Abstract
High-entropy oxides (HEOs) have attracted more and more attention because of their unique structures and potential applications. In this work, (FeCoCrMnZn)3O4 HEO powders were synthesized via a facile solid-state reaction route. The confirmation of phase composition, the observation of microstructure, and the analysis of crystal structure, distribution of elements, and valences of elements were conducted by X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy-dispersive X-ray spectroscopy (EDS), and X-ray photoelectron spectroscopy (XPS), respectively. Furthermore, a (FeCoCrMnZn)3O4/nickel foam ((FeCoCrMnZn)3O4/NF) electrode was prepared via a coating method, followed by the investigation of its supercapacitor performance. The results show that, after calcining (FeCoCrMnZn)3O4 powders at 900 °C for 2 h, a single spinel structure (FCC, Fd-3m, a = 0.8399 nm) was obtained with uniform distribution of Fe, Co, Cr, Mn, and Zn elements, the typical characteristic of a high-entropy oxide. In addition, the mass specific capacitance of the (FeCoCrMnZn)3O4/NF composite electrode was 340.3 F·g−1 (with 1 M KOH as the electrolyte and 1 A·g−1 current density), which indicates that the (FeCoCrMnZn)3O4 HEO can be regarded as a prospective candidate for an electrode material in the field of supercapacitor applications.
1. Introduction
The concept of “high entropy” is a new material design concept originally developed from high-entropy alloys in recent years, and it has become a hotspot in the field of material research. In 2004, Ye et al. first proposed the concept of high-entropy alloys, in which a variety of alloying elements were removed in an equimolar or near-molar ratio to obtain a solid solution with a single crystal structure [1]. Owing to the difference in alloy elements and the radius of each atom, unexpected effects are produced in highly disordered multicomponent systems: the high-entropy effect, cocktail effect, sluggish diffusion effect, and lattice distortion effect [2,3,4]. The synergy of these four effects leads to alloys exhibiting high strength, high hardness, and excellent corrosion resistance [5,6,7].
With the continuous deepening of research, Rost et al. applied “high entropy” to ceramic materials in 2015 [8]. Recently, this concept was extended to new high-entropy materials such as high-entropy nitride [9], high-entropy carbide [10,11,12], high-entropy boride [13,14,15], and high-entropy sulfide [16], which show unique physical properties and good application prospects in a range of applications.
Initially, Rost et al. prepared a (CoCuMgNiZn)O solid solution with a rock-salt structure (Fm-3m) via a solid-state reaction route [8]. Moreover, they pointed out that the high-entropy oxide (HEO) system has a higher configuration entropy (S) when the metal atoms are in equimolar concentration, resulting in lower Gibbs free energy (G), which indicates that a higher configuration entropy can keep the HEO more stable at high temperature. So far, researchers have synthesized a variety of single-phase HEOs with different crystal structures, such as rock-salt type [8,17,18], spinel type [19,20,21,22], fluorite type [23,24], and perovskite type [25,26,27]. Mao et al. [19,21] prepared spinel-type high-entropy oxides (CrFeMnNiZn)3O4 and (CrFeMnNiZn)3O4 via a solution combustion synthesis method with complicated procedures. Up to now, only a small number of HEO-related performances have been reported [18,28,29,30]. Recently, works from Sarkar [18] and Berardan [31] showed that HEOs have great application potential in energy storage. The high configuration entropy can maintain the stability of the original crystal structure of HEOs during charging and discharging such that the cycle stability of HEOs is much higher than that of traditional transition-metal oxides.
In this study, (FeCoCrMnZn)3O4 HEO powder with a single spinel structure was prepared via a facile solid-state reaction route (900 °C, 2 h). In addition, the supercapacitor performances of a (FeCoCrMnZn)3O4/Ni foam (NF) electrode were investigated.
2. Experiments and Procedures
2.1. Synthesis of (FeCoCrMnZn)3O4 Powders
In this work, a facile solid-phase reaction method was used to synthesize (FeCoCrMnZn)3O4 HEO powders. First, Fe2O3 (99.0%), Co2O3 (99.0%), Cr2O3 (99.0%), MnO2 (91.0%), and ZnO (99.99%) powders (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), with a molar ratio of 1:1:1:2:2, were mixed using pot mill (GMJ-5, Xianyang Jinhong General Machinery Ltd., Xianyan, China) for 12 h in distilled water, with ZrO2 balls as the mill medium. Second, the mixed powders were calcined at 800–1000 °C in the atmosphere (with a heating rate of 10 °C/min) for 2 h, before grinding and sieving after furnace cooling.
2.2. Preparation of (FeCoCrMnZn)3O4/NF Electrode
To prepare the (FeCoCrMnZn)3O4/NF electrode, as-synthesized (FeCoCrMnZn)3O4 powders, binder (polytetrafluoroethylene, PTFE), and acetylene black (with a mass ratio of 8:1:1) were mixed in a mixture of ethanol and distilled water via ultrasonic separation for 15 min. Then, the NF was immersed into the as-mixed slurry with ultrasonic treatment to make sure that the NF was covered uniformly by (FeCoCrMnZn)3O4 powder. Finally, the (FeCoCrMnZn)3O4/NF electrode was vacuum-dried at 60 °C.
2.3. Material Characterization
The phase analysis was conducted using an X-ray diffractometer (XRD; D8 ADVANCE, Bruker-AXES, Karlsruhe, Germany). The particle size distribution was tested using a laser particle size analyzer (BT-9300H). The microstructure of (FeCoCrMnZn)3O4 powders was observed using a field-emission scanning electron microscope (FE-SEM; Nova Nano SEM 450, FEI, Brno, Czech). The high-resolution transmission electron microscopy images, selected area electron diffraction (SAED) patterns, and energy-dispersive X-ray spectroscopy (EDS) elemental mapping images were obtained using a high-resolution transmission electron microscope (HR-TEM; Talos F200X, FEI, Brno, Czech). The element valence state was determined via X-ray photoelectron spectroscopy (XPS; Axis Ultra DLD, KRATOS, Manchester, UK).
2.4. Electrochemical Measurements
The supercapacitor performance (cyclic voltammetry (CV) curves, galvanostatic charge-discharge (GCD) curves and electrochemical impedance spectra (EIS)) was measured by an electrochemical workstation (CHI660E, Shanghai Chenhua Instrument Co., LTD, Shanghai, China) with 1 M KOH electrolyte. (FeCoCrMnZn)3O4/NF, Ag/AgCl, and Pt foil served as the working electrode, reference electrode, and counter electrode, respectively. The mass specific capacitance (Cm) was calculated by the following equations:
where Cm (F·g−1) is the mass specific capacitance, i is the discharging current (A), t is the discharge time (s), v is the scan rate (mV·s−1), Δu is the discharge voltage range (V), and m is the mass (g) of (FeCoCrMnZn)3O4 HEO active materials.
3. Result and Discussion
3.1. X-Ray Diffraction Analysis
Figure 1 shows the XRD patterns of (FeCoCrMnZn)3O4 powders calcined at 800–1000 °C for 2 h. Figure 1a illustrates that the major diffraction peaks of (FeCoCrMnZn)3O4 powders calcined at 800 °C could be identified as a spinel structure. However, other minor phases, eskolaite, cobalt manganese oxide, hetaerolite, and Co3O4-like spinel, were also observed. When the calcining temperature was increased to 900 °C, the diffraction peaks of the minor phases vanished. Notably, all the diffraction peaks of (FeCoCrMnZn)3O4 powders calcined at 900 °C and 1000 °C could be identified as a spinel structure with the space group of Fd-3m (PDF#34-0140). The diffraction peaks at 2θ = 18.32°, 30.15°, 35.51°, 37.15°, 43.16°, 53.53°, 57.07°, and 62.67° were consistent with the (111), (220), (311), (222), (400), (422), (511), and (440) planes, respectively. In addition, as the calcining temperature increased, the diffraction peaks of (FeCoCrMnZn)3O4 powders became sharper and narrower, indicating higher crystallinity. The enlarged XRD patterns in the range of 32–38° are presented in Figure 1b. Notably, with the increase in calcining temperature, the diffraction peak corresponding to the (311) crystal plane gradually shifted to a higher scattering angle, suggesting a constriction of the lattice constant [32]. Moreover, it is apparent that the full width at half maximum (FWHM) corresponding to the (311) crystal plane decreased with the increase in calcining temperature, indicating an increase in average grain size. Figure 1c shows the Rietveld refined XRD pattern of (FeCoCrMnZn)3O4 powders synthesized at 900 °C. The initial structural model was established on the basis of the spinel structure (Fd-3m), whereby all the observed XRD patterns fit well with the calculated data of the spinel structure, and the calculated lattice constant was 0.8399 nm.
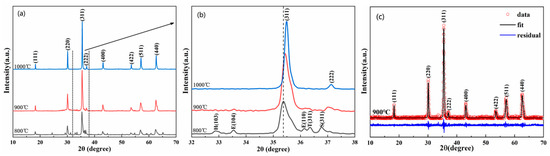
Figure 1.
(a) X-ray diffraction (XRD) pattern of (FeCoCrMnZn)3O4 powders calcined at 800, 900, and 1000 °C, respectively; (b) partially enlarged XRD patterns (32–38°), where peaks indexed with (E), (H), (T), and (S) correspond to eskolaite, hetaerolite, cobalt manganese oxide, and Co3O4-like spinel phases, respectively; (c) Rietveld refinement XRD pattern of the powders calcined at 900 °C.
3.2. Micromorphology and Structure Analysis
Figure 2 demonstrates the SEM image and particle size distribution diagram of the (FeCoCrMnZn)3O4 powders calcined at 900 °C for 2 h. Irregular particles with some agglomeration can be observed from Figure 2a. It can be seen in Figure 2b that the particle size of the (FeCoCrMnZn)3O4 powders was mainly between 0.5 μm and 1 μm with a normal distribution, and the average particle size was 0.65 μm.
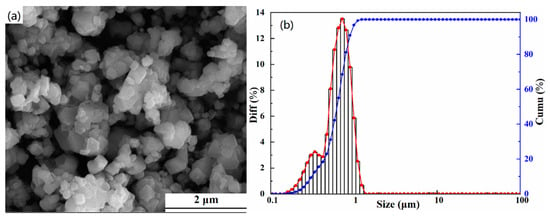
Figure 2.
SEM image (a) and particle size distribution (b) of (FeCoCrMnZn)3O4 powders (900 °C, 2 h).
In order to better understand the crystal structure of (FeCoCrMnZn)3O4 powder, it was further determined by TEM (Figure 3). Figure 3b–g show the energy-dispersive X-ray spectroscopy (EDS) analysis of O, Fe, Co, Cr, Mn, and Zn elements. We can find that all metal elements in the grain were uniformly dispersed, without obvious reunion. All results indicate that (FeCoCrMnZn)3O4 powder was chemically and structurally homogeneous. In addition, the HR-TEM image of (FeCoCrMnZn)3O4 powder is shown in Figure 3h. The interplanar space value (0.294 nm) obtained from the HR-TEM images is very close to that of the (220) crystal plane of the spinel structure (PDF#34-0140, 0.296 nm), which matches with the results of XRD analysis (Figure 1a). The fast Fourier transfer (FFT) generated in the same area shows that the powder possessed a typical face-centered cubic (fcc) structure, and the diffraction spots are also marked in Figure 3i. The diffraction pattern could be identified as a face-centered cubic spinel structure, which is in accordance with the XRD pattern.
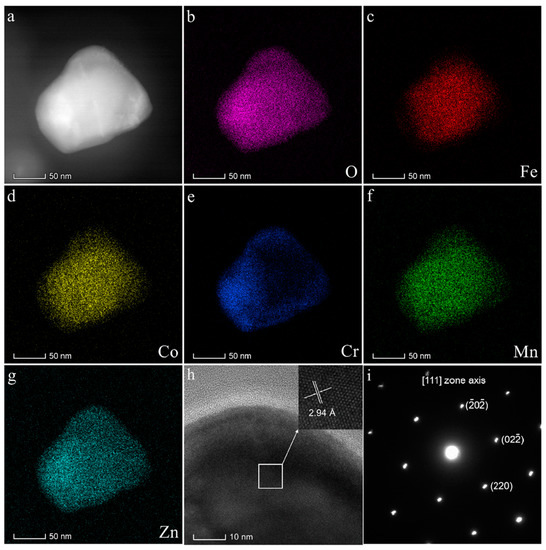
Figure 3.
Characterization of (FeCoCrMnZn)3O4 powder: (a) STEM image, (b–g) energy-dispersive X-ray spectroscopy (EDS) analysis of O, Fe, Co, Cr, Mn, Zn, (h) high-resolution transmission electron microscope (HR-TEM) image, and (i) corresponding fast Fourier transform (FFT) pattern aligned along the zone axis.
Figure 4 is the XPS peak spectrum of each element in the (FeCoCrMnZn)3O4 powder. The peak spectrum of O 1s in Figure 4a shows that the peaks at 530, 531.5, and 532.8 eV corresponded to metal oxygen bonds, surface-adsorbed oxyhydroxide, and surface physical/chemically adsorbed H2O, respectively. Figure 4b–f show the XPS spectra of Fe, Co, Cr, Mn, and Zn metal elements. The XPS analysis indicates that Fe, Co, and Mn cations in (FeCoCrMnZn)3O4 HEO were at the valences of both +2 and +3 states, while Cr was in the +3 state and Zn was in the +2 state.
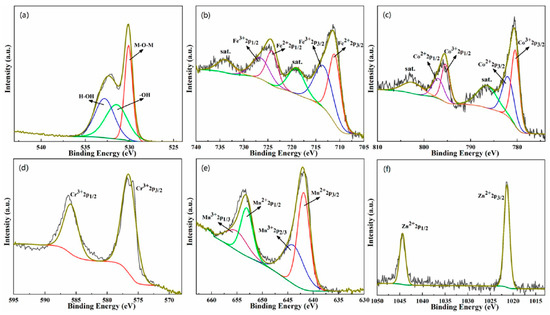
Figure 4.
X-ray photoelectron spectroscopy (XPS) patterns of (FeCoCrMnZn)3O4 powders: (a) O 1s, (b) Fe 2p, (c) Co 2p, (d) Cr 2p, (e) Mn 2p, and (f) Zn 2p.
3.3. Electrochemical Performance
Due to the unique structural characteristics of the HEO, it has special significance as a supercapacitor. Cyclic voltammetry (CV) measurements were conducted in a three-electrode cell to investigate the pseudocapacitive performance of (FeCoCrMnZn)3O4/NF electrode. Figure 5a shows CV curves of the (FeCoCrMnZn)3O4/NF electrode in the potential range of 0.15–0.5 V at a scan rate from 5 to 100 mV·s−1. Apparently, there was a clear symmetric redox peak in the CV curve due to the conversion of MO and MO–OH (M represents the metal elements Co, Ni) during the charge–discharge process [33]. In addition, the shape of the CV curves did not change significantly with the increasing scan rate, which manifests that the structure of the (FeCoCrMnZn)3O4/NF electrode is helpful for the rapid redox reaction [34]. Moreover, as the scan rate increased, the oxidation and reduction peaks of the CV curve moved to high and low potentials, respectively, demonstrating the limitation of charge transfer [35,36]. According to Equation (1), the mass specific capacitance (Cm) of the (FeCoCrMnZn)3O4/NF electrode at a scan rate of 5 mV·s−1 was 352.9 F·g−1.
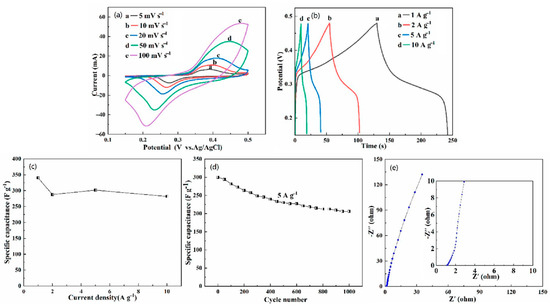
Figure 5.
Electrochemical characteristics of the (FeCoCrMnZn)3O4/nickel foam (NF) composites: (a) cyclic voltammetry (CV) curves, (b) galvanostatic charge-discharge (GCD) curves, (c) corresponding specific capacitance (SCs) at different current densities, (d) cycling performance at a current density of 5 A·g−1, and (e) electrochemical impedance spectra (EIS).
The performance of the (FeCoCrMnZn)3O4/NF electrode for supercapacitors was studied with chronopotentiometry tests (0.15–0.48 V) at different current densities (Figure 5b). The Cm calculated by Equation (2) at current densities of 1, 2, 5, and 10 A·g−1 were 340.3, 287.9, 301.5, and 281.8 F·g−1, respectively. The results show that 82.8% remained of the primal Cm even at a high current density of 10 A·g−1, which indicates the prominent rate performance of (FeCoCrMnZn)3O4/NF electrode. Furthermore, the relatively smooth shape of charge–discharge curves indicates that the (FeCoCrMnZn)3O4/NF electrode is in accordance with the characteristics of pseudocapacitance (nonlinear charge–discharge), which is triggered by the redox reaction of the active material of the electrode during the rapid charge–discharge process. Additionally, the two slow stages of charge/discharge curves in Figure 5b correspond to the redox peaks in CV curves [37]. As shown in Figure 5c, the discharge specific capacitance featured a downward trend with the increase in current density. This is because the reversible redox reaction is a diffusion process. As a result, at a large current density, there was an obvious diminution in the electrochemical utilization of (FeCoCrMnZn)3O4 HEO powders [38,39]. The cycling performance of the (FeCoCrMnZn)3O4 powder material supported on NF was tested at a current density of 5 A·g−1 (Figure 5d). The result shows that about 69% of the initial specific capacitance remained even after 1000 cycles at a current density of 5 A·g−1. To explore the characteristics of the (FeCoCrMnZn)3O4/NF electrode for supercapacitor performance, EIS measurements were conducted at open-circuit potential over the frequency range of 0.01 Hz and 100 kHz for the (FeCoCrMnZn)3O4/NF electrode. It can be seen from Figure 5e that the slope of the straight line was large, indicating that the (FeCoCrMnZn)3O4 HEO had good pseudocapacitance performance. Moreover, there were no observable semicircular arcs in the high-frequency region, manifesting that the charge transfer was dominated by diffusion of ions, instead of an interface reaction [40]. Hence, the newly developed (FeCoCrMnZn)3O4 HEO has outstanding application prospects in supercapacitor applications.
4. Conclusions
In summary, a (FeCoCrMnZn)3O4 HEO with a single spinel structure was prepared via a facile and easily industrialized route (900 °C, 2 h). The (FeCoCrMnZn)3O4 HEO exhibited a face-centered cubic crystal structure with five highly dispersed metal elements (Fe, Co, Cr, Mn, and Zn). In addition, the (FeCoCrMnZn)3O4 HEO can be used as a prospective candidate material for supercapacitors. The mass specific capacitance was 340.3 F·g−1 at a current density of 1 A·g−1 in the electrolyte of 1 M KOH. This research provides not only a facile route for preparing an HEO, but also a new path for the application of HEOs to the field of supercapacitors.
Author Contributions
Methodology, investigation, and data curation, B.L., Y.W, and M.L.; writing—original draft preparation, Y.W.; writing—review and editing, B.L.; supervision, C.L.; formal analysis, S.O.; validation and resources, Y.A. All the authors discussed the data and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (51664043), the Natural Science Foundation of Jiangxi Province (20192BAB206007), and the Key Laboratory for Microstructural Control of Metallic Materials of Jiangxi Province (Nanchang Hangkong University) Open Fund (EJ201901455).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Yeh, J.W. Recent progress in high-entropy alloys. Ann. Chim. Sci. Mat. 2006, 31, 633–648. [Google Scholar] [CrossRef]
- Kim, K.B.; Warren, P.J.; Cantor, B.; Eckert, J. Devitrification of nano-scale icosahedral phase in multicomponent alloys. Mat. Sci. Eng. A 2007, 449, 983–986. [Google Scholar] [CrossRef]
- Tsai, K.Y.; Tsai, M.H.; Yeh, J.W. Sluggish diffusion in Co-Cr-Fe-Mn-Ni high-entropy alloys. Acta Mater. 2013, 61, 4887–4897. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, H.; Fan, Y.Z.; Wei, R.; Zhang, W.W.; Wang, T.; Zhang, T.; Wu, K.; Li, F.S.; Guan, S.K.; et al. Improvement of corrosion resistance and magnetic properties of FeCoNiAl0.2Si0.2 high entropy alloy via rapid-solidification. Intermetallics 2020, 122, 106778. [Google Scholar] [CrossRef]
- Niu, C.N.; LaRosa, C.R.; Miao, J.S.; Mills, M.J.; Ghazisaeidi, M. Magnetically-driven phase transformation strengthening in high entropy alloys. Nat. Commun. 2018, 9, 1363. [Google Scholar] [CrossRef] [PubMed]
- Edalati, P.; Floriano, R.; Tang, Y.P.; Mohammadi, A.; Pereira, K.D.; Luchessi, A.D.; Edalati, K. Ultrahigh hardness and biocompatibility of high-entropy alloy TiAlFeCoNi processed by high-pressure torsion. Mater. Sci. Eng. C 2020, 112, 110908. [Google Scholar] [CrossRef] [PubMed]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J. Entropy-stabilized oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Sang, X.H.; Unocic, R.R.; Kinch, R.T.; Liu, X.F.; Hu, J.; Liu, H.L.; Dai, S. Mechanochemical-assisted synthesis of high-entropy metal nitride via a soft urea strategy. Adv. Mater. 2018, 30, 1707512. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.F.; Liu, J.X.; Li, F.; Qin, Y.; Liang, Y.C.; Zhang, G.J. High entropy carbide ceramics from different starting materials. J. Eur. Ceram. Soc. 2019, 39, 2989–2994. [Google Scholar] [CrossRef]
- Demirskyi, D.; Borodianska, H.; Suzuki, T.S.; Sakka, Y.; Yoshimi, K.; Vasylkiv, O. High-temperature flexural strength performance of ternary high-entropy carbide consolidated via spark plasma sintering of TaC, ZrC and NbC. Scr. Mater. 2019, 164, 12–16. [Google Scholar] [CrossRef]
- Harrington, T.J.; Gild, J.; Sarker, P.; Toher, C.; Rost, C.M.; Dippo, O.F.; McElfresh, C.; Kaufmann, K.; Marin, E.; Borowski, L.; et al. Phase stability and mechanical properties of novel high entropy transition metal carbides. Acta Mater. 2019, 166, 271–280. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, S.K.; Zhang, W.; You, Y.; Guo, W.M.; Chen, Z.W.; Yuan, J.H.; Lin, H. Improved densification and hardness of high-entropy diboride ceramics from fine powders synthesized via borothermal reduction process. Ceram. Int. 2020, 46, 14299–14303. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Z.B.; Sun, S.K.; Guo, W.M.; Chen, Q.S.; Qiu, J.X.; Plucknett, K.; Lin, H.T. Microstructure and mechanical properties of high-entropy borides derived from boro/carbothermal reduction. J. Eur. Ceram. Soc. 2019, 39, 3920–3924. [Google Scholar] [CrossRef]
- Liu, D.; Wen, T.Q.; Ye, B.L.; Chu, Y.H. Synthesis of superfine high-entropy metal diboride powders. Scr. Mater. 2019, 167, 110–114. [Google Scholar] [CrossRef]
- Zhang, R.Z.; Gucci, F.; Zhu, H.Y.; Chen, K.; Reece, M.J. Data-driven design of ecofriendly thermoelectric high-entropy sulfides. Inorg. Chem. 2018, 57, 13027–13033. [Google Scholar] [CrossRef]
- Berardan, D.; Meena, A.K.; Franger, S.; Herrero, C.; Dragoe, N. Controlled Jahn-Teller distortion in (MgCoNiCuZn)O-based high entropy oxides. J. Alloys Compd. 2017, 704, 693–700. [Google Scholar] [CrossRef]
- Sarkar, A.; Velasco, L.; Wang, D.; Wang, Q.S.; Talasila, G.; Biasi, L.D.; Kübel, C.; Brezesinski, T.; Bhattacharya, S.S.; Hahn, H.; et al. High entropy oxides for reversible energy storage. Nat. Commun. 2018, 9, 3400. [Google Scholar] [CrossRef]
- Mao, A.Q.; Xiang, H.Z.; Zhang, Z.G.; Kuramoto, K.; Zhang, H.; Jia, Y.G. A new class of spinel high-entropy oxides with controllable magnetic properties. J. Magn. Magn. Mater. 2020, 497, 165884. [Google Scholar]
- Grzesik, Z.; Smoła, G.; Miszczak, M.; Stygar, M.; Dąbrowa, J.; Zajusz, M.; Świerczek, K.; Danielewski, M. Defect structure and transport properties of (Co,Cr,Fe,Mn,Ni)3O4 spinel-structured high entropy oxide. J. Eur. Ceram. Soc. 2020, 40, 835–839. [Google Scholar] [CrossRef]
- Mao, A.Q.; Quan, F.; Zheng, H.Z.; Zhang, Z.G.; Kuramoto, K.; Xia, A.L. Facile synthesis and ferrimagnetic property of spinel (CoCrFeMnNi)3O4 high-entropy oxide nanocrystalline powder. J. Mol. Struct. 2019, 1194, 11–18. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Stygar, M.; Mikuła, A.; Knapik, A.; Mroczka, K.; Tejchman, W.; Danielewski, M.; Martin, M. Synthesis and microstructure of the (Co,Cr,Fe,Mn,Ni)3O4 high entropy oxide characterized by spinel structure. Mater. Lett. 2018, 216, 32–36. [Google Scholar] [CrossRef]
- Chen, K.P.; Pei, X.T.; Tang, L.; Cheng, H.R.; Li, Z.M.; Li, C.W.; Zhang, X.W.; An, L.N. A five-component entropy-stabilized fluorite oxide. J. Eur. Ceram. Soc. 2018, 38, 4161–4164. [Google Scholar] [CrossRef]
- Gild, J.; Samiee, M.; Braun, J.L.; Harrington, T.; Vega, H.; Hopkins, P.E.; Vecchio, K.; Luo, J. High-entropy fluorite oxides. J. Eur. Ceram. Soc. 2018, 38, 3578–3584. [Google Scholar] [CrossRef]
- Jiang, S.C.; Hu, T.; Gild, J.; Zhou, N.X.; Nie, J.Y.; Qin, M.D.; Harrington, T.; Vecchio, K.S.; Luo, J. A new class of high-entropy perovskite oxides. Scr. Mater. 2018, 142, 116–120. [Google Scholar] [CrossRef]
- Witte, R.; Sarkar, A.; Kruk, R.; Eggert, B.; Brand, R.A.; Wende, H.; Hahn, H. High-entropy oxides: An emerging prospect for magnetic rare-earth transition metal perovskites. Phys. Rev. Mater. 2019, 3, 034406. [Google Scholar] [CrossRef]
- Sarkar, A.; Djenadic, R.; Wang, D.; Hein, C.; Kautenburger, R.; Clemens, O.; Hahn, H. Rare earth and transition metal based entropy stabilised perovskite type oxides. J. Eur. Ceram. Soc. 2018, 38, 2318–2327. [Google Scholar] [CrossRef]
- Qiu, N.; Chen, H.; Yang, Z.M.; Sun, S.; Wang, Y.; Cui, Y.H. A high entropy oxide (Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O with superior lithium storage performance. J. Alloys Compd. 2019, 777, 767–774. [Google Scholar] [CrossRef]
- Berardan, D.; Franger, S.; Dragoe, D.; Meena, A.K.; Dragoe, N. Colossal dielectric constant in high entropy oxides. Phys. Status Solidi Rapid Res. Lett. 2016, 10, 328–333. [Google Scholar] [CrossRef]
- Mao, A.Q.; Xiang, H.Z.; Zhang, Z.G.; Kuramoto, K.; Yu, H.Y.; Ran, S.L. Solution combustion synthesis and magnetic property of rock-salt (Co0.2Cu0.2Mg0.2Ni0.2Zn0.2)O high-entropy oxide nanocrystalline powder. J. Magn. Magn. Mater. 2019, 484, 245–252. [Google Scholar] [CrossRef]
- Bérardan, D.; Franger, S.; Meena, A.K.; Dragoe, N. Room temperature lithium superionic conductivity in high entropy oxides. J. Mater. Chem. 2016, 4, 9536–9541. [Google Scholar] [CrossRef]
- Tian, Z.M.; Zhu, C.M.; Wang, J.F.; Xia, Z.C.; Liu, Y.; Yuan, S.L. Size dependence of structure and magnetic properties of CoCr2O4 nanoparticles synthesized by hydrothermal technique. J. Magn. Magn. Mater. 2015, 377, 176–182. [Google Scholar] [CrossRef]
- Wang, H.L.; Gao, Q.M.; Jiang, L. Facile approach to prepare nickel cobaltite nanowire materials for supercapacitors. Small 2011, 7, 2454–2459. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Fang, Y.; Shi, B.; Huang, F.F.; Rong, F.; Que, R.H. Three-dimensional NiCo2O4@NiCo2O4 core–shell nanocones arrays for high-performance supercapacitors. Chem. Eng. J. 2018, 344, 311–319. [Google Scholar] [CrossRef]
- Wei, W.F.; Cui, X.W.; Chen, W.X.; Ivey, D.G. Electrochemical cyclability mechanism for MnO2 electrodes utilized as electrochemical supercapacitors. J. Power Sources 2009, 186, 543–550. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, J.W.; Wu, X.D.; Han, Q.F.; Wang, X. Graphene oxide-MnO2 nanocomposites for supercapacitors. ACS Nano 2010, 4, 2822–2830. [Google Scholar] [CrossRef]
- Kumar, D.R.; Prakasha, K.R.; Prakash, A.S.; Shim, J.J. Direct growth of honeycomb-like NiCo2O4@Ni foam electrode for pouch-type high-performance asymmetric supercapacitor. J. Alloys Compd. 2020, 836, 155370. [Google Scholar] [CrossRef]
- Subramanian, V.; Zhu, H.W.; Vajtai, R.; Ajayan, P.M.; Wei, B.Q. Hydrothermal synthesis and pseudocapacitance properties of MnO2 nanostructures. J. Phys. Chem. B 2005, 109, 20207–20214. [Google Scholar] [CrossRef]
- Xu, M.W.; Zhao, D.D.; Bao, S.J.; Li, H.L. Mesoporous amorphous MnO2 as electrode material for supercapacitor. J. Solid State Electr. 2007, 11, 1101–1107. [Google Scholar] [CrossRef]
- Patil, U.M.; Nam, M.S.; Sohn, J.S.; Kulkarni, S.B.; Shin, R.; Kang, S.; Lee, S.; Kim, J.H.; Jun, S.C. Controlled electrochemical growth of Co(OH)2 flakes on 3D multilayered graphene foam for high performance supercapacitors. J. Mater. Chem. 2014, 2, 19075–19083. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).