Direct Synthesis of Fe-Al Alloys from Elemental Powders Using Laser Engineered Net Shaping
Abstract
1. Introduction
2. Methods
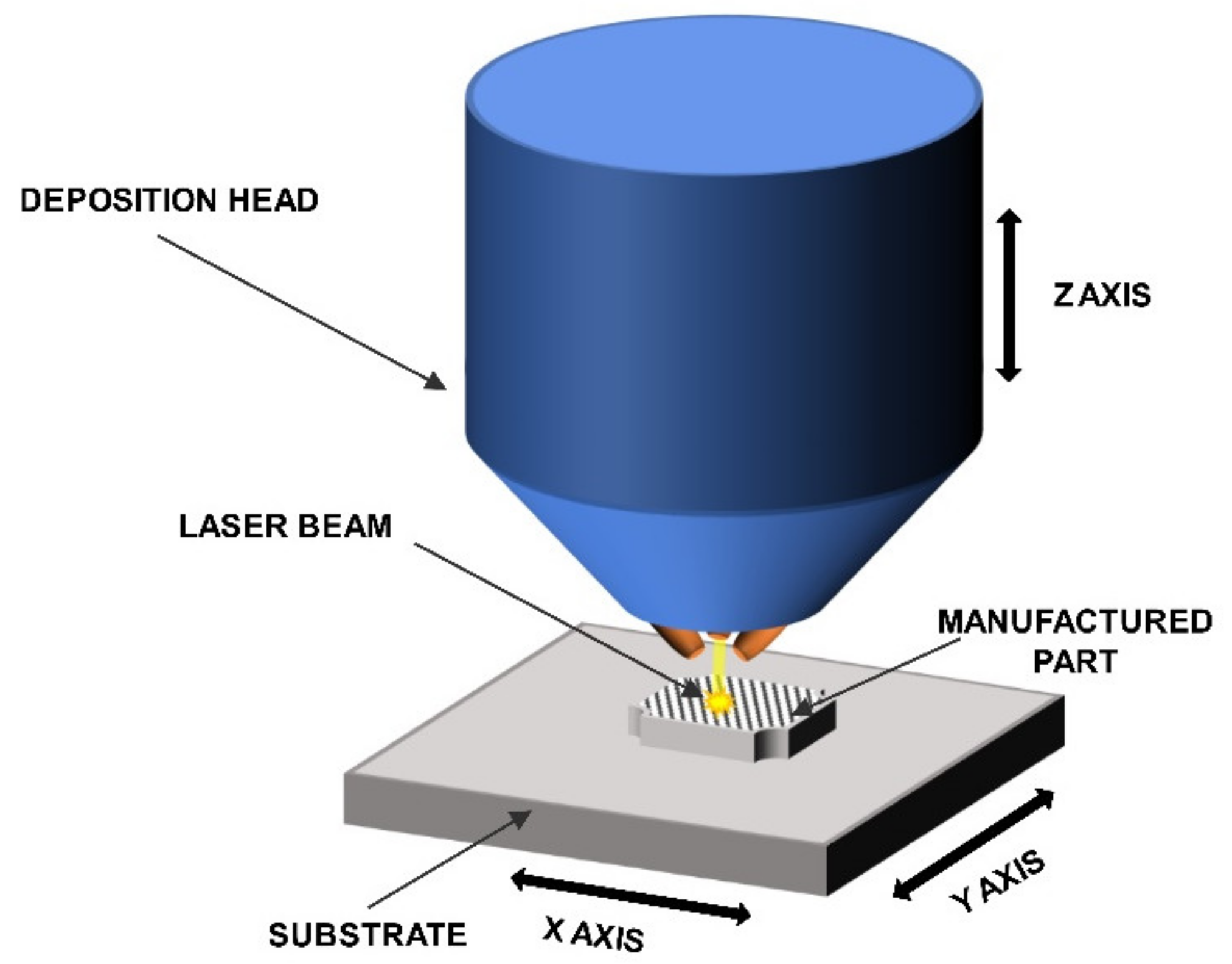
2.1. Samples Manufacturing
2.2. Samples Characterization
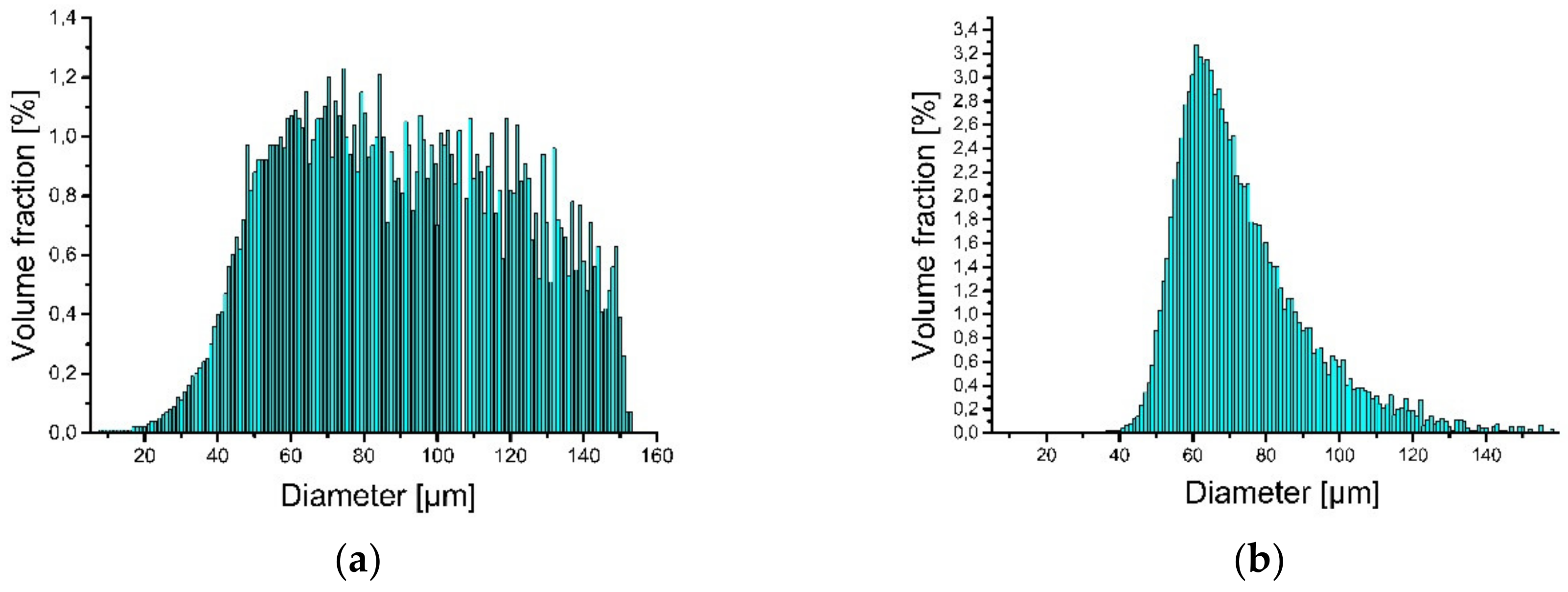
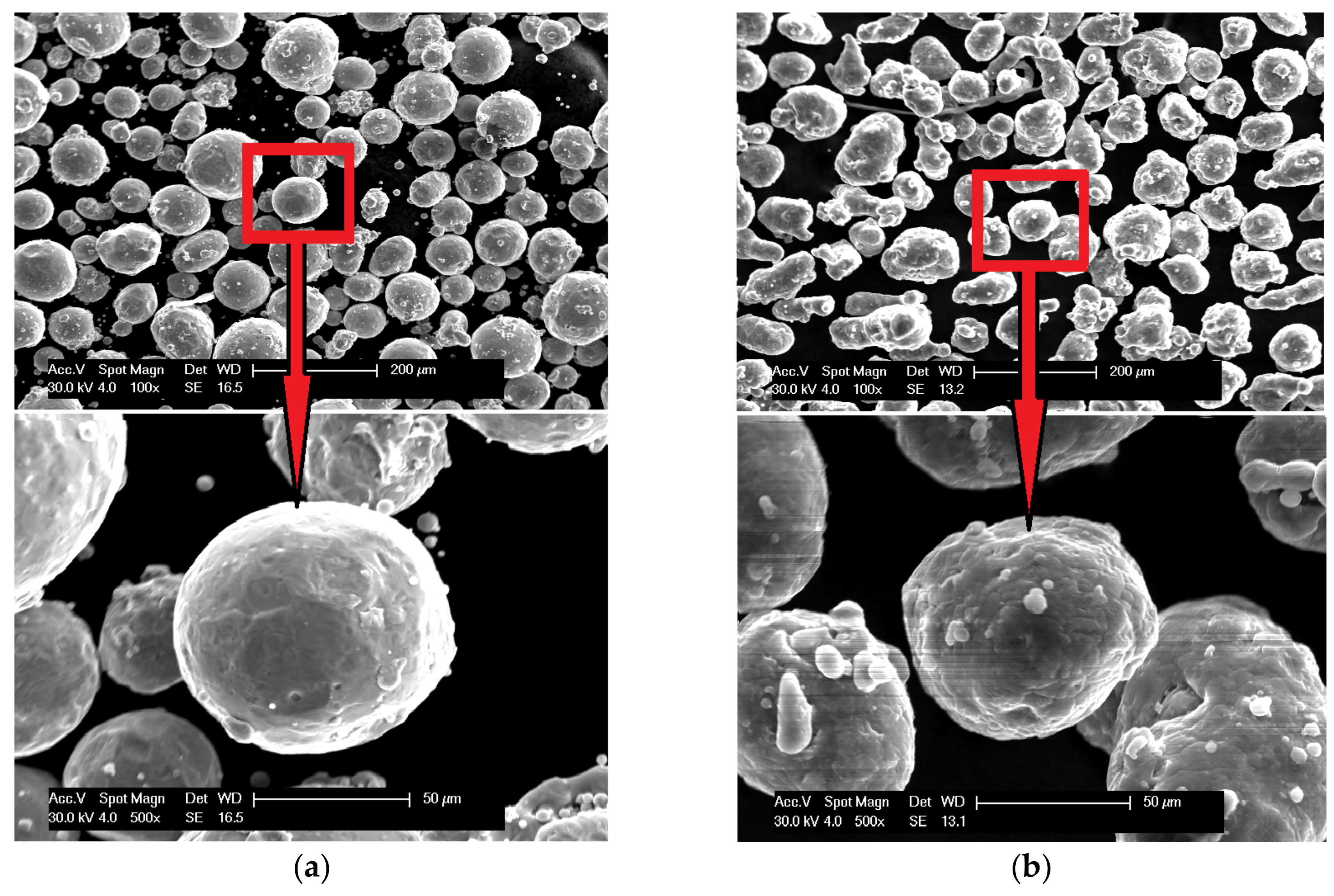
3. Materials and Preliminary Research
4. Experimental Procedure
5. Results
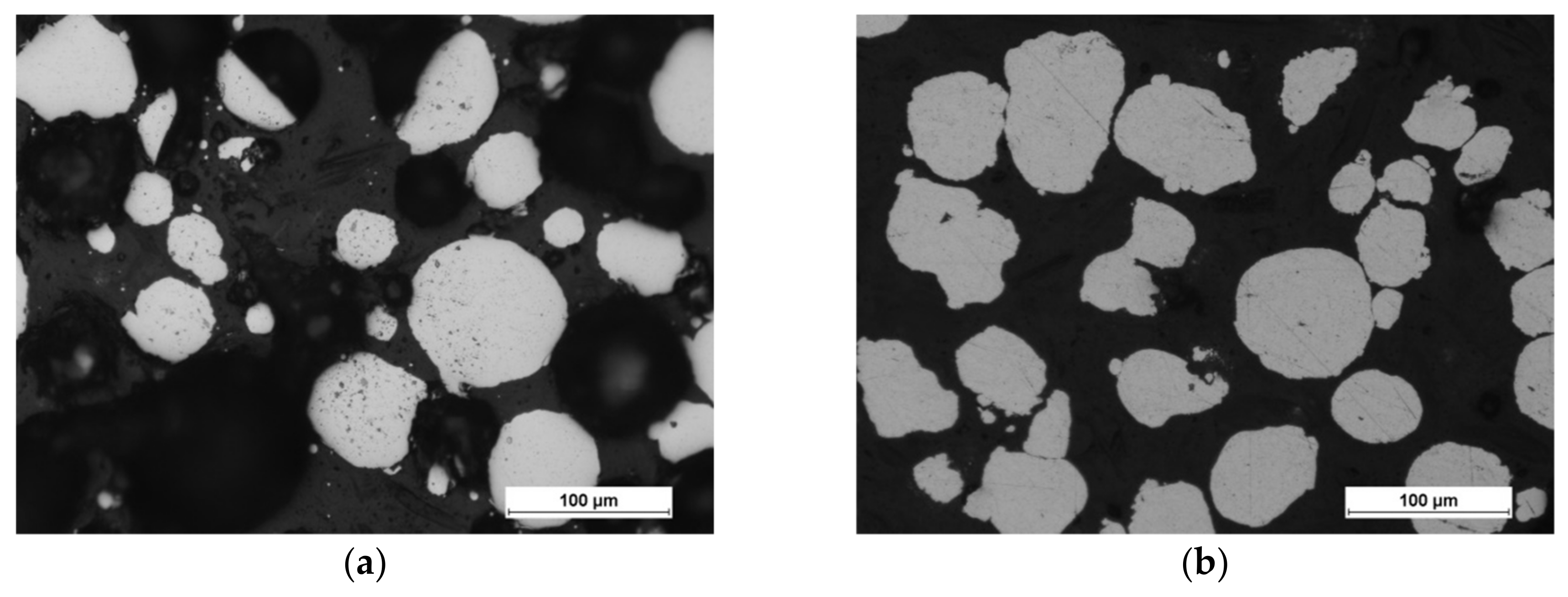
5.1. Chemical Composition
5.2. Microanalysis
5.3. X-ray Diffraction Phase Analysis
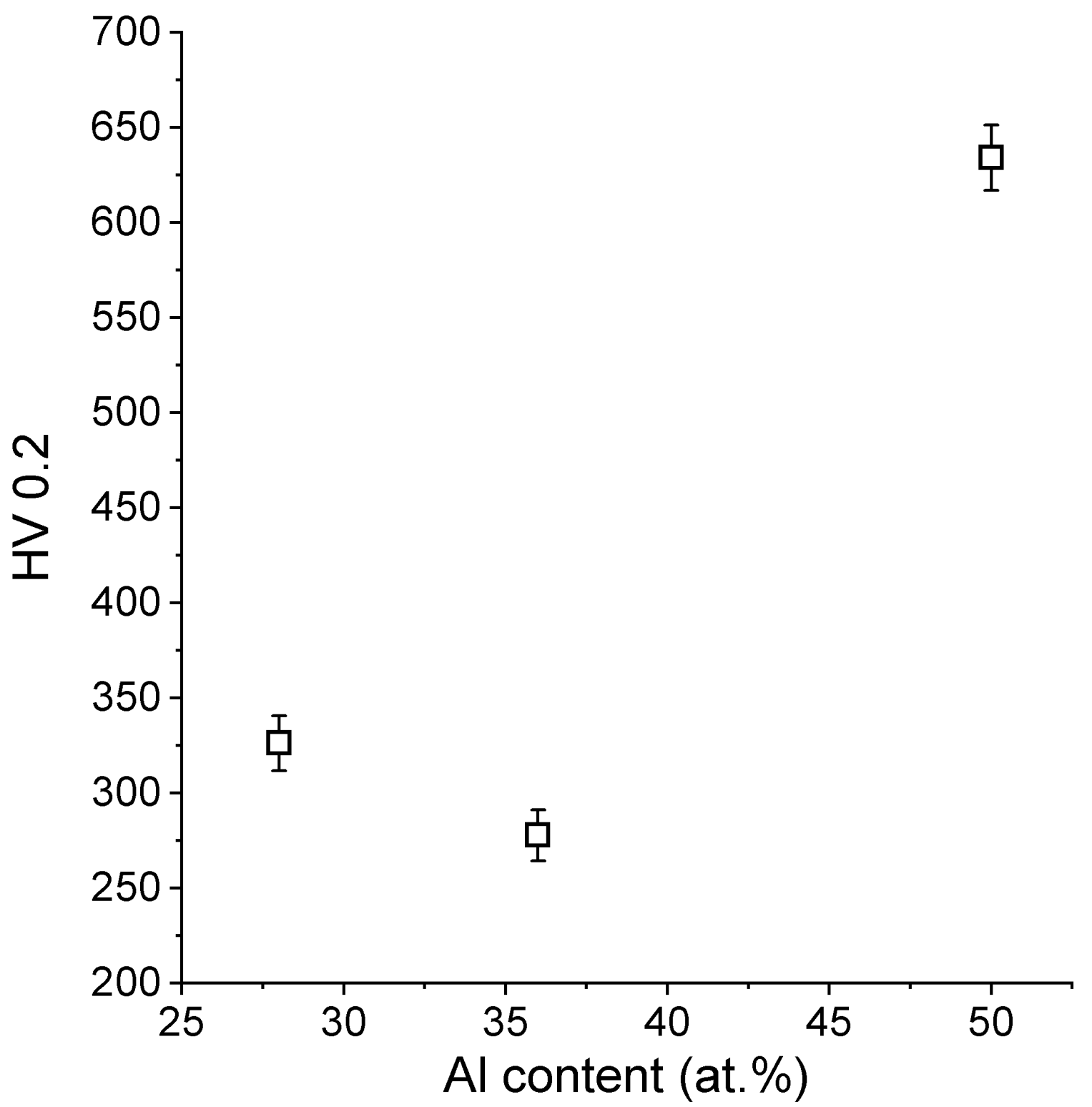
5.4. Microhardness Test
6. Conclusions
- The LENS technique can be used as an alternative approach to conventional alloying methods for the direct fabrication of Fe-Al alloys of the desired chemical and phase composition.
- The application of mixtures of elemental powders (as a feedstock) allows the for production of alloys with desired chemical and phase compositions.
- Thermal expansion during the process, as well as limited heat dissipation, results in cracks formation and delamination of the samples in some cases, despite the same chemical composition of the substrate and deposited layer.
- The hardness of the manufactured alloys is very similar to the alloys that were produced by classical methods, and no direct linear correlation between the aluminum content and the hardness was found.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Łyszkowski, R. High-temperature oxidation of Fe(3)Al intermetallic alloy prepared by additive manufacturing LENS. Materials 2015, 8, 1499–1512. [Google Scholar] [CrossRef] [PubMed]
- PalDey, S.; Deevi, S.C. Cathodic arc deposited FeAl coatings: Properties and oxidation characteristics. Mater. Sci. Eng. A 2003, 355, 208–215. [Google Scholar] [CrossRef]
- Pedraza, F.; Grosseau-Poussard, J.L.; Dinhut, J.F. Evolution of oxide scales on an ODS FeAl intermetallic alloy during high temperature exposure in air. Intermetallics 2005, 13, 27–33. [Google Scholar] [CrossRef][Green Version]
- Pint, B.A.; Schneibel, J.H. The effect of carbon and reactive element dopants on oxidation lifetime of FeAl. Scr. Mater. 2005, 52, 1199–1204. [Google Scholar] [CrossRef]
- Rao, V.S. The influence of temperature on the oxidation behaviour of Fe3Al–Fe3AlC0.69 and FeAl–Fe3AlC0.69 intermetallics. Intermetallics 2003, 11, 713–719. [Google Scholar] [CrossRef]
- Novák, P.; Zelinková, M.; Šerák, J.; Michalcová, A.; Novák, M.; Vojtěch, D. Oxidation resistance of SHS Fe–Al–Si alloys at 800 °C in air. Intermetallics 2011, 19, 1306–1312. [Google Scholar] [CrossRef]
- Deevi, S.C.; Swindeman, R.W. Yielding, hardening and creep behavior of iron aluminides. Mater. Sci. Eng. A 1998, 258, 203–210. [Google Scholar] [CrossRef]
- Jimenez, J.A.; Frommeyer, G. Creep behavior of intermetallic FeAl and FeAlCr alloys. Mater. Sci. Eng. A 1996, 220, 93–99. [Google Scholar] [CrossRef]
- Morris, D.G.; Gutierrez-Urrutia, I.; Muñoz-Morris, M.A. High temperature creep behaviour of an FeAl intermetallic strengthened by nanoscale oxide particles. Int. J. Plast. 2008, 24, 1205–1223. [Google Scholar] [CrossRef]
- Schmitt, A.; Kumar, K.S.; Kauffmann, A.; Li, X.; Stein, F.; Heilmaier, M. Creep of binary Fe-Al alloys with ultrafine lamellar microstructures. Intermetallics 2017, 90, 180–187. [Google Scholar] [CrossRef]
- Sundar, R.S.; Deevi, S.C. High-temperature strength and creep resistance of FeAl. Mater. Sci. Eng. A 2003, 357, 124–133. [Google Scholar] [CrossRef]
- Luu, W.C.; Wu, J.K. Moisture and hydrogen-induced embrittlement of Fe3Al alloys. Mater. Chem. Phys. 2001, 70, 236–241. [Google Scholar] [CrossRef]
- Radhakrishna, A.; Baligidad, R.G.; Sarma, D.S. Effect of carbon on structure and properties of FeAl based intermetallic alloy. Scripta Mater 2001, 45, 1077–1082. [Google Scholar] [CrossRef]
- Liu, Y.; Chong, X.; Jiang, Y.; Zhou, R.; Feng, J. Mechanical properties and electronic structures of Fe-Al intermetallic. Physica B 2017, 506, 1–11. [Google Scholar] [CrossRef]
- Ruan, Y.; Zan, N.; Zhu, H.Z.; Zhou, K.; Wei, B. Thermal performance determination of binary Fe-Al alloys at elevated temperatures. J. Alloys Compd. 2017, 701, 676–681. [Google Scholar] [CrossRef]
- Shen, C.; Liss, K.-D.; Pan, Z.; Wang, Z.; Li, X.; Li, H. Thermal cycling of Fe3Al based iron aluminide during the wire-arc additive manufacturing process: An in-situ neutron diffraction study. Intermetallics 2018, 92, 101–107. [Google Scholar] [CrossRef]
- McKamey, C.G.; DeVan, J.H.; Tortorelli, P.F.; Sikka, V.K. A review of recent developments in Fe3Al-based alloys. J. Mater. Res. 1991, 6, 1179–1805. [Google Scholar] [CrossRef]
- Jiao, Z.B.; Luan, J.H.; Liu, C.T. Strategies for improving ductility of ordered intermetallics. Prog. Nat. Sci. Mater. Int. 2016, 26, 1–12. [Google Scholar] [CrossRef]
- Mendiratta, M.G.; Ehlers, S.K.; Dimiduk, D.M.; Kerr, W.R.; Mazdiyasni, S.; Lipsit, H.A. A review of recent developments in iron aluminides. Mater. Res. Soc. Symp. Proc. 1987, 81, 393–404. [Google Scholar] [CrossRef]
- Łyszkowski, R.; Bystrzycki, J. Hot deformation and processing maps of an Fe3Al intermetallic alloy. Intermetallics 2006, 14, 1231–1237. [Google Scholar] [CrossRef]
- Łyszkowski, R.; Bystrzycki, J. Hot deformation and processing maps of a Fe–Al intermetallic alloy. Mater. Charact. 2014, 96, 196–205. [Google Scholar] [CrossRef]
- Łyszkowski, R.; Bystrzycki, J.; Płociński, T. Processing maps for hot working of FeAl-based alloy. Intermetallics 2010, 18, 1344–1347. [Google Scholar] [CrossRef]
- Łyszkowski, R.; Bystrzycki, J. Influence of temperature and strain rate on the microstructure and flow stress of iron aluminides. Arch. Mater. Metall. 2007, 52, 347–350. [Google Scholar]
- Polkowski, W.; Jóźwik, P.; Łyszkowski, R. Effect of hot differential speed rolling on microstructure and mechanical properties of Fe3Al-based intermetallic alloy. Int. J. Mater. Res. 2016, 107, 867–871. [Google Scholar] [CrossRef]
- Łyszkowski, R.; Czujko, T.; Varin, R.A. Multi-axial forging of Fe3Al-base intermetallic alloy and its mechanical properties. Int. J. Mater. Res. 2017, 52, 2902–2914. [Google Scholar] [CrossRef]
- Łyszkowski, R.; Polkowski, W.; Czujko, T. Severe plastic deformation of Fe-22Al-5Cr alloy by cross-channel extrusion with back pressure. Materials 2018, 11, 2214. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lin, D.; Lin, T.L.; Liu, Y. Effect of temperature on the tensile properties and dislocation structures of FeAl alloys. Mater. Sci. Eng. 1998, A249, 206–216. [Google Scholar] [CrossRef]
- García-Alonso, M.C.; López, M.F.; Escudero, M.L.; González-Carrasco, J.L.; Morris, D.G. Corrosion behaviour of an Fe3Al-type intermetallic in a chloride containing solution. Intermetallics 1999, 7, 185–191. [Google Scholar] [CrossRef]
- Deevi, S.C.; Sikka, V.K.; Liu, C.T. Processing, properties, and applications of nickel and iron aluminides. Prog. Mater. Sci. 1997, 42, 177–192. [Google Scholar] [CrossRef]
- Sundar, R.S.; Baligid, R.G.; Prasad, Y.V.R.K.; Sastry, D.H. Processing of iron aluminides. Mater. Sci. Eng. 1998, A258, 219–228. [Google Scholar] [CrossRef]
- Deevi, S.C.; Sikka, V.K. Nickel and iron aluminides: An overview on properties, processing, and applications. Intermetallics 1996, 4, 357–375. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, W.; Gao, H.; Shen, W.; He, Y. Suppression of the SHS reactions during synthesis of porous FeAlintermetallics by introducing silicon. J. Alloys Compd. 2018, 735, 1435–1438. [Google Scholar] [CrossRef]
- Josh, D.L.; Easton, D.S.; Liu, C.T.; Babu, S.S.; David, S.A. Processing of FesAl and FeAl alloys by reaction synthesis. Intermetallics 1995, 3, 467–481. [Google Scholar]
- Siemiaszko, D.; Mościcki, R. Kinetics study on the SHS reaction in massive samples with high heating rate in the Fe–Al system. J. Alloys Compd. 2015, 632, 335–342. [Google Scholar] [CrossRef]
- Baligidad, R.G.; Prakash, U.; Rao, R.V.; Rao, P.K.; Ballal, N.B. Processing of Fe3Al based intermetallic alloys through electroslag remelting. ISIJ Int. 1996, 36, 1448–1452. [Google Scholar] [CrossRef]
- Sikka, V.K.; Wilkening, D.; Liebetrau, J.; Mackey, B. Melting and casting of FeAl-based cast alloy. Mater. Sci. Eng. 1998, A258, 229–235. [Google Scholar] [CrossRef]
- Karczewski, K.; Jóźwiak, S. Influence of aluminum oxides on abrasive wear resistance of Fe–50 at.% Al intermetallic sinters. J. Alloys Compd. 2009, 482, 405–411. [Google Scholar] [CrossRef]
- Kang, H.-Z.; Hu, C.-T. Swelling behavior in reactive sintering of Fe–Al mixtures. Mater. Chem. Phys. 2004, 8, 264–272. [Google Scholar] [CrossRef]
- Novák, P.; Knotek, V.; Voděrová, M.; Kubásek, J.; Šerák, J.; Michalcová, A.; Vojtěch, D. Intermediary phases formation in Fe–Al–Si alloys during reactive sintering. J. Alloys Compd. 2010, 497, 90–94. [Google Scholar] [CrossRef]
- Novák, P.; Michalcová, A.; Marek, I.; Mudrová, M.; Saksl, K.; Bednarčík, J.; Zikmund, P.; Vojtěch, D. On the formation of intermetallics in Fe–Al system – An in situ XRD study. Intermetallics 2013, 32, 127–136. [Google Scholar] [CrossRef]
- Školáková, A.; Průša, F.; Novák, P. Thermal analysis of FeAl intermetallic compound sintered at heating rate of 300 °C/min. J. Alloys Compd. 2019. [Google Scholar] [CrossRef]
- Mossino, P. Some aspects in self-propagating high-temperature synthesis. Ceram. Int. 2004, 30, 311–332. [Google Scholar] [CrossRef]
- Azem, S.; Nechiche, M.; Taibi, K. Development of copper matrix composite reinforced with FeAl particles produced by combustion synthesis. Powder Technol. 2011, 208, 515–520. [Google Scholar] [CrossRef]
- Lazinska, M.; Durejko, T.; Lipinski, S.; Polkowski, W.; Czujko, T.; Varin, R.A. Porous graded FeAl intermetallic foams fabricated by sintering process using NaCl space holders. Mater. Sci. Eng. Struct. Mater. Prop. Microstruct. Process. 2015, 636, 407–414. [Google Scholar] [CrossRef]
- Song, B.; Dong, S.; Coddet, P.; Liao, H.; Coddet, C. Fabrication and microstructure characterization of selective laser-melted FeAl intermetallic parts. Surf. Coat. Technol. 2012, 206, 4704–4709. [Google Scholar] [CrossRef]
- Durejko, T.; Żuchowska, E.; Łazińska, M. Application of lens method in Fe40Al + N-Al2O3 composite materials fabrication. Compos. Theory Pract. 2012, 12, 81–85. [Google Scholar]
- Attaran, M. The rise of 3-D printing: The advantages of additive manufacturing over traditional manufacturing. Bus. Horiz. 2017, 60, 677–688. [Google Scholar] [CrossRef]
- Dilip, J.J.S.; Miyanaji, H.; Lassell, A.; Starr, T.L.; Stucker, B. A novel method to fabricate TiAl intermetallic alloy 3D parts using additive manufacturing. Def. Technol. 2017, 13, 72–76. [Google Scholar] [CrossRef]
- Loeber, L.; Biamino, S.; Ackelid, U.; Sabbadini, S.; Epicoco, P.; Fino, P.; Al, E. Comparison of selective laser and electron beam melted titanium aluminides. In Proceedings of the 22nd International Symposium Solid Freeform Fabrication, University of Texas, Austin, TX, USA, 8–10 August 2011. [Google Scholar]
- Umaras, E.; Tsuzuki, M.S.G. Additive manufacturing—Considerations on geometric accuracy and factors of influence. IFAC PapersOnLine 2017, 50, 14940–14945. [Google Scholar] [CrossRef]
- Foteinopoulos, P.; Papacharalampopoulos, A.; Stavropoulos, P. On thermal modeling of additive manufacturing processes. CIRP J. Manuf. Sci. Technol. 2018, 20, 66–83. [Google Scholar] [CrossRef]
- Srinivas, M.; Sridhar Babu, B. A critical review on recent research methodologies in additive manufacturing. Mater. Today Proc. 2017, 4, 9049–9059. [Google Scholar] [CrossRef]
- Singh, S.; Ramakrishna, S.; Singh, R. Material issues in additive manufacturing: A review. J. Manuf. Process. 2017, 35, 185–200. [Google Scholar] [CrossRef]
- Dehoff, R.R.; Sarosi, P.M.; Collins, P.C.; Fraser, H.L.; Mills, M.J. Microstructures of LENS™ deposited Nb-Si alloys. In Proceedings of the Materials Research Society Symposium, 29 November–1 December 2004; pp. 285–290. [Google Scholar]
- Banerjee, R.; Nag, S.; Samuel, S.; Fraser, H.L. Laser-deposited Ti-Nb-Zr-Ta orthopedic alloys. J. Biomed. Mater. Res. Part A 2006, 78, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Brice, C.A.; Banerjee, S.; Fraser, H.L. Microstructural evolution in laser deposited Ni–25at.% Mo alloy. Mater. Sci. Eng. A 2003, 347, 1–4. [Google Scholar] [CrossRef]
- Schwendner, K.I.; Banerjee, R.; Collins, P.C.; Brice, C.A.; Fraser, H.L. Direct laser deposition of alloys from elemental powder blends. Scr. Mater. 2001, 45, 1123–1129. [Google Scholar] [CrossRef]
- Catchpole-Smith, S.; Clare, A.T. In-Situ synthesis of titanium aluminides by direct metal deposition. J. Mater. Process. Technol. 2017, 239, 230–239. [Google Scholar] [CrossRef]
- Collins, P.C.; Banerjee, R.; Fraser, H.L. The influence of the enthalpy of mixing during the laser deposition of complex titanium alloys using elemental blends. Scr. Mater. 2003, 48, 1445–1450. [Google Scholar] [CrossRef]
- Banerjee, R.; Genç, A.; Collins, P.C.; Fraser, H.L. Comparison of microstructural evolution in laser-deposited and arc-melted in-situ Ti-TiB composites. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. A 2004, 35, 2143–2152. [Google Scholar] [CrossRef]
- Grigoriev, A.; Polozov, I.; Sufiiarov, V.; Popovich, A. In-situ synthesis of Ti2AlNb-based intermetallic alloy by selective laser melting. J. Alloys Compd. 2017, 704, 434–442. [Google Scholar] [CrossRef]
- Polozov, I.; Sufiiarov, V.; Kantyukov, A.; Popovich, A. Selective laser melting of Ti2AlNb-based intermetallic alloy using elemental powders: Effect of process parameters and post-treatment on microstructure, composition, and properties. Intermetallics 2019, 112. [Google Scholar] [CrossRef]
- Nazarov, A.; Safronov, V.A.; Khmyrov, R.S.; Shishkovsky, I. Fabrication of Gradient Structures in the Ni–Al System via SLM Process. Procedia IUTAM 2017, 23, 161–166. [Google Scholar] [CrossRef]
- Shishkovskii, I.V.; Makarenko, A.G.; Petrov, A.L. Conditions for SHS of intermetallic compounds with selective laser sintering of powdered compositions. Combust.Explos. Shock Waves 1999, 35. [Google Scholar] [CrossRef]
- Shishkovsky, I.V.; Scherbakov, V.I.; Morozov, Y.G.; Kuznetsov, M.V.; Parkin, I.P. Surface laser sintering of exothermic powder compositions A thermal and SEM/EDX study. J. Therm. Anal. Calorim. 2008, 91, 427–436. [Google Scholar] [CrossRef]
- Durejko, T.; Lipiński, S.; Bojar, Z.; Bystrzycki, J. Processing and characterization of graded metal/intermetallic materials: The example of Fe/FeAlintermetallics. Mater. Des. 2011, 32, 2827–2834. [Google Scholar] [CrossRef]
- Durejko, T.; Zietala, M.; Lazinska, M.; Lipinski, S.; Polkowski, W.; Czujko, T.; Varin, R.A. Structure and properties of the Fe3Al-type intermetallic alloy fabricated by laser engineered net shaping (LENS). Mater. Sci. Eng. Struct. Mater. Prop. Microstruct. Process. 2016, 650, 374–381. [Google Scholar] [CrossRef]
- Łazińska, M.; Durejko, T.; Zasada, D.; Bojar, Z. Microstructure and mechanical properties of a Fe-28%Al-5%Cr-1%Nb-2%B alloy fabricated by laser engineered net shaping. Mater. Lett. 2017, 196, 87–90. [Google Scholar] [CrossRef]
- Karczewski, K.; Dąbrowska, M.; Ziętala, M.; Polański, M. Fe-Al thin walls manufactured by Laser Engineered Net Shaping. J. Alloys Compd. 2017, 696, 1105–1112. [Google Scholar] [CrossRef]
- Shishkovsky, I.V.; Yadroitsev, I.; Smurov, I. Direct selective laser melting of nitinol powder. Phys. Procedia 2012, 39, 447–454. [Google Scholar] [CrossRef]
- Baran, A.; Polanski, M. Microstructure and properties of LENS (laser engineered net shaping) manufactured Ni-Ti shape memory alloy. J. Alloys Compd. 2018, 750, 863–870. [Google Scholar] [CrossRef]
- Shishkovsky, I.V.; Missemer, F.; Kakovkina, N.; Smurov, I. Intermetallics synthesis in the Fe–Al system via layer by layer 3D laser cladding. Crystals 2013, 3, 517–529. [Google Scholar] [CrossRef]
- Available online: https://www.optomec.com/3d-printed-metals/lens-materials/ (accessed on 20 November 2019).
- Kunce, I.; Polanski, M.; Bystrzycki, J. Microstructure and hydrogen storage properties of a TiZrNbMoV high entropy alloy synthesized using Laser Engineered Net Shaping (LENS). Int. J. Hydrogen Energy 2014, 39, 9904–9910. [Google Scholar] [CrossRef]
- Kwiatkowska, M.; Zasada, D.; Bystrzycki, J.; Polański, M. Synthesis of Fe-Al-Ti based intermetallics with the use of laser engineered net shaping (LENS). Materials 2015, 8, 2311–2331. [Google Scholar] [CrossRef]
- Polanski, M.; Kwiatkowska, M.; Kunce, I.; Bystrzycki, J. Combinatorial synthesis of alloy libraries with a progressive composition gradient using laser engineered net shaping (LENS): Hydrogen storage alloys. Int. J. Hydrogen Energy 2013, 38, 12159–12171. [Google Scholar] [CrossRef]
- Karczewski, K.; Jóźwiak, S.; Bojar, Z. Mechanisms of strength properties anomaly of Fe-Al sinters by compression tests at elevated temperature. Arch. Metall. Mater. 2007, 52, 361–366. [Google Scholar]
- Siemiaszko, D.; Kuzia, J. The influence of large particles of iron powder on the microstructure and properties of FeAl intermetallic phase. Intermetallics 2019, 104, 16–23. [Google Scholar] [CrossRef]
- Cottrell, A.H. Vacancies in FeAl and NiAl. Intermetallics 1997, 5, 467–469. [Google Scholar] [CrossRef]
- Jordan, J.L.; Deevi, S.C. Vacancy formation and effects in FeAl. Intermetallics 2003, 11, 507–528. [Google Scholar] [CrossRef]
- Frutos, E.; Morris, D.G.; Muñoz-Morris, M.A. Evaluation of elastic modulus and hardness of Fe–Al base intermetallics by nano-indentation techniques. Intermetallics 2013, 38, 1–3. [Google Scholar] [CrossRef]
- Karczewski, K.; Durejko, T.; Czujko, T. The Microstructure Evolution of a Fe3Al Alloy during the LENS process. Materials 2018, 11, 390. [Google Scholar] [CrossRef]
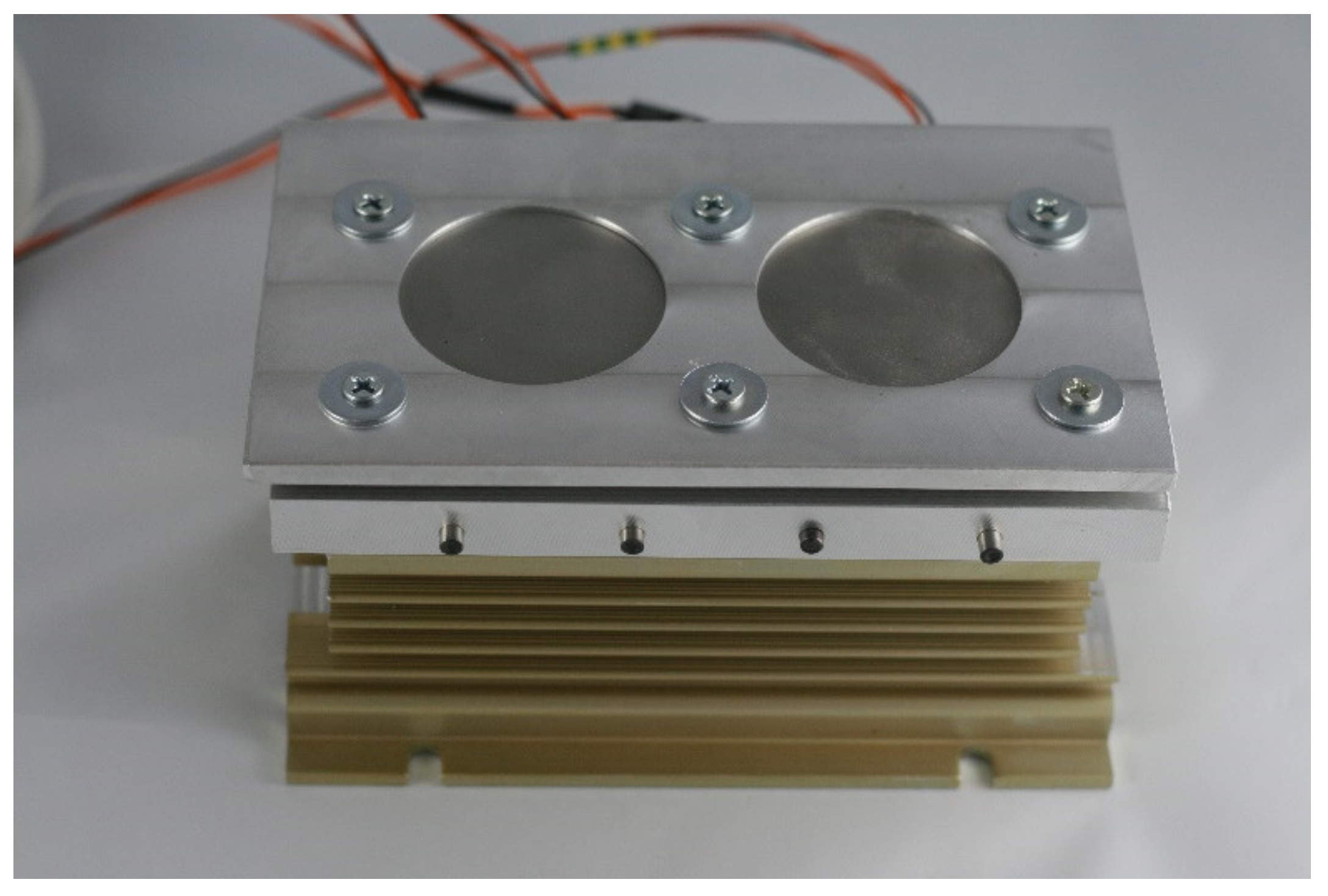

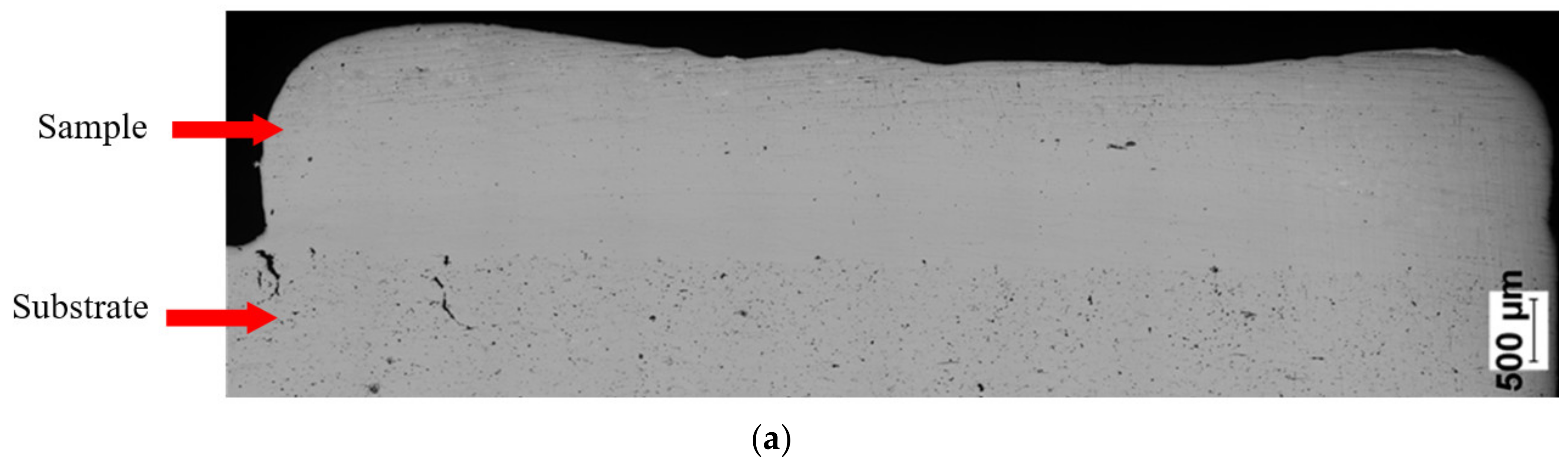
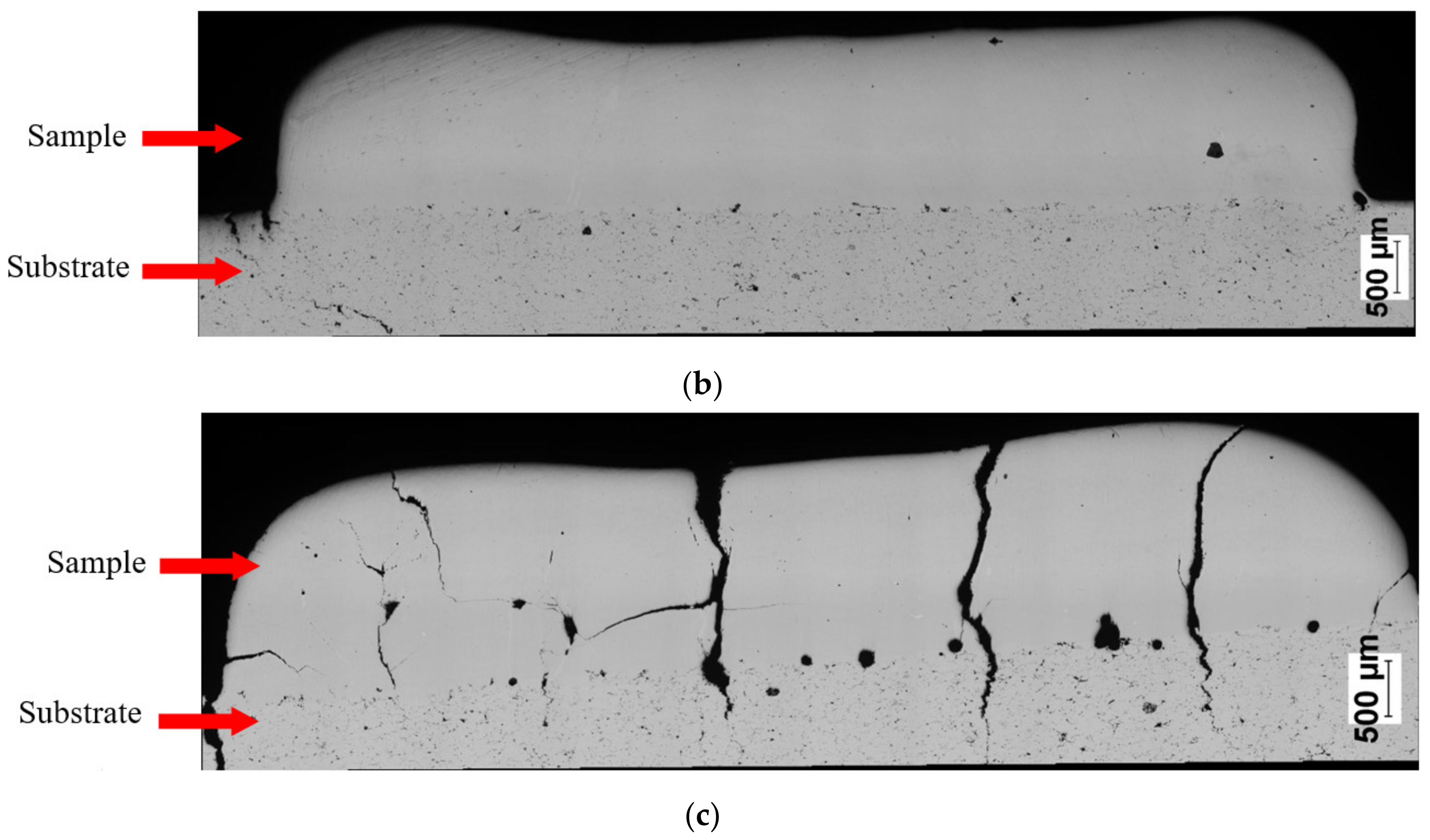
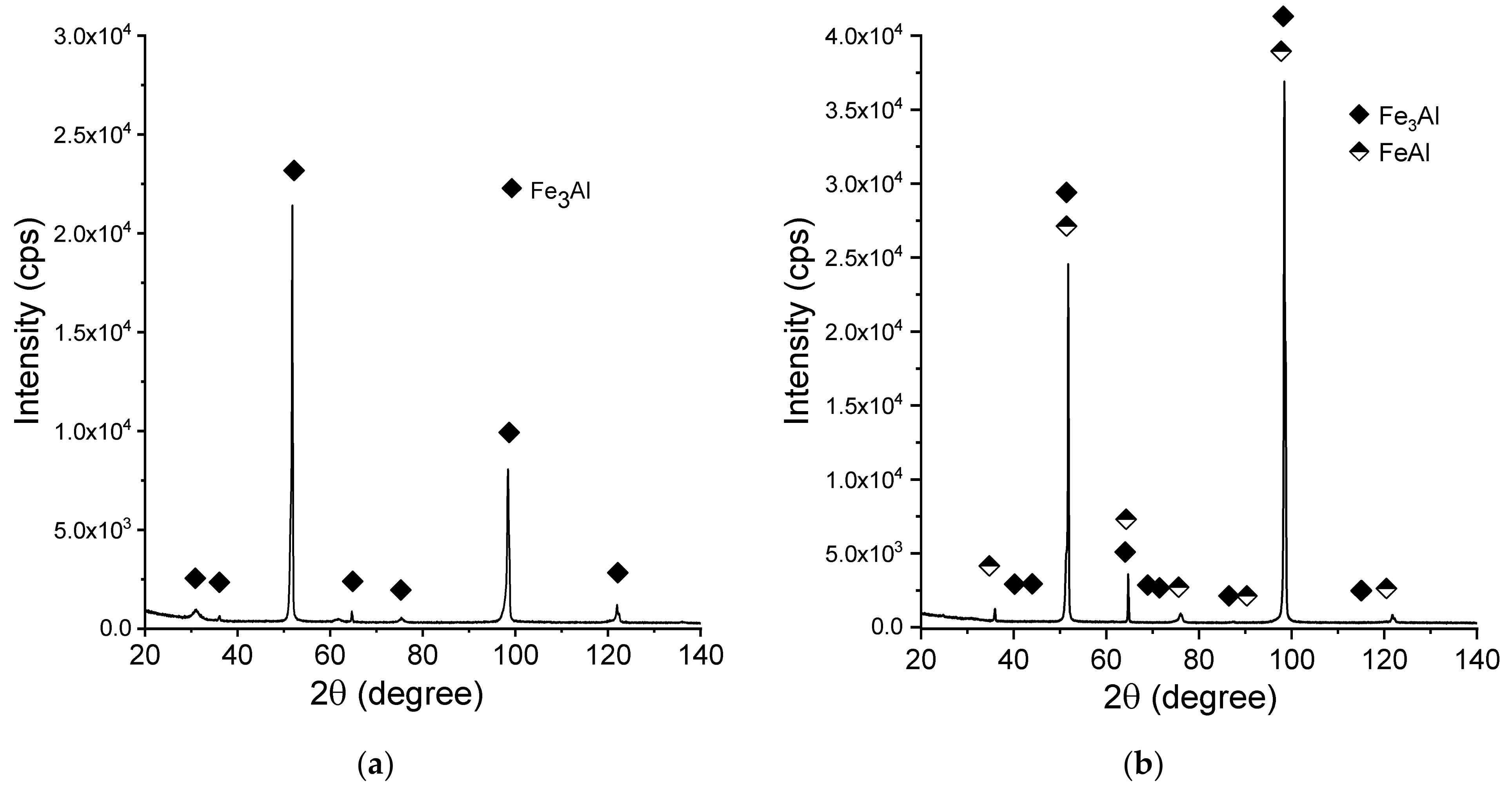

| Sample No. | Powder | Laser Power (W) | Feedrate (mm/s) | Powder Output (Rpm) | SubstrateTemperature (°C) |
|---|---|---|---|---|---|
| 6.1 | Mixture (94at.%Fe+6at.% Al) | 200 | 5 | 4 | 200 |
| 6.2 | 200 | 2.5 | 2 | 200 | |
| 6.3 | 250 | 7.6 | 6 | 200 | |
| 6.4 | 200 | 7.6 | 6 | 200 | |
| 28.1 | Mixture (72at.%Fe+28at.% Al) | 200 | 5 | 4 | 200 |
| 28.2 | 200 | 7.5 | 6 | 200 | |
| 28.3 | 250 | 10 | 8 | 200 | |
| 28.4 | 250 | 12.5 | 10 | 200 | |
| 28.5 | 200 | 12.5 | 10 | 200 | |
| 36.1 | Mixture (64at.%Fe+36at.% Al) | 200 | 5 | 4 | 200 |
| 36.2 | 200 | 5 | 6 | 200 | |
| 50.1 | Mixture (50at.%Fe+50at.% Al) | 200 | 5 | 6 | 200 |
| Sample | Fe | Al | ||
|---|---|---|---|---|
| wt.% | at.% | wt.% | at.% | |
| Fe6Al | 96.9 | 93.9 | 3.1 | 6.1 |
| Fe28Al | 83.7 | 71.3 | 16.3 | 28.7 |
| Fe36Al | 80.7 | 66.8 | 19.3 | 33.2 |
| Fe50Al | 62.9 | 45.1 | 37.1 | 54.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pęska, M.; Karczewski, K.; Rzeszotarska, M.; Polański, M. Direct Synthesis of Fe-Al Alloys from Elemental Powders Using Laser Engineered Net Shaping. Materials 2020, 13, 531. https://doi.org/10.3390/ma13030531
Pęska M, Karczewski K, Rzeszotarska M, Polański M. Direct Synthesis of Fe-Al Alloys from Elemental Powders Using Laser Engineered Net Shaping. Materials. 2020; 13(3):531. https://doi.org/10.3390/ma13030531
Chicago/Turabian StylePęska, Magda, Krzysztof Karczewski, Magdalena Rzeszotarska, and Marek Polański. 2020. "Direct Synthesis of Fe-Al Alloys from Elemental Powders Using Laser Engineered Net Shaping" Materials 13, no. 3: 531. https://doi.org/10.3390/ma13030531
APA StylePęska, M., Karczewski, K., Rzeszotarska, M., & Polański, M. (2020). Direct Synthesis of Fe-Al Alloys from Elemental Powders Using Laser Engineered Net Shaping. Materials, 13(3), 531. https://doi.org/10.3390/ma13030531






