The Influence of Environmentally Friendly Flame Retardants on the Thermal Stability of Phase Change Polyurethane Foams
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Micro-PCMs
2.3. Fabrication of PUF
2.4. Characterization of Flame Retardant PUF
3. Results and Discussion
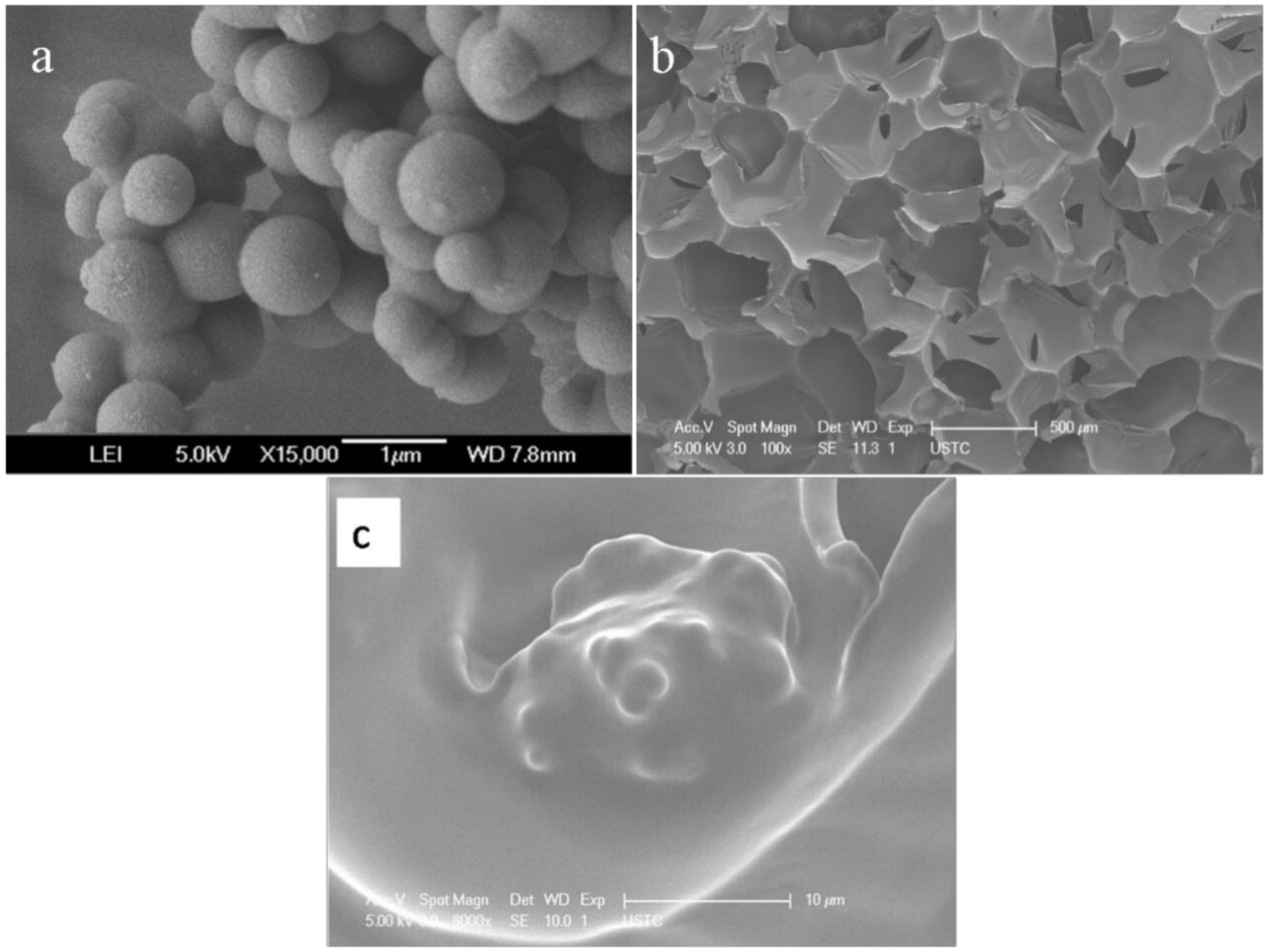
3.1. SEM Photos of Micro-PCMs and Pure PUF Structure
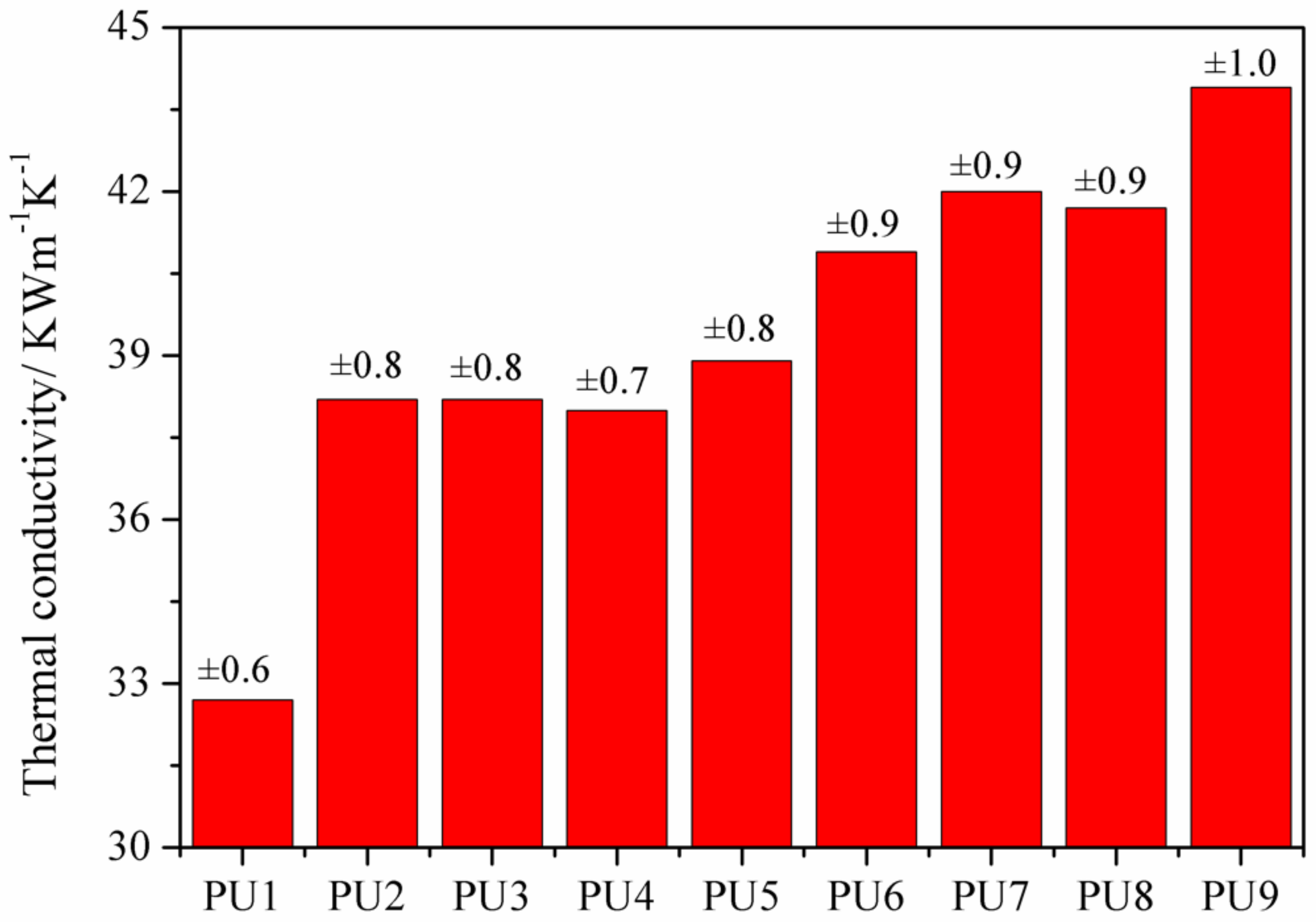
3.2. The Thermal Conductivities of the PUFs
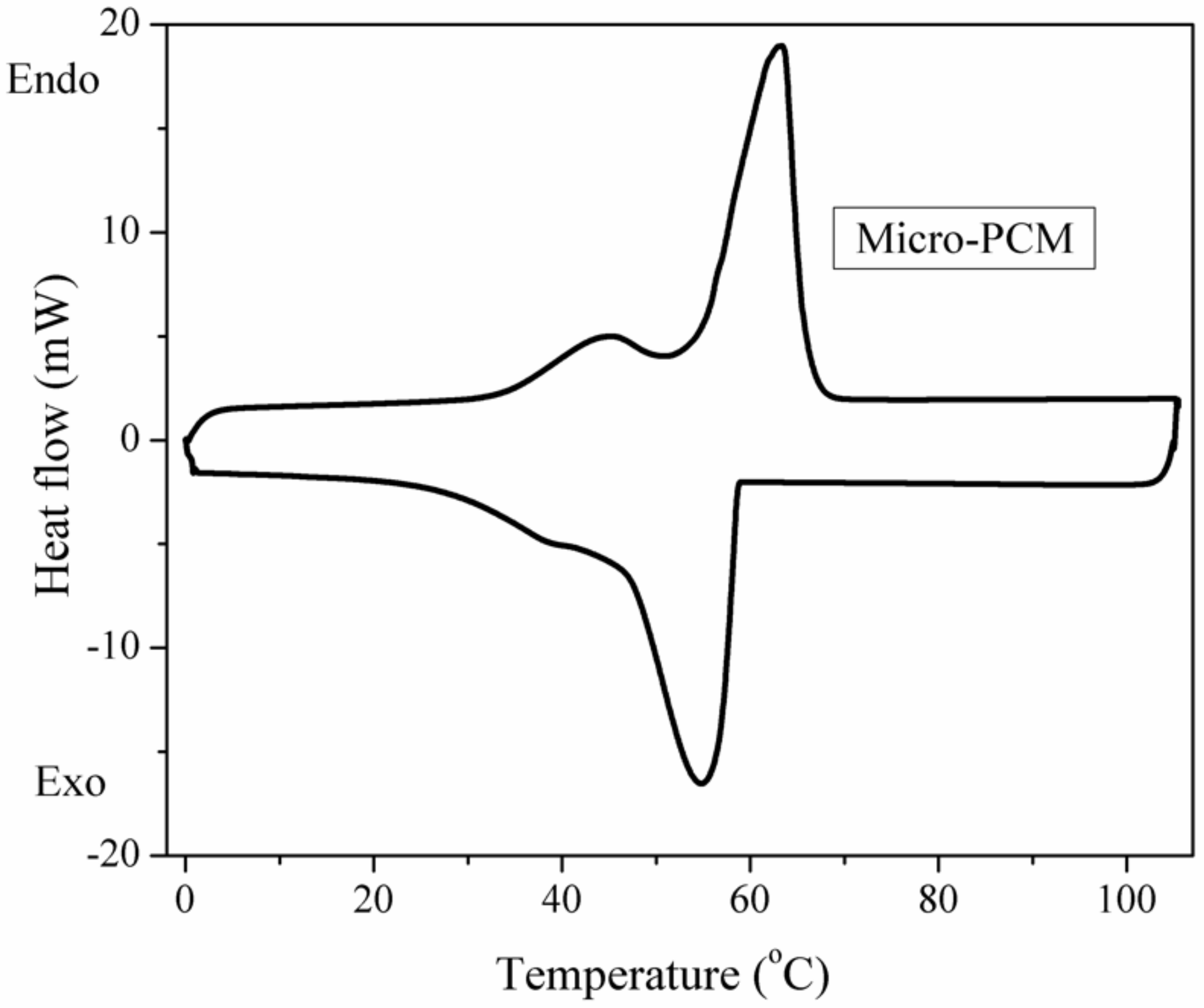
3.3. The Latent Heats of the PUFs
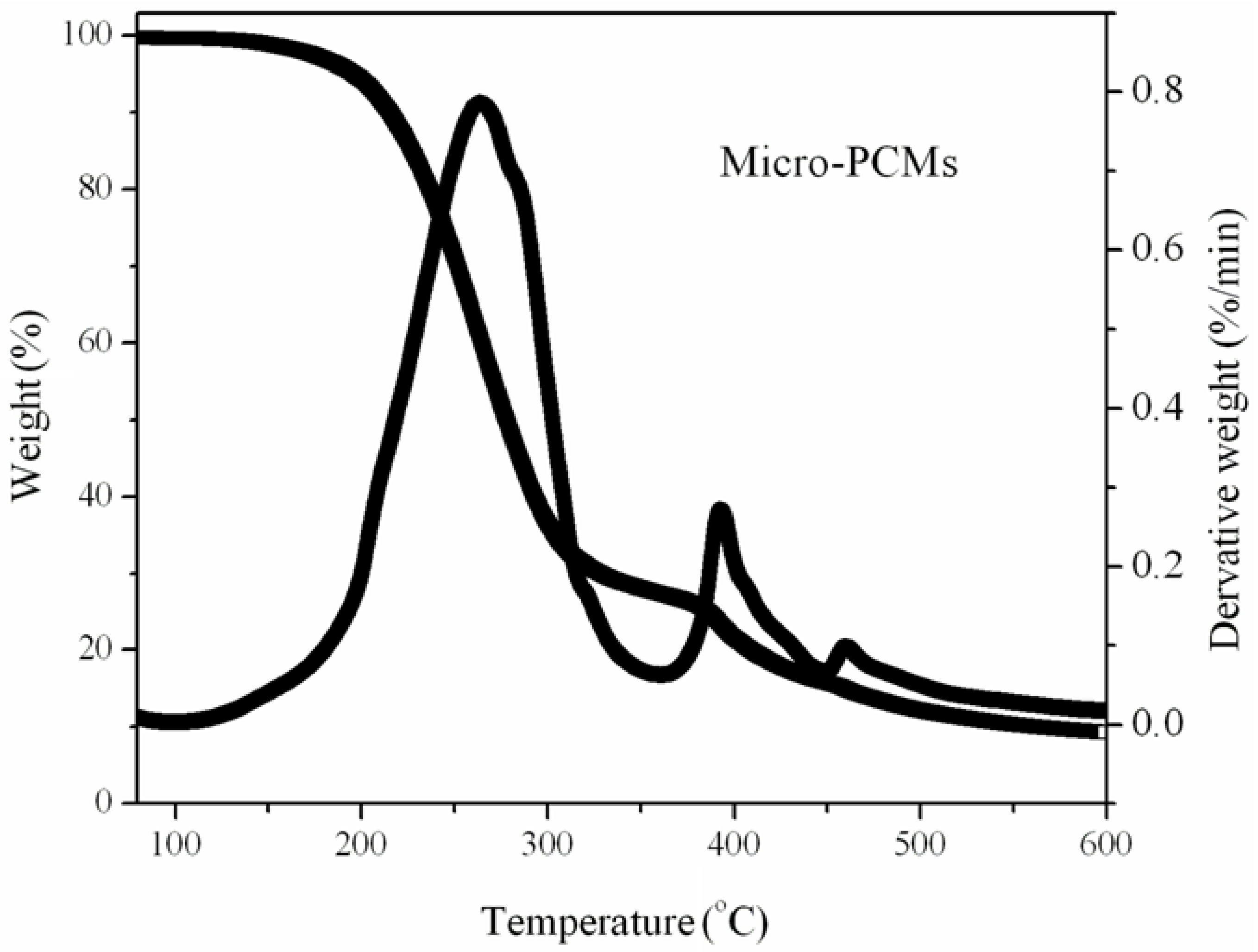
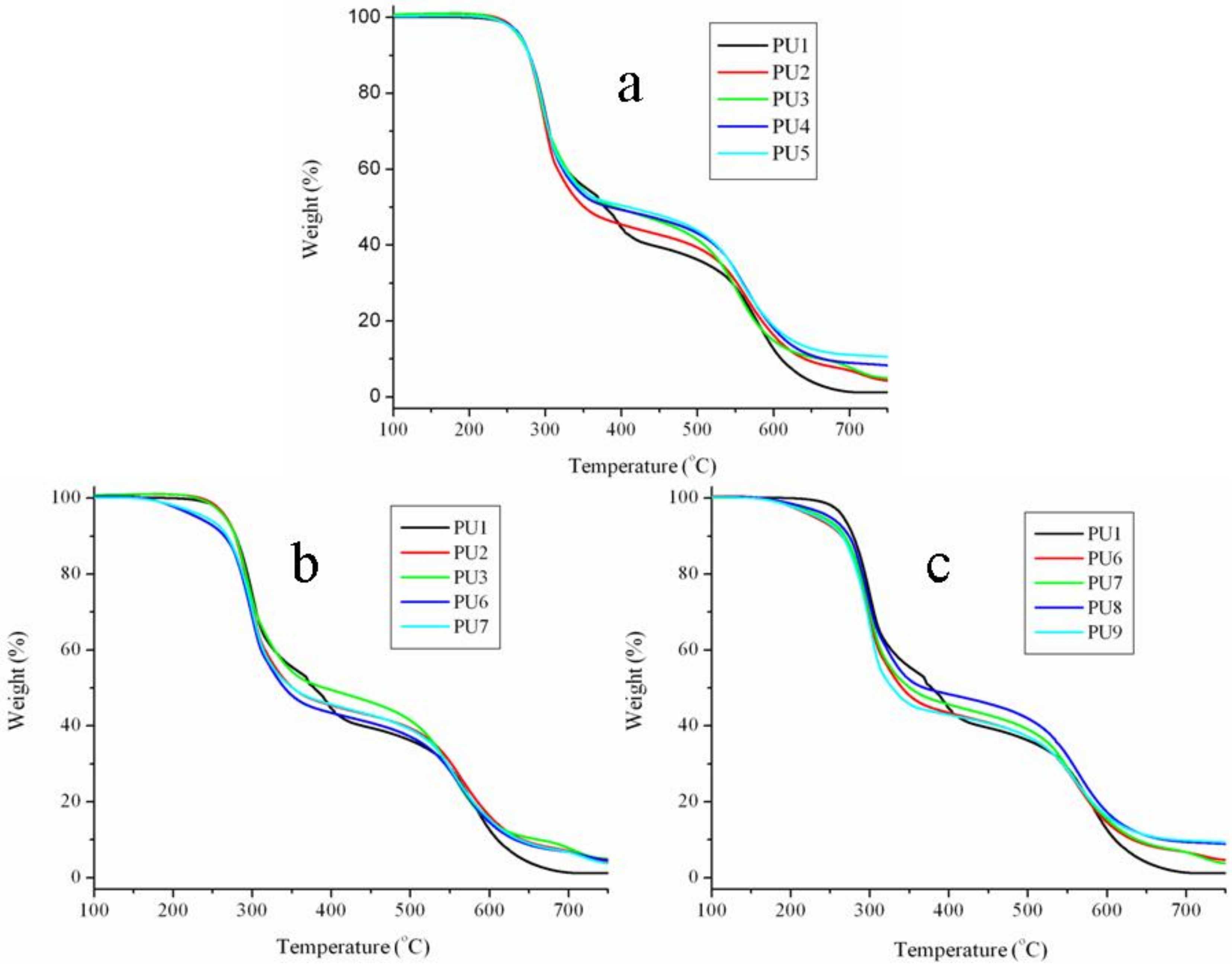
3.4. The Thermal Stabilities of PUFs
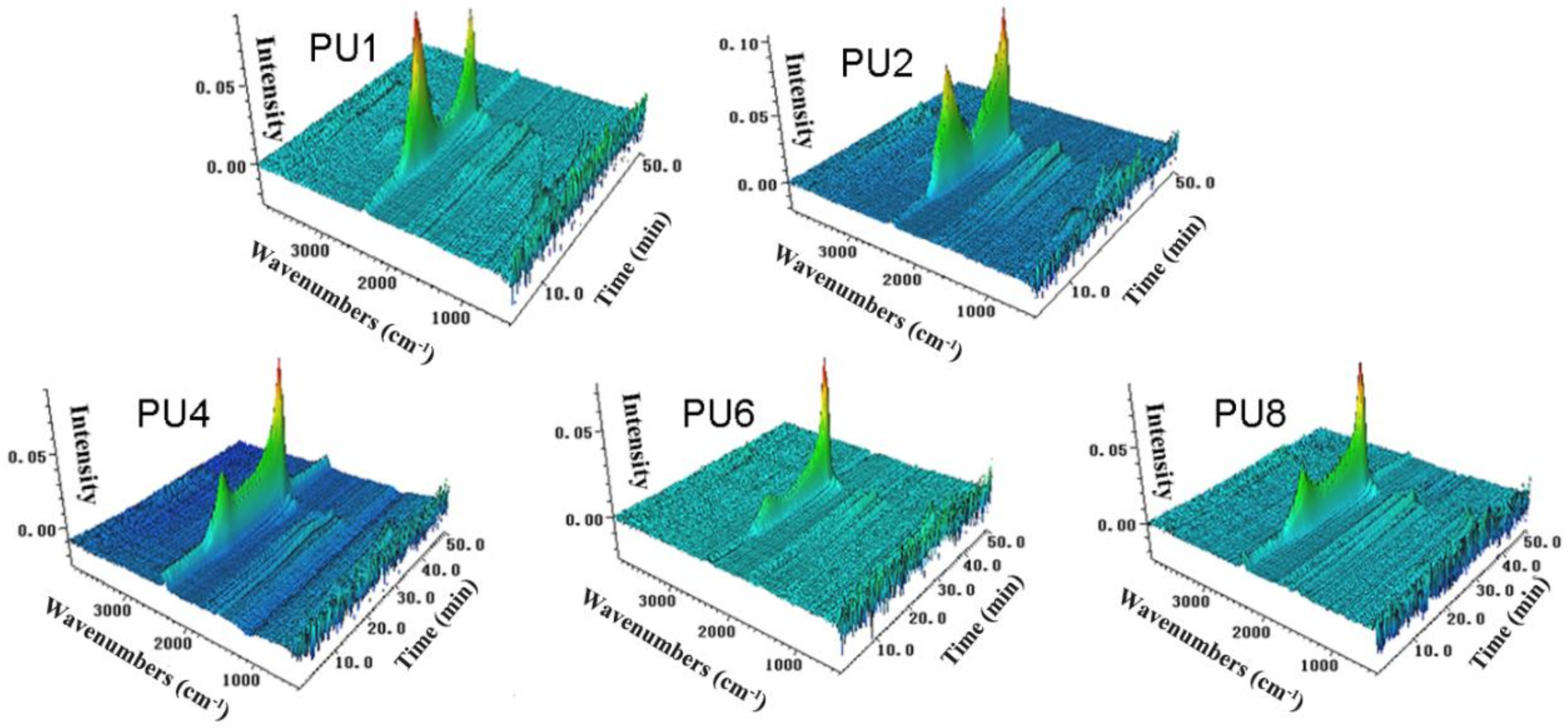
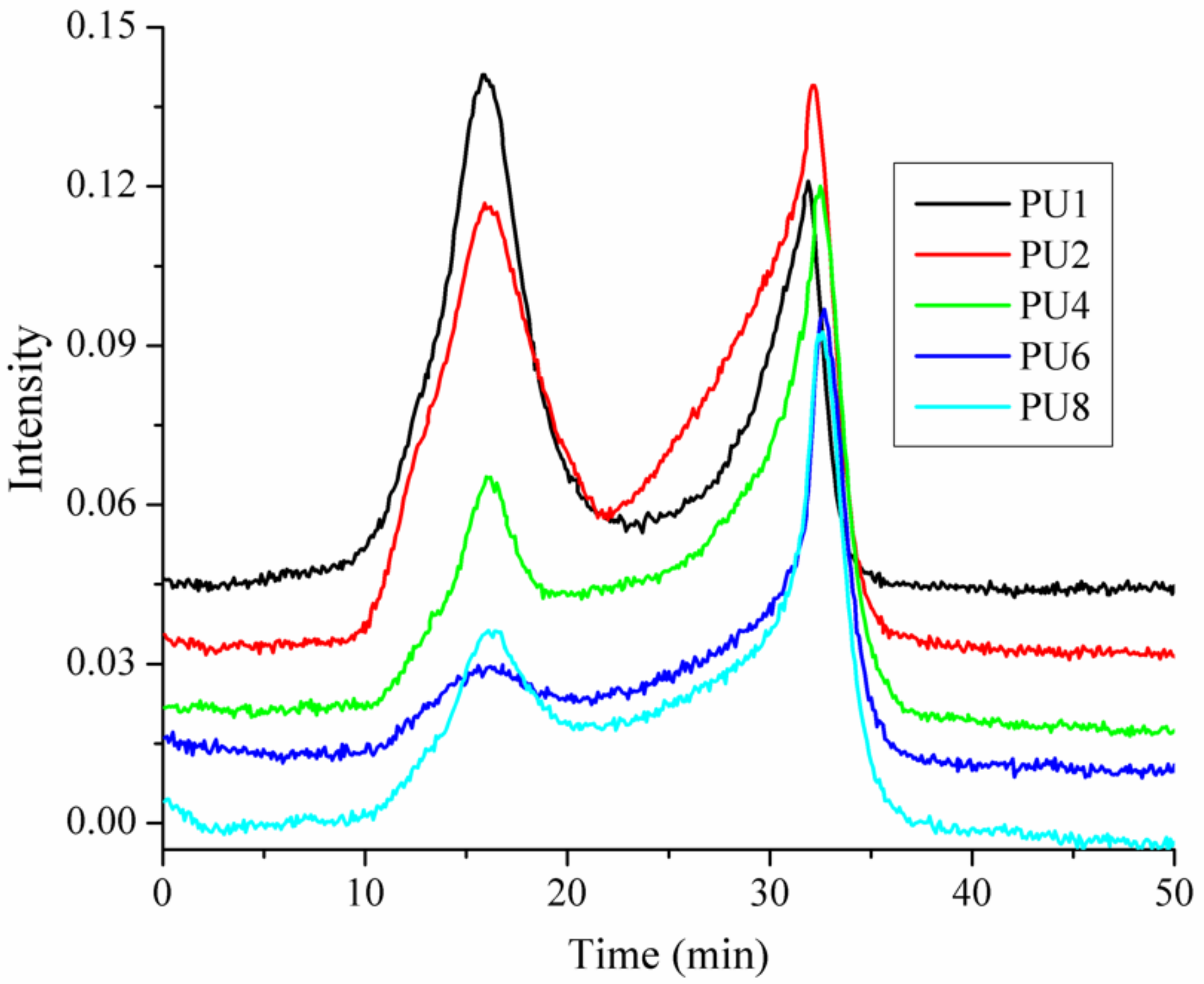
3.5. The Volatilized Products of the PUFs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, T.T.; Zhang, X.; Wang, H.Y.; Dai, W.N.; Huang, S.Y.; Shiu, B.C.; Lou, C.W.; Lin, J.H. Sound absorption and compressive property of PU foam-filled composite sandwiches: Effects of needle-punched fabric structure, porous structure, and fabric-foam interface. Polym. Adv. Technol. 2019. [Google Scholar] [CrossRef]
- Tiuc, A.E.; Nemes, O.; Vermesan, H.; Toma, A.C. New sound absorbent composite materials based on sawdust and polyurethane foam. Compos. Part B Eng. 2019, 165, 120–130. [Google Scholar] [CrossRef]
- Park, M.; Park, H.K.; Shin, H.K.; Kang, D.; Pant, B.; Kim, H.; Song, J.K.; Kim, H.Y. Sound absorption and insulation properties of a polyurethane foam Mixed with electrospun nylon-6 and polyurethane nanofibre Mats. J. Nanosci. Nanotechnol. 2019, 19, 3558–3563. [Google Scholar] [CrossRef]
- Cooper, E.M.; Kroeger, G.; Davis, K.; Clark, C.R.; Ferguson, P.L.; Stapleton, H.M. Results from screening polyurethane foam based consumer products for flame retardant chemicals: Assessing impacts on the change in the furniture flammability standards. Environ. Sci. Technol. 2016, 50, 10653–10660. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Z.Z.; Zhu, M.H. Flame retardant, mechanical and thermal insulating properties of rigid polyurethane foam modified by nano zirconium amino-tris-(methylenephosphonate) and expandable graphite. Polym. Degrad. Stabil. 2019, 170, 108997. [Google Scholar] [CrossRef]
- Harikrishnan, G.; Singh, S.N.; Kiesel, E.; Macosko, C.W. Nanodispersions of carbon nanofiber for polyurethane foaming. Polym. 2010, 51, 3349–3353. [Google Scholar] [CrossRef]
- Dementyev, A.G.; Dementyev, M.A.; Zinger, P.A.; Metlyakova, I.R. Effect of the cellular structure on thermal conductivity of rigid closed-cell foam polymers during long-term aging. Mech. Compos. Mater. 1999, 35, 129–138. [Google Scholar] [CrossRef]
- Ye, L.; Meng, X.Y.; Liu, X.M.; Tang, J.H.; Li, Z.M. Structure and magnetic properties of regenerated cellulose/Fe3O4 nanocomposite films. J. Appl. Polym. Sci. 2009, 111, 2372–2380. [Google Scholar] [CrossRef]
- Bian, X.C.; Tang, J.H.; Li, Z.M.; Lu, Z.Y.; Lu, A. Dependence of flame-retardant properties on density of expandable graphite filled rigid polyurethane foam. J. Appl. Polym. Sci. 2007, 104, 3347–3355. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Singha, N.K.; Manjunath, B.S.; Naik, Y.P. A facile approach to incorporate silver nanoparticles into dextran-based hydrogels for antibacterial and catalytical application. J. Macromol. Sci. A. 2009, 46, 704–712. [Google Scholar] [CrossRef]
- Mulligan, J.C.; Colvin, D.P.; Bryant, Y.G. Microencapsulated phase-change material suspensions for heat transfer in spacecraft thermal systems. J. Spacecr. Rockets. 1996, 33, 278–284. [Google Scholar]
- Sarier, N.; Onder, E. Thermal characteristics of polyurethane foams incorporated with phase change materials. Thermochim. Acta. 2007, 454, 90–98. [Google Scholar] [CrossRef]
- You, M.; Zhang, X.X.; Li, W.; Wang, X.C. Effects of MicroPCMs on the fabrication of MicroPCMs/polyurethane composite foams. Thermochim. Acta. 2008, 472, 20–24. [Google Scholar] [CrossRef]
- Pasupathy, A.; Athanasius, L.; Velraj, R.; Seeniraj, R.V. Experimental investigation and numerical simulation analysis on the thermal performance of a building roof incorporating phase change material (PCM) for thermal management. Appl. Therm. Eng. 2008, 28, 556–565. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Z.H.; Zeng, X.R. Preparation characterization and thermal properties of microPCMs containing n-dodecanol by using different types of styrene-maleic anhydride as emulsifier. Colloid Polym. Sci. 2009, 287, 549–560. [Google Scholar] [CrossRef]
- Su, J.F.; Wang, L.X.; Ren, L. Synthesis of polyurethane microPCMs containing n-octadecane by interfacial polycondensation: Influence of styrene-maleic anhydride as a surfactant. Colloid Surface A. 2007, 299, 268–275. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, M.N.; Zhang, Y.C.; Yang, W.M.; Jiao, Z.W.; Yang, L.P. EG/TPU Composites with enhanced flame retardancy and mechanical properties prepared by microlayer coextrusion technology. RSC Adv. 2019, 9, 23944–23956. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Gui, Z.; Yuan, B.H.; Hu, Y.; Zheng, Y.Y.J. Flammability of polystyrene/aluminim phosphinate composites containing modified ammonium polyphosphate. Therm. Anal. Calorim. 2018, 131, 1067–1077. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, P.; Song, L.X.; Li, J.P.; Ji, B.Q.; Li, J.J.; Chen, L. Polyethylene glycol supported by phosphorylated polyvinyl alcohol/graphene aerogel as a high thermal stability phase change material. Compos. Part B Eng. 2019, 179, 107545. [Google Scholar] [CrossRef]
- Gao, M.; Li, J.F.; Zhou, X. A flame retardant rigid polyurethane foam system including functionalized graphene oxide. Polym. Compos. 2019, 40, 1274–1282. [Google Scholar] [CrossRef]
- Elbasuney, S. Surface engineering of layered double hydroxide (LDH) nanoparticles for polymer flame retardancy. Powder Technol. 2015, 277, 63–73. [Google Scholar] [CrossRef]
- Santiago-Calvo, M.; Tirado-Mediavilla, J.; Ruiz-Herrero, J.L.; Villafane, F.; Rodriguez-Perez, M.A. Long-term thermal conductivity of cyclopentane¿water blown rigid polyurethane foams reinforced with different types of fillers. Polym. Int. 2019, 68, 1826–1835. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, P.; Mou, Y.R.; Kang, M.; Liu, M.; Song, L.X.; Lu, A.; Rong, J.Z. Synthesis of the polyethylene glycol solid-solid phase change materials with a functionalized graphene oxide for thermal energy storage. Polym. Test. 2017, 63, 494–504. [Google Scholar] [CrossRef]
- Yan, Y.J.; Xia, H.; Qiu, Y.P.; Xu, Z.Z.; Ni, Q.Q. Fabrication of gradient vapor grown carbon fiber based polyurethane foam for shape memory driven microwave shielding. RSC Adv. 2019, 9, 9401–9409. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal analysis of polyurethane dispersions based on different polyols. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Tang, Z.; Maroto-Valer, M.M.; Andresen, J.M. Thermal degradation behavior of rigid polyurethane foams prepared with different fire retardant concentrations and blowing agents. Polym. 2002, 43, 6471–6479. [Google Scholar] [CrossRef]
- Duquesne, S.; Le Bras, M.; Bourbigot, S.; Delobel, R.; Vezin, H.; Camino, G.; Eling, B.; Lindsay, C.; Roels, T. Expandable graphite: A fire retardant additive for polyurethane coatings. Fire Mater. 2003, 27, 103–117. [Google Scholar] [CrossRef]
- Duquesne, S.; Delobel, R.; Le Bras, M.; Camino, G. A comparative study of the mechanism of action of ammonium polyphosphate and expandable graphite in polyurethane. Polym. Degrad. Stabil. 2002, 77, 333–344. [Google Scholar] [CrossRef]
- Zheng, Z.H.; Liu, Y.; Zhang, L.; Wang, H.Y. Synergistic effect of expandable graphite and intumescent flame retardants on the flame retardancy and thermal stability of polypropylene. J. Mater. Sci. 2016, 51, 5857–5871. [Google Scholar] [CrossRef]
- Karimpour-Motlagh, N.; Khonakdar, H.A.; Jafari, S.H.; Panahi-Sarmad, M.; Javadi, A.; Shojaei, S.; Goodarzi, V. An experimental and theoretical mechanistic analysis of thermal degradation of polypropylene/polylactic acid/clay nanocomposites. Polym. Adv. Technol. 2019, 30, 2695–2706. [Google Scholar] [CrossRef]

| MDI (g) | YD-4450 (g) | EG (g) | APP (g) | Micro-PCM (g) | Energy Storage Capacity (J/g) | Energy Release Capacity (J/g) | |
|---|---|---|---|---|---|---|---|
| PU1 | 50 | 50 | 0 | 0 | 0 | 0 | 0 |
| PU2 | 50 | 50 | 10 | 0 | 0 | 0 | 0 |
| PU3 | 50 | 50 | 15 | 0 | 0 | 0 | 0 |
| PU4 | 50 | 50 | 8 | 2 | 0 | 0 | 0 |
| PU5 | 50 | 50 | 13 | 2 | 0 | 0 | 0 |
| PU6 | 50 | 50 | 10 | 0 | 10 | 7.73 | 5.87 |
| PU7 | 50 | 50 | 15 | 0 | 10 | 6.58 | 5.55 |
| PU8 | 50 | 50 | 8 | 2 | 10 | 8.21 | 7.12 |
| PU9 | 50 | 50 | 13 | 2 | 10 | 6.66 | 6.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Hu, A. The Influence of Environmentally Friendly Flame Retardants on the Thermal Stability of Phase Change Polyurethane Foams. Materials 2020, 13, 520. https://doi.org/10.3390/ma13030520
Liu D, Hu A. The Influence of Environmentally Friendly Flame Retardants on the Thermal Stability of Phase Change Polyurethane Foams. Materials. 2020; 13(3):520. https://doi.org/10.3390/ma13030520
Chicago/Turabian StyleLiu, Dong, and Anjie Hu. 2020. "The Influence of Environmentally Friendly Flame Retardants on the Thermal Stability of Phase Change Polyurethane Foams" Materials 13, no. 3: 520. https://doi.org/10.3390/ma13030520
APA StyleLiu, D., & Hu, A. (2020). The Influence of Environmentally Friendly Flame Retardants on the Thermal Stability of Phase Change Polyurethane Foams. Materials, 13(3), 520. https://doi.org/10.3390/ma13030520






