, 13, 243–264. [Google Scholar] [CrossRef]
- Topçu, İ.B.; Canbaz, M. Properties of concrete containing waste glass. Cem. Concr. Res. 2004, 34, 267–274. [Google Scholar] [CrossRef]
- Rugele, K.; Lehmhus, D.; Hussainova, I.; Peculevica, J.; Lisnanskis, M.; Shishkin, A. Effect of Fly-Ash Cenospheres on Properties of Clay-Ceramic Syntactic Foams. Materials 2017, 10, 828. [Google Scholar] [CrossRef] [Green Version]
- Švinka, R.; Švinka, V. Silikātu materiālu ķīmija un tehnoloģija; Saknes: Riga, Latvia, 1997. [Google Scholar]
- Csáki, Š.; Štubňa, I.; Dobroň, P.; Minárik, P.; Záleská, M.; Václavů, T.; Vozár, L. Influence of mechanical treatment on thermophysical processes in illitic clay during firing. Appl. Clay Sci. 2017, 141, 240–247. [Google Scholar] [CrossRef]
- Csáki, Š.; Štubňa, I.; Trnovcová, V.; Ondruška, J.; Vozár, L.; Dobroň, P. Evolution of AC conductivity of wet illitic clay during drying. IOP Conf. Ser. Mater. Sci. Eng. 2017, 175, 012041. [Google Scholar] [CrossRef]
- Silva, R.V.; de Brito, J.; Lye, C.Q.; Dhir, R.K. The role of glass waste in the production of ceramic-based products and other applications: A review. J. Clean. Prod. 2018, 167, 346–364. [Google Scholar] [CrossRef]
- Djangang, C.N.; Kamseu, E.; Elimbi, A.; Lecomte, G.L.; Blanchart, P. Net-Shape Clay Ceramics with Glass Waste Additive. Mater. Sci. Appl. 2014, 05, 592–602. [Google Scholar] [CrossRef] [Green Version]
- Húlan, T.; Kaljuvee, T.; Štubňa, I.; Trník, A. Investigation of elastic and inelastic properties of Estonian clay from a locality in Kunda during thermal treatment. J. Therm. Anal. Calorim. 2016, 124, 1153–1159. [Google Scholar] [CrossRef]
- Abuh, M.A.; Agulanna, C.A.; Chimezie, P.E.; Bethel-Wali, J.U. Implications and characterization of waste glass cullet – kaolinite clay ceramics. J. Appl. Sci. Environ. Manag. 2019, 23, 513. [Google Scholar] [CrossRef]
- Costa, F.B.; Teixeira, S.R.; Souza, A.E.; Santos, G.T.A. Recycling of glass cullet as aggregate for clays used to produce roof tiles. Rev. Mater. 2009, 14, 1146–1153. [Google Scholar]
- Ondruška, J.; Csáki, Š.; Štubňa, I. Influence of waste glass addition on thermal properties of kaolin and illite. AIP Conf. Proc. 2019, 020028. [Google Scholar] [CrossRef]
- Goel, G.; Kalamdhad, A.S.; Agrawal, A. Parameter optimisation for producing fired bricks using organic solid wastes. J. Clean. Prod. 2018, 205, 836–844. [Google Scholar] [CrossRef]
- Goel, G.; Kalamdhad, A.S. A practical proposal for utilisation of water hyacinth: Recycling in fired bricks. J. Clean. Prod. 2018, 190, 261–271. [Google Scholar] [CrossRef]
- Goel, G.; Kalamdhad, A.S. Degraded municipal solid waste as partial substitute for manufacturing fired bricks. Constr. Build. Mater. 2017, 155, 259–266. [Google Scholar] [CrossRef]
- Goel, G.; Kalamdhad, A.S. An investigation on use of paper mill sludge in brick manufacturing. Constr. Build. Mater. 2017, 148, 334–343. [Google Scholar] [CrossRef]
- Phonphuak, N.; Kanyakam, S.; Chindaprasirt, P. Utilization of waste glass to enhance physical–mechanical properties of fired clay brick. J. Clean. Prod. 2016, 112, 3057–3062. [Google Scholar] [CrossRef]
- Bernardo, E. Micro- and macro-cellular sintered glass-ceramics from wastes. J. Eur. Ceram. Soc. 2007, 27, 2415–2422. [Google Scholar] [CrossRef]
- Jimenez-Millan, J.; Abad, I.; Jimenez-Espinosa, R.; Yebra-Rodriguez, A. Assessment of solar panel waste glass in the manufacture of sepiolite based clay bricks. Mater. Lett. 2018, 218, 346–348. [Google Scholar] [CrossRef]
- Andreola, F.; Barbieri, L.; Lancellotti, I.; Leonelli, C.; Manfredini, T. Recycling of industrial wastes in ceramic manufacturing: State of art and glass case studies. Ceram. Int. 2016, 42, 13333–13338. [Google Scholar] [CrossRef]
- Shishkin, A.; Mironov, V.; Zemchenkov, V.; Antonov, M.; Hussainova, I. Hybrid Syntactic Foams of Metal –Fly Ash Cenosphere–Clay. Key Eng. Mater. 2016, 674, 35–40. [Google Scholar] [CrossRef]
- Souza, M.T.; Maia, B.G.O.; Teixeira, L.B.; de Oliveira, K.G.; Teixeira, A.H.B.; Novaes de Oliveira, A.P. Glass foams produced from glass bottles and eggshell wastes. Process Saf. Environ. Prot. 2017, 111, 60–64. [Google Scholar] [CrossRef]
- Goljandin, D.; Sarjas, H.; Kulu, P.; Käerdi, H.; Mikli, V. Metal-Matrix Hardmetal/Cermet Reinforced Composite Powders for Thermal Spray. Mater. Sci. 2012, 18, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Bumanis, G.; Goljandin, D.; Bajare, D. The Properties of Mineral Additives Obtained by Collision Milling in Disintegrator. Key Eng. Mater. 2016, 721, 327–331. [Google Scholar] [CrossRef]
- Zimakov, S.; Goljandin, D.; Peetsalu, P.; Kulu, P. Metallic powders produced by the disintegrator technology. Int. J. Mater. Prod. Technol. 2007, 28, 226. [Google Scholar] [CrossRef]
- Mironov, V.; Indriksone, E.; Goljandin, D.; Shishkin, A. Obtaining of SiC Fine Powder from the Used Heating Elements by Milling and Grinding by High-Energy Disintegrator. In Proceedings of the 23rd International Baltic Conference on Materials Engineering, Kaunas, Lithuania, 26–27 October 2014; 2014; Volume 1, p. 31. [Google Scholar]
- Shishkin, A.; Mironov, V.; Goljandin, D.; Lapkovsky, V. Recycling of Al-W-B Composite Material. Key Eng. Mater. 2012, 527, 143–147. [Google Scholar] [CrossRef]
- Keramikas flīzes -3. daļa. Ūdens absorbcijas, šķietamās porainības, šķietamā un patiesā blīvuma noteikšana LVS. LVS EN 10545-3:2002; Latvian Standard: Riga, Latvia, 2002.
- Muter, O.; Potapova, K.; Nikolajeva, V.; Petrina, Z.; Griba, T.; Patmalnieks, A.; Svinka, R.; Svinka, V. Comparative study on bacteria colonization onto ceramic beads originated from two Devonian clay deposits in Latvia. Mater. Sci. Appl. Chem. 2012, 134–1401. [Google Scholar]
- Fluegel, A. Glass viscosity calculation based on a global statistical modelling approach. Glas. Technol. Eur. J. Glas. Sci. Technol. Part A 2007, 48, 13–30. [Google Scholar]
- Felisberto, R.; Santos, M.C.; Arcaro, S.; Basegio, T.M.; Bergmann, C.P. Assessment of environmental compatibility of glass–ceramic materials obtained from galvanic sludge and soda–lime glass residue. Process Saf. Environ. Prot. 2018, 120, 72–78. [Google Scholar] [CrossRef]
- Ondruška, J.; Trnovcová, V.; Štubňa, I.; Csáki, Š.; Vozár, L. Influence of texture on DC conductivity and dimensional changes of kaolin and illitic clay. Ceram. Int. 2019, 45, 2425–2431. [Google Scholar] [CrossRef]
- Csáki, Š.; Ondruška, J.; Trnovcová, V.; Štubňa, I.; Dobroň, P.; Vozár, L. Temperature dependence of the AC conductivity of illitic clay. Appl. Clay Sci. 2018, 157, 19–23. [Google Scholar] [CrossRef]
- Ondro, T.; Húlan, T.; Al-Shantir, O.; Csáki, Š.; Václavů, T.; Trník, A. Kinetic analysis of the formation of high-temperature phases in an illite-based ceramic body using thermodilatometry. J. Therm. Anal. Calorim. 2019, 138, 2289–2294. [Google Scholar] [CrossRef]
- Ibrahim, D.M.; Helmy, M. Crystallite growth of rice husk ash silica. Thermochim. Acta 1981, 45, 79–85. [Google Scholar] [CrossRef]
- Loryuenyong, V.; Panyachai, T.; Kaewsimork, K.; Siritai, C. Effects of recycled glass substitution on the physical and mechanical properties of clay bricks. Waste Manag. 2009, 29, 2717–2721. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, E.; Bonomo, E.; Dattoli, A. Optimisation of sintered glass–ceramics from an industrial waste glass. Ceram. Int. 2010, 36, 1675–1680. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, X.; Liu, Y.; Wu, D.; Song, C. Three-dimensional mesoscale modelling of concrete composites by using random walking algorithm. Compos. Sci. Technol. 2017, 149, 235–245. [Google Scholar] [CrossRef]
- Lin, K.L.; Lee, T.C.; Hwang, C.L. Effects of sintering temperature on the characteristics of solar panel waste glass in the production of ceramic tiles. J. Mater. Cycles Waste Manag. 2015, 17, 194–200. [Google Scholar] [CrossRef]
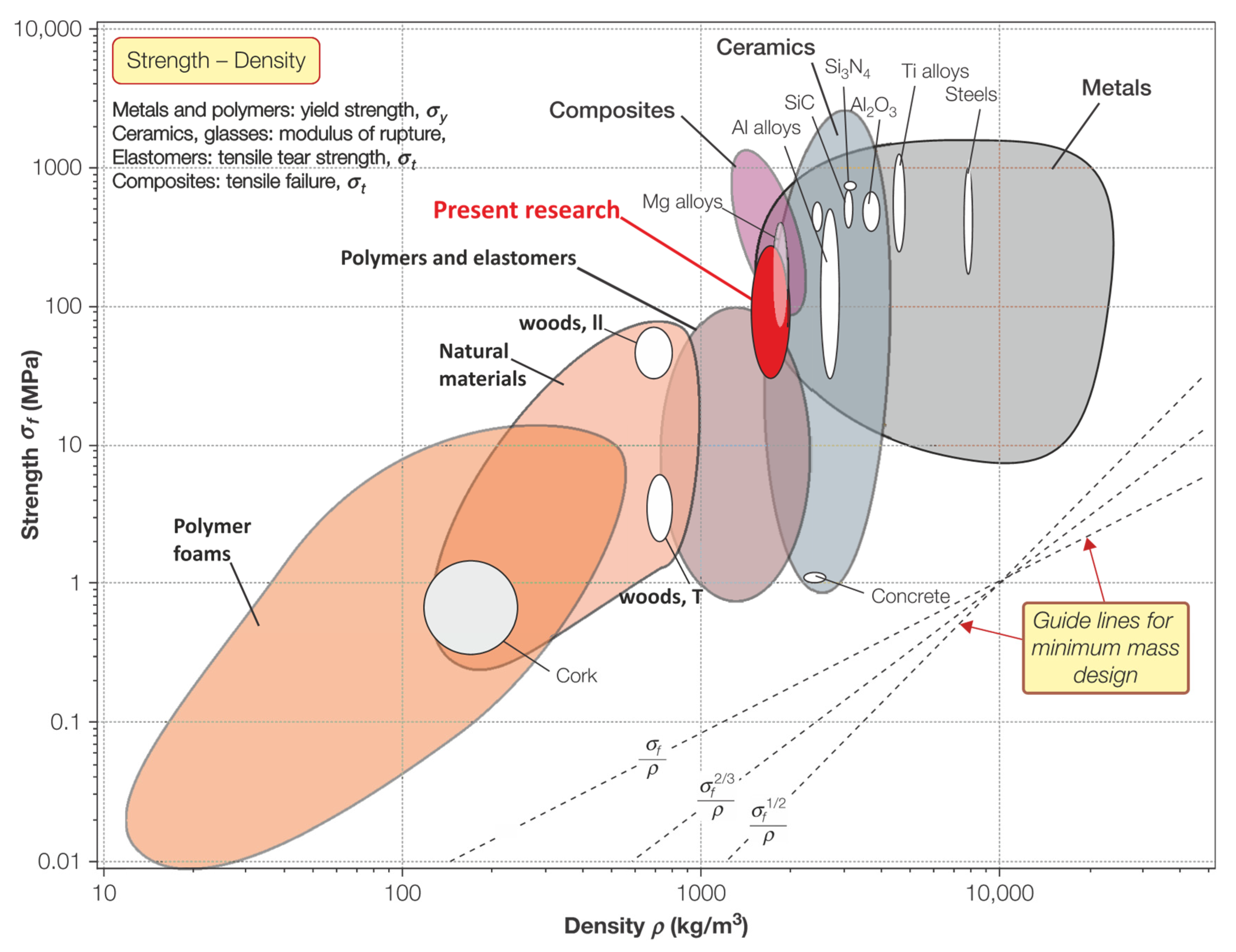
- Ashby, M.F. Materials Selection in Mechanical Design, 4th ed.; Butterworth Heinemann: Oxford, UK, 2010; ISBN 978-1-85617-663-7. [Google Scholar]
| Material | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | TiO2 | LOI * |
|---|---|---|---|---|---|---|---|---|---|
| Clay | 62.8 ± 0.5 | 15.4 ± 0.7 | 6.8 ± 0.2 | 0.7 ± 0.2 | 1.4 ± 0.2 | 0.1 ± 0.1 | 4.2 ± 0.1 | 1.9 ± 0.1 | 4.7 ± 0.1 |
| Glass | 70.20 | 2.10 | 0.10 | 9.50 | – | 16.60 | – | – | 1.50 |
| Phase, % | CLWG 0% | CLWG 15% | Glass 100% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 °C | 700 °C | 800 °C | 900 °C | 1000 °C | 700 °C | 800 °C | 900 °C | 1000 °C | 20 °C | 1000 °C | |
| Quartz | 40.8 | 60.9 | 60.3 | 71.0 | 72.6 | 57.7 | 58.7 | 69.1 | 67.9 | ||
| Illite | 45.8 | 28.7 | 18.5 | 30.8 | 26.9 | 6.8 | |||||
| Haematite | 3.1 | 1.5 | 1.8 | 3.0 | 5.3 | 4.6 | 5.3 | 5.0 | 4.6 | ||
| Microcline | 3.4 | 29.2 | 8.7 | 7.1 | 8.3 | 6.9 | 3.6 | 6.8 | 8.6 | ||
| Kaolinite | 6.9 | ||||||||||
| Magnetite | 0.51 | 0.49 | |||||||||
| Diopside | 1.7 | 5.5 | 4.8 | 5.3 | 60.4 | ||||||
| Spinel | 12.2 | 3.9 | 7.3 | ||||||||
| Cristobalite | 3.5 | 6.4 | |||||||||
| Wollastonite | 39.6 | ||||||||||
| Amorphous phase, % | 13.0 | 13.0 | 14.0 | 21.0 | 26.0 | 16.0 | 15.0 | 21.0 | 26.0 | 100 | 85.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shishkin, A.; Baronins, J.; Mironovs, V.; Lukáč, F.; Štubňa, I.; Ozolins, J. Influence of Glass Additions on Illitic Clay Ceramics. Materials 2020, 13, 596. https://doi.org/10.3390/ma13030596
Shishkin A, Baronins J, Mironovs V, Lukáč F, Štubňa I, Ozolins J. Influence of Glass Additions on Illitic Clay Ceramics. Materials. 2020; 13(3):596. https://doi.org/10.3390/ma13030596
Chicago/Turabian StyleShishkin, Andrei, Janis Baronins, Viktors Mironovs, František Lukáč, Igor Štubňa, and Jurijs Ozolins. 2020. "Influence of Glass Additions on Illitic Clay Ceramics" Materials 13, no. 3: 596. https://doi.org/10.3390/ma13030596
APA StyleShishkin, A., Baronins, J., Mironovs, V., Lukáč, F., Štubňa, I., & Ozolins, J. (2020). Influence of Glass Additions on Illitic Clay Ceramics. Materials, 13(3), 596. https://doi.org/10.3390/ma13030596






