Microstructure of Coatings on Nickel and Steel Platelets Obtained by Co-Milling with NiAl and CrB2 Powders
Abstract
1. Introduction
2. Experimental Procedure
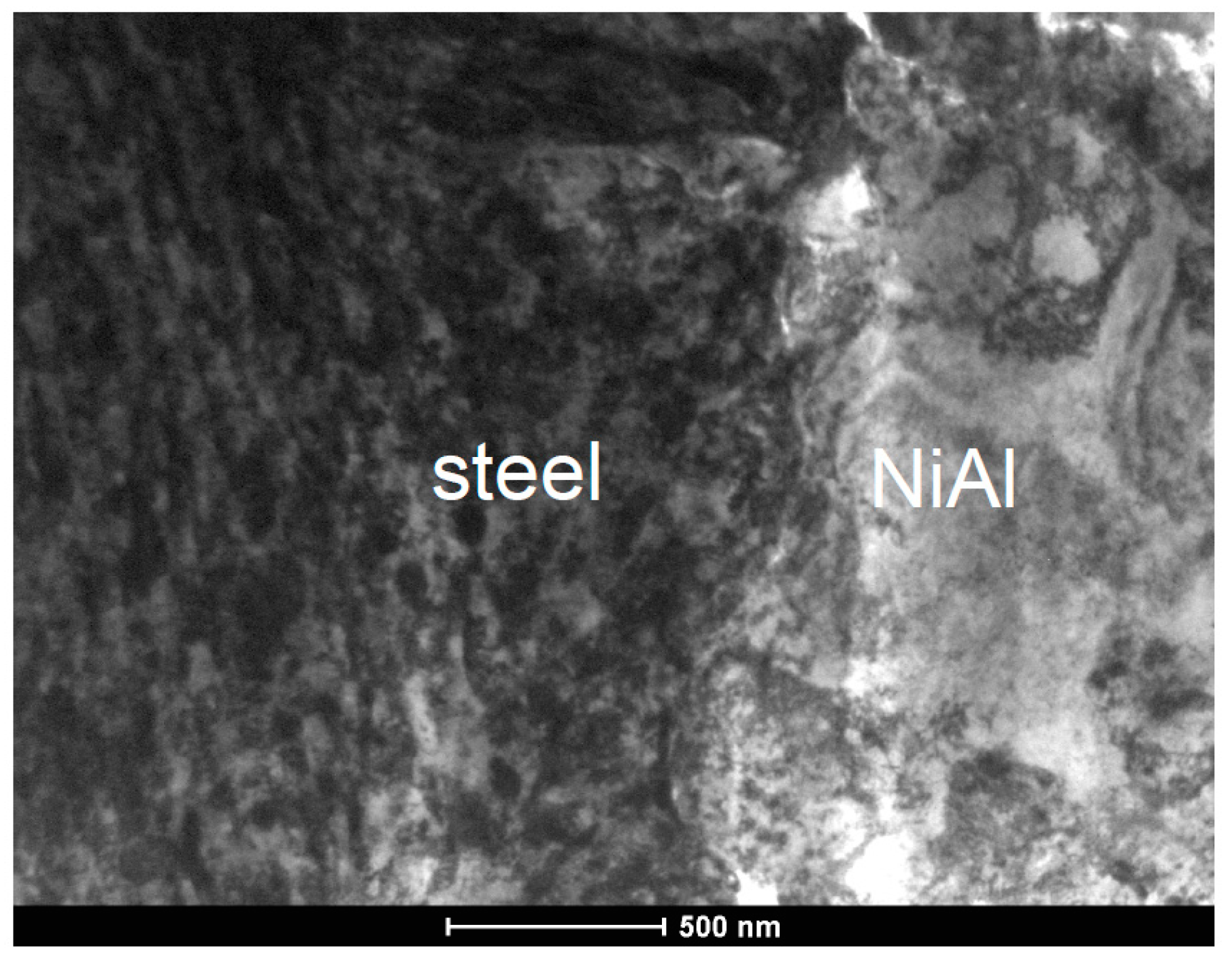
3. Results
4. Discussion
5. Summary
- increasing the energy transfer from steel balls to particles pressed against substrates widens the intermixing zone and raises the deposition rate of the coatings, but only at the early stage of this process;
- substituting nickel with a harder substrate, like stainless steel, narrows the coating intermixing zone, but its deposition rate remains roughly the same; and
- the coating final thickness is limited by the brittleness of the milled nanocrystalline NiAl matrix and remains approximately the same for the investigated milling parameters.
Author Contributions
Funding
Conflicts of Interest
References
- Sierra, C.; Vazquez, A.J. NiAl coating on carbon steel with an intermediate Ni gradient layer. Surf. Coat. Technol. 2006, 200, 4383–4388. [Google Scholar] [CrossRef]
- Prevot, G.; Schmausa, D.; Le Moal, S. Epitaxial alloyed films out of the bulk stability domain: Surface structure and composition of Ni3Al and NiAl films. Surf. Sci. 2010, 604, 770–777. [Google Scholar] [CrossRef][Green Version]
- Krasnowski, M.; Gierlotka, S.; Ciołek, S.; Kulig, T. Nanocrystalline NiAl intermetallic alloy with high hardness produced mechanical alloying and hot-pressing consolidation. Adv. Powder Technol. 2019, 30, 1312–1318. [Google Scholar] [CrossRef]
- Umanskyi, O.; Poliarus, O.; Ukrainets, M.; Martsenyuk, I. Effect of ZrB2, CrB2 and TiB2 additives on the tribological characteristics of NiAl-based plasma coatings. Key Eng. Mat. 2014, 604, 20–23. [Google Scholar] [CrossRef]
- Poliarus, O.; Morgiel, J.; Umanskyi, O.; Szlezynger, M.; Pomorska, M.; Bobrowski, P.; Szczerba, M. Microstructure and phase composition of NiAl-CrB2 composite powders used for plasma spraying. Compos. Theory Pract. 2018, 18, 121–124. [Google Scholar]
- Umanskyi, O.; Poliarus, O.; Ukrainets, M.; Antonov, M.; Hussainova, I. High temperature sliding wear of NiAl-based coating reinforced by borides. Mater. Sci. 2016, 22. [Google Scholar] [CrossRef]
- Umanskyi, O.; Poliarus, O.; Ukrainets, M.; Kostenko, O.; Terentyev, O. Influence of CrB2 additives into NiAl intermetallics on tribological properties of thermal spray coatings at high temperature friction. In Proceedings of the Conference MET-2013: Materials, Environment, Technology, Riga, Latvia, 19–20 June 2013; pp. 37–43. [Google Scholar]
- Zadorozhnyy, V.; Kaloshkin, S.; Kaevitser, E.; Romankov, S. Coating of metals with intermetallics by mechanical alloying. J. Alloys Compd. 2011, 509, S507–S509. [Google Scholar] [CrossRef]
- Zadorozhnyy, V.; Kaloshkin, S.; Tcherdyntsev, V.; Gorshenkov, M.; Komissarov, A.; Zadorozhnyy, M. Formation of intermetallic Ni-Al coatings by mechanical alloying on the different hardness substrates. J. Alloys Compd. 2014, 586, S373–S376. [Google Scholar] [CrossRef]
- Kubaski, E.T.; Cintho, O.M.; Antoniassi, J.L.; Kahn, H. Obtaining NiAl intermetallic compound using different milling devices. Adv. Powder Tech. 2012, 23, 667–672. [Google Scholar] [CrossRef]
- Mohammadnezhad, M.; Shamanian, M.; Enayati, M.H. Formation of nano structured NiAl coating on carbon steel by using mechanical alloying. Appl. Surf. Sci. 2012, 263, 730–736. [Google Scholar] [CrossRef]
- Pouriamanesh, R.; Vahdati-Khaki, J.; Mohammadi, Q. Coating of Al substrate by metallic Ni through mechanical alloying. J. Alloys Compd. 2009, 488, 430–436. [Google Scholar] [CrossRef]
- Yazdani, A.; Zakeri, A. An insight into formation of nanostructured coatings on metallic substrates by planetary ball milling. Powder Technol. 2014, 278, 196–203. [Google Scholar] [CrossRef]
- Tian, Y.; Lu, C.; Shen, Y.; Feng, X. Microstructure and corrosion property of crMnFeCoNi high entropy alloy coating on Q235 substrate via mechanical alloying method. Surf. Interfaces 2019, 15, 135–140. [Google Scholar] [CrossRef]
- Szlezynger, M.; Morgiel, J.; Rogal, L.; Poliarus, O.; Kurtyka, P. Microstructure of NiAl + 15 wt. % CrB2 nano-crystalline composite coatings obtained through co-milling of NiAl and CrB2 powders. Compos. Theory Pract. 2018, 149–155. [Google Scholar]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Kobayashi, K. Formation of coating film on milling balls for mechanical alloying. Mater. Trans. JIM 1995, 36, 134–137. [Google Scholar] [CrossRef]
- Mason, B.A.; Sippel, T.R.; Groven, L.J.; Gunduz, I.E.; Son, S.F. Combustion of mechanically activated Ni/Al reactive composites with microstructural refinement tailored using two-step milling. Intermetallics 2015, 66, 88–95. [Google Scholar] [CrossRef]
- Romankov, S.; Kaloshkin, S.D.; Hayasaka, Y.; Hayasaka, N.; Kasai, E.; Komarov, S.V. Effect of process parameters on the formation of Ti-Al coatings fabricated by mechanical milling. J. Alloys Compd. 2009, 484, 665–673. [Google Scholar] [CrossRef]
- Revesz, A.; Takacs, L. Coating metals by surface mechanical attrition treatment. J. Alloys Compd. 2007, 441, 111–114. [Google Scholar] [CrossRef]
- Sytschev, A.E.; Busurin, S.M.; Kovalev, D.Y.; Vrel, D.; Boyarchenko, O.D.; Sachkova, N.V. Deposition of Ni-Al coatings onto copper by mechanical/heat treatment. Int. J. Self-Propag. High-Temp. Synth. 2013, 22, 103–109. [Google Scholar] [CrossRef]
- Mitka, M. Development of Quasicrystalline Al-Cu-Fe Phase Formation by Mechanical Alloying as Reinforcement in Composites and Their Characterization. Ph.D. Thesis, Institute of Metallurgy and Materials Science Polish Academy of Sciences, Cracow, Poland, 2017. [Google Scholar]
- Sanchez, E.; Bannier, E.; Cantavella, V.; Salvador, M.D.; Klyatskina, E.; Morgiel, J.; Grzonka, J.; Boccaccini, A.R. Deposition of Al2O3-TiO2 nanostructured powder by atmospheric plasma spraying. J. Therm. Spray Technol. 2007, 17, 329–337. [Google Scholar] [CrossRef]
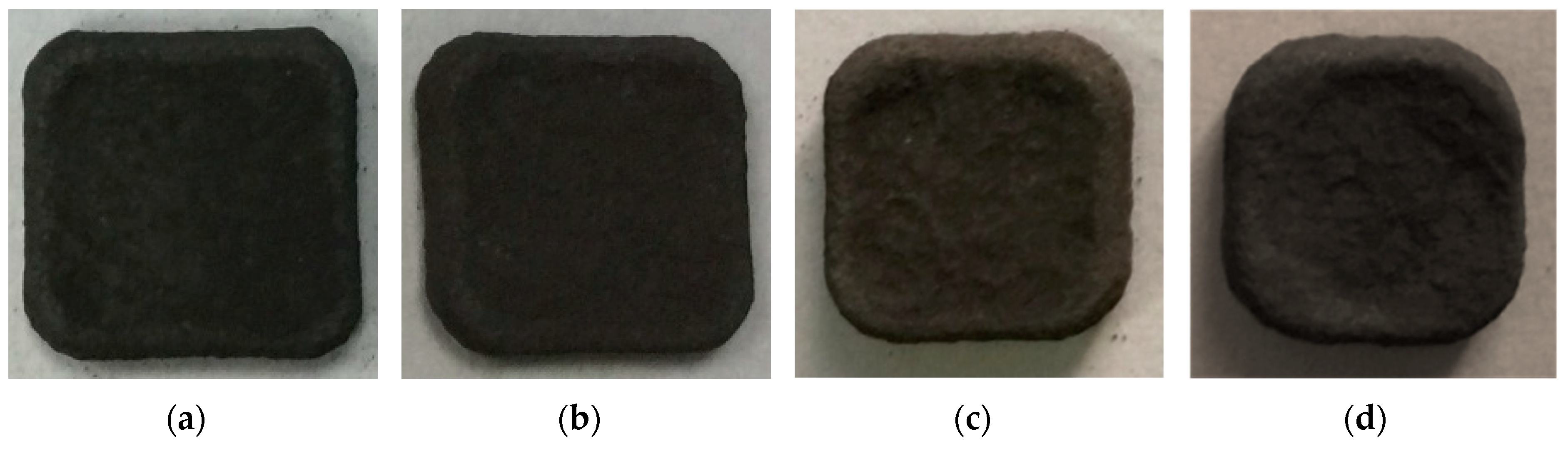
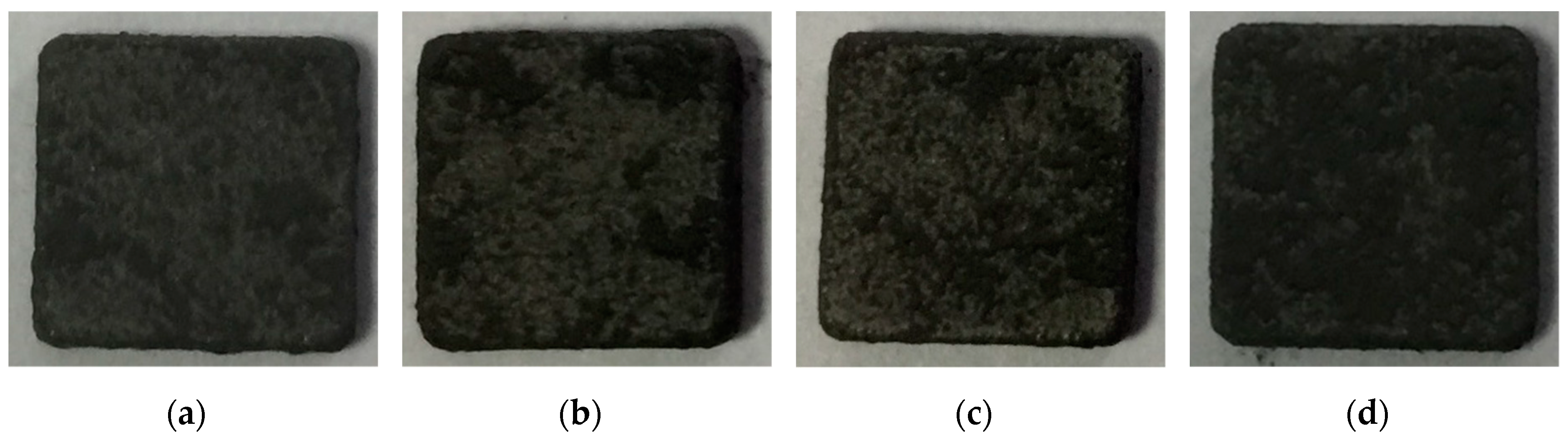
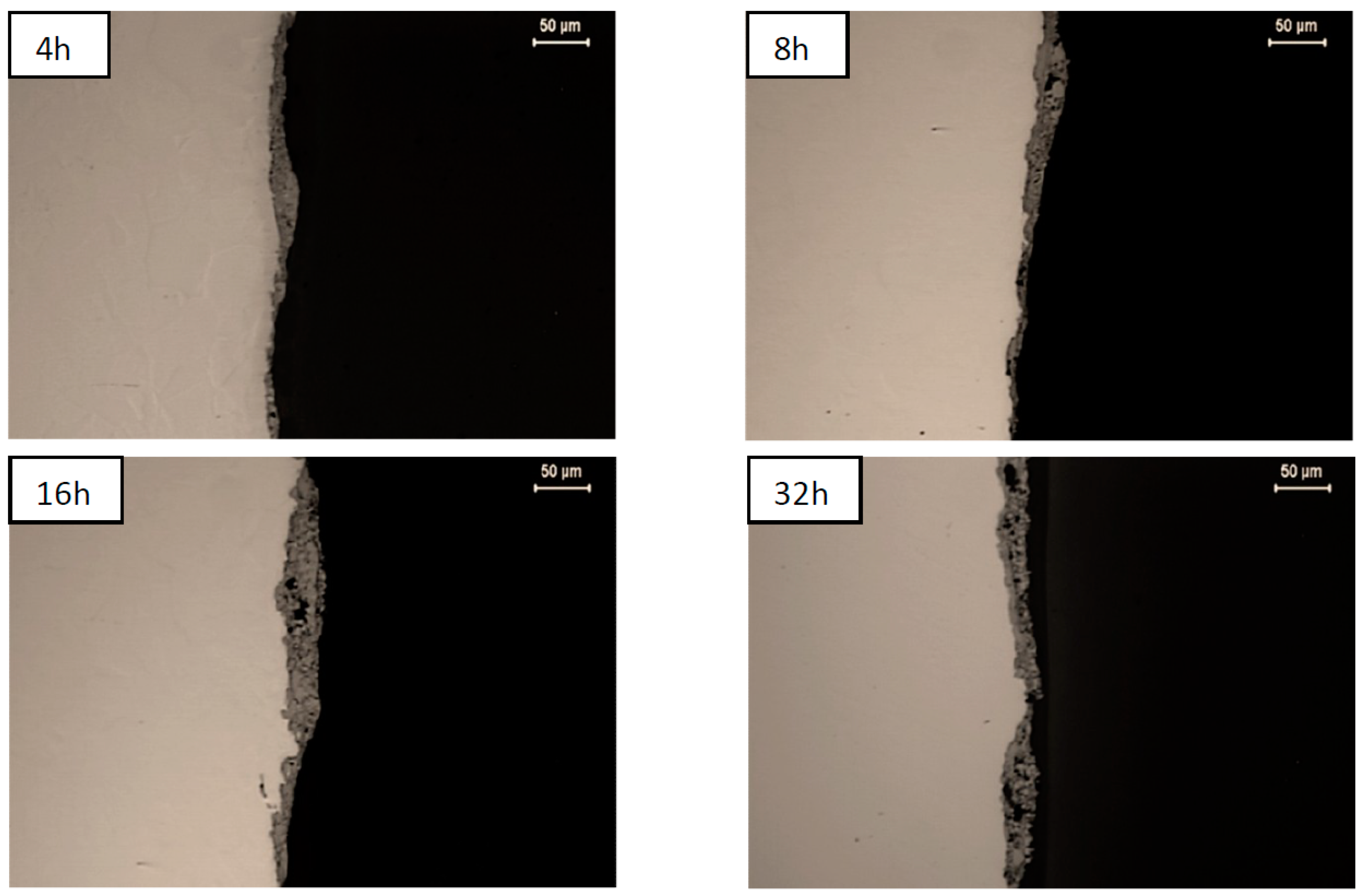
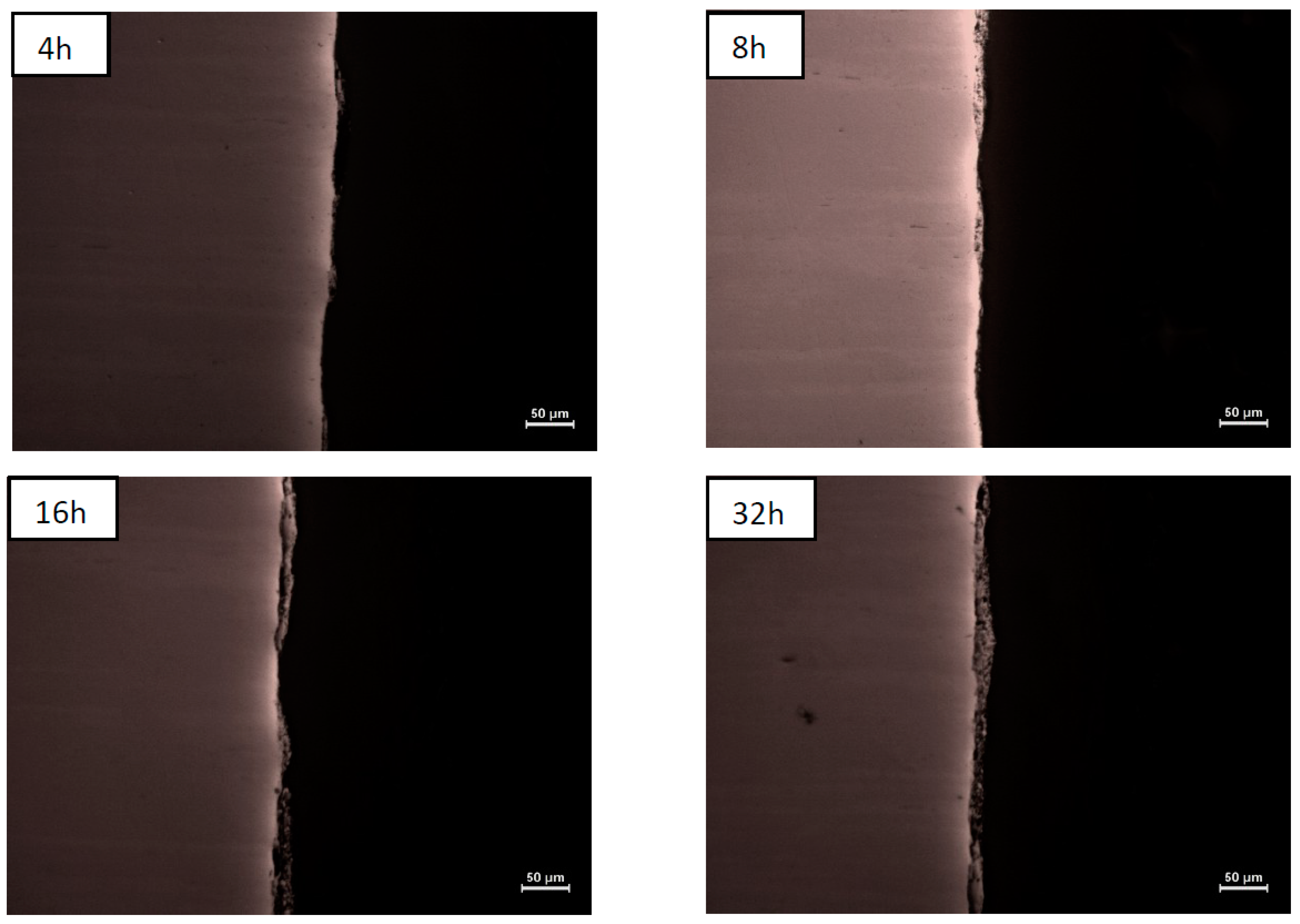
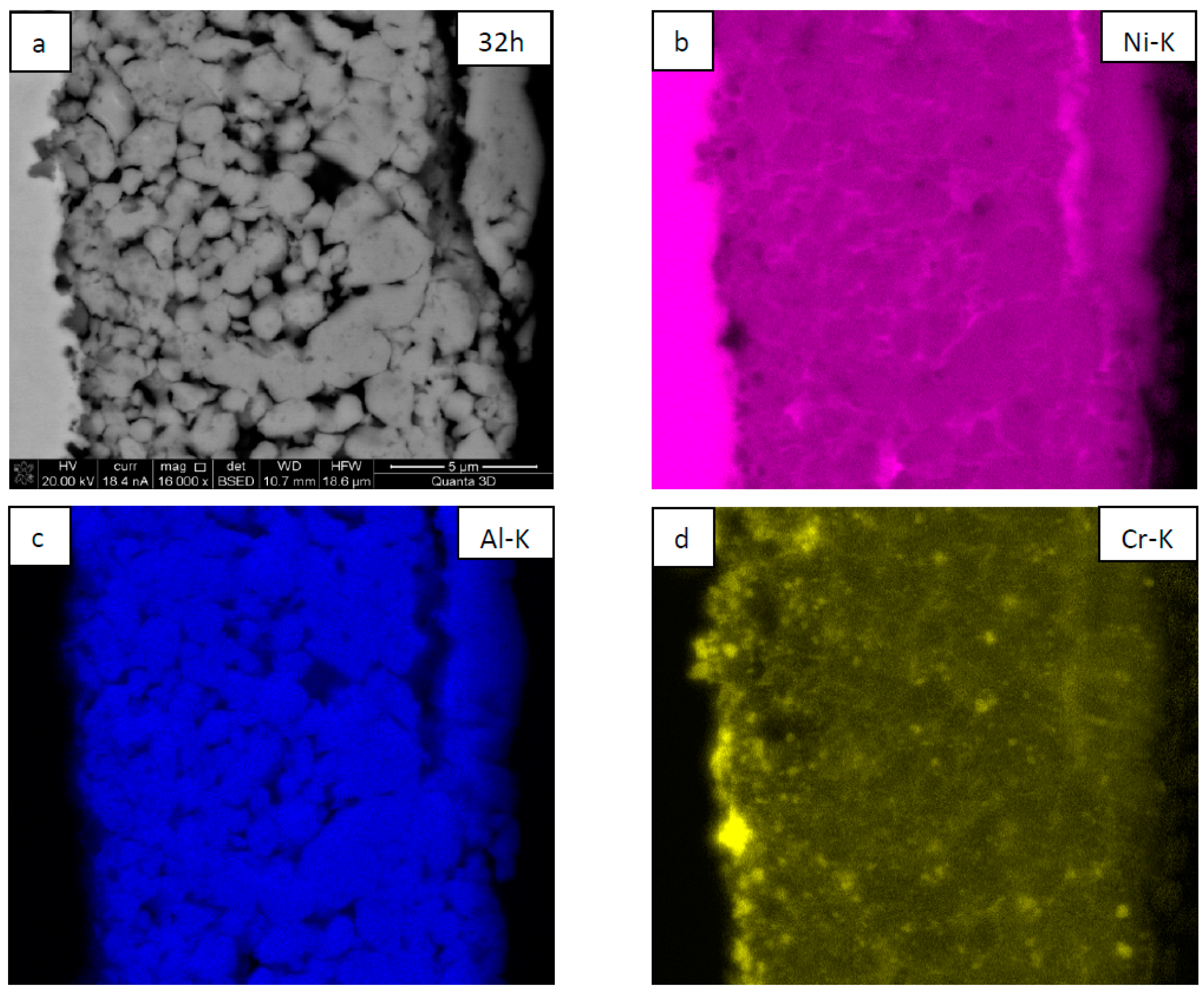
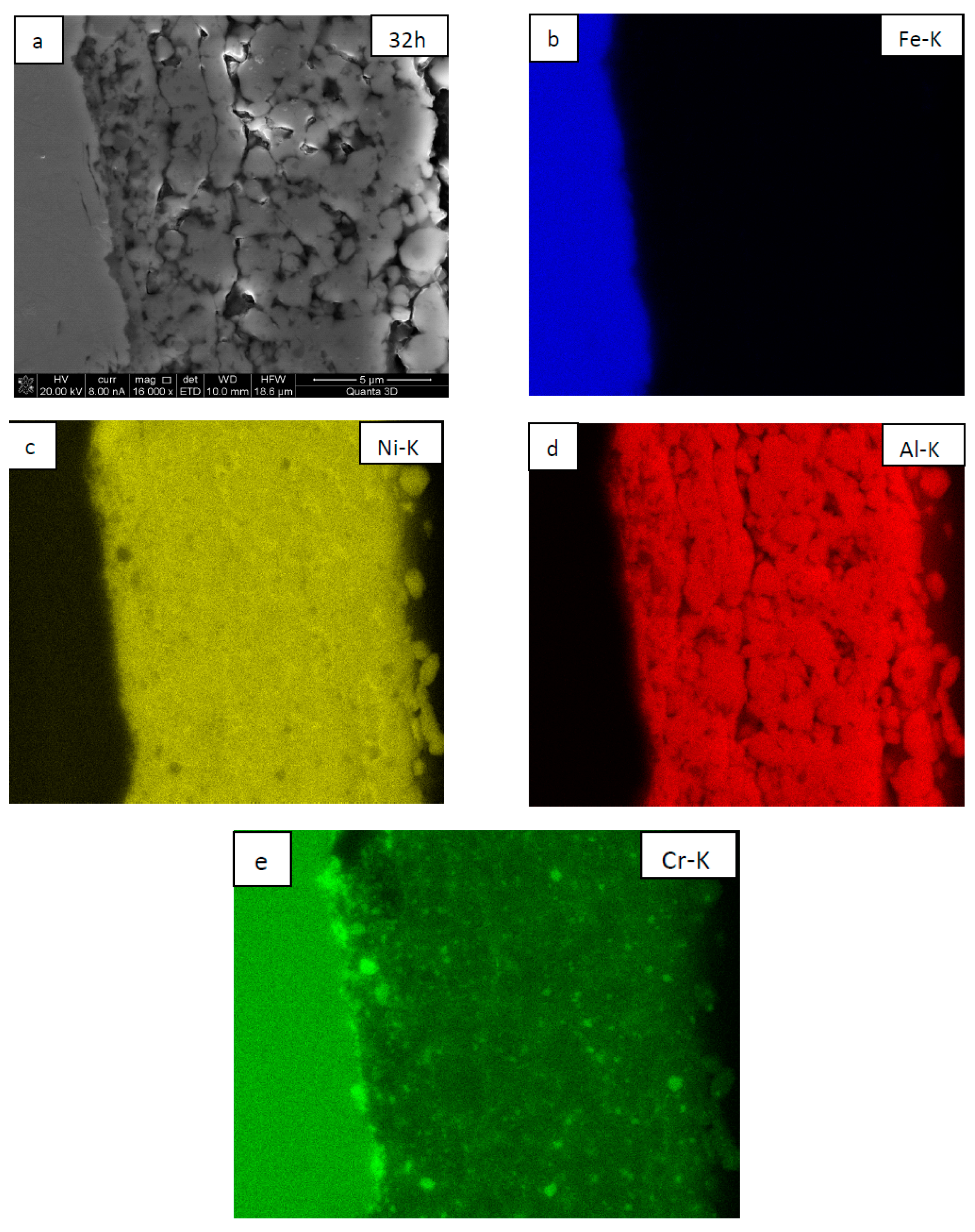
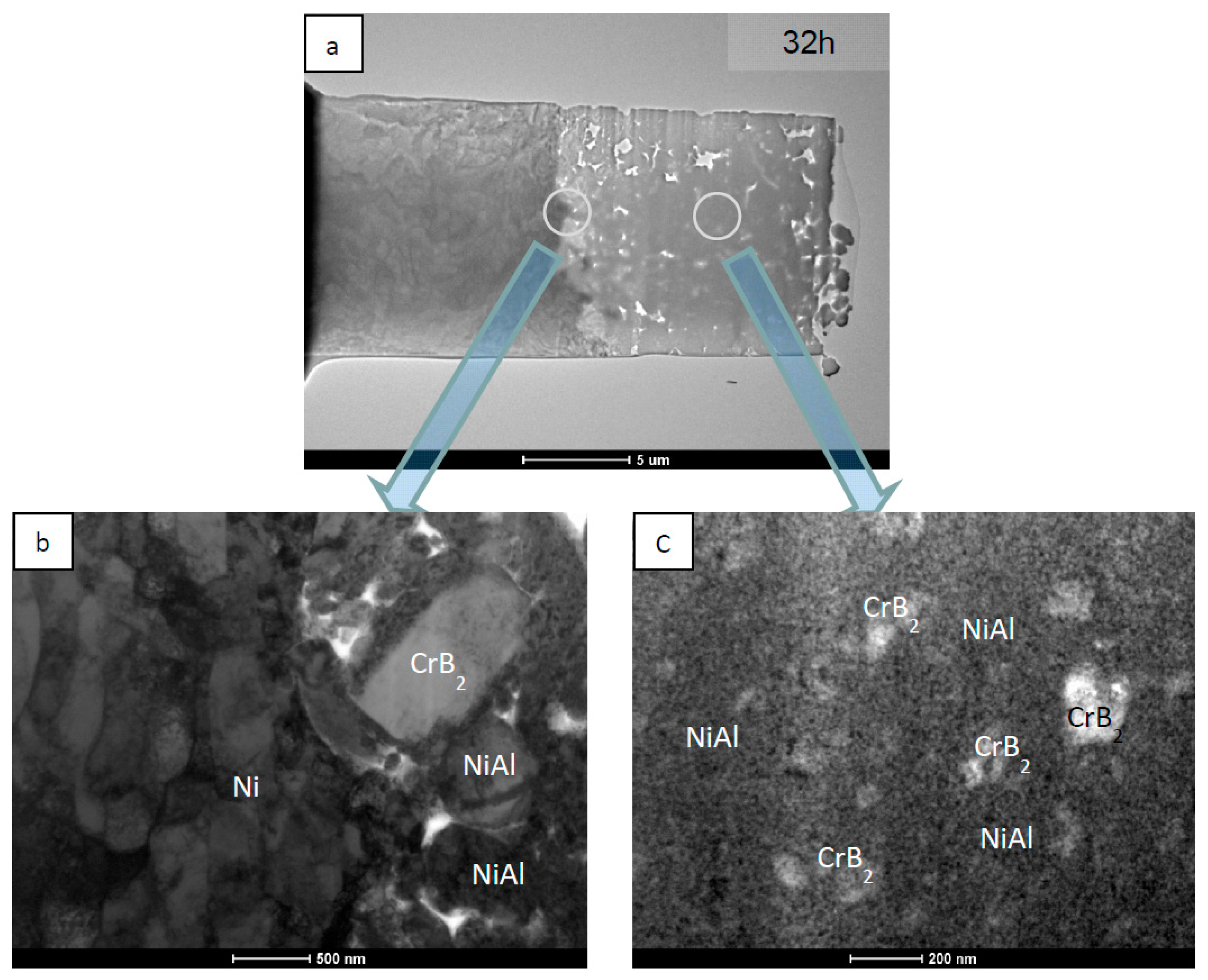
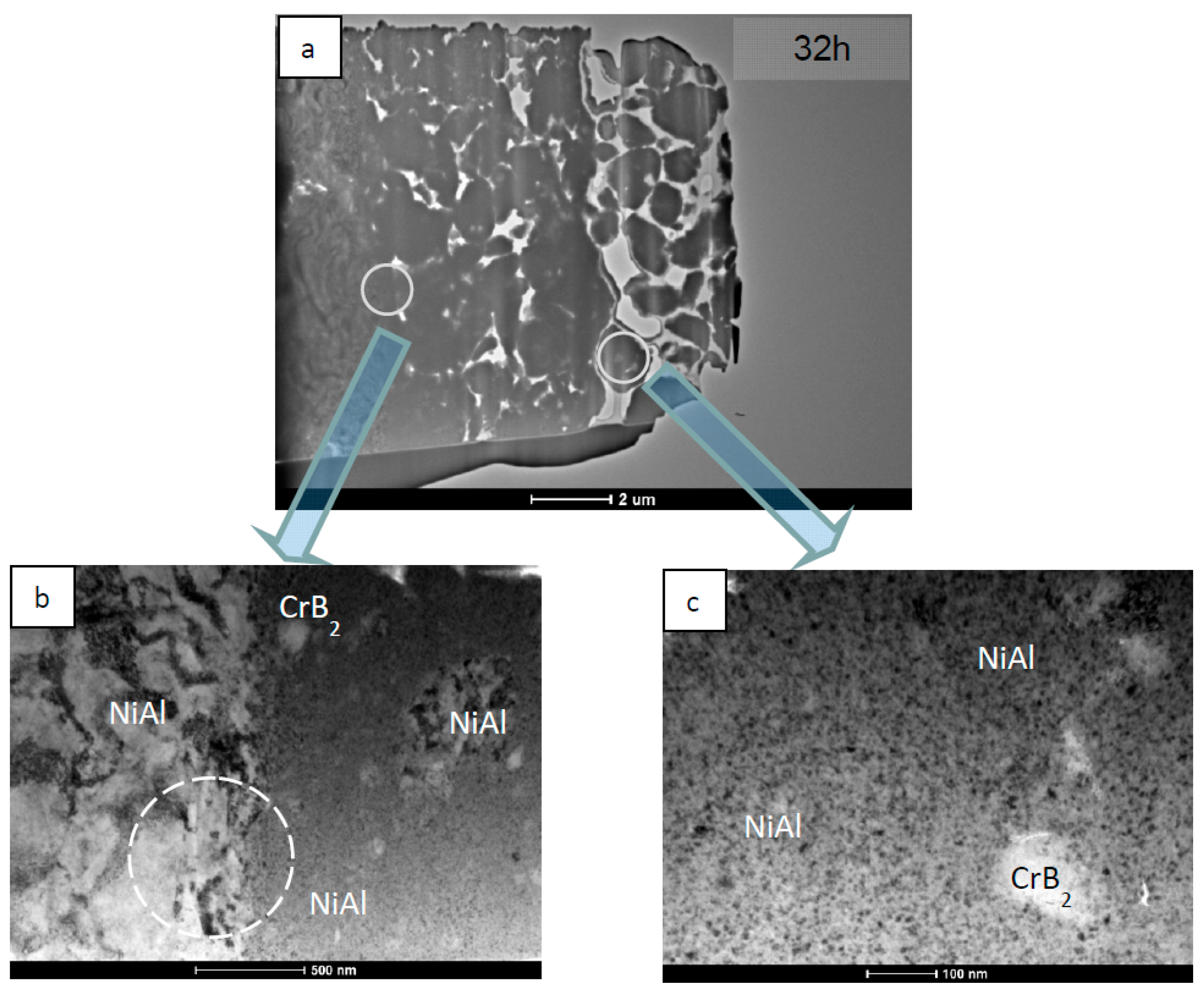
| NiAl + CrB2 Coatings | Milling Time (h) | |||
|---|---|---|---|---|
| 4 | 8 | 16 | 32 | |
| Ni substrate | 9.8 ± 3.5 µm | 14.6 ± 5.5 µm | 21.0 ± 10 µm | 21.6 ± 6.3 µm |
| Steel substrate | 7.8 ± 3.9 µm | 8.9 ± 4.4 µm | 13.5 ± 4.0 µm | 19.2 ± 9.8 µm |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szlezynger, M.; Morgiel, J.; Maj, Ł.; Poliarus, O.; Czaja, P. Microstructure of Coatings on Nickel and Steel Platelets Obtained by Co-Milling with NiAl and CrB2 Powders. Materials 2019, 12, 2593. https://doi.org/10.3390/ma12162593
Szlezynger M, Morgiel J, Maj Ł, Poliarus O, Czaja P. Microstructure of Coatings on Nickel and Steel Platelets Obtained by Co-Milling with NiAl and CrB2 Powders. Materials. 2019; 12(16):2593. https://doi.org/10.3390/ma12162593
Chicago/Turabian StyleSzlezynger, Maciej, Jerzy Morgiel, Łukasz Maj, Olena Poliarus, and Paweł Czaja. 2019. "Microstructure of Coatings on Nickel and Steel Platelets Obtained by Co-Milling with NiAl and CrB2 Powders" Materials 12, no. 16: 2593. https://doi.org/10.3390/ma12162593
APA StyleSzlezynger, M., Morgiel, J., Maj, Ł., Poliarus, O., & Czaja, P. (2019). Microstructure of Coatings on Nickel and Steel Platelets Obtained by Co-Milling with NiAl and CrB2 Powders. Materials, 12(16), 2593. https://doi.org/10.3390/ma12162593





