Identification of Phenolic Compounds and Determination of Antioxidant Activity in Extracts and Infusions of Salvia Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Plant Material and Growth Conditions
2.3. Extracts and Infusions Preparation
2.4. Essential Oil (Hydrodestilation)
2.5. Ferric Reducing Antioxidant Power (FRAP) Determination
2.6. Measurement of DPPH Radical-Scavenging Activity
2.7. Total Polyphenols Content (TPC)
2.8. Statistical Analysis
- The levels of the factors do not influence differentially the values of the dependent variables (experiment results),
- There are no interactions between the factors (the response of the studied dependent variable to one factor is the same at all levels of the other factor).
- -
- normal distribution of the dependent variable within groups—the Shapiro-Wilk test was used
- -
- homogeneity (homogeneity) of variance—Levene’s test was used
2.9. LC-MS/MS Conditions
3. Results
3.1. Results for Essential Oils
3.2. Antioxidant Parameter Results (FRAP, DPPH, TPC)
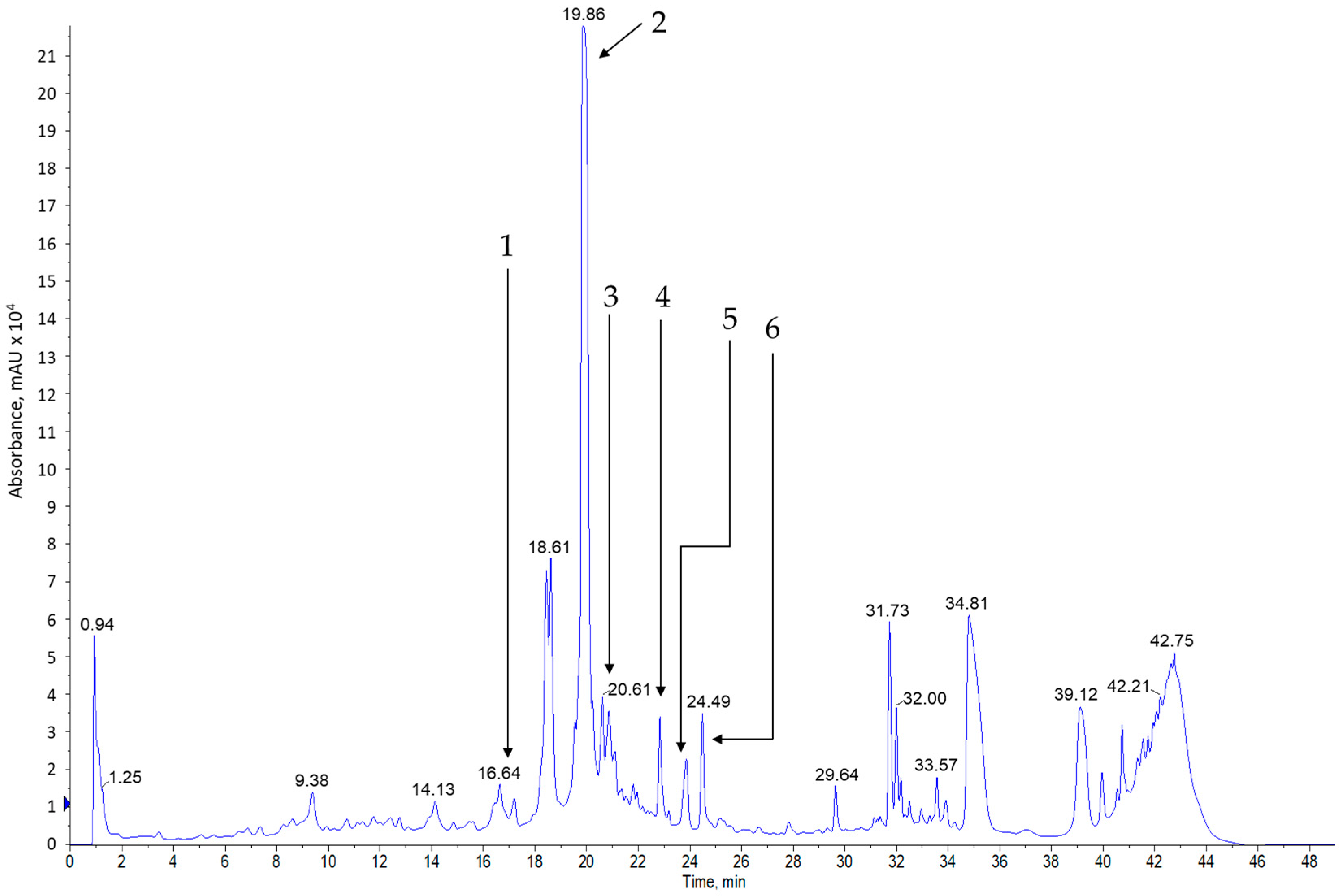
3.3. The Results of the Chromatographic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boutebouhart, H.; Didaoui, L.; Tata, S.; Sabaou, N. Effect of Extraction and Drying Method on Chemical Composition, and Evaluation of Antioxidant and Antimicrobial Activities of Essential Oils from Salvia officinalis L. J. Essent. Oil-Bear. Plants 2019, 22, 717–727. [Google Scholar] [CrossRef]
- Kandil, M.A.M.; Sabry, R.M.; Ahmed, S.S. Influence of drying methods on the quality of sage (Salvia officinalis), parsley (Petroselinum crispum) and nasturtium (Tropaeolum majus). Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1112–1123. [Google Scholar]
- Zawiślak, G.; Dyduch, J. The analysis of the content and chemical composition of essential oil in the leaves of sage (Salvia officinalis L.) cv. ‘bona’ in the second year of cultivation. J. Essent. Oil Res. 2006, 18, 402–404. [Google Scholar] [CrossRef]
- Perry, E.K.; Pickering, A.T.; Wang, W.W.; Houghton, P.J.; Perry, N.S.L. Medicinal plants and Alzheimer’s disease: From ethnobotany to phytotherapy. J. Pharm. Pharmacol. 1999, 51, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Kikuzaki, H.; Zhu, N.; Sang, S.; Nakatani, N.; Ho, C.-T. Isolation and structural elucidation of two new glycosides from sage (Salvia officinalis L.). J. Agric. Food Chem. 2000, 48, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.S.C.; Menti, C.; Lambert, A.P.F.; Barcellos, T.; Moura, S.; Calloni, C.; Branco, C.S.; Salvador, M.; Roesch-Ely, M.; Henriques, J.A.P. Pharmacological perspectives from brazilian Salvia officinalis (Lamiaceae): Antioxidant, and antitumor in mammalian cells. Ann. Acad. Bras. Cienc. 2016, 88, 281–292. [Google Scholar] [CrossRef]
- Adams, M.; Gmünder, F.; Hamburger, M. Plants traditionally used in age related brain disorders-A survey of ethnobotanical literature. J. Ethnopharmacol. 2007, 113, 363–381. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-G.; Shibamoto, T. Determination of antioxidant potential of volatile extracts isolated from various herbs and spices. J. Agric. Food Chem. 2002, 50, 4947–4952. [Google Scholar] [CrossRef]
- Horváthová, E.; Srančíková, A.; Regendová-Sedláčková, E.; Melušová, M.; Meluš, V.; Netriová, J.; Krajčovičová, Z.; Slameňová, D.; Pastorek, M.; Kozics, K. Enriching the drinking water of rats with extracts of Salvia officinalis and Thymus vulgaris increases their resistance to oxidative stress. Mutagenesis 2016, 31, 51–59. [Google Scholar] [CrossRef]
- European Pharmacopoeia. Determination of essential oils in herbal drugs. In European Pharmacopoeia 6. 2.8.12; Council of Europe: Strasbourg Cedex, France, 2008; pp. 251–256. [Google Scholar]
- Hamrouni-Sellami, I.; Rebey, I.B.; Sriti, J.; Rahali, F.Z.; Limam, F.; Marzouk, B. Drying Sage (Salvia officinalis L.) Plants and Its Effects on Content, Chemical Composition, and Radical Scavenging Activity of the Essential Oil. Food Bioprocess Technol. 2012, 5, 2978–2989. [Google Scholar] [CrossRef]
- Angelova, V.R.; Ivanova, R.V.; Todorov, G.M.; Ivanov, K.I. Potential of Salvia sclarea L. for phytoremediation of soils contaminated with heavy metals. Int. J. Agric. Biosyst. Eng. 2016, 10, 780–790. [Google Scholar]
- Zheljazkov, V.; Nielsen, N. Growing clary sage (Salvia Sclarea L.) In heavy metal-polluted areas. Acta Hortic. 1996, 426, 309–328. [Google Scholar] [CrossRef]
- Sung, Y.-H.; Honermeier, B. Effect of cultivation method and harvest time on root yield and content of tanshinones of Salvia miltiorrhiza Bunge [Einfluss von Anbaumethode und Erntetermin auf den Wurzelertrag und den Gehalt an Tanshinonen von Chinesischem Salbei (Salvia miltiorrhiza Bunge)]. Z. Arznei Gewurzpflanzen 2013, 18, 34–41. [Google Scholar]
- Mameli, M.G.; Zucca, L.; Maxia, M.; Manca, G.; Satta, M. Effects of different irrigation management on biomass and essential oil production of Thymus vulgaris L., Salvia officinalis L. and Rosmarinus officinalis L., cultivated in the Southern Sardinian climate (Italy). Acta Hortic. 2011, 889, 469–474. [Google Scholar] [CrossRef]
- Moustakas, M.; Hanć, A.; Dobrikova, A.; Sperdouli, I.; Adamakis, I.-D.S.; Apostolova, E. Spatial Heterogeneity of Cadmium Effects on Salvia sclarea Leaves Revealed by Chlorophyll Fluorescence Imaging Analysis and Laser Ablation Inductively Coupled Plasma Mass Spectrometry. Materials 2019, 12, 2935. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1998, 299, 15–27. [Google Scholar] [CrossRef]
- Akter, M.S.; Ahmed, M.; Eun, J.-B. Solvent effects on antioxidant properties of persimmon (Diospyros kaki L. cv. Daebong) seeds. Int. J. Food Sci. Technol. 2010, 45, 2258–2264. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Brown, A.M. A new software for carrying out one-way ANOVA post hoc tests. Comput. Methods Progr. Biomed. 2005, 79, 89–95. [Google Scholar] [CrossRef]
- Francik, R.; Kryczyk-Kozioł, J.; Francik, S.; Gryboś, R.; Krośniak, M. Bis(4,4′-dimethyl-2,2′-bipyridine)oxidovanadium(IV) Sulfate Dehydrate: Potential Candidate for Controlling Lipid Metabolism? BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef]
- Hwang, S.; Chung, S.H.; Lee, J.T.; Kim, Y.T.; Kim, Y.J.; Oh, S.; Yeo, I.S.L. Influence of acid, ethanol, and anthocyanin pigment on the optical and mechanical properties of a nanohybrid dental composite resin. Materials 2018, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, R.A.; Ferreira, G.; Delfino, M.M.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M.; Sasso-Cerri, E.; Filho, I.B.; Cerri, P.S. Physical Properties, Antimicrobial Activity and In Vivo Tissue Response to Apexit Plus. Materials 2020, 13, 1171. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.M.; Linhares, T.S.; Lima, S.N.L.; Carvalho, E.M.; Loguercio, A.D.; Bauer, J.; Carvalho, C.N. Effect of sonic application of self-adhesive resin cements on push-out bond strength of glass fiber posts to root dentin. Materials 2019, 12, 1930. [Google Scholar] [CrossRef] [PubMed]
- Dvořáková, M.; Guido, L.F.; Dostálek, P.; Skulilová, Z.; Moreira, M.M.; Barros, A.A. Antioxidant properties of free, soluble ester and insoluble-bound phenolic compounds in different barley varieties and corresponding malts. J. Inst. Brew. 2008, 114, 27–33. [Google Scholar] [CrossRef]
- Lu, J.; Zhao, H.; Chen, J.; Fan, W.; Dong, J.; Kong, W.; Sun, J.; Cao, Y.; Cai, G. Evolution of phenolic compounds and antioxidant activity during malting. J. Agric. Food Chem. 2007, 55, 10994–11001. [Google Scholar] [CrossRef]
- Kopeć, A.; Skoczylas, J.; Jędrszczyk, E.; Francik, R.; Bystrowska, B.; Zawistowski, J. Chemical composition and concentration of bioactive compounds in garlic cultivated from air bulbils. Agriculture 2020, 10, 40. [Google Scholar] [CrossRef]
- Verardo, V.; Arráez-Román, D.; Segura-Carretero, A.; Marconi, E.; Fernández-Gutiérrez, A.; Caboni, M.F. Identification of buckwheat phenolic compounds by reverse phase high performance liquid chromatography-electrospray ionization-time of flight-mass spectrometry (RP-HPLC-ESI-TOF-MS). J. Cereal Sci. 2010, 52, 170–176. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L. Polyphenolic in Salvia. Phytochemistry 2002, 59, 117–140. [Google Scholar]
- Chen, C.Y.; Li, H.; Yuan, Y.N.; Dai, H.Q.; Yang, B. Antioxidant activity and components of a traditional chinese medicine formula consisting of Crataegus pinnatifida and Salvia miltiorrhiza. BMC Complement. Altern. Med. 2013, 13, 99. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Miura, K.; Kikuzaki, H.; Nakatani, N. Antioxidant activity of chemical components from sage (Salvia officinalis L.) and thyme (Thymus vulgaris L.) measured by the oil stability index method. J. Agric. Food Chem. 2002, 50, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Luximon-Ramma, A.; Bahorun, T.; Soobrattee, M.A.; Aruoma, O.I. Antioxidant activities of phenolic, proanthocyanidin, and flavonoid components in extracts of Cassia fistula. J. Agric. Food Chem. 2002, 50, 5042–5047. [Google Scholar] [CrossRef] [PubMed]
- Santos-Gomes, P.C.; Fernandes-Ferreira, M. Essential oils produced by in vitro shoots of sage (Salvia officinalis L.). J. Agric. Food Chem. 2003, 51, 2260–2266. [Google Scholar] [CrossRef] [PubMed]
- Nurmi, A.; Nurmi, T.; Mursu, J.; Hiltunen, R.; Voutilainen, S. Ingestion of oregano extract increases excretion of urinary phenolic metabolites in humans. J. Agric. Food Chem. 2006, 54, 6916–6923. [Google Scholar] [CrossRef] [PubMed]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Jovin, E. Antimicrobial and antioxidant properties of Rosemary and Sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) essential oils. J. Agric. Food Chem. 2007, 55, 7879–7885. [Google Scholar] [CrossRef]
- Koşar, M.; Göger, F.; Başer, K.H.C. In vitro antioxidant properties and phenolic composition of Salvia virgata Jacq. from Turkey. J. Agric. Food Chem. 2008, 56, 2369–2374. [Google Scholar] [CrossRef]
- Moon, J.-K.; Shibamoto, T. Antioxidant assays for plant and food components. J. Agric. Food Chem. 2009, 57, 1655–1666. [Google Scholar] [CrossRef]
- Miroddi, M.; Navarra, M.; Quattropani, M.C.; Calapai, F.; Gangemi, S.; Calapai, G. Systematic review of clinical trials assessing pharmacological properties of Salvia species on memory, cognitive impairment and Alzheimer’s disease. CNS Neurosci. Ther. 2014, 20, 485–495. [Google Scholar] [CrossRef]
- Moss, M.; Rouse, M.; Moss, L. Aromas of Salvia species enhance everyday prospective memory performance in healthy young adults. Adv. Chem. Eng. Sci. 2014, 4, 339–346. [Google Scholar] [CrossRef]
- Moss, L.; Rouse, M.; Wesnes, K.A.; Moss, M. Differential effects of the aromas of Salvia species on memory and mood. Hum. Psychopharmacol. 2010, 25, 388–396. [Google Scholar] [CrossRef]
- Scholey, A.B.; Tildesley, N.T.J.; Ballard, C.G.; Wesnes, K.A.; Tasker, A.; Perry, E.K.; Kennedy, D.O. An extract of Salvia (sage) with anticholinesterase properties improves memory and attention in healthy older volunteers. Psychopharmacology 2008, 198, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Cuvelier, M.-E.; Richard, H.; Berset, C. Antioxidative activity and phenolic composition of pilot-plant and commercial extracts of sage and rosemary. JAOCS J. Am. Oil Chem. Soc. 1996, 73, 645–652. [Google Scholar] [CrossRef]
- Jiang, R.-W.; Lau, K.-M.; Hon, P.-M.; Mak, T.; Woo, K.; Fung, K.-P. Chemistry and Biological Activities of Caffeic Acid Derivatives from Salvia miltiorrhiza. Curr. Med. Chem. 2005, 12, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.P.; Kang, M.Y.; Nam, S.H.; Friedman, M. Dietary rice bran component γ-oryzanol inhibits tumor growth in tumor-bearing mice. Mol. Nutr. Food Res. 2012, 56, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Wang, O.; Liu, J.; Cheng, Q.; Guo, X.; Wang, Y.; Zhao, L.; Zhou, F.; Ji, B. Effects of ferulic acid and γ-Oryzanol on high-fat and high-fructose diet-induced metabolic syndrome in rats. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Engelsma, G. Effect of Daylength on Phenol Metabolism in the Leaves of Salvia occidentalis. Plant Physiol. 1979, 63, 765–768. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Katan, M.B. Absorption, metabolism and health effects of dietary flavonoids in man. Biomed. Pharmacother. 1997, 51, 305–310. [Google Scholar] [CrossRef]
- Hanganu, D.; Olah, N.K.; Pop, C.E.; Vlase, L.; Oniga, I.; Ciocarlan, N.; Matei, A.; Pușcaș, C.; Silaghi-Dumitrescu, R.; Benedec, D. Evaluation of polyphenolic profile and antioxidant activity for some Salvia species. Farmacia 2019, 67, 801–805. [Google Scholar] [CrossRef]
- Santos-Gomes, P.C.; Fernandes-Ferreira, M. Organ- and season-dependent variation in the essential oil composition of Salvia officinalis L. cultivated at two different sites. J. Agric. Food Chem. 2001, 49, 2908–2916. [Google Scholar] [CrossRef]
- Baydar, H.; Özkan, G.; Erbaş, S.; Altindal, D. Yield, chemical composition and antioxidant properties of extracts and essential oils of sage and rosemary depending on seasonal variations. Acta Hortic. 2009, 826, 383–390. [Google Scholar] [CrossRef]
- Bettaieb, I.; Zakhama, N.; Wannes, W.A.; Kchouk, M.E.; Marzouk, B. Water deficit effects on Salvia officinalis fatty acids and essential oils composition. Sci. Hortic. 2009, 120, 271–275. [Google Scholar] [CrossRef]
- Farhat, M.B.; Jordán, M.J.; Chaouech-Hamada, R.; Landoulsi, A.; Sotomayor, J.A. Variations in essential oil, phenolic compounds, and antioxidant activity of tunisian cultivated Salvia officinalis L. J. Agric. Food Chem. 2009, 57, 10349–10356. [Google Scholar] [CrossRef] [PubMed]
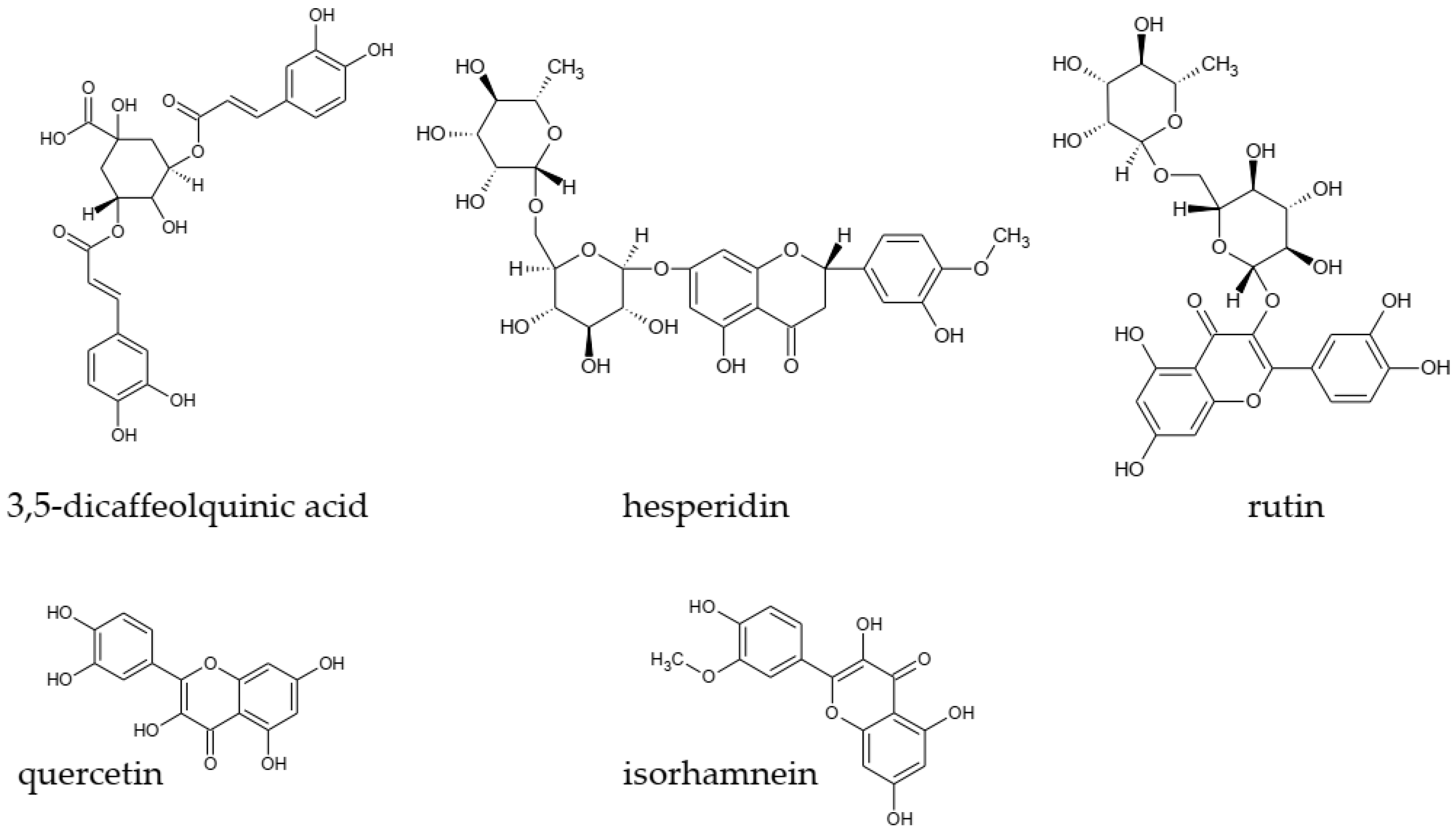


| Compound | M * [g/mol] | m/z [M-H+]− | m/z MRM * | RT [min] | DP ** | FP *** | CE # | CXP ## |
|---|---|---|---|---|---|---|---|---|
| 3,5-dCQA 1 | 516.45 | 515.40 | 352.80 | 22.36 | −70 | −100 | −25 | −10 |
| sinapinic acid | 223.21 | 222.10 | 207.80 | 20.88 | −31 | −160 | −12 | −15 |
| p-coumaric acid | 163.16 | 162.80 | 119.10 | 19.81 | −31 | −320 | −9 | −22 |
| ferulic acid | 193.18 | 192.80 | 134.00 | 20.70 | −36 | −250 | −10 | −8 |
| hesperidin | 609.56 | 608.14 | 163.80 | 24.36 | −86 | −50 | −15 | −6 |
| isorhamnetin | 315.26 | 314.70 | 300.00 | 24.80 | −91 | −340 | −16 | −10 |
| catechin | 290.27 | 289.27 | a 2 | 15.15 | −20 | −200 | −15 | −15 |
| rutin | 610.52 | 609.14 | 301.00 | 20.64 | −20 | −200 | −29 | −15 |
| quercetin | 302.24 | 301.03 | 178.90 | 24.30 | −20 | −200 | −15 | −15 |
| Treatment | Essential Oils [mL/100 g] |
|---|---|
| Harvest June, dried naturally | 1.68 ± 0.06 BC |
| Harvest June dried at 35 °C | 1.58 ± 0.06 C |
| Harvest July, dried naturally | 1.95 ± 0.01 A |
| Harvest July, dried at 35 °C | 1.78 ± 0.05 B |
| Solution | p FRAP [mM Fe2+/L] | p DPPH [%] | p TPC [mg GAE/g DW] | |||
|---|---|---|---|---|---|---|
| Solution | 0.00000 | * | 0.00001 | * | 0.00000 | * |
| Harvest Term | 0.00015 | * | 0.06366 | 0.00002 | * | |
| Drying | 0.00118 | * | 0.08957 | 0.00516 | * | |
| Solution * Harvest Term | 0.00106 | * | 0.11727 | 0.01057 | * | |
| Solution * Drying | 0.30904 | 0.70735 | 0.88394 | |||
| Harvest Term * Drying | 0.22673 | 0.11428 | 0.94231 | |||
| Solution * Harvest Term * Drying | 0.62503 | 0.96881 | 0.22557 |
| Solution | Harvest Term | Drying Method | FRAP [mM Fe2+/L] | DPPH [%] | TPC [mg GAE/g DW] |
|---|---|---|---|---|---|
| extract | June | natural | 381 ± 64 B,C | 57.5 ± 4.4 B | 11.6 ± 5.6 A,B |
| extract | June | 35 °C | 300 ± 26 A,B | 50.9 ± 9.4 A,B | 12.4 ± 6.1 B,C |
| extract | July | natural | 496 ± 21 D | 60.5 ± 8.8 A,B | 14.9 ± 4.1 C,D |
| extract | July | 35 °C | 436 ± 10 C,D | 56.2 ± 10.0 A,B | 17.1 ± 7.1 D |
| infusion | June | natural | 276 ± 45 A | 36.0 ± 7.0 A,C | 8.9 ± 6.8 A |
| infusion | June | 35 °C | 211 ± 38 A | 24.3 ± 5.2 C | 10.2 ± 5.9 A,B |
| infusion | July | natural | 265 ± 17 A | 42.2 ± 4.9 A,B,C | 10.6 ± 4.9 A,B |
| infusion | July | 35 °C | 249 ± 22 A | 40.4 ± 8.1 A,B,C | 11.6 ± 1.9 A,B |
| Solution-Harvest Term-Drying Method | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound | Extract -June- Natural | Extract -June- 35 °C | Extract -July- Natural | Extract -July- 35 °C | Infusion -June- Natural | Infusion -June- 35 °C | Infusion -July- Natural | Infusion -July- 35 °C |
| 3,5-dCQA 1 [ng/g DW] | 9.198 | 29.286 | 18.333 | 40.834 | 33.659 | 35.06 | 47.944 | 48.643 |
| sinapinic acid [μg/g DW] | 0.199 | 0.358 | 0.539 | 0.621 | 0.514 | 0.711 | 0.588 | 0.782 |
| p-coumaric acid [μg/g DW] | 0.21 | 0.258 | 0.564 | 0.602 | 0.517 | 0.711 | 0.486 | 0.442 |
| Ferulic acid [μg/g DW] | 6.185 | 2.897 | 19.396 | 9.236 | 1.884 | 2.59 | 4.177 | 5.588 |
| hesperidin [ng/g DW] | 1.667 | 1.049 | 3.866 | 3.53 | 3.601 | 6.459 | 1.072 | 5.352 |
| isorhamnetin [ng/g DW] | 3.617 | 5.558 | 2.333 | 3.161 | 0.595 | 0.731 | 0.451 | 0.521 |
| catechin [ng/g DW] | 0.384 | 0.419 | 0.265 | 0.314 | 0.537 | 0.439 | 0.344 | 0.297 |
| rutin [μg/g DW] | 11.49 | 6.712 | 9.13 | 6.653 | 13.293 | 7.758 | 10.131 | 5.925 |
| quercetin [μg/g DW] | 0.272 | 0.21 | 0.184 | 0.228 | 0.269 | 0.193 | 0.061 | 0.034 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francik, S.; Francik, R.; Sadowska, U.; Bystrowska, B.; Zawiślak, A.; Knapczyk, A.; Nzeyimana, A. Identification of Phenolic Compounds and Determination of Antioxidant Activity in Extracts and Infusions of Salvia Leaves. Materials 2020, 13, 5811. https://doi.org/10.3390/ma13245811
Francik S, Francik R, Sadowska U, Bystrowska B, Zawiślak A, Knapczyk A, Nzeyimana A. Identification of Phenolic Compounds and Determination of Antioxidant Activity in Extracts and Infusions of Salvia Leaves. Materials. 2020; 13(24):5811. https://doi.org/10.3390/ma13245811
Chicago/Turabian StyleFrancik, Sławomir, Renata Francik, Urszula Sadowska, Beata Bystrowska, Agnieszka Zawiślak, Adrian Knapczyk, and Abdul Nzeyimana. 2020. "Identification of Phenolic Compounds and Determination of Antioxidant Activity in Extracts and Infusions of Salvia Leaves" Materials 13, no. 24: 5811. https://doi.org/10.3390/ma13245811
APA StyleFrancik, S., Francik, R., Sadowska, U., Bystrowska, B., Zawiślak, A., Knapczyk, A., & Nzeyimana, A. (2020). Identification of Phenolic Compounds and Determination of Antioxidant Activity in Extracts and Infusions of Salvia Leaves. Materials, 13(24), 5811. https://doi.org/10.3390/ma13245811








