Photopolymerization-Based Synthesis of Uniform Magnetic Hydrogels and Colorimetric Glucose Detection
Abstract
1. Introduction
2. Materials and Methods
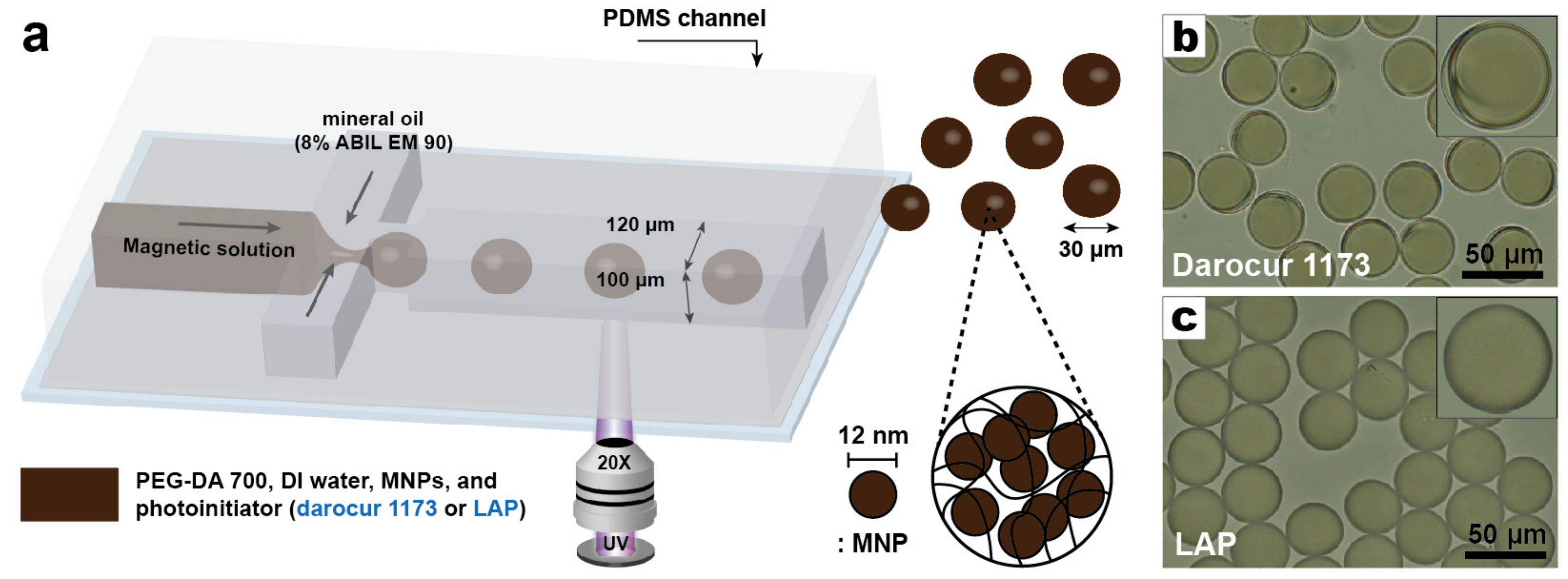
2.1. Microfluidic Device
2.2. Materials
2.3. Characterization
2.4. Generation of Emulsion Droplets
2.5. Vibrating Sample Magnetometer Measurement
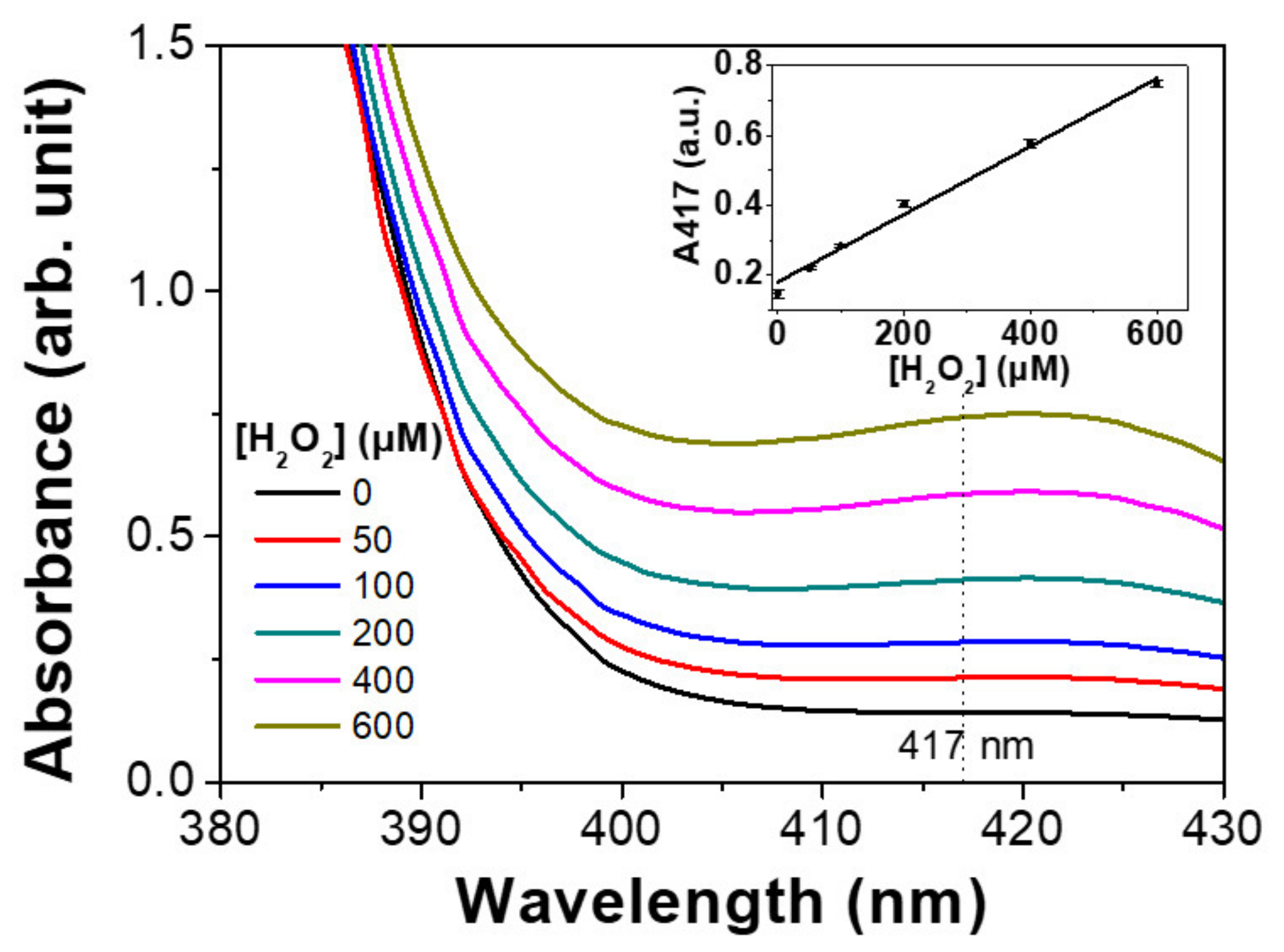
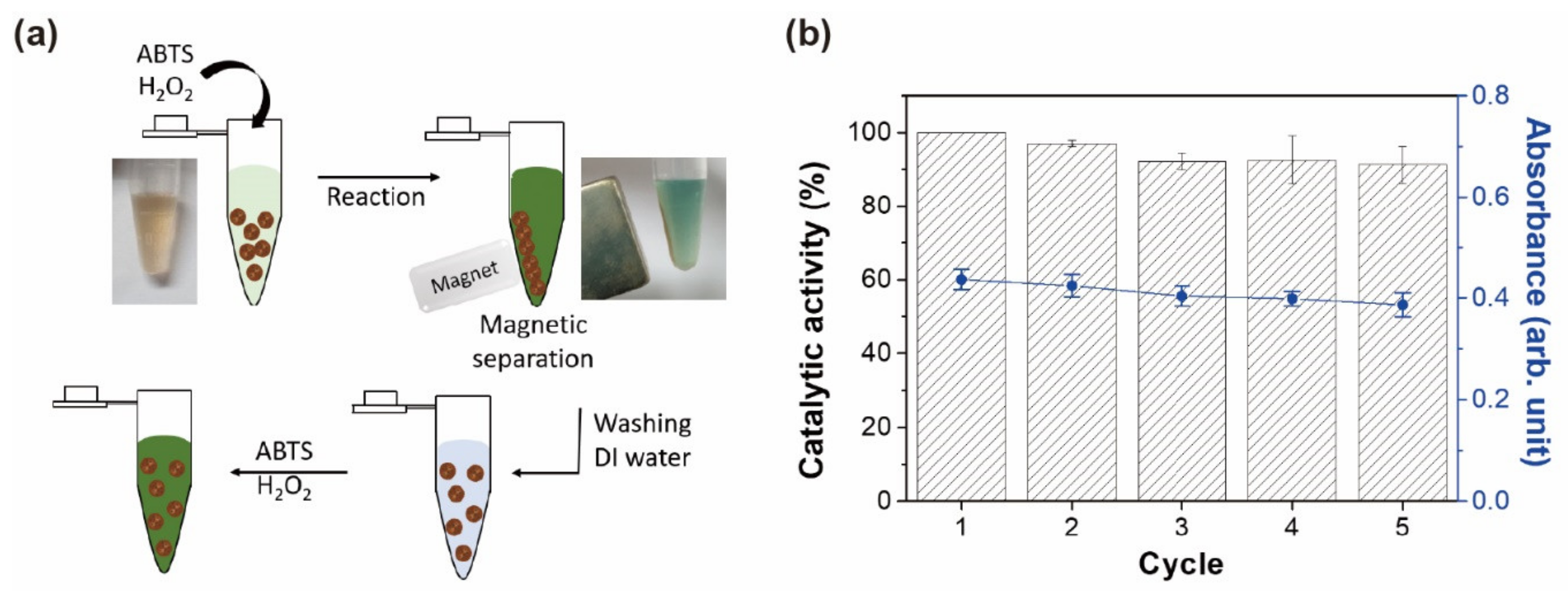
2.6. Colorimetric Detection of H2O2 and Glucose
3. Results and Discussion
3.1. Synthesis of Magnetic Hydrogels
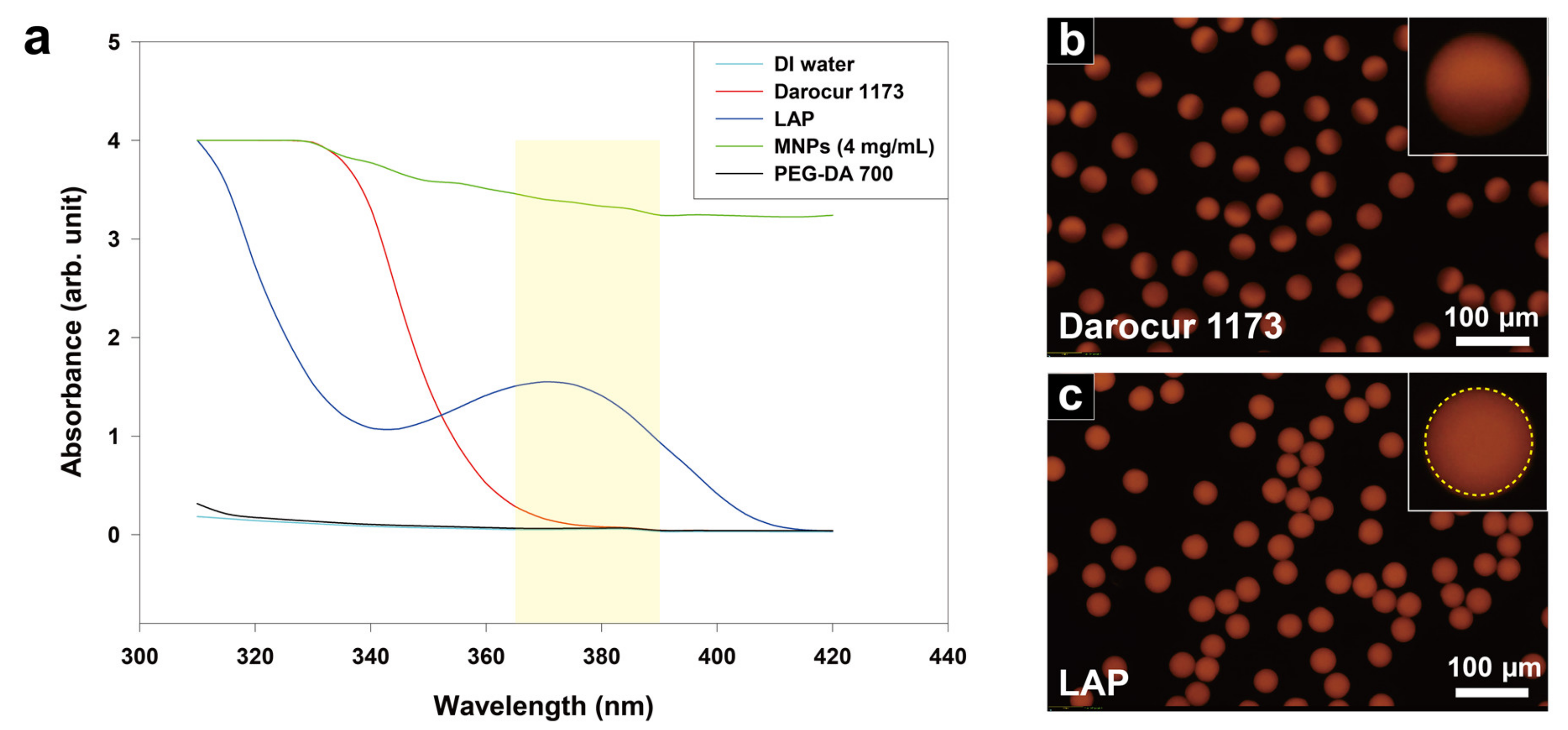
3.2. Absorbance Characterization
3.3. Uniformity of the Magnetic Hydrogel
3.4. Magnetic Characterization of Magnetic Hydrogels
3.5. Colorimetric Detection of H2O2 Based on Magnetic Hydrogels
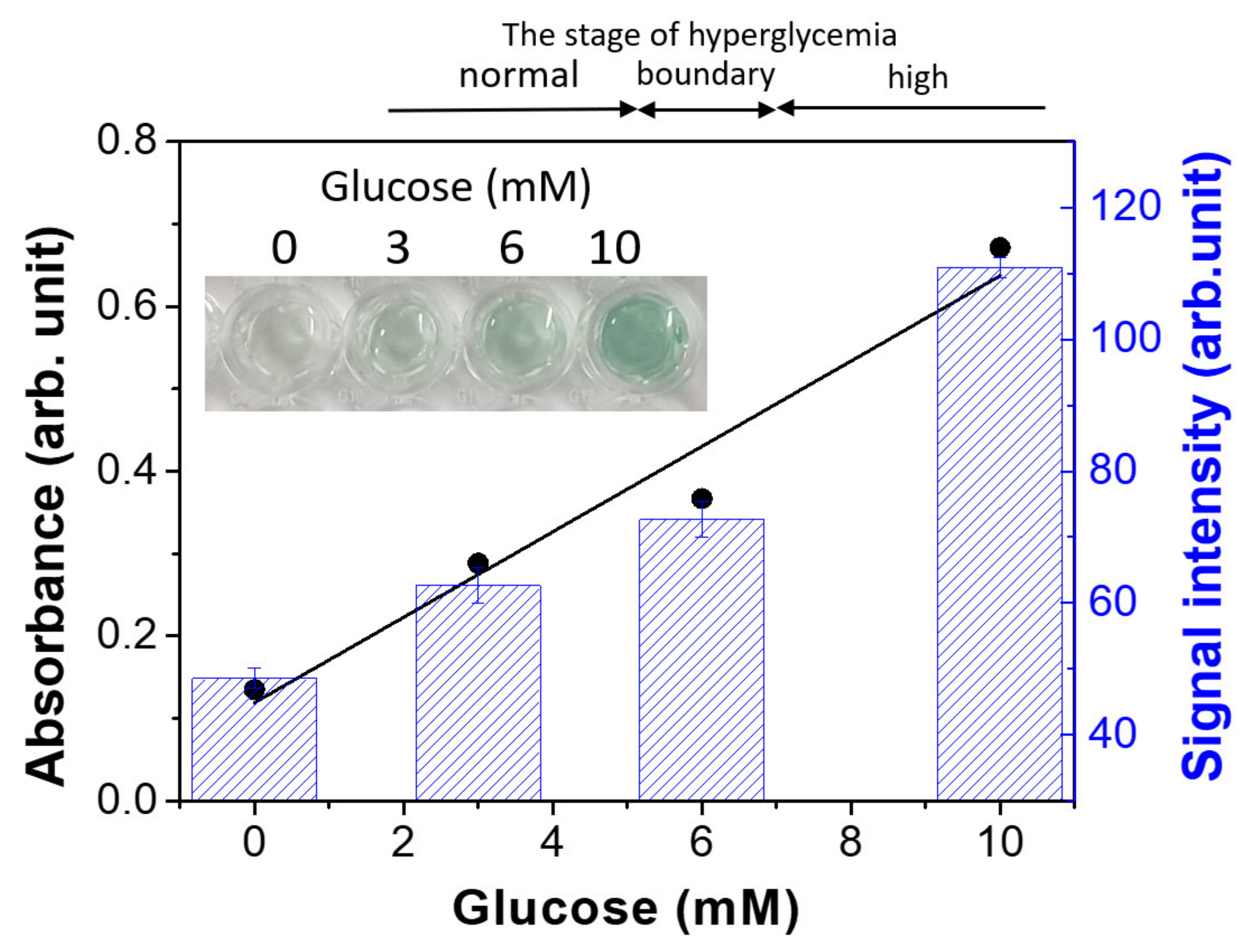
3.6. Colorimetric Detection of Glucose Based on Magnetic Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Giani, G.; Fedi, S.; Barbucci, R. Hybrid Magnetic Hydrogel: A Potential System for Controlled Drug Delivery by Means of Alternating Magnetic Fields. Polymers 2012, 4, 1157–1169. [Google Scholar] [CrossRef]
- Satarkar, N.; Hilt, J.Z. Magnetic hydrogel nanocomposites for remote controlled pulsatile drug release. J. Control. Release 2008, 130, 246–251. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, L.H.; Wang, D.; Zhao, L.; Wan, B. Light-induced efficient molecular oxygen activation on a Cu (II)-grafted TiO2/graphene photocatalyst for phenol degradation. ACS Appl. Mater. Interfaces 2015, 7, 1816–1823. [Google Scholar] [CrossRef]
- Meenach, S.A.; Hilt, J.Z.; Anderson, K.W. Poly(ethylene glycol)-based magnetic hydrogel nanocomposites for hyperthermia cancer therapy. Acta Biomater. 2010, 6, 1039–1046. [Google Scholar] [CrossRef]
- Wu, H.; Liu, L.; Song, L.; Ma, M.; Gu, N.; Zhanga, Y. Enhanced Tumor Synergistic Therapy by Injectable Magnetic Hydrogel Mediated Generation of Hyperthermia and Highly Toxic Reactive Oxygen Species. ACS Nano 2019, 13, 14013–14023. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, L.; Xu, Y.; Du, W.; Cai, X.; Wang, F.; Ling, Y.; Chen, H.; Wang, Z.; Hu, B.; et al. A pH and magnetic dual-response hydrogel for synergistic chemo-magnetic hyperthermia tumor therapy. RSC Adv. 2018, 8, 9812–9821. [Google Scholar] [CrossRef]
- Bong, K.W.; Chapin, S.C.; Doyle, P.S. Magnetic Barcoded Hydrogel Microparticles for Multiplexed Detection. Langmuir 2010, 26, 8008–8014. [Google Scholar] [CrossRef]
- Song, S.; Liu, Y.; Song, A.; Zhao, Z.; Lu, H.; Hao, J. Peroxidase mimetic activity of Fe3O4 nanoparticle prepared based on magnetic hydrogels for hydrogen peroxide and glucose detection. J. Colloid Interface Sci. 2017, 506, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, Y.; Zhang, P.; Li, Y.; Ding, L. A temperature-triggered fiber optic biosensor based on hydrogel-magnetic immobilized enzyme complex for sequential determination of cholesterol and glucose. Biochem. Eng. J. 2017, 125, 123–128. [Google Scholar] [CrossRef]
- Curtis, L.H.; Dember, L.M.; Vazquez, M.A.; Murray, D.; DeBar, L.; Staman, K.L.; Septimus, E.; Mor, V.; Volandes, A.; Wells, B.L.; et al. Addressing guideline and policy changes during pragmatic clinical trials. Clin. Trials 2019, 16, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Juthani, N.; Doyle, P.S. A platform for multiplexed colorimetric microRNA detection using shape-encoded hydrogel particles. Analyst 2020, 145, 5134–5140. [Google Scholar] [CrossRef] [PubMed]
- Roh, Y.H.; Lee, H.J.; Kim, J.Y.; Kim, H.U.; Kim, S.M.; Bong, K.W. Precipitation-based colorimetric multiplex immunoassay in hydrogel particles. Lab Chip 2020. [Google Scholar] [CrossRef] [PubMed]
- Won, B.Y.; Lee, D.W.; Shin, S.C.; Cho, D.-Y.; Lee, S.S.; Yoon, H.C.; Park, H.G. A DNA intercalation-based electrochemical method for detection of Chlamydia trachomatis utilizing peroxidase-catalyzed signal amplification. Biosens. Bioelectron. 2008, 24, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New horizons for responsive biomedical applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.Y.; Kim, M.I.; Cho, S.; Lee, J.; Na, H.B. Visual determination of hydrogen peroxide and glucose by exploiting the peroxidase-like activity of magnetic nanoparticles functionalized with a poly(ethylene glycol) derivative. Microchim. Acta 2017, 42, 2115–2122. [Google Scholar] [CrossRef]
- Zhu, X.; Mao, X.; Wang, Z.; Feng, C.; Chen, G.; Li, G. Fabrication of nanozyme@DNA hydrogel and its application in biomedical analysis. Nano Res. 2016, 10, 959–970. [Google Scholar] [CrossRef]
- Panda, P.; Bong, K.W.; Hatton, T.A.; Doyle, P.S. Branched Networks by Directed Assembly of Shape Anisotropic Magnetic Particles. Langmuir 2011, 27, 13428–13435. [Google Scholar] [CrossRef]
- Kim, H.U.; Lim, Y.J.; Lee, H.J.; Lee, N.J.; Bong, K.W. Degassed micromolding lithography for rapid fabrication of anisotropic hydrogel microparticles with high-resolution and high uniformity. Lab Chip 2020, 20, 74–83. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Gao, Q.; Liu, X.; Tong, Z. Magnetic hydrogels with supracolloidal structures prepared by suspension polymerization stabilized by Fe2O3 nanoparticles. Acta Biomater. 2010, 6, 275–281. [Google Scholar] [CrossRef]
- Shah, R.K.; Shum, H.C.; Rowat, A.C.; Lee, D.-J.; Agresti, J.J.; Utada, A.S.; Chu, L.-Y.; Kim, J.W.; Fernandez-Nieves, A.; Martinez, C.J.; et al. Designer emulsions using microfluidics. Mater. Today 2008, 11, 18–27. [Google Scholar] [CrossRef]
- Ménager, C.; Sandre, O.; Mangili, J.; Cabuil, V. Preparation and swelling of hydrophilic magnetic microgels. Polymers 2004, 45, 2475–2481. [Google Scholar] [CrossRef]
- Landfester, K.; Rez, L.P.R. Encapsulated magnetite particles for biomedical application. J. Phys. Condens. Matter 2003, 15, S1345–S1361. [Google Scholar] [CrossRef]
- Mason, T.G.; Bibette, J. Shear Rupturing of Droplets in Complex Fluids. Langmuir 1997, 13, 4600–4613. [Google Scholar] [CrossRef]
- Perrin, P. Amphiphilic Copolymers: A New Route to Prepare Ordered Monodisperse Emulsions. Langmuir 1998, 14, 5977–5979. [Google Scholar] [CrossRef]
- Nakashima, T.; Shimizu, M.; Kukizaki, M. Particle control of emulsion by membrane emulsification and its applications. Adv. Drug Deliv. Rev. 2000, 45, 47–56. [Google Scholar] [CrossRef]
- Nakashima, T.; Shimizu, M.; Kukizaki, M. Membrane Emulsification by Microporous Glass. Key Eng. Mater. 1992, 61, 513–516. [Google Scholar] [CrossRef]
- Gañán-Calvo, A.M. Generation of Steady Liquid Microthreads and Micron-Sized Monodisperse Sprays in Gas Streams. Phys. Rev. Lett. 1998, 80, 285–288. [Google Scholar] [CrossRef]
- Teh, S.-Y.; Lin, R.; Hung, L.-H.; Lee, A.P. Droplet microfluidics. Lab Chip 2008, 8, 198. [Google Scholar] [CrossRef]
- Guo, M.T.; Rotem, A.; Heyman, J.A.; Weitz, D.A. Droplet microfluidics for high-throughput biological assays. Lab Chip 2012, 12, 2146–2155. [Google Scholar] [CrossRef]
- Dendukuri, D.; Tsoi, K.; Hatton, T.A.; Doyle, P.S. Controlled Synthesis of Nonspherical Microparticles Using Microfluidics. Langmuir 2005, 21, 2113–2116. [Google Scholar] [CrossRef]
- Xu, S.; Nie, Z.; Seo, M.; Lewis, P.; Kumacheva, E.; Stone, H.A.; Garstecki, P.; Weibel, D.B.; Gitlin, I.; Whitesides, G.M. Generation of Monodisperse Particles by Using Microfluidics: Control over Size, Shape, and Composition. Angew. Chem. 2005, 117, 734–738. [Google Scholar] [CrossRef]
- Gañán-Calvo, A.M.; González-Prieto, R.; Chueca, P.R.; Herrada, M.A.; Flores-Mosquera, M. Focusing capillary jets close to the continuum limit. Nat. Phys. 2007, 3, 737–742. [Google Scholar] [CrossRef]
- Hwang, D.K.; Dendukuri, D.; Doyle, P.S. Microfluidic-based synthesis of non-spherical magnetic hydrogel microparticles. Lab Chip 2008, 8, 1640–1647. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; An, K.; Hwang, Y.; Park, J.-G.; Noh, H.-J.; Kim, J.-Y.; Park, J.-H.; Hwang, N.-M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Na, H.B.; Lee, I.S.; Seo, H.; Park, Y.I.; Lee, J.H.; Kim, S.-W.; Hyeon, T.; Lee, J.S. Versatile PEG-derivatized phosphine oxide ligands for water-dispersible metal oxide nanocrystals. Chem. Commun. 2007, 5167. [Google Scholar] [CrossRef]
- Dendukuri, D.; Panda, P.; Haghgooie, R.; Kim, J.M.; Hatton, T.A.; Doyle, P.S. Modeling of Oxygen-Inhibited Free Radical Photopolymerization in a PDMS Microfluidic Device. Macromolecules 2008, 41, 8547–8556. [Google Scholar] [CrossRef]
- Krutkramelis, K.; Xia, B.; Oakey, J.S. Monodisperse polyethylene glycol diacrylate hydrogel microsphere formation by oxygen-controlled photopolymerization in a microfluidic device. Lab Chip 2016, 16, 1457–1465. [Google Scholar] [CrossRef]
- Bae, M.; Gemeinhart, R.A.; Divan, R.; Suthar, K.J.; Mancini, D.C. Fabrication of poly(ethylene glycol) hydrogel structures for pharmaceutical applications using electron beam and optical lithography. J. Vac. Sci. Technol. B 2010, 28, C6P24–C6P29. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Lalevée, J. Photoinitiators for Polymer Synthesis; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Kızılel, S.; Pérez-Luna, V.H.; Teymour, F. Mathematical Model for Surface-Initiated Photopolymerization of Poly(ethylene glycol) Diacrylate. Macromol. Theory Simul. 2006, 15, 686–700. [Google Scholar] [CrossRef]
- Bishop, T.E.; Elgin, I. Multiple Photoinitiators for Improved Performance. In Proceedings of the Radtech NA Conference Proceedings, Elgin, IL, USA, 4–7 May 2008. [Google Scholar]
- Majima, T.; Schnabel, W.; Weber, W. Phenyl-2,4,6-trimethylbenzoylphosphinates as water-soluble photoinitiators. Generation and reactivity O=Ṗ(C6H5)(O−)radical anions. Macromol. Chem. Phys. 1991, 192, 2307–2315. [Google Scholar] [CrossRef]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Weiss, P.; Forrer, R. La saturation absolue des ferromagnétiques et les lois d’approche en fonction du champ et de la température. Ann. Phys. 1929, 10, 279–372. [Google Scholar] [CrossRef]
- Mo, S.; Bødker, F.; Hendriksen, P.; Linderoth, S. Spin-glass-like ordering of the magnetic moments of interacting nanosized maghemite particles. Phys. Rev. B 1995, 52, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.P.; Veintemillas-Verdaguer, S.; Montero, M.I.; Serna, C.J.; Roig, A.; Casas, L.; Martinez, B.; Sandiumenge, F. Surface and Internal Spin Canting in γ-Fe2O3 Nanoparticles. Chem. Mater. 1999, 11, 3058–3064. [Google Scholar] [CrossRef]
- Oh, S.; Kim, J.; Tran, V.T.; Lee, D.K.; Ahmed, S.R.; Hong, J.C.; Lee, J.; Park, E.Y.; Lee, J. Magnetic Nanozyme-Linked Immunosorbent Assay for Ultrasensitive Influenza A Virus Detection. ACS Appl. Mater. Interfaces 2018, 10, 12534–12543. [Google Scholar] [CrossRef]
- Ge, J.; Hu, Y.; Biasini, M.; Beyermann, W.P.; Yin, Y. Superparamagnetic Magnetite Colloidal Nanocrystal Clusters. Angew. Chem. Int. Ed. 2007, 46, 4342–4345. [Google Scholar] [CrossRef]
- Kang, T.; Kim, Y.G.; Kim, D.; Hyeon, T. Inorganic nanoparticles with enzyme-mimetic activities for biomedical applications. Co-ord. Chem. Rev. 2020, 403, 213092. [Google Scholar] [CrossRef]
- Shen, L.; Hagen, J.A.; Papautsky, I. Point-of-care colorimetric detection with a smartphone. Lab Chip 2012, 12, 4240–4243. [Google Scholar] [CrossRef]
- Umans-Eckenhausen, M.A.; Defesche, J.C.; Sijbrands, E.J.; Kastelein, J.J. Familial hypercholesterolaemia. Lancet 2001, 357, 1712. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mun, S.J.; Ko, D.; Kim, H.U.; Han, Y.; Roh, Y.H.; Kim, B.-G.; Na, H.B.; Bong, K.W. Photopolymerization-Based Synthesis of Uniform Magnetic Hydrogels and Colorimetric Glucose Detection. Materials 2020, 13, 4401. https://doi.org/10.3390/ma13194401
Mun SJ, Ko D, Kim HU, Han Y, Roh YH, Kim B-G, Na HB, Bong KW. Photopolymerization-Based Synthesis of Uniform Magnetic Hydrogels and Colorimetric Glucose Detection. Materials. 2020; 13(19):4401. https://doi.org/10.3390/ma13194401
Chicago/Turabian StyleMun, Seok Joon, Donghyun Ko, Hyeon Ung Kim, Yujin Han, Yoon Ho Roh, Bong-Geun Kim, Hyon Bin Na, and Ki Wan Bong. 2020. "Photopolymerization-Based Synthesis of Uniform Magnetic Hydrogels and Colorimetric Glucose Detection" Materials 13, no. 19: 4401. https://doi.org/10.3390/ma13194401
APA StyleMun, S. J., Ko, D., Kim, H. U., Han, Y., Roh, Y. H., Kim, B.-G., Na, H. B., & Bong, K. W. (2020). Photopolymerization-Based Synthesis of Uniform Magnetic Hydrogels and Colorimetric Glucose Detection. Materials, 13(19), 4401. https://doi.org/10.3390/ma13194401




