Effect of Multi-Phosphonate Coating of Titanium Surfaces on Osteogenic Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Titanium Discs Characteristics
2.2. Cell Culture
2.2.1. MG-63 Osteoblast-Like Cells
2.2.2. Human Periodontal Ligament Stem Cells (hPDLSCs)
2.2.3. Bone Marrow Mesenchymal Stem Cells (BM-MSC)
2.3. Fluorescence Microscopy
2.4. Cell Proliferation/Viability
2.5. Quantitative Real-Time PCR
2.6. Statistical Analysis
3. Results
3.1. Surface Characteristics
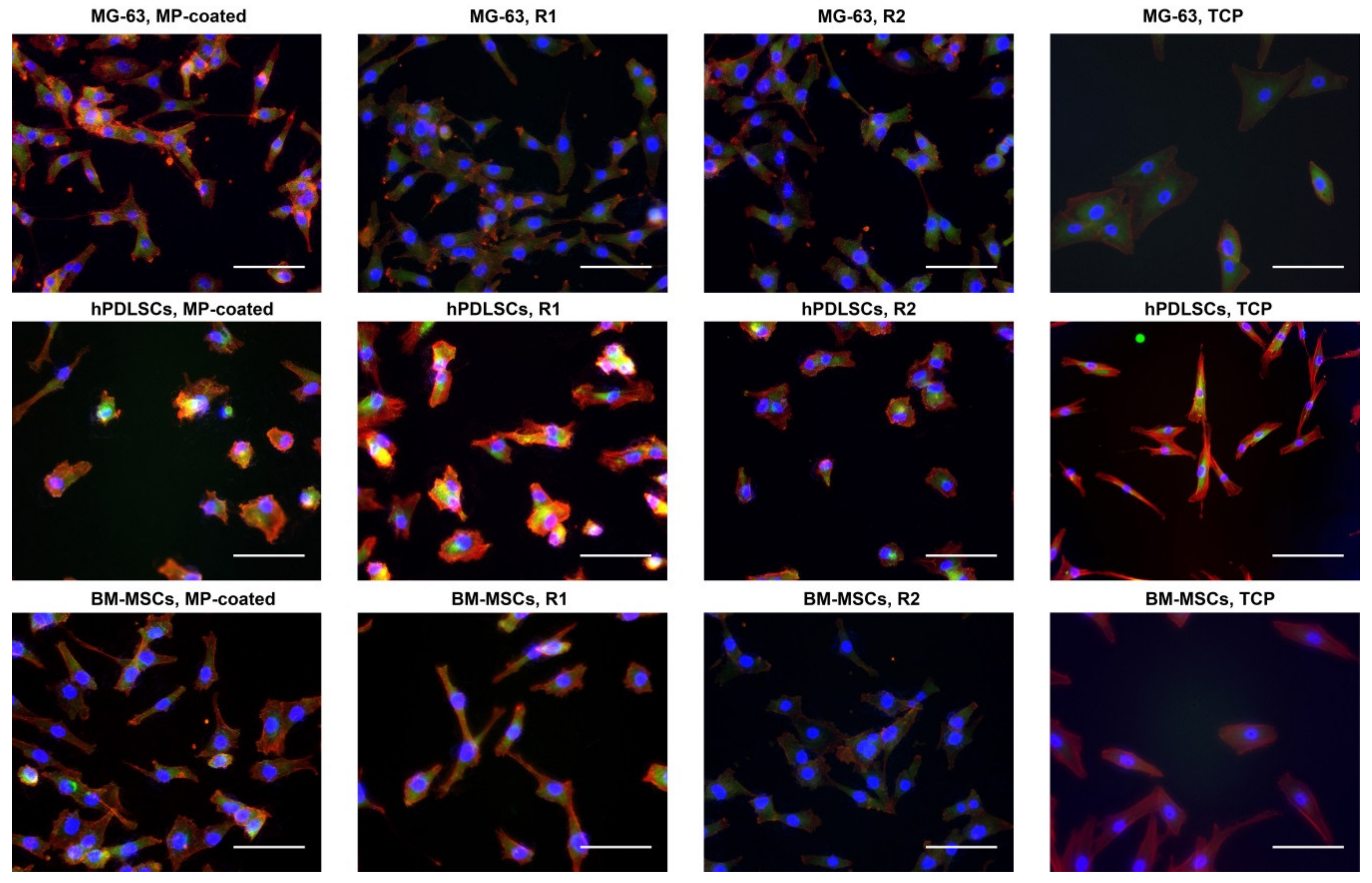
3.2. Fluorescence Microscopy
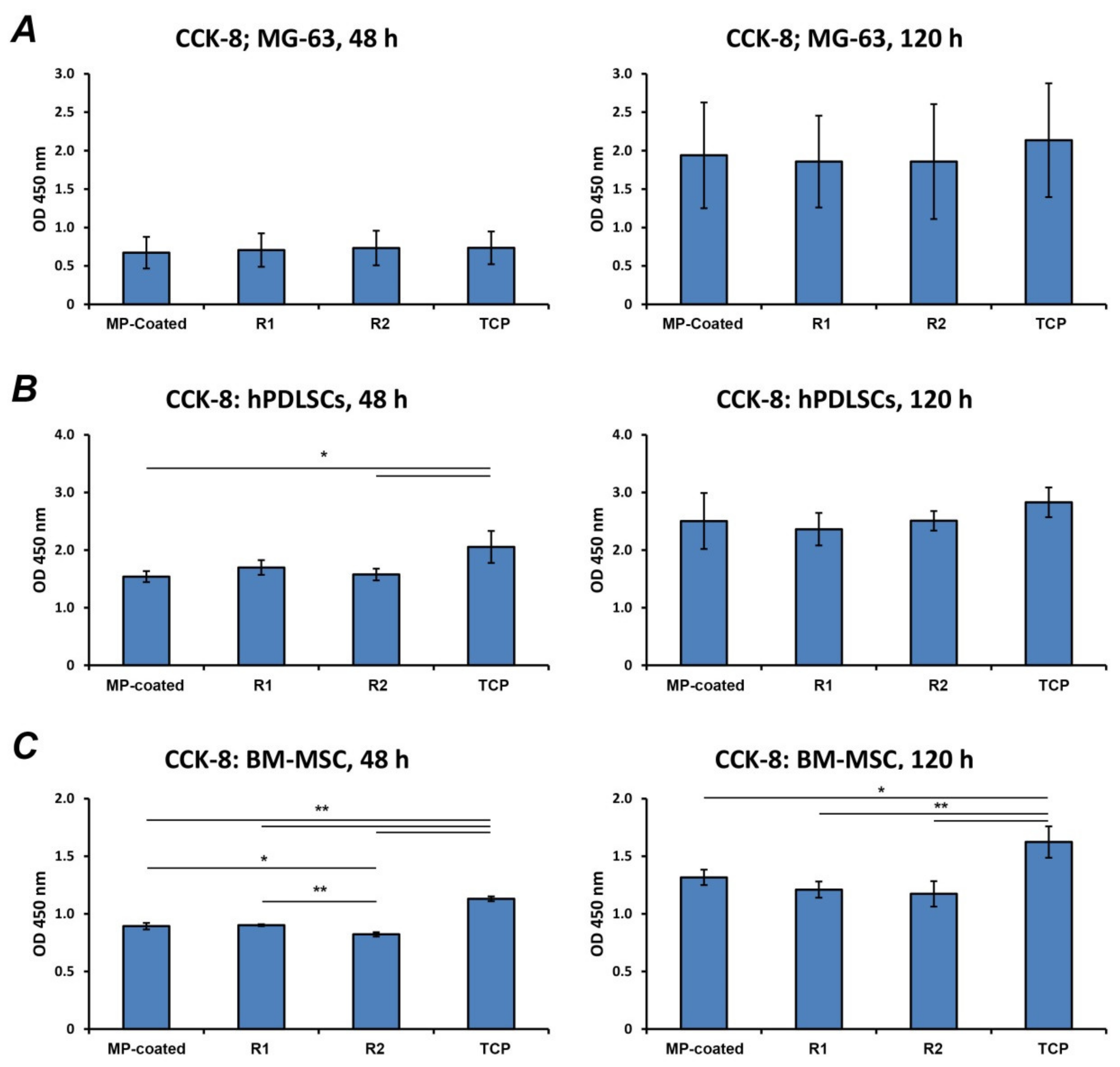
3.3. Cell Proliferation/Viability
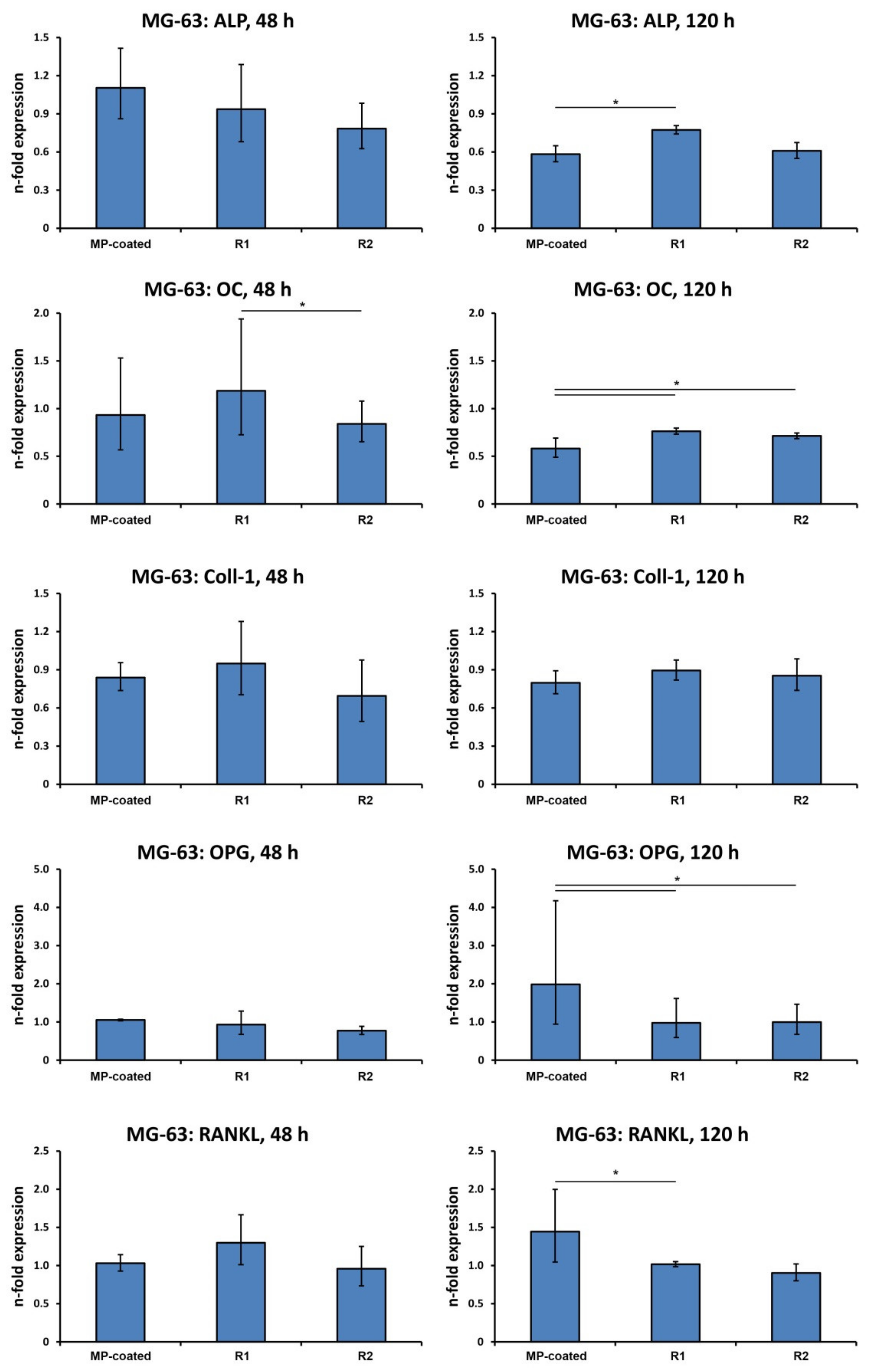
3.4. Gene Expression in MG-63 Cells
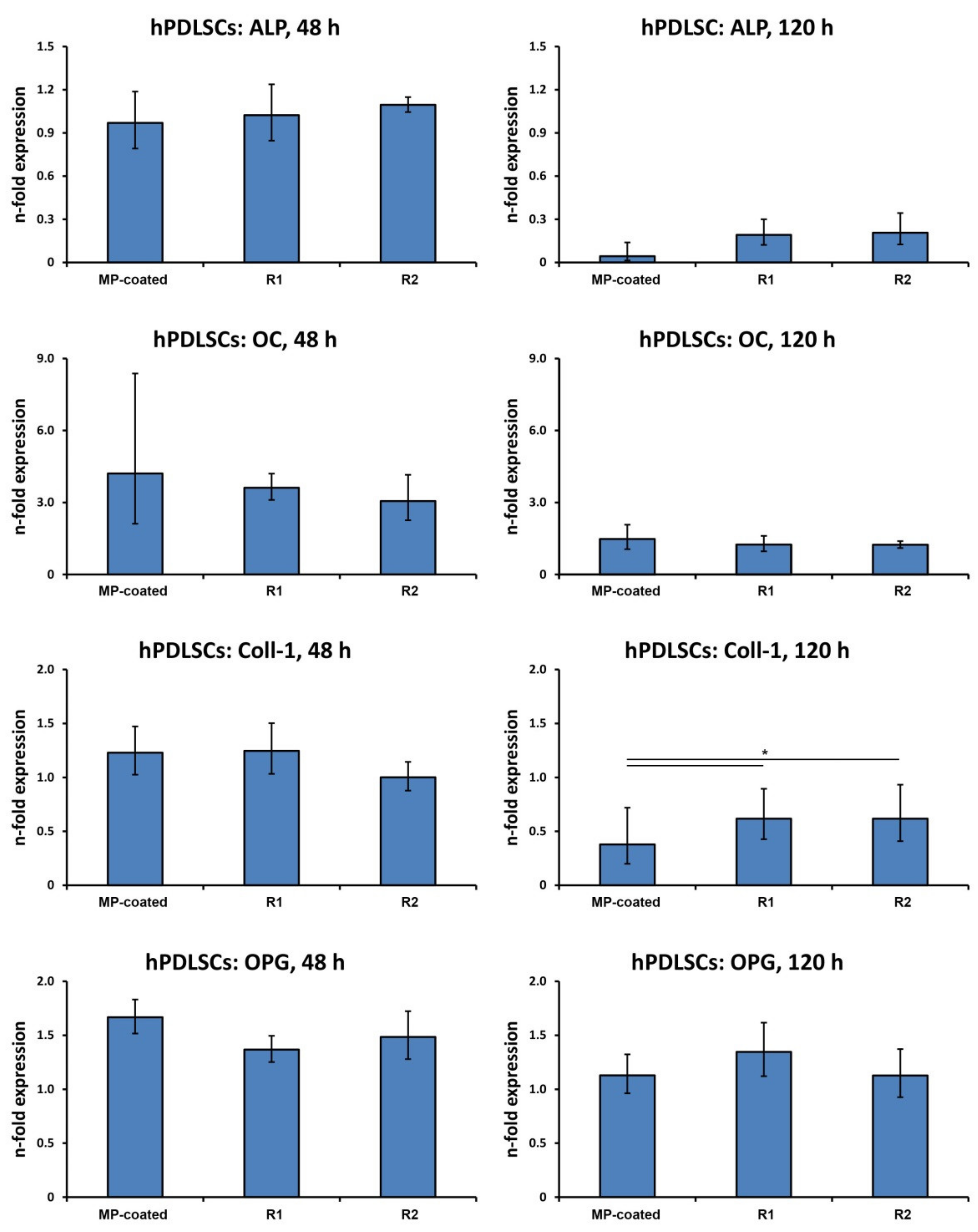
3.5. Gene Expression in hPDLSCs
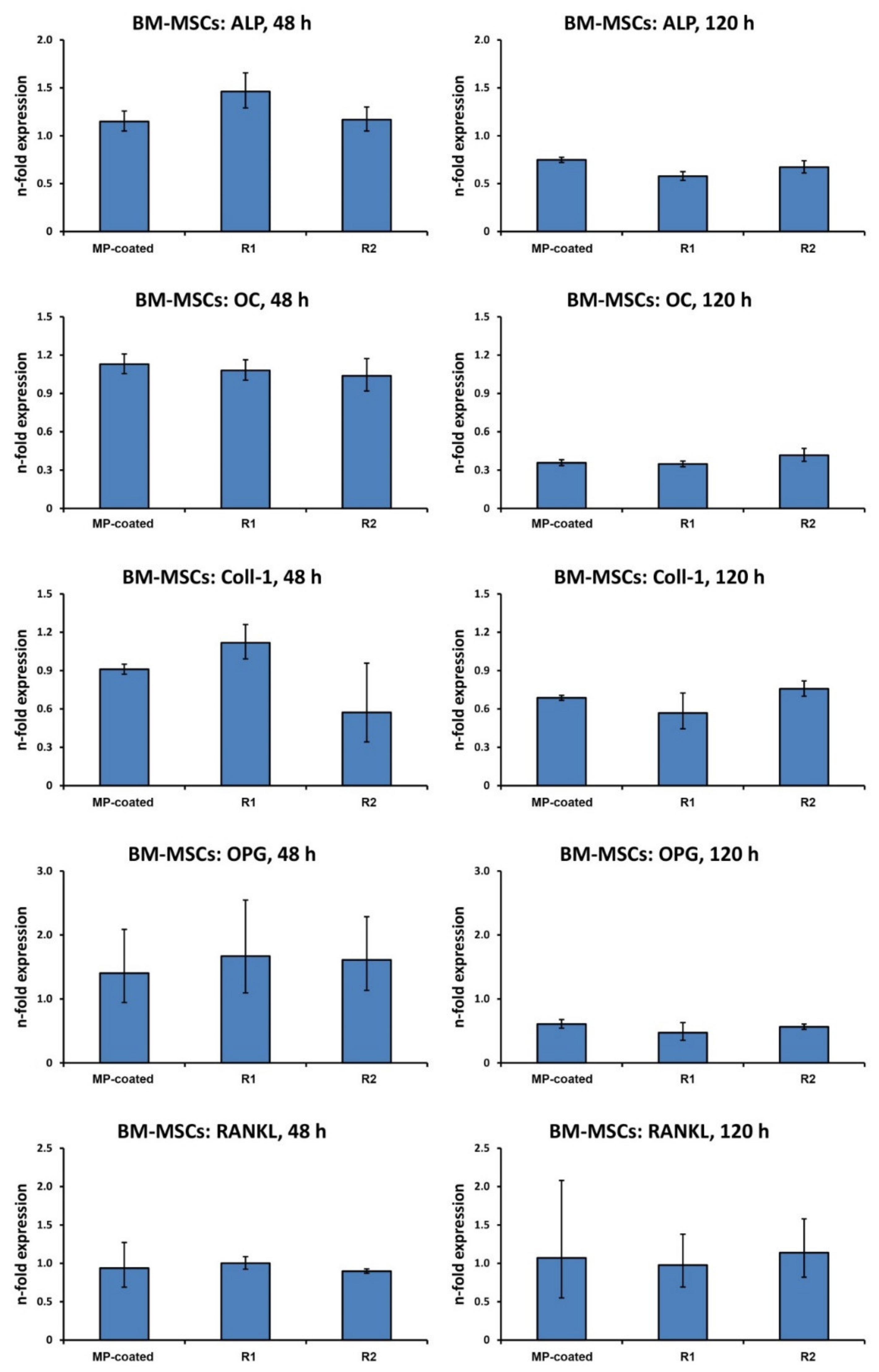
3.6. Gene Expression in BM-MSCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. Biomed. Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 1—Review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int. J. Prosthodont. 2004, 17, 536–543. [Google Scholar]
- Diamanti, M.V.; Del Curto, B.; Pedeferri, M. Anodic oxidation of titanium: From technical aspects to biomedical applications. J. Appl. Biomater. Biomech. 2011, 9, 55–69. [Google Scholar] [CrossRef]
- Rongo, R.; Ametrano, G.; Gloria, A.; Spagnuolo, G.; Galeotti, A.; Paduano, S.; Valletta, R.; D’Antò, V. Effects of intraoral aging on surface properties of coated nickel-titanium archwires. Angle Orthod. 2014, 84, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Jorge, J.R.; Barão, V.A.; Delben, J.A.; Faverani, L.P.; Queiroz, T.P.; Assunção, W.G. Titanium in dentistry: Historical development, state of the art and future perspectives. J. Indian Prosthodont. Soc. 2013, 13, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Von Salis-Soglio, M.; Stübinger, S.; Sidler, M.; Klein, K.; Ferguson, S.J.; Kämpf, K.; Zlinszky, K.; Buchini, S.; Curno, R.; Péchy, P.; et al. A novel multi-phosphonate surface treatment of titanium dental implants: A study in sheep. J. Funct. Biomater. 2014, 5, 135–157. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Dojcinovic, I.; Germon, L.; Lévy, N.; Curno, R.; Buchini, S.; Péchy, P.; Aronsson, B.O. Safety and efficacy of a biomimetic monolayer of permanently bound multi-phosphonic acid molecules on dental implants: 1 year post-loading results from a pilot quadruple-blinded randomised controlled trial. Eur. J. Oral Implantol. 2013, 6, 227–236. [Google Scholar] [PubMed]
- Russell, R.G.; Watts, N.B.; Ebetino, F.H.; Rogers, M.J. Mechanisms of action of bisphosphonates: Similarities and differences and their potential influence on clinical efficacy. Osteoporos. Int. 2008, 19, 733–759. [Google Scholar] [CrossRef]
- Banerjee, S.; Wehbi, M.; Manseri, A.; Mehdi, A.; Alaaeddine, A.; Hachem, A.; Ameduri, B. Poly(vinylidene fluoride) Containing Phosphonic Acid as Anticorrosion Coating for Steel. ACS Appl. Mater. Interfaces 2017, 9, 6433–6443. [Google Scholar] [CrossRef] [PubMed]
- Donos, N.; Hamlet, S.; Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Bosshardt, D.D.; Ivanovski, S. Gene expression profile of osseointegration of a hydrophilic compared with a hydrophobic microrough implant surface. Clin. Oral Impl. Res. 2011, 22, 365–372. [Google Scholar] [CrossRef]
- Schwarz, F.; Herten, M.; Sager, M.; Wieland, M.; Dard, M.; Becker, J. Histological and immunohistochemical analysis of initial and early osseous integration at chemically modified and conventional SLA titanium implants: Preliminary results of a pilot study in dogs. Clin. Oral Impl. Res. 2007, 18, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef] [PubMed]
- Alsabeeha, N.H.; Ma, S.; Atieh, M.A. Hydroxyapatite-coated oral implants: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Implant. 2012, 27, 1123–1130. [Google Scholar]
- Trisi, P.; Keith, D.J.; Rocco, S. Human histologic and histomorphometric analyses of hydroxyapatite-coated implants after 10 years of function: A case report. Int. J. Oral Maxillofac. Implant. 2005, 20, 124–130. [Google Scholar]
- Albrektsson, T. Hydroxyapatite-coated implants: A case against their use. J. Oral Maxillofac. Surg. 1998, 56, 1312–1326. [Google Scholar] [CrossRef]
- Tîlmaciu, C.M.; Mathieu, M.; Lavigne, J.P.; Toupet, K.; Guerrero, G.; Ponche, A.; Amalric, J.; Noël, D.; Mutin, P.H. In vitro and in vivo characterization of antibacterial activity and biocompatibility: A study on silver-containing phosphonate monolayers on titanium. Acta Biomater. 2015, 15, 266–277. [Google Scholar] [CrossRef]
- Ayre, W.N.; Scott, T.; Hallam, K.; Blom, A.W.; Denyer, S.; Bone, H.K.; Mansell, J.P. Fluorophosphonate-functionalised titanium via a pre-adsorbed alkane phosphonic acid: A novel dual action surface finish for bone regenerative applications. J. Mater. Sci. Mater. Med. 2016, 27, 36. [Google Scholar] [CrossRef]
- Marco, F.; Milena, F.; Gianluca, G.; Vittoria, O. Peri-implant osteogenesis in health and osteoporosis. Micron 2005, 36, 630–644. [Google Scholar] [CrossRef]
- Puleo, D.A.; Nanci, A. Understanding and controlling the bone-implant interface. Biomaterials 1999, 20, 2311–2321. [Google Scholar] [CrossRef]
- Pautke, C.; Schieker, M.; Tischer, T.; Kolk, A.; Neth, P.; Mutschler, W.; Milz, S. Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2 OS in comparison to human osteoblasts. Anticancer Res. 2004, 24, 3743–3748. [Google Scholar]
- Andrukhov, O.; Huber, R.; Shi, B.; Berner, S.; Rausch-Fan, X.; Moritz, A.; Spencer, N.D.; Schedle, A. Proliferation, behavior, and differentiation of osteoblasts on surfaces of different microroughness. Dent. Mater. 2016, 32, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Bosshardt, D.D.; Lang, N.P.; Abrahamsson, I.; Berglundh, T.; Lindhe, J.; Ivanovski, S.; Donos, N. Temporal sequence of hard and soft tissue healing around titanium dental implants. Periodontol. 2000 2015, 68, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Lotz, E.M.; Berger, M.B.; Schwartz, Z.; Boyan, B.D. Regulation of osteoclasts by osteoblast lineage cells depends on titanium implant surface properties. Acta Biomater. 2018, 68, 296–307. [Google Scholar] [CrossRef]
- Stiehler, M.; Lind, M.; Mygind, T.; Baatrup, A.; Dolatshahi-Pirouz, A.; Li, H.; Foss, M.; Besenbacher, F.; Kassem, M.; Bünger, C. Morphology, proliferation, and osteogenic differentiation of mesenchymal stem cells cultured on titanium, tantalum, and chromium surfaces. J. Biomed. Mater. Res. A 2008, 86, 448–458. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Suggested guidelines for the topographic evaluation of implant surfaces. Int. J. Oral Maxillofac. Implant. 2000, 15, 331–344. [Google Scholar]
- Andrukhov, O.; Andrukhova, O.; Hulan, U.; Tang, Y.; Bantleon, H.P.; Rausch-Fan, X. Both 25-hydroxyvitamin-D3 and 1,25-dihydroxyvitamin-D3 reduces inflammatory response in human periodontal ligament cells. PLoS ONE 2014, 9, e90301. [Google Scholar] [CrossRef]
- Jayasuriya, A.C.; Bhat, A. Mesenchymal stem cell function on hybrid organic/inorganic microparticles in vitro. J. Tissue Eng. Regen. Med. 2010, 4, 340–348. [Google Scholar] [CrossRef]
- Van Peer, G.; Mestdagh, P.; Vandesompele, J. Accurate RT-qPCR gene expression analysis on cell culture lysates. Sci. Rep. 2012, 2, 222. [Google Scholar] [CrossRef]
- Brånemark, P.I.; Adell, R.; Breine, U.; Hansson, B.O.; Lindström, J.; Ohlsson, A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef]
- Cheng, G.; Davoudi, Z.; Xing, X.; Cheng, X.; Li, Z.; Deng, H.; Wng, Q. Advanced Silk Fibroin Biomaterials for Cartilage Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 2704–2715. [Google Scholar] [CrossRef]
- Chen, J.; Zhan, Y.; Wang, Y.; Han, D.; Tao, B.; Luo, Z.; Ma, S.; Wang, Q.; Li, X.; Fan, L.; et al. Chitosan/silk fibroin modified nanofibrous patches with mesenchymal stem cells prevent heart remodeling post-myocardial infarction in rats. Acta Biomater. 2018, 80, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Coelho, P.G.; Kang, B.S.; Sul, Y.T.; Albrektsson, T. Classification of osseointegrated implant surfaces: Materials, chemistry and topography. Trends Biotechnol. 2010, 28, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.H.; Ben-Nissan, B.; Matinlinna, J.P.; Conway, R.C. Current perspectives: Calcium phosphate nanocoatings and nanocomposite coatings in dentistry. J. Dent. Res. 2013, 92, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Huang, X.; Hang, R.; Zhang, X.; Tang, B. Effect of a biomimetic titania mesoporous coating doped with Sr on the osteogenic activity. Mater. Sci Eng. C Mater. Biol. Appl. 2018, 91, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, K.; Yoshida, Y.; Mine, A.; Fujisawa, T.; Van Meerbeek, B.; Suzuki, K.; Kuboki, T. Chemical interaction of polyphosphoric acid with titanium and its effect on human bone marrow derived mesenchymal stem cell behavior. J. Biomed. Mater. Res. A 2007, 82, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, K.; Shimono, K.; Oshima, M.; Yoshida, Y.; Van Meerbeek, B.; Suzuki, K.; Kuboki, T. Polyphosphoric acid treatment promotes bone regeneration around titanium implants. J. Oral Rehabil. 2009, 36, 362–367. [Google Scholar] [CrossRef]
- Esposito, M.; Dojcinovic, I.; Buchini, S.; Péchy, P.; Aronsson, B.O. Safety and efficacy of a biomimetic monolayer of permanently bound multiphosphonic acid molecules on dental implants: 3 years post-loading results from a pilot quadruple-blinded randomised controlled trial. Eur. J. Oral Implantol. 2017, 10, 43–54. [Google Scholar]
- Owen, T.A.; Holthuis, J.; Markose, E.; van Wijnen, A.J.; Wolfe, S.A.; Grimes, S.R.; Lian, J.B.; Stein, G.S. Modifications of protein-DNA interactions in the proximal promoter of a cell-growth-regulated histone gene during onset and progression of osteoblast differentiation. Proc. Natl. Acad. Sci. USA 1990, 87, 5129–5133. [Google Scholar] [CrossRef]
- Shi, S.; Kirk, M.; Kahn, A.J. The role of type I collagen in the regulation of the osteoblast phenotype. J. Bone Miner. Res. 1996, 11, 1139–1145. [Google Scholar] [CrossRef]
- Boskey, A.L.; Wians, F.H.; Hauschka, P.V. The effect of osteocalcin on in vitro lipid-induced hydroxyapatite formation and seeded hydroxyapatite growth. Calcif. Tissue Int. 1985, 37, 57–62. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Bostanci, N. The RANKL-OPG system in clinical periodontology. J. Clin. Periodontol. 2012, 39, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Kaspar, D.; Sarkar, M.R.; Claes, L.E.; Ignatius, A.A. A scanning electron microscopy study of human osteoblast morphology on five orthopedic metals. J. Biomed. Mater. Res. 2002, 63, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Liangjiao, C.; Ping, Z.; Ruoyu, L.; Yanli, Z.; Ting, S.; Yanjun, L.; Longquan, S. Potential proinflammatory and osteogenic effects of dicalcium silicate particles in vitro. J. Mech. Behav. Biomed. Mater. 2015, 44, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Borsari, V.; Giavaresi, G.; Fini, M.; Torricelli, P.; Salito, A.; Chiesa, R.; Chiusoli, L.; Volpert, A.; Rimondini, L.; Giardino, R. Physical characterization of different-roughness titanium surfaces, with and without hydroxyapatite coating, and their effect on human osteoblast-like cells. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 75, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Filová, E.; Suchý, T.; Sucharda, Z.; Supová, M.; Zaloudková, M.; Balík, K.; Lisá, V.; Slouf, M.; Bačáková, L. Support for the initial attachment, growth and differentiation of MG-63 cells: A comparison between nano-size hydroxyapatite and micro-size hydroxyapatite in composites. Int. J. Nanomed. 2014, 9, 3687–3706. [Google Scholar] [CrossRef] [PubMed]
- Rausch-fan, X.; Qu, Z.; Wieland, M.; Matejka, M.; Schedle, A. Differentiation and cytokine synthesis of human alveolar osteoblasts compared to osteoblast-like cells (MG63) in response to titanium surfaces. Dent. Mater. 2008, 24, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, K.B.; Frost, A.; Nilsson, O.; Ljunghall, S.; Ljunggren, O. Three isolation techniques for primary culture of human osteoblast-like cells: A comparison. Acta Orthop. Scand. 1999, 70, 365–373. [Google Scholar] [CrossRef]
- Martinez, M.E.; del Campo, M.T.; Medina, S.; Sanchez, M.; Sanchez-Cabezudo, M.J.; Esbrit, P.; Martinez, P.; Moreno, I.; Rodrigo, A.; Garces, M.V.; et al. Influence of skeletal site of origin and donor age on osteoblastic cell growth and differentiation. Calcif. Tissue Int. 1999, 64, 280–286. [Google Scholar] [CrossRef]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Kapila, Y.; Lotz, J.; Kapila, S. Multiple differentiation capacity of STRO-1+/CD146+ PDL mesenchymal progenitor cells. Stem Cells Dev. 2009, 18, 487–496. [Google Scholar] [CrossRef]
- Heo, Y.Y.; Um, S.; Kim, S.K.; Park, J.M.; Seo, B.M. Responses of periodontal ligament stem cells on various titanium surfaces. Oral Dis. 2011, 17, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Marconi, G.D.; Diomede, F.; Pizzicannella, J.; Fonticoli, L.; Merciaro, I.; Pierdomenico, S.D.; Mazzon, E.; Piattelli, A.; Trubiani, O. Enhanced VEGF/VEGF-R and RUNX2 Expression in Human Periodontal Ligament Stem Cells Cultured on Sandblasted/Etched Titanium Disk. Front. Cell Dev. Biol. 2020, 8, 315. [Google Scholar] [CrossRef] [PubMed]
- Kilian, K.A.; Bugarija, B.; Lahn, B.T.; Mrksich, M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 4872–4877. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.S.; Carley, A.; Fleming, G.J.; Crean, S.J.; Sloan, A.J.; Waddington, R.J. Osteogenic potential of bone marrow stromal cells on smooth, roughened, and tricalcium phosphate-modified titanium alloy surfaces. Int. J. Oral Maxillofac. Implant. 2012, 27, 1029–1042. [Google Scholar]
- Lin, L.; Chow, K.L.; Leng, Y. Study of hydroxyapatite osteoinductivity with an osteogenic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. A 2009, 89, 326–335. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Hyzy, S.L.; Hutton, D.L.; Erdman, C.P.; Wieland, M.; Boyan, B.D.; Schwartz, Z. Direct and indirect effects of microstructured titanium substrates on the induction of mesenchymal stem cell differentiation towards the osteoblast lineage. Biomaterials 2010, 31, 2728–2735. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 172–184. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wehner, C.; Behm, C.; Husejnagic, S.; Moritz, A.; Rausch-Fan, X.; Andrukhov, O. Effect of Multi-Phosphonate Coating of Titanium Surfaces on Osteogenic Potential. Materials 2020, 13, 5777. https://doi.org/10.3390/ma13245777
Wehner C, Behm C, Husejnagic S, Moritz A, Rausch-Fan X, Andrukhov O. Effect of Multi-Phosphonate Coating of Titanium Surfaces on Osteogenic Potential. Materials. 2020; 13(24):5777. https://doi.org/10.3390/ma13245777
Chicago/Turabian StyleWehner, Christian, Christian Behm, Selma Husejnagic, Andreas Moritz, Xiaohui Rausch-Fan, and Oleh Andrukhov. 2020. "Effect of Multi-Phosphonate Coating of Titanium Surfaces on Osteogenic Potential" Materials 13, no. 24: 5777. https://doi.org/10.3390/ma13245777
APA StyleWehner, C., Behm, C., Husejnagic, S., Moritz, A., Rausch-Fan, X., & Andrukhov, O. (2020). Effect of Multi-Phosphonate Coating of Titanium Surfaces on Osteogenic Potential. Materials, 13(24), 5777. https://doi.org/10.3390/ma13245777




