Engineering of TeO2-ZnO-BaO-Based Glasses for Mid-Infrared Transmitting Optics
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
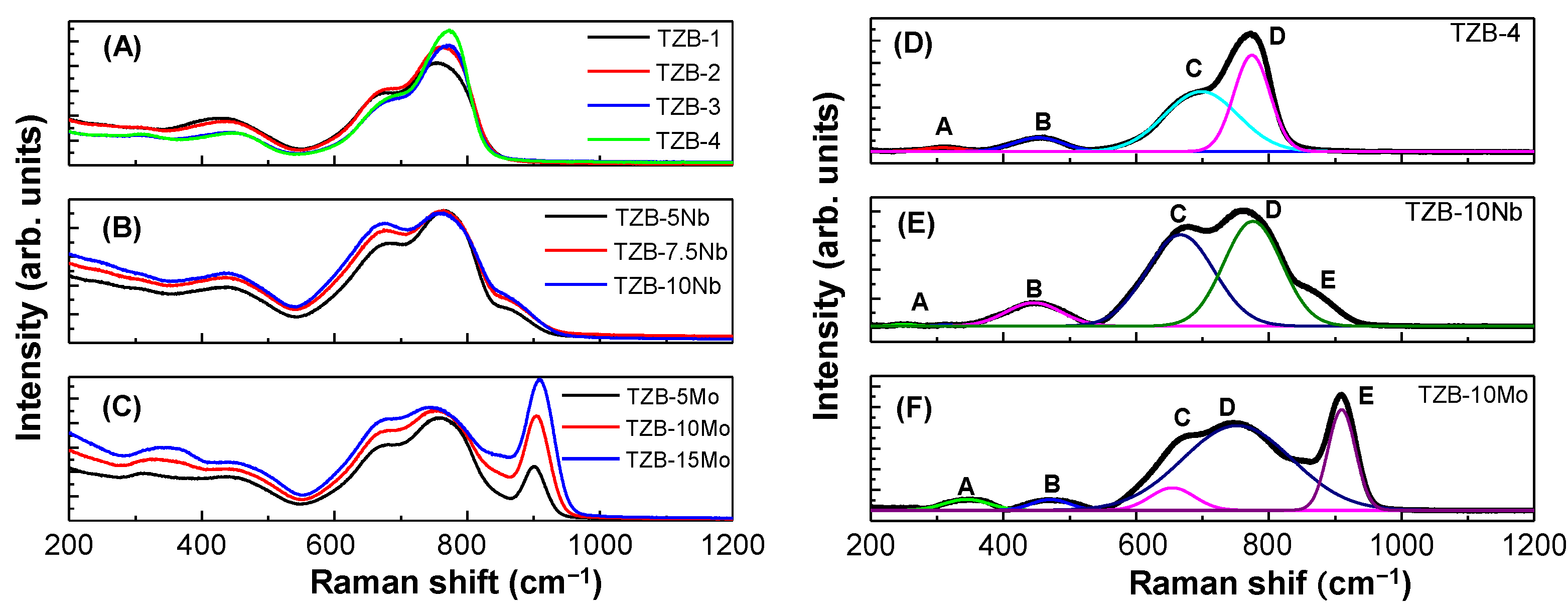
3.1. Structural Analysis—Raman Spectra
3.2. Optical Studies
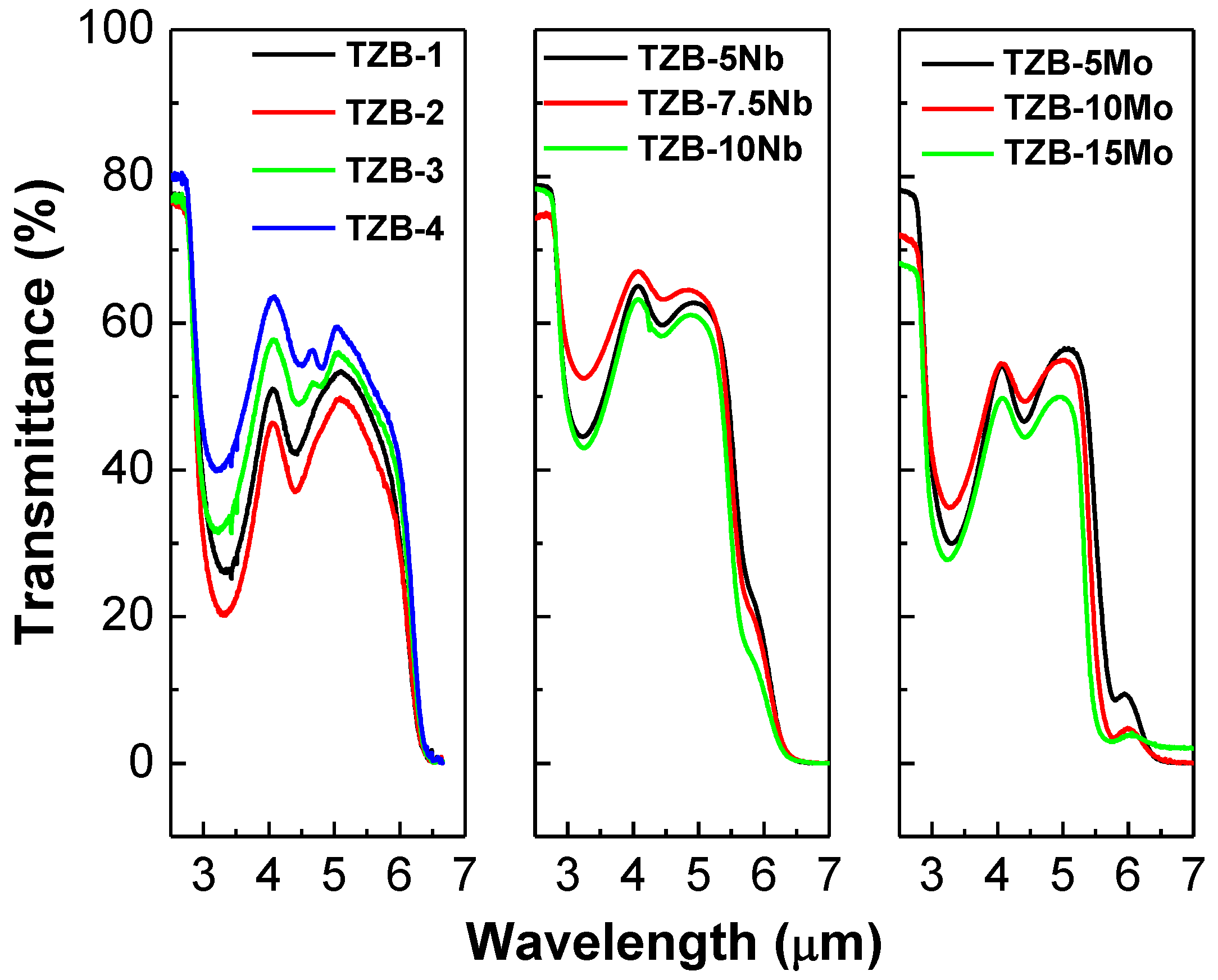
3.2.1. Mid-Infrared Optical Transmittance
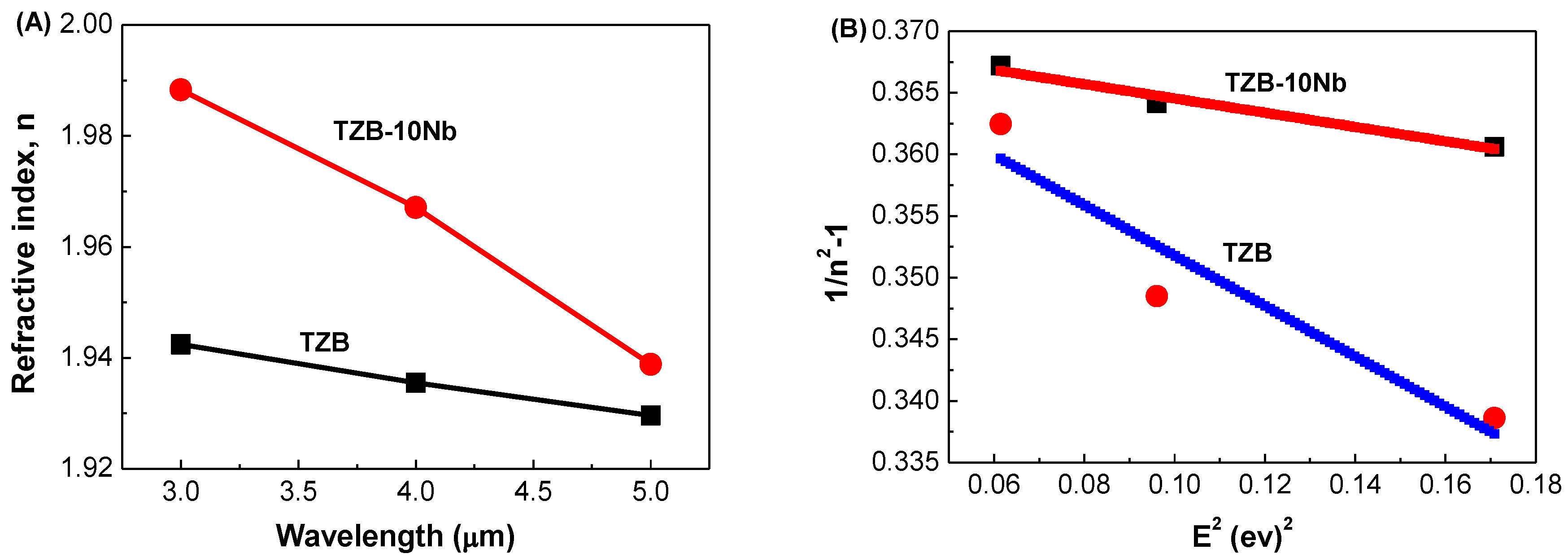
3.2.2. Refractive Index and Optical Dispersion
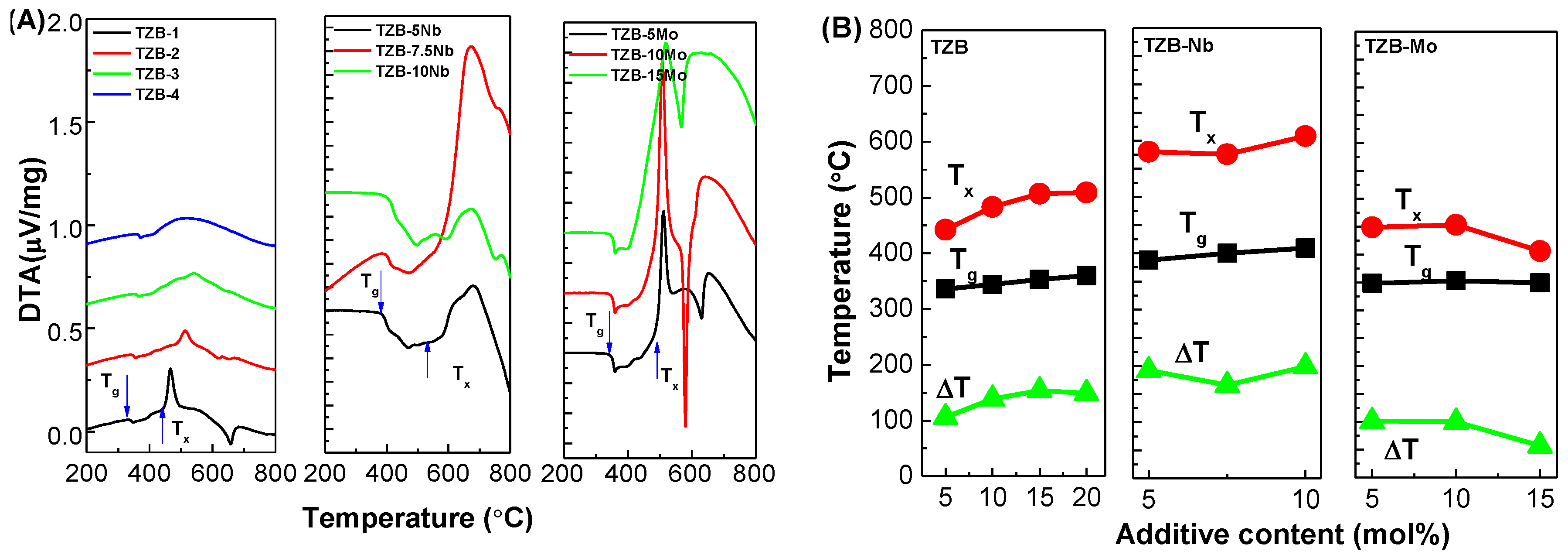
3.3. Thermal Studies
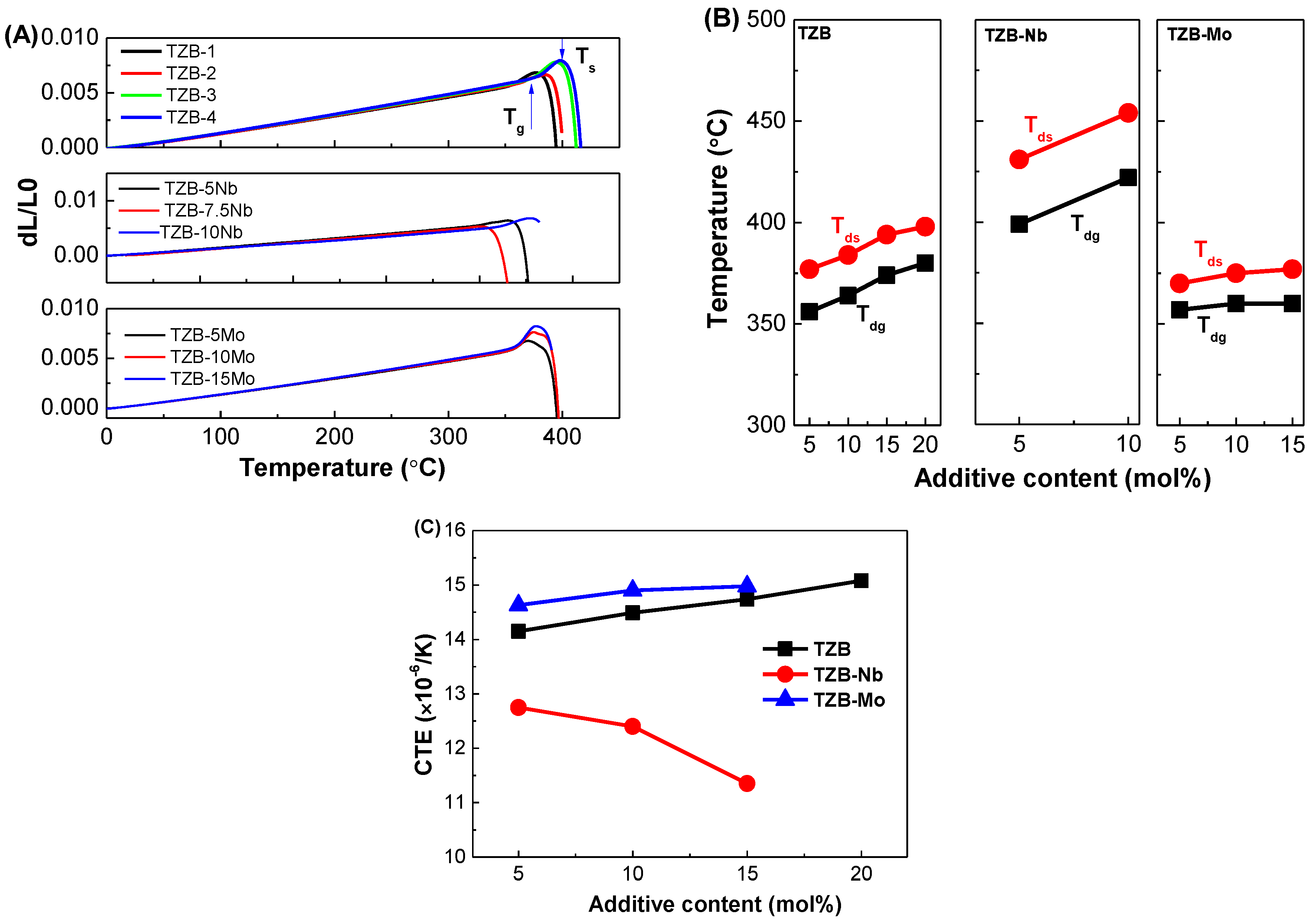
3.4. Thermo-Mechanical Studies
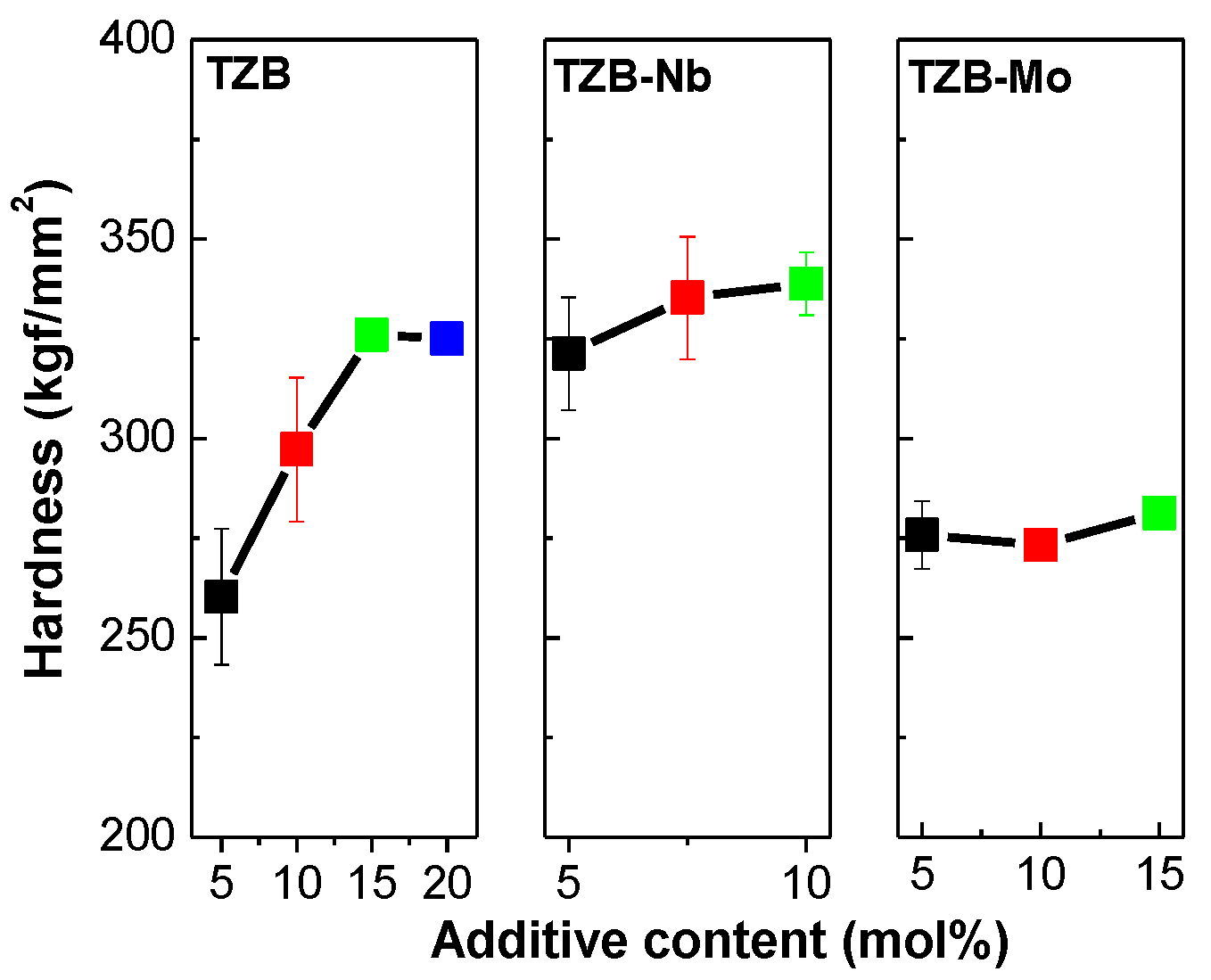
3.5. Mechanical Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bureau, B.; Zhang, X.H.; Smektala, F.; Adam, J.-L.; Troles, J.; Ma, H.; Boussard-Pledel, C.; Lucas, J.; Lucas, P.; Le Coq, D.; et al. Recent advances in chalcogenide glasses. J. Non-Cryst. Solids. 2004, 345, 276–283. [Google Scholar] [CrossRef]
- Zhang, X.H.; Bureau, B.; Lucas, P.; Boussard-Pledel, C.; Lucas, J. Glasses for seeing beyond visible. Chem. Eur. J. 2008, 14, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Bureau, B.; Boussard-Pledel, C.; Lucas, P.; Zhang, X.H.; Lucas, J. Forming glasses from Se and Te. Molecules 2009, 14, 4337–4350. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Chahal, R.; Boussard-Plédel, C.; Nazabal, V.; Doualan, J.-L.; Troles, J.; Lucas, J.; Bureau, B. From selenium-to tellurium-based glass optical fibers for infrared spectroscopies. Molecules 2013, 18, 5373–5388. [Google Scholar] [CrossRef] [PubMed]
- Umicore Germanium Optics Leading the Way in Infrared Optics. Available online: http://pdf.directindustry.com/pdf/umicore-electronic-materials/germanium-infrared-optics-brochure/59063-507001.html (accessed on 31 January 2019).
- Ueno, T.; Hasegawa, M.; Yoshimura, M.; Okada, H.; Nishioka, T.; Teraoka, K.; Fujii, A.; Nakayama, S. Development of ZnS lenses for FIR cameras. SEI Tech. Rev. 2009, 69, 48–53. [Google Scholar]
- Corsetti, J.A.; McCarthy, P.; Moore, D.T. Color correction in the infrared using gradient-index materials. Opt. Eng. 2013, 52, 112109. [Google Scholar] [CrossRef]
- Huddleston, J.; Novak, J.; Moreshead, W.V.; Symmons, A. Investigation of As40Se60 chalcogenide glass in precision glass molding for high-volume thermal imaging lenses. Proc. SPIE 2015, 9451, 94511O. [Google Scholar]
- Zhou, T.; Zhu, Z.; Liu, X.; Liang, Z.; Wang, X. A Review of the Precision Glass Molding of Chalcogenide Glass (ChG) for Infrared Optics. Micromachines 2018, 9, 337. [Google Scholar] [CrossRef]
- Manikandan, N.; Ryasnyanskiy, A.; Toulouse, J. Thermal and optical properties of TeO2–ZnO–BaO glasses. J. Non-Cryst. Solids 2012, 358, 947–951. [Google Scholar] [CrossRef]
- Jose, R.; Suzuki, T.; Ohishi, Y. Thermal and optical properties of TeO2–BaO–SrO–Nb2O5 based glasses: New broadband Raman gain media. J. Non-Cryst. Solids 2006, 352, 5564–5571. [Google Scholar] [CrossRef]
- Manning, S.; Ebendorff-Heidepriem, H.; Monro, T.M. Ternary tellurite glasses for the fabrication of nonlinear optical fibres. Opt. Mater. Express 2012, 2, 140–152. [Google Scholar] [CrossRef]
- Murugan, G.S.; Ohishi, Y. Structural and physical properties of a novel TeO2–BaO–SrO–Ta2O5 glass system for photonic device applications. J. Non-Cryst. Solids 2005, 351, 364–371. [Google Scholar] [CrossRef]
- Jha, A.; Joshi, P.; Shen, S.; Huang, L. Spectroscopic characterization of signal gain and pump ESA in short-lengths of RE-doped tellurite fibers. J. Non-Cryst. Solids 2007, 353, 1407–1413. [Google Scholar] [CrossRef]
- Bell, M.J.V.; Anjos, V.; Moreira, L.M.; Falci, R.F.; Kassab, L.R.P.; da Silva, D.S.; Doualan, J.L.; Camy, P.; Moncorgé, R. Laser emission of a Nd-doped mixed tellurite and zinc oxide glass. J. Opt. Soc. Am. B 2014, 31, 1590–1594. [Google Scholar] [CrossRef]
- Rivera, V.A.G.; Manzani, D. Technological advances in tellurite glasses. In Springer Series in Material Science, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Muravyev, S.V.; Anashkina, E.A.; Andrianov, A.V.; Dorofeev, V.V.; Motorin, S.E.; Koptev, M.Y.; Kim, A.V. Dual-band Tm3+-doped tellurite fiber amplifier and laser at 1.9 μm and 2.3 μm. Sci. Rep. 2018, 8, 16164. [Google Scholar] [CrossRef] [PubMed]
- Narro-García, R.; Chillcce, E.F.; Miranda, A.R.; Giehl, J.M.; Barbosa, L.C.; Rodriguez, E.; Arronte, M. Optical and physical properties of Er3+-Yb3+ co-doped tellurite fibers. Proc. SPIE 2011, 8120, 812005. [Google Scholar]
- Shen, S.; Jha, A.; Liu, X.; Naftaly, M.; Bindra, K.; Bookey, H.J.; Kar, A.K. Tellurite Glasses for Broadband Amplifiers and Integrated Optics. J. Am. Ceram. Soc. 2002, 85, 1391–1395. [Google Scholar] [CrossRef]
- Mori, A.; Ohishi, Y.; Sudo, S. Erbium-doped tellurite glass fibre laser and amplifier. Electron. Lett. 1997, 33, 863–864. [Google Scholar] [CrossRef]
- Dolhen, M.; Tanaka, M.; Couderc, V.; Chenu, S.; Delaizir, G.; Hayakawa, T.; Cornette, J.; Brisset, F.; Colas, M.; Thomas, P.; et al. Nd3+-doped transparent tellurite ceramics bulk lasers. Sci. Rep. 2018, 8, 4640. [Google Scholar] [CrossRef]
- Tang, J.; Sun, M.; Huang, Y.; Gou, J.; Zhang, Y.; Li, G.; Li, Y.; Man, Y.; Yang, J. Study on optical properties and upconversion luminescence of Er3+/Yb3+ co-doped tellurite glass for highly sensitive temperature measuring. Opt. Mater. Express 2017, 7, 3238–3250. [Google Scholar] [CrossRef]
- Jakutis, J.; Gomes, L.; Amancio, C.T.; Kassab, L.R.P.; Martinelli, J.R.; Wetter, N.U. Increased Er3+ upconversion in tellurite fibers and glasses by co-doping with Yb3+. Opt. Mater. 2010, 33, 107–111. [Google Scholar] [CrossRef]
- O’Donnell, M.D.; Richardson, K.; Stolen, R.; Seddon, A.B.; Furniss, D.; Tikhomirov, V.K.; Rivero, C.; Ramme, M.; Stegeman, R.; Stegeman, G.; et al. Tellurite and Fluorotellurite Glasses for Fiberoptic Raman Amplifiers: Glass Characterization, Optical Properties, Raman Gain, Preliminary Fiberization, and Fiber Characterization. J. Am. Ceram. Soc. 2007, 90, 1448–1457. [Google Scholar] [CrossRef]
- De Andrade, G.D.; Rocha, H.D.O.; Segatto, M.E.V.; Pontes, M.J.; Castellani, C.E.S. Study and Optimization of Raman Amplifiers in Tellurite-Based Optical Fibers for Wide-Band Telecommunication Systems. J. Microw. Optoelectron. 2019, 18, 219–226. [Google Scholar] [CrossRef]
- Richards, B.D.O.; Jha, A.; Jose, G.; Teddy-Fernandez, T.; Binks, D.; Tsang, Y. Tellurite glass as a solid-state mid-infrared laser host material. In Proceedings of the Advanced Solid-State Lasers Congress OSA Technical Digest, Paris, France, 27 October–1 November 2013. [Google Scholar]
- Feng, X.; Shi, J.; Segura, M.; White, N.M.; Kannan, P.; Calvez, L.; Zhang, X.; Brilland, L.; Loh, W.H. Towards Water-Free Tellurite Glass Fiber for 2–5 μm Nonlinear Applications. Fibers 2013, 1, 70–81. [Google Scholar] [CrossRef]
- Canioni, L.; Martin, M.-O.; Bousquet, B.; Sarger, L. Precise measurements and analysis of linear and nonlinear optical properties of glass materials near 1.5 μm. Opt. Commun. 1998, 151, 241–246. [Google Scholar] [CrossRef]
- Sekiya, T.; Mochida, N.; Ohtsuka, A. Raman spectra of MO-TeO2 (M = Mg, Sr, Ba and Zn) glasses. J. Non-Cryst. Solids 1994, 168, 106–114. [Google Scholar] [CrossRef]
- Jha, A.; Richards, B.D.O.; Jose, G.; Toney Fernandez, T.; Hill, C.J.; Lousteau, J.; Joshi, P. Review on structural, thermal, optical and spectroscopic properties of tellurium oxide based glasses for fibre optic and waveguide applications. Int. Mater. Rev. 2012, 57, 357–382. [Google Scholar] [CrossRef]
- Jackson, J.; Smith, C.; Massera, J.; Rivero-Baleine, C.; Bungay, C.; Petit, L.; Richardson, K. Estimation of peak Raman gain coefficients for Barium-Bismuth-Tellurite glasses from spontaneous Raman cross-section experiments. Opt. Express 2009, 17, 9071–9079. [Google Scholar] [CrossRef]
- Hill, C.J.; Jha, A. Development of novel ternary tellurite glasses for high temperature fiber optic mid-IR chemical sensing. J. Non-Cryst. Solids 2007, 353, 1372–1376. [Google Scholar] [CrossRef]
- Lin, J.; Huang, W.; Sun, Z.; Ray, C.S.; Day, D.E. Structure and non-linear optical performance of TeO2–Nb2O5–ZnO glasses. J. Non-Cryst. Solids 2004, 336, 189–194. [Google Scholar] [CrossRef]
- Bachvarova-Nedelcheva, A.; Iordanova, R.; Ganev, S.; Dimitriev, Y. Glass formation and structural studies of glasses in the TeO2–ZnO–Bi2O3–Nb2O5 system. J. Non-Cryst. Solids 2019, 503–504, 224–231. [Google Scholar] [CrossRef]
- Kaur, A.; Khanna, A.; Sathe, V.G.; Gonzalez, F.; Ortiz, B. Optical, thermal, and structural properties of Nb2O5–TeO2 and WO3–TeO2 glasses. Phase Transit. 2013, 86, 598–619. [Google Scholar] [CrossRef]
- Moraes, J.C.S.; Nardi, J.A.; Sidel, S.M.; Mantovani, B.G.; Yukimitu, K.; Reynoso, V.C.S.; Malmonge, L.F.; Ghofraniha, N.; Ruocco, G.; Andrade, L.H.C.; et al. Relation among optical, thermal and thermo-optical properties and niobium concentration in tellurite glasses. J. Non-Cryst. Solids 2010, 356, 2146–2150. [Google Scholar] [CrossRef]
- Chen, D.D.; Liu, Y.H.; Zhang, Q.Y.; Deng, Z.D.; Jiang, Z.H. Thermal stability and spectroscopic properties of Er3+-doped niobium tellurite glasses for broadband amplifiers. Mater. Chem. Phys. 2005, 90, 78–82. [Google Scholar] [CrossRef]
- Elkhoshkhany, N.; Abbas, R.; El-Mallawany, R.; Humoud Sharba, K.S.H. Thermal properties of quaternary TeO2–ZnO–Nb2O5–Gd2O3 glasses. Ceram. Int. 2014, 40, 11985–11994. [Google Scholar] [CrossRef]
- Hoppe, U.; Yousef, E.; Russel, C.; Neuefeind, J.; Hannon, A.C. Structure of zinc and niobium tellurite glasses by neutron and X-ray diffraction. J. Phys. Condens. Matter 2004, 16, 1645–1663. [Google Scholar] [CrossRef]
- Sekiya, T.; Mochida, N.; Ogawa, S. Structural study of MoO3-TeO2 glasses. J. Non-Cryst. Solids 1995, 185, 135–144. [Google Scholar] [CrossRef]
- Liu, J.L.; Wang, W.C.; Xiao, Y.B.; Huang, S.J.; Mao, L.Y.; Zhang, Q.Y. Nd3+-doped TeO2–MoO3–ZnO tellurite glass for a diode-pump 1.06μm laser. J. Non-Cryst. Solids 2019, 506, 32–38. [Google Scholar] [CrossRef]
- Kaur, A.; Khanna, A.; González, F.; Pesquera, C.; Chen, B. Structural, optical, dielectric and thermal properties of molybdenum tellurite and borotellurite glasses. J. Non-Cryst. Solids 2016, 444, 1–10. [Google Scholar] [CrossRef]
- Yuan, J.; Yang, Q.; Chen, D.D.; Qian, Q.; Shen, S.X.; Zhang, Q.Y.; Jiang, Z.H. Compositional effect of WO3, MoO3, and P2O5 on Raman spectroscopy of tellurite glass for broadband and high gain Raman amplifier. J. Appl. Phys. 2012, 111, 103511. [Google Scholar] [CrossRef]
- Feng, X.; Tanabe, S.; Hanada, T. Hydroxyl groups in erbium-doped germanotellurite glasses. J. Non-Cryst. Solids 2001, 281, 48–54. [Google Scholar] [CrossRef]
- O’Donnell, M.D.; Miller, C.A.; Furniss, D.; Tikhomirov, V.K.; Seddon, A.B. Fluorotellurite glasses with improved mid-infrared transmission. J. Non-Cryst. Solids 2003, 331, 48–57. [Google Scholar] [CrossRef]
- Chen, F.; Wei, T.; Jing, X.; Tian, Y.; Zhang, J.; Xu, S. Investigation of mid-infrared emission characteristics and energy transfer dynamics in Er3+ doped oxyfluoride tellurite glass. Sci. Rep. 2015, 5, 10676. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.F.; Zhang, W.J.; Yuan, J.; Chen, D.D.; Qian, Q.; Zhang, Q.Y. Enhanced 2.7 μm emission from Er3+ doped oxyfluoride tellurite glasses for a diode-pump mid-infrared laser. AIP Adv. 2014, 4, 047101. [Google Scholar] [CrossRef]
- Chen, F.; Xu, S.; Wei, T.; Wang, F.; Cai, M.; Tian, Y.; Xu, S. Mid-infrared emission and Raman spectra analysis of Er3+-doped oxyfluorotellurite glasses. Appl. Opt. 2015, 54, 3345–3352. [Google Scholar] [CrossRef]
- Gomes, L.; Oermann, M.; Ebendorff-Heidepriem, H.; Ottaway, D.; Monro, T.; Librantz, A.F.H.; Jackson, S.D. Energy level decay and excited state absorption processes in erbium-doped tellurite glass. J. Appl. Phys. 2011, 110, 083111. [Google Scholar] [CrossRef]
- Dimitrov, V.; Komatsu, T. An interpretation of optical properties of oxides and oxide glasses in terms of the electronic ion polarizability and average single bond strengths. J. Univ. Chem. Technol. Metall. 2010, 45, 219–250. [Google Scholar]
- Ghosh, G. Sellmeier Coefficients and Chromatic Dispersions for Some Tellurite Glasses. J. Am. Ceram. Soc. 1995, 78, 2828–2830. [Google Scholar] [CrossRef]
- Wemple, S.H.; DiDomenico, M., Jr. Behavior of the Electronic Dielectric Constant in Covalent and Ionic Materials. Phys. Rev. B 1971, 3, 1338–1351. [Google Scholar] [CrossRef]
- Vijaya Prakash, G.; Narayana Rao, D.; Bhatnagar, A.K. Linear optical properties of niobium-based tellurite glasses. Solid State Commun. 2001, 119, 39–44. [Google Scholar] [CrossRef]
- Terashima, K.; Kim, S.-H.; Yoko, T. Nonlinear Optical Properties of B2O3-Based Glasses: M2O-B2O3(M = Li, Na, K, Rb, Cs, and Ag) Binary Borate Glasses. J. Am. Ceram. Soc. 1995, 78, 1601–1605. [Google Scholar] [CrossRef]
- Noda, J.; Okamoto, K.; Sasaki, Y. Polarization-maintaining fibers and their applications. J. Lightwave Technol. 1986, 4, 1071–1089. [Google Scholar] [CrossRef]
- Dragic, P.; Cavillon, M.; Ballato, J. On the thermo-optic coefficient of P2O5 in SiO2. Opt. Mater. Express 2017, 7, 3654–3661. [Google Scholar] [CrossRef]
- Komatsu, T.; Ito, N.; Honma, T.; Dimitrov, V. Temperature dependence of refractive index and electronic polarizability of RO–TeO2 glasses (R=Mg, Ba, Zn). Solid State Sci. 2012, 14, 1419–1425. [Google Scholar] [CrossRef]
- Silva, K.C.; Sakai, O.A.; Steimacher, A.; Pedrochi, F.; Baesso, M.L.; Bento, A.C.; Medina, A.N.; Lima, S.M.; Oliveira, R.C.; Moraes, J.C.S.; et al. Temperature and wavelength dependence of the thermo-optical properties of tellurite and chalcogenide glasses. J. Appl. Phys. 2007, 102, 073507. [Google Scholar] [CrossRef]
- Yoshida, S.; Matsuoka, J.; Soga, N. Crack growth behavior of zinc tellurite glass with or without sodium oxide. J. Non-Cryst. Solids 2001, 279, 44–50. [Google Scholar] [CrossRef]
- Watanabe, T.; Benino, Y.; Ishizaki, K.; Komatsu, T. Temperature dependence of Vickers hardness for TeO2-based and soda-lime silicate glasses. J. Ceram. Soc. Jpn. 1999, 107, 1140–1145. [Google Scholar] [CrossRef]
- Knoop, F.; Peters, C.G.; Emerson, W.B. A sensitive pyramidal-diamond tool for indentation measurements. J. Res. NBS 1939, 23, 39–61. [Google Scholar] [CrossRef]
| S. No. | Glass Label | Molar Composition (mol%) |
|---|---|---|
| 1 | TZB1 | 65TeO2–30ZnO–5BaO |
| 2 | TZB2 | 60TeO2–30ZnO–10BaO |
| 3 | TZB3 | 55TeO2–30ZnO–15BaO |
| 4 | TZB4 | 50TeO230ZnO–20BaO |
| 5 | TZB–5Nb | 60TeO2–25ZnO–10BaO–5Nb2O5 |
| 6 | TZB–7.5Nb | 60TeO2–22.5ZnO–10BaO–7.5Nb2O5 |
| 7 | TZB–10Nb | 60TeO2–20ZnO–10BaO–10Nb2O5 |
| 8 | TZB–5Mo | 60TeO2–25ZnO–10BaO–5MoO3 |
| 9 | TZB–10Mo | 60TeO2–20ZnO–10BaO–10MoO3 |
| 10 | TZB–15Mo | 60TeO2–15ZnO–10BaO–15MoO3 |
| Optical Phonon Frequency, ν (cm−1) | Assigned Vibrational Modes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TZB | TZB–Nb | TZB–Mo | ||||||||
| 1 | 2 | 3 | 4 | 5 Nb | 7.5 Nb | 10 Nb | 5 Mo | 10 Mo | 15 Mo | |
| 309 | 300 | 301 | 304 | 308 | 310 | 310 | 304 | 326 | 338 | ν(Te–O) stretching and bending vibrations in TeO3+δ and TeO3 networks |
| 428 | 438 | 437 | 437 | 439 | 440 | 440 | 433 | 436 | 436 | νs(Te–O–Te) stretching and bending vibrations |
| 671 | 675 | 683 | 683 | 681 | 674 | 676 | 678 | 678 | 679 | νas(Te–O) stretching vibrations in TeO4 networks |
| 754 | 759 | 769 | 769 | 771 | 762 | 758 | 754 | 750 | 745 | ν(Te–O) stretching vibrations in TeO3 and TeO3+δ networks |
| - | - | - | - | 865 | 860 | 857 | 900 | 905 | 909 | νs(Nb–O) stretching vibrations of bonds in NbO6 octahedra |
| Glass Sample | Tg ±2 (°C) | Tx ±2 (°C) | ∆T (°C) | Tdg ±2 (°C) | Tds ±2 (°C) | α ±0.1 (×10−6/K) | HK (kgf/mm2) | Reference |
|---|---|---|---|---|---|---|---|---|
| TZB1 | 336 | 442 | 106 | 356 | 377 | 14.15 | 260 | This work |
| TZB2 | 344 | 483 | 139 | 364 | 384 | 14.49 | 297 | This work |
| TZB3 | 353 | 507 | 154 | 374 | 394 | 14.74 | 326 | This work |
| TZB4 | 360 | 509 | 149 | 380 | 398 | 15.08 | 325 | This work |
| TZB–5Nb | 388 | 580 | 192 | 399 | 431 | 12.75 | 321 | This work |
| TZB–7.5Nb | 400 | 576 | 176 | 384 | 399 | 12.40 | 335 | This work |
| TZB–10Nb | 409 | 598 | 189 | 422 | 454 | 11.35 | 339 | This work |
| TZB–5Mo | 350 | 487 | 137 | 357 | 370 | 14.63 | 276 | This work |
| TZB–10Mo | 351 | 471 | 120 | 360 | 375 | 14.90 | 273 | This work |
| TZB–15Mo | 352 | 408 | 56 | 360 | 377 | 14.98 | 281 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linganna, K.; In, J.-H.; Kim, S.H.; Han, K.; Choi, J.H. Engineering of TeO2-ZnO-BaO-Based Glasses for Mid-Infrared Transmitting Optics. Materials 2020, 13, 5829. https://doi.org/10.3390/ma13245829
Linganna K, In J-H, Kim SH, Han K, Choi JH. Engineering of TeO2-ZnO-BaO-Based Glasses for Mid-Infrared Transmitting Optics. Materials. 2020; 13(24):5829. https://doi.org/10.3390/ma13245829
Chicago/Turabian StyleLinganna, Kadathala, Jung-Hwan In, Seon Hoon Kim, Karam Han, and Ju Hyeon Choi. 2020. "Engineering of TeO2-ZnO-BaO-Based Glasses for Mid-Infrared Transmitting Optics" Materials 13, no. 24: 5829. https://doi.org/10.3390/ma13245829
APA StyleLinganna, K., In, J.-H., Kim, S. H., Han, K., & Choi, J. H. (2020). Engineering of TeO2-ZnO-BaO-Based Glasses for Mid-Infrared Transmitting Optics. Materials, 13(24), 5829. https://doi.org/10.3390/ma13245829





