Use of Coal Bottom Ash and CaO-CaCl2-Activated GGBFS Binder in the Manufacturing of Artificial Fine Aggregates through Cold-Bonded Pelletization
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Sample Preparation and Tests
2.2.1. Mortar Samples
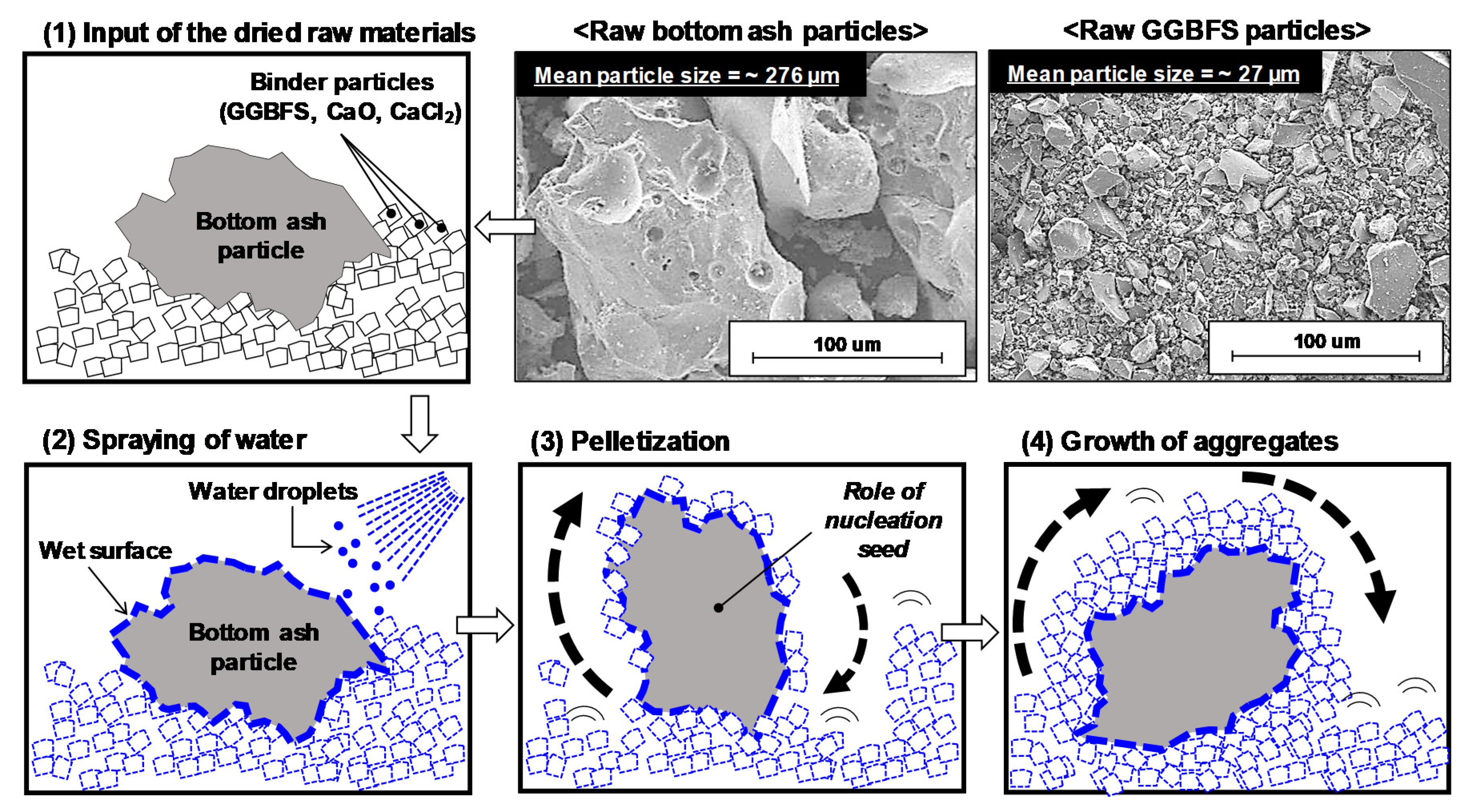
2.2.2. Aggregate Samples
3. Results and Discussion
3.1. Test Results for Mortar Samples
3.1.1. Compressive Strength Test
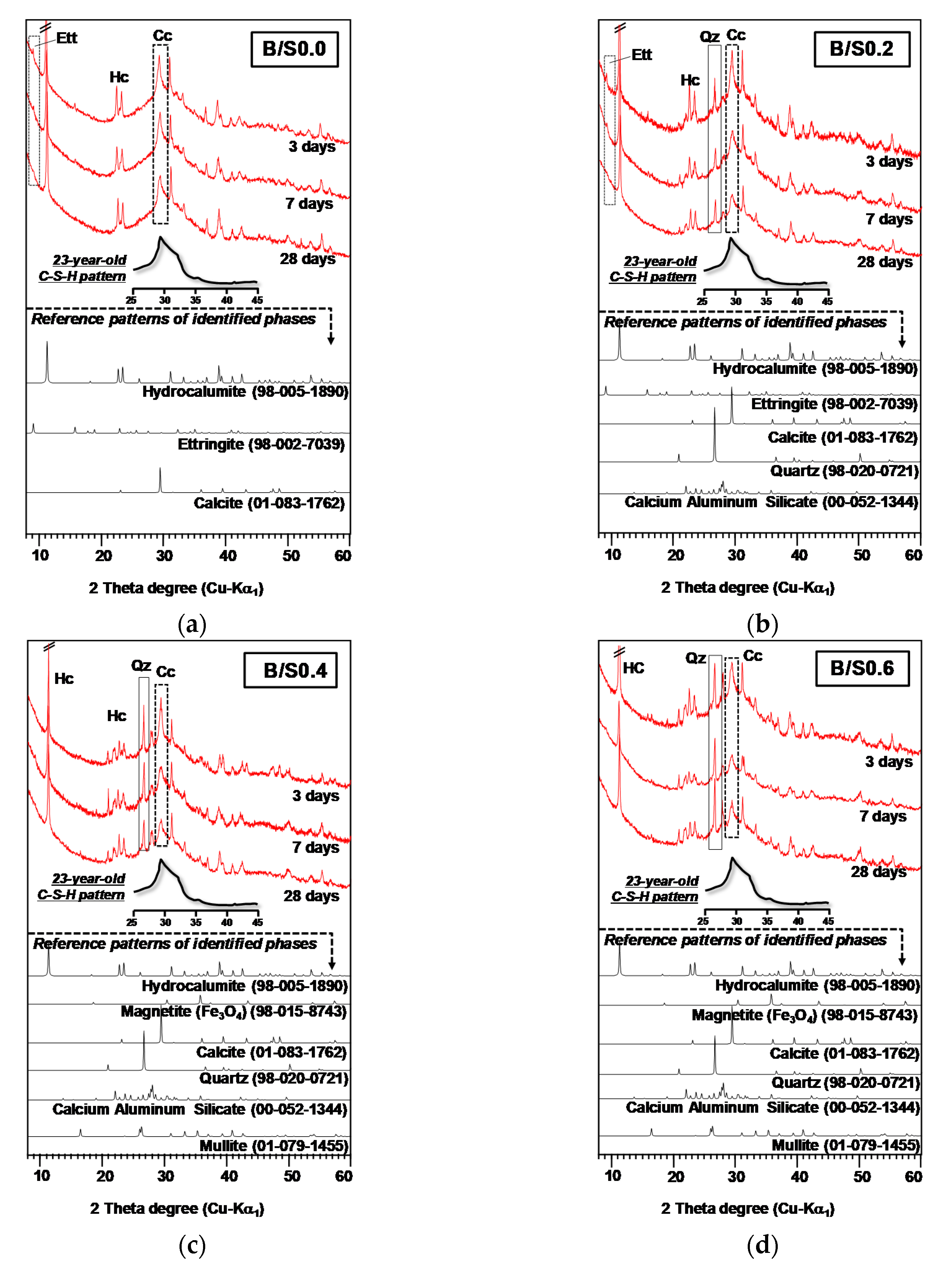
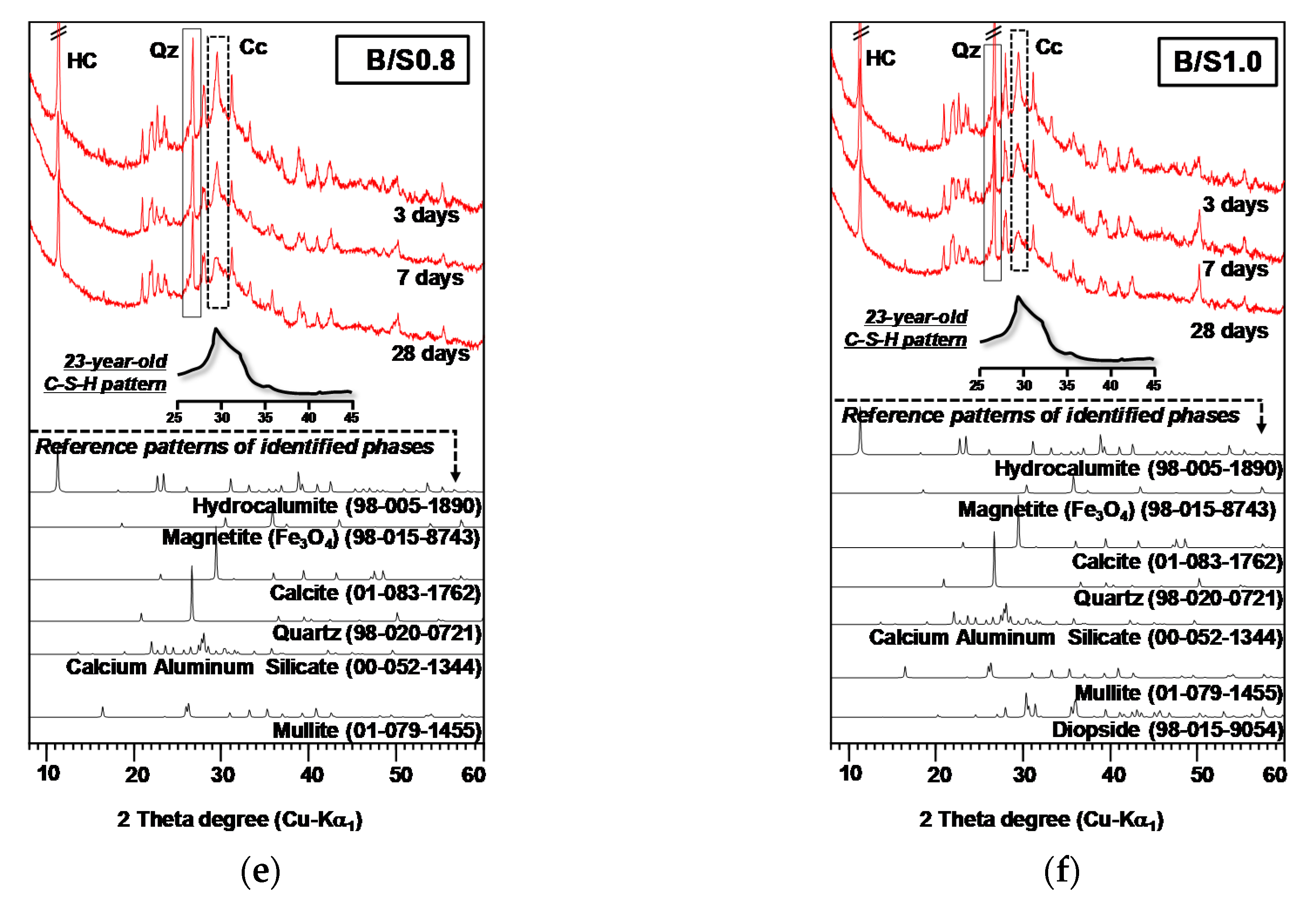
3.1.2. XRD
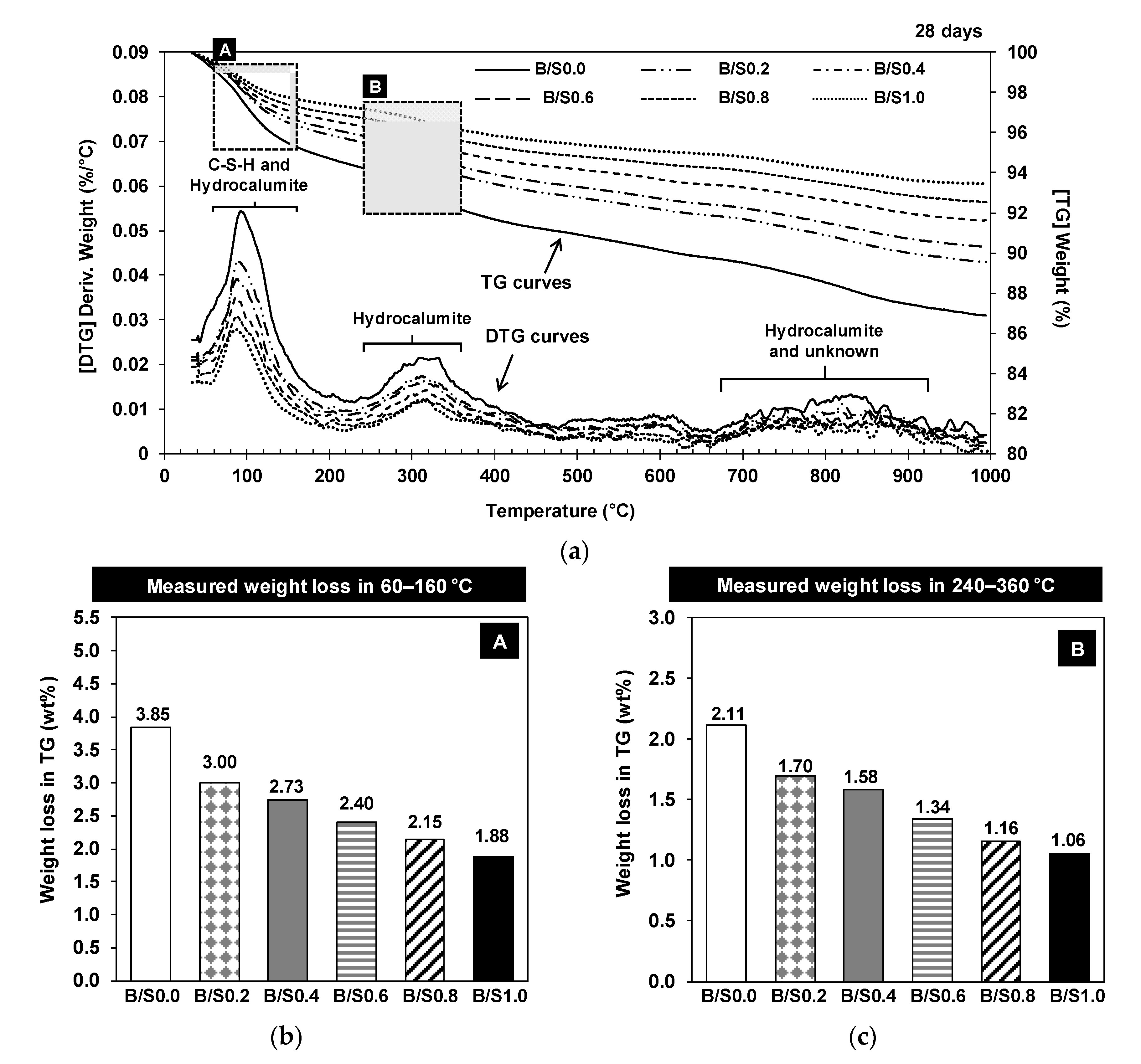
3.1.3. TG
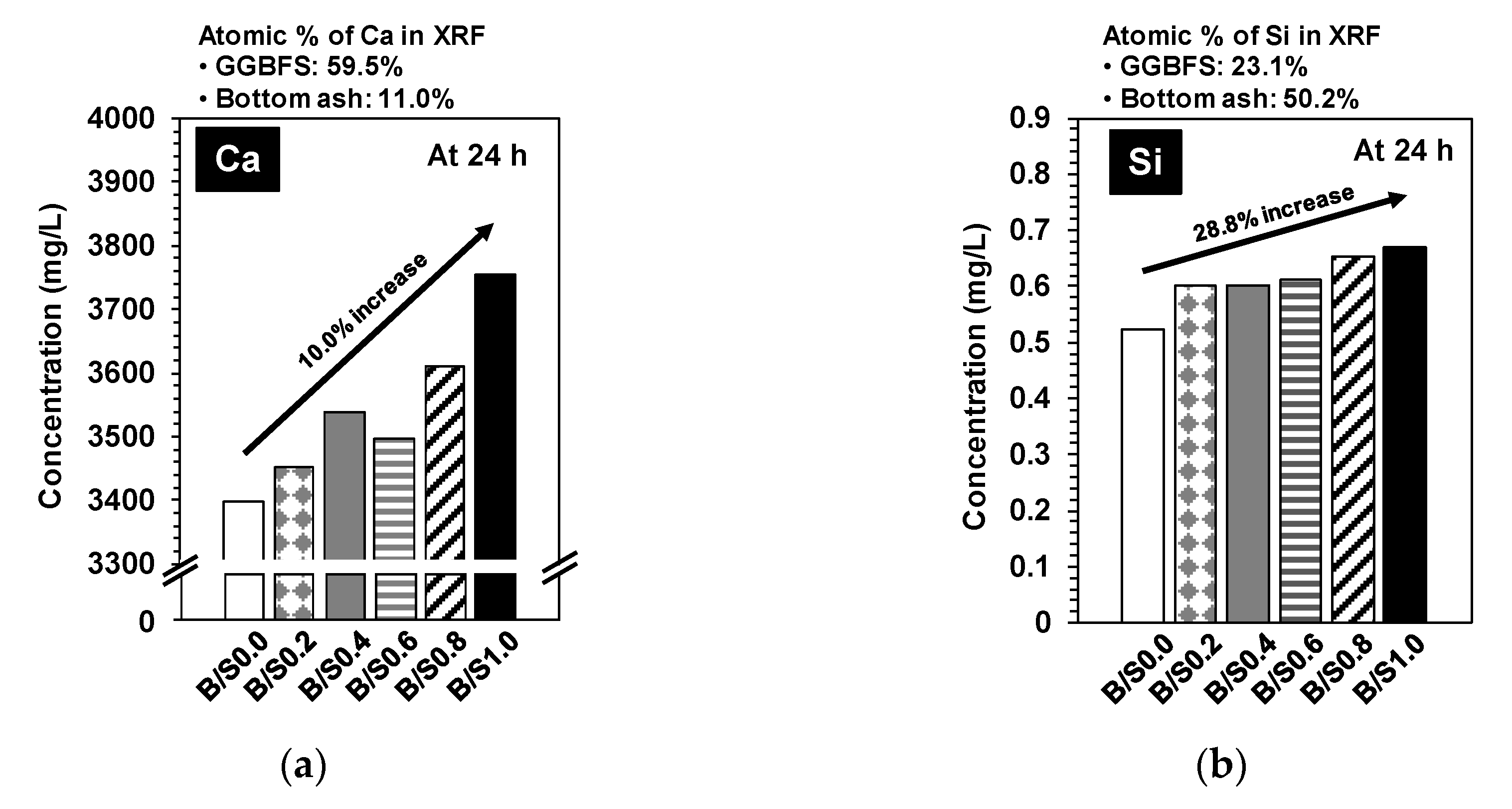
3.1.4. ICP-OES and IC
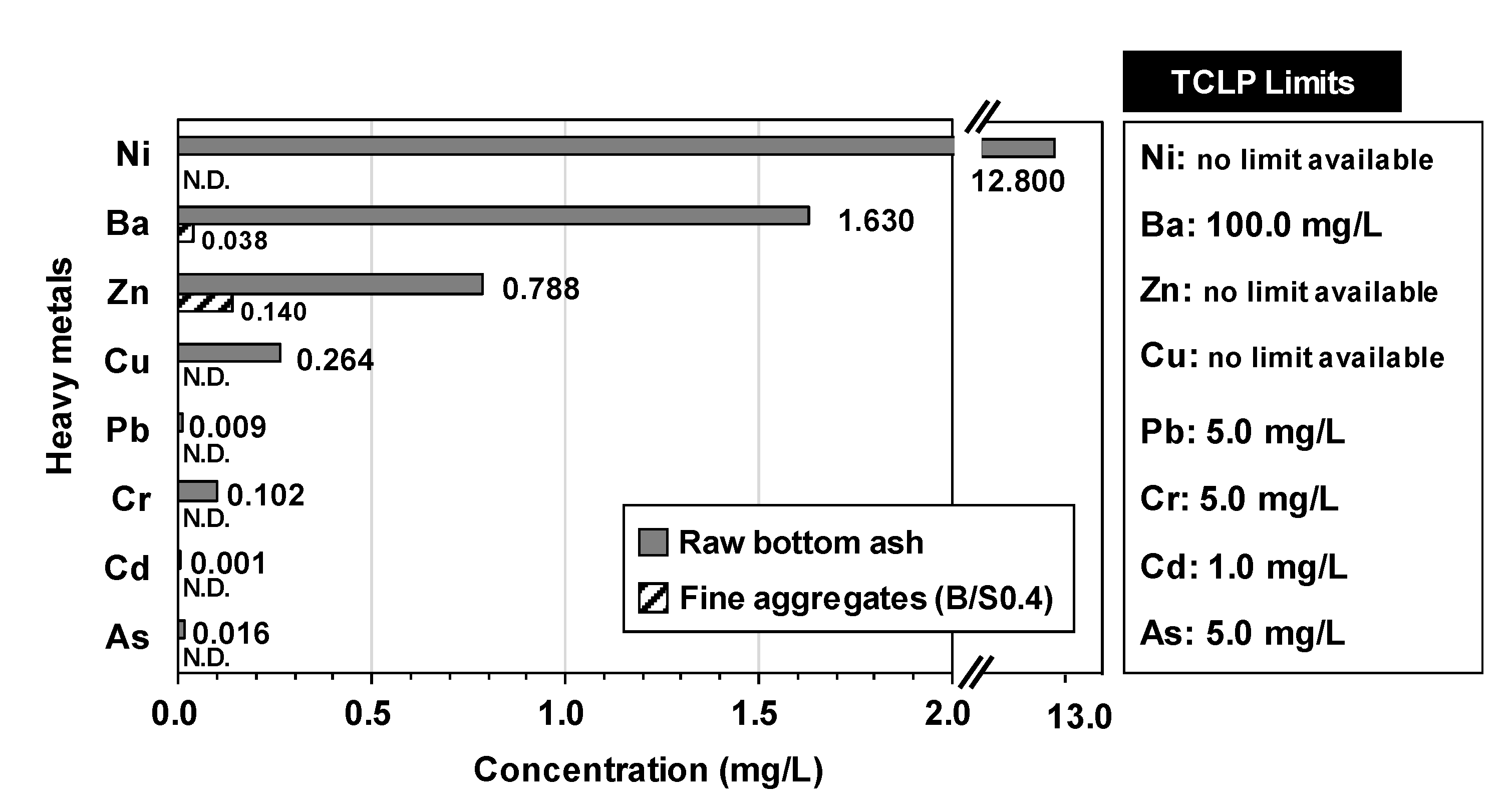
3.2. Results of Aggregate Production
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Metha, K.M.; Paulo, M. Concrete: Microstructure, Properties, and Materials; McGraw-Hill Publishing: New York, NY, USA, 2006. [Google Scholar]
- Wang, H.; Wang, L.; Li, L.; Cheng, B.; Zhang, Y.; Wei, Y. The Study on the Whole Stress–Strain Curves of Coral Fly Ash-Slag Alkali-Activated Concrete under Uniaxial Compression. Materials 2020, 13, 4291. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.; Ramli, M. Engineering properties of treated recycled concrete aggregate (RCA) for structural applications. Constr. Build. Mater. 2013, 44, 464–476. [Google Scholar] [CrossRef]
- Frigione, M. Recycling of PET bottles as fine aggregate in concrete. Waste Manag. 2010, 30, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, İ.; Bilir, T.; Özkan, Ö. Durability of concrete incorporating non-ground blast furnace slag and bottom ash as fine aggregate. Build. Environ. 2007, 42, 2651–2659. [Google Scholar] [CrossRef]
- Ghassemi, M.; Andersen, P.K.; Ghassemi, A.; Chianelli, R.R. Hazardous Waste from Fossil Fuels. In Encyclopedia of Energy; Cleveland, C.J., Ed.; Elsevier: New York, NY, USA, 2004; pp. 119–131. [Google Scholar] [CrossRef]
- Hashemi, S.S.G.; Mahmud, H.B.; Ghuan, T.C.; Chin, A.B.; Kuenzel, C.; Ranjbar, N. Safe disposal of coal bottom ash by solidification and stabilization techniques. Constr. Build. Mater. 2019, 197, 705–715. [Google Scholar] [CrossRef]
- Cheriaf, M.; Rocha, J.C.; Péra, J. Pozzolanic properties of pulverized coal combustion bottom ash. Cem. Concr. Res. 1999, 29, 1387–1391. [Google Scholar] [CrossRef]
- Wyrzykowski, M.; Ghourchian, S.; Sinthupinyo, S.; Chitvoranund, N.; Chintana, T.; Lura, P. Internal curing of high performance mortars with bottom ash. Cem. Concr. Compos. 2016, 71, 1–9. [Google Scholar] [CrossRef]
- Ong, S.K.; Mo, K.H.; Alengaram, U.J.; Jumaat, M.Z.; Ling, T.-C. Valorization of Wastes from Power Plant, Steel-Making and Palm Oil Industries as Partial Sand Substitute in Concrete. Waste Biomass Valorization 2018, 9, 1645–1654. [Google Scholar] [CrossRef]
- Siddique, R. Utilization of Industrial By-products in Concrete. Procedia Eng. 2014, 95, 335–347. [Google Scholar] [CrossRef]
- Gooi, S.; Mousa, A.A.; Kong, D. A critical review and gap analysis on the use of coal bottom ash as a substitute constituent in concrete. J. Clean. Prod. 2020, 268, 121752. [Google Scholar] [CrossRef]
- Kim, H.; Ha, K.; Lee, H.-K. Internal-curing efficiency of cold-bonded coal bottom ash aggregate for high-strength mortar. Constr. Build. Mater. 2016, 126, 1–8. [Google Scholar] [CrossRef]
- Gesoğlu, M.; Özturan, T.; Güneyisi, E. Shrinkage cracking of lightweight concrete made with cold-bonded fly ash aggregates. Cem. Concr. Res. 2004, 34, 1121–1130. [Google Scholar] [CrossRef]
- Bui, L.A.-T.; Hwang, C.-L.; Chen, C.-T.; Lin, K.-L.; Hsieh, M.-Y. Manufacture and performance of cold bonded lightweight aggregate using alkaline activators for high performance concrete. Constr. Build. Mater. 2012, 35, 1056–1062. [Google Scholar] [CrossRef]
- Gesoğlu, M.; Özturan, T.; Güneyisi, E. Effects of fly ash properties on characteristics of cold-bonded fly ash lightweight aggregates. Constr. Build. Mater. 2007, 21, 1869–1878. [Google Scholar] [CrossRef]
- Ferone, C.; Colangelo, F.; Messina, F.; Iucolano, F.; Liguori, B.; Cioffi, R. Coal combustion wastes reuse in low energy artificial aggregates manufacturing. Materials 2013, 6, 5000–5015. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.K.; Bordoloi, D.; Borthakur, P.C. Investigation on reduction of cement binder in cold bonded pelletization of iron ore fines. Int. J. Min. Process. 1997, 49, 97–105. [Google Scholar] [CrossRef]
- Geetha, S.; Ramamurthy, K. Environmental friendly technology of cold-bonded bottom ash aggregate manufacture through chemical activation. J. Clean. Prod. 2010, 18, 1563–1569. [Google Scholar] [CrossRef]
- Kockal, N.U.; Ozturan, T. Effects of lightweight fly ash aggregate properties on the behavior of lightweight concretes. J. Hazard. Mater. 2010, 179, 954–965. [Google Scholar] [CrossRef]
- Yum, W.S.; Jeong, Y.; Yoon, S.; Jeon, D.; Jun, Y.; Oh, J.E. Effects of CaCl2 on hydration and properties of lime (CaO)-activated slag/fly ash binder. Cem. Concr. Compos. 2017, 84, 111–123. [Google Scholar] [CrossRef]
- Jeong, Y.; Yum, W.S.; Moon, J.; Oh, J.E. Utilization of precipitated CaCO3 from carbon sequestration of industrially emitted CO2 in cementless CaO-activated blast-furnace slag binder system. J. Clean. Prod. 2017, 166, 649–659. [Google Scholar] [CrossRef]
- Pert HighScore Plus Software; Version 3.0 e; Malvern Panalytical: Malvern, The Netherlands, 2012.
- Andersen, M.D.; Jakobsen, H.J.; Skibsted, J. Incorporation of aluminum in the calcium silicate hydrate (C-S-H) of hydrated Portland cements: A high-field 27Al and 29Si MAS NMR investigation. Inorg. Chem. 2003, 42, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Allmann, R.; Hinek, R. The introduction of structure types into the Inorganic Crystal Structure Database ICSD. Acta Crystallogr. Sect. A Found. Crystallogr. 2007, 63, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Jeon, D.; Yum, W.S.; Jeong, Y.; Oh, J.E. Properties of quicklime (CaO)-activated Class F fly ash with the use of CaCl2. Cem. Concr. Res. 2018, 111, 147–156. [Google Scholar] [CrossRef]
- Jeon, D.; Yum, W.S.; Song, H.; Sim, S.; Oh, J.E. The temperature-dependent action of sugar in the retardation and strength improvement of Ca(OH)2-Na2CO3-activated fly ash systems through calcium complexation. Constr. Build. Mater. 2018, 190, 918–928. [Google Scholar] [CrossRef]
- Baykal, G.; Döven, A.G. Utilization of fly ash by pelletization process; theory, application areas and research results. Resour. Conserv. Recycl. 2000, 30, 59–77. [Google Scholar] [CrossRef]
- Bassani, M.; Diaz Garcia, J.C.; Meloni, F.; Volpatti, G.; Zampini, D. Recycled coarse aggregates from pelletized unused concrete for a more sustainable concrete production. J. Clean. Prod. 2019, 219, 424–432. [Google Scholar] [CrossRef]
- Standard Test Method for Density, Relative Density (Specific Gravity), and Absorption of Fine Aggregate; ASTM International (ASTM): West Conshohocken, PA, USA, 2015.
- Method 1311 Toxicity Characteristic Leaching Procedure (TCLP); Agency, E.P., Ed.; Environmental Protection Agency (EPA): Washington, DC, USA, 1992. [Google Scholar]
- Taylor, H.F. Cement Chemistry; Thomas Telford London: London, UK, 1997; Volume 2. [Google Scholar]
- Oh, J.E.; Monteiro, P.J.; Jun, S.S.; Choi, S.; Clark, S.M. The evolution of strength and crystalline phases for alkali-activated ground blast furnace slag and fly ash-based geopolymers. Cem. Concr. Res. 2010, 40, 189–196. [Google Scholar] [CrossRef]
- Song, H.; Jeong, Y.; Bae, S.; Jun, Y.; Yoon, S.; Oh, J.E. A study of thermal decomposition of phases in cementitious systems using HT-XRD and TG. Constr. Build. Mater. 2018, 169, 648–661. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Birnin-Yauri, U.; Glasser, F. Friedel’s salt, Ca2Al(OH)6 (Cl, OH) 2H2O: Its solid solutions and their role in chloride binding. Cem. Concr. Res. 1998, 28, 1713–1723. [Google Scholar] [CrossRef]
- Wang, K.; Shah, S.P.; Mishulovich, A. Effects of curing temperature and NaOH addition on hydration and strength development of clinker-free CKD-fly ash binders. Cem. Concr. Res. 2004, 34, 299–309. [Google Scholar] [CrossRef]
- Vieille, L.; Rousselot, I.; Leroux, F.; Besse, J.-P.; Taviot-Guého, C. Hydrocalumite and its polymer derivatives. 1. Reversible thermal behavior of Friedel’s salt: A direct observation by means of high-temperature in situ powder X-ray diffraction. Chem. Mater. 2003, 15, 4361–4368. [Google Scholar] [CrossRef]
- Petrucci, R.H.; Herring, F.G.; Bissonnette, C.; Madura, J.D. General Chemistry: Principles and Modern Applications, 11th ed.; Pearson: London, UK, 2016. [Google Scholar]
- Concrete Aggregate (KS F 2527); Korean Standards Association: Seoul, Korea, 2016.
- Beddu, S.; Abd Manan, T.S.B.; Zainoodin, M.M.; Khan, T.; Wan Mohtar, W.H.M.; Nurika, O.; Jusoh, H.; Yavari, S.; Kamal, N.L.M.; Ghanim, A.A.; et al. Dataset on leaching properties of coal ashes from Malaysian coal power plant. Data Brief 2020, 31, 105843. [Google Scholar] [CrossRef] [PubMed]
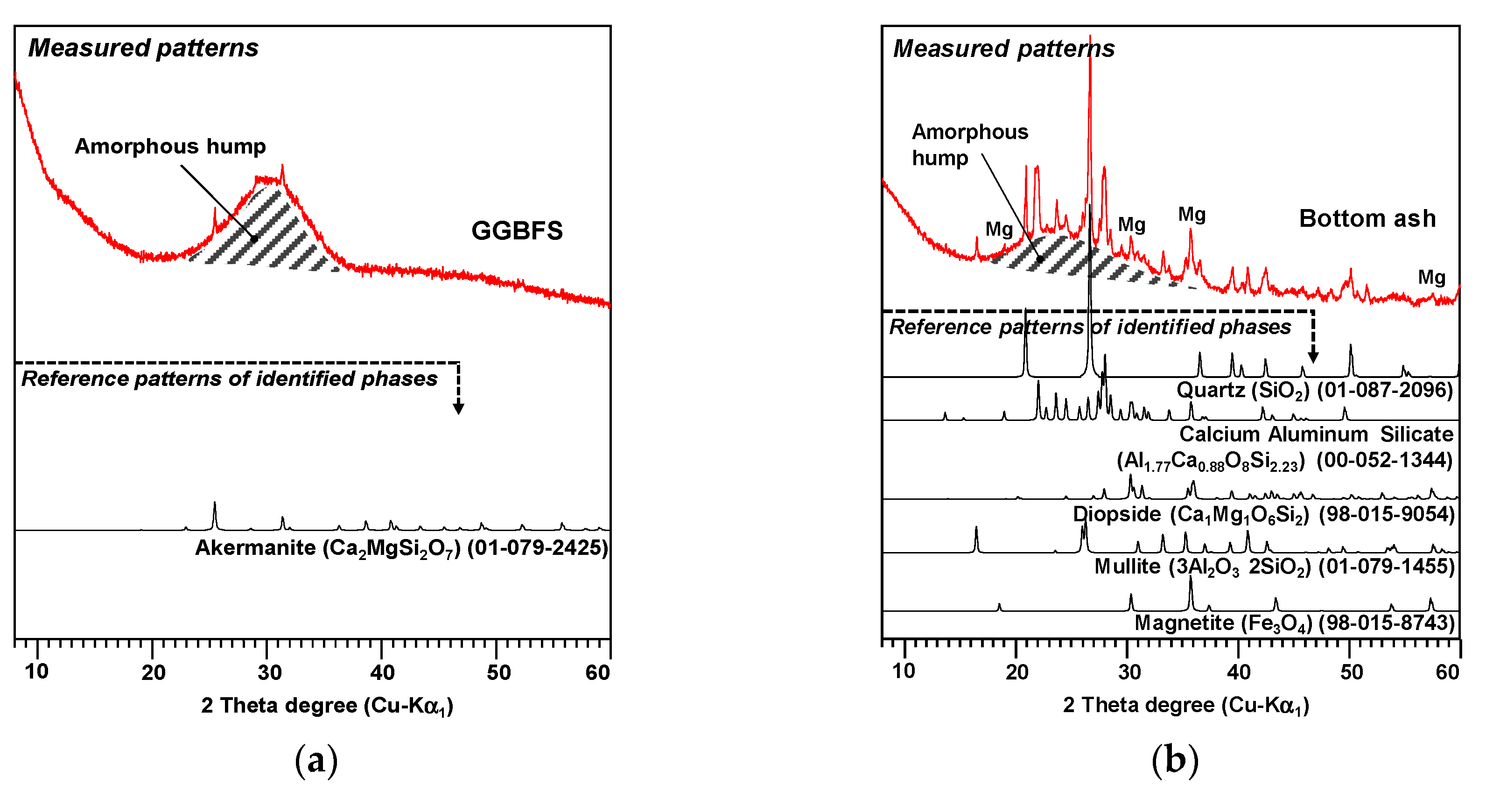

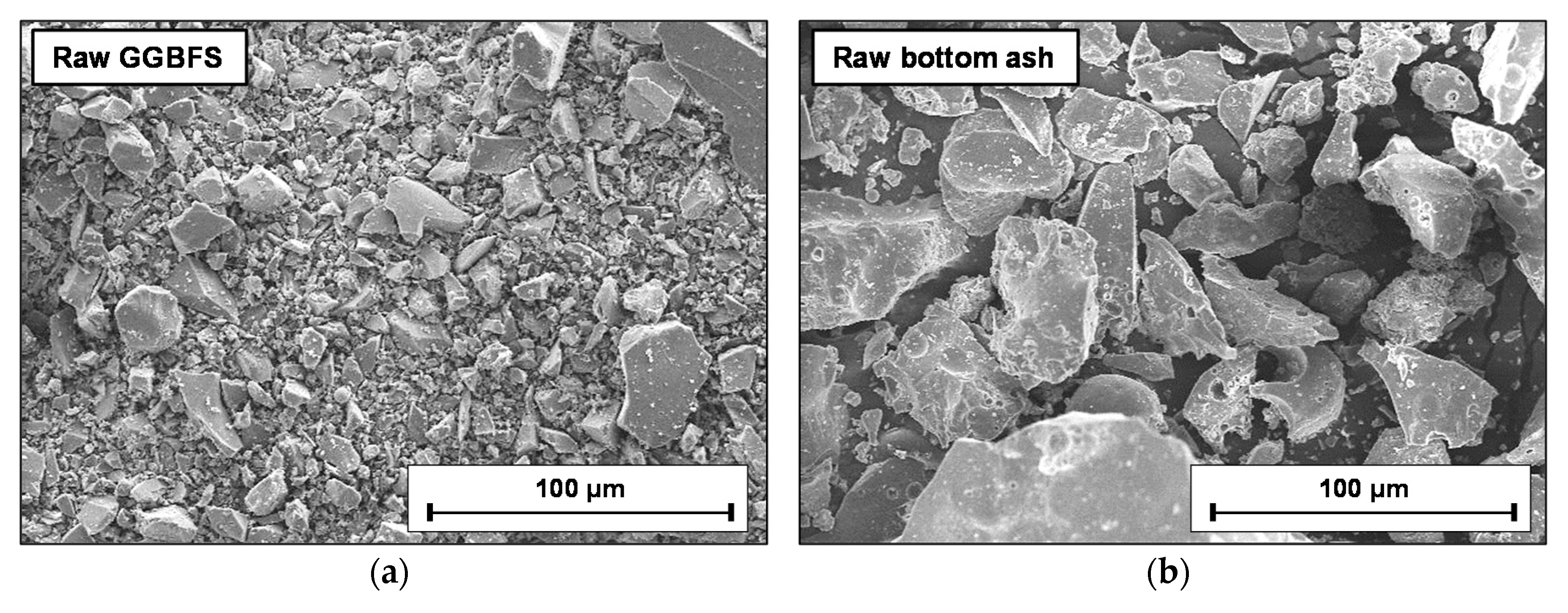
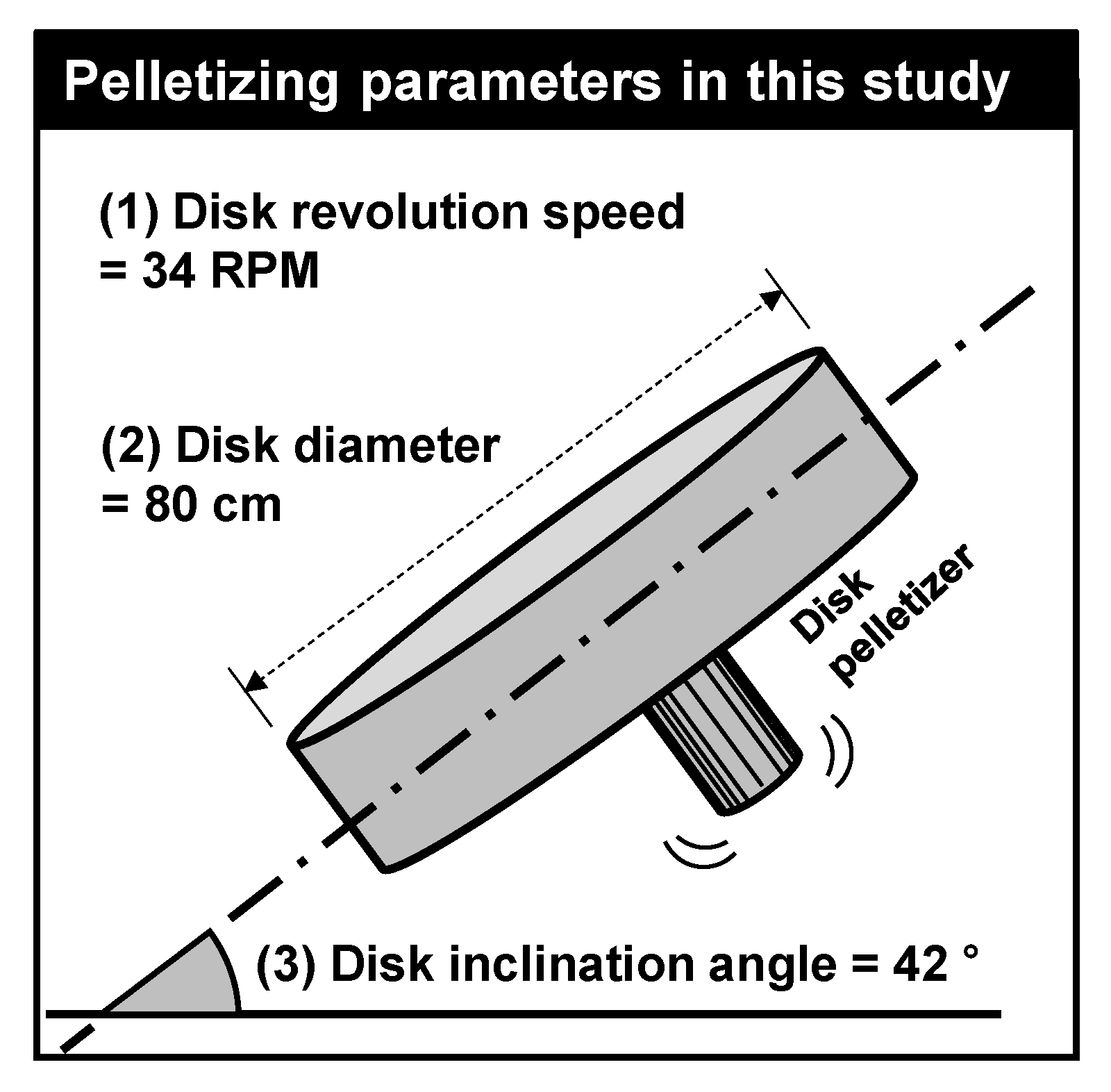
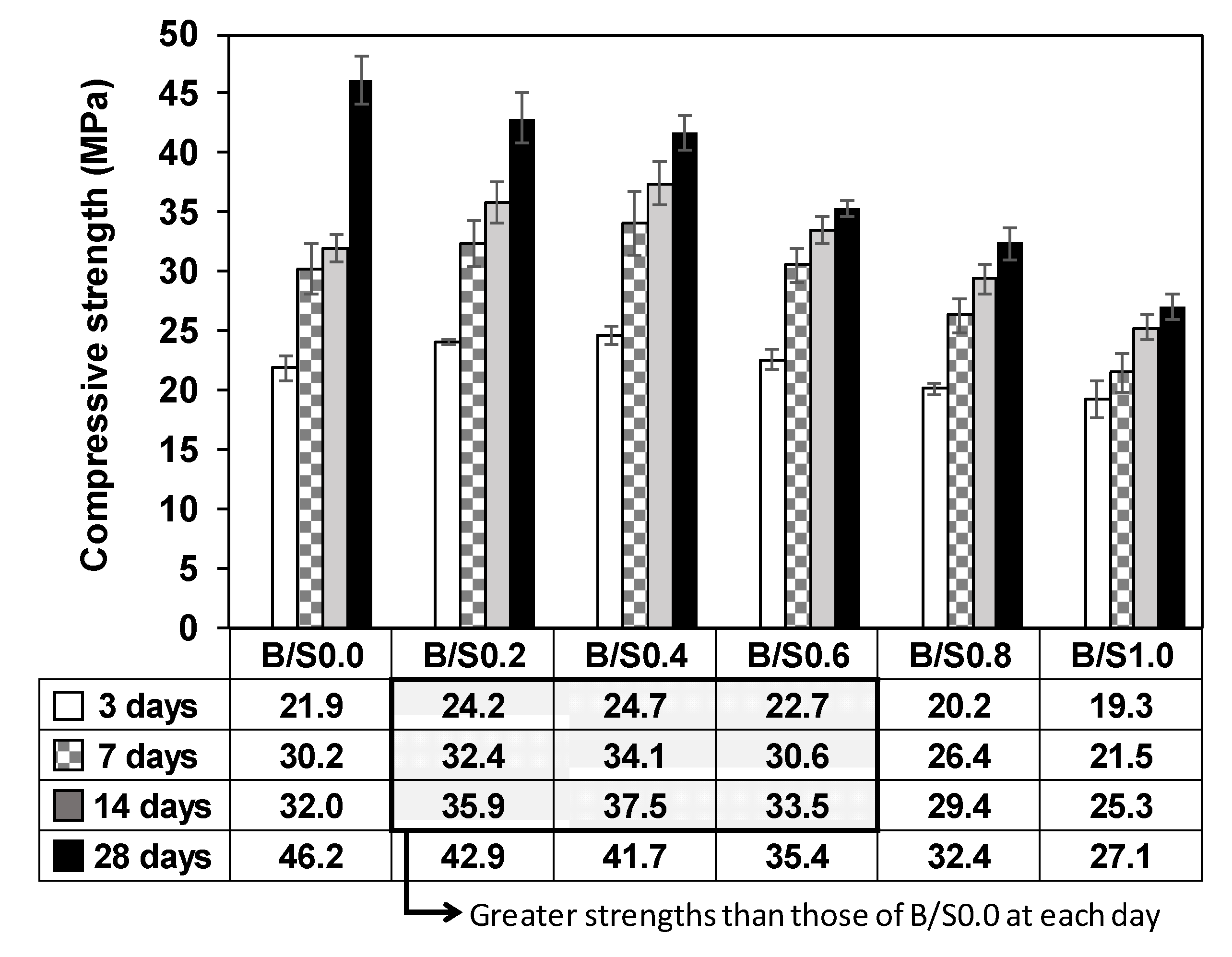



| GGBFS | Bottom Ash | ||||||
|---|---|---|---|---|---|---|---|
| Element (atomic%) | Oxide (wt %) | Element (atomic%) | Oxide (wt %) | ||||
| Ca | 59.5 | CaO | 45.1 | Si | 50.2 | SiO2 | 60.4 |
| Si | 23.1 | SiO2 | 33.6 | Al | 15.4 | Al2O3 | 18.4 |
| Al | 9.7 | Al2O3 | 13.3 | Fe | 13.3 | Fe2O3 | 7.4 |
| Mg | 2.5 | MgO | 3.2 | Ca | 11.0 | CaO | 6.8 |
| S | 1.4 | SO3 | 2.1 | K | 2.0 | MgO | 1.6 |
| Ti | 1.0 | TiO2 | 0.8 | Ti | 1.7 | Na2O | 1.4 |
| Mn | 0.8 | MnO | 0.5 | Na | 1.5 | TiO2 | 1.2 |
| Fe | 0.7 | K2O | 0.5 | Mg | 1.5 | K2O | 1.1 |
| K | 0.7 | Fe2O3 | 0.4 | Sr | 0.8 | MoO3 | 0.4 |
| Na | 0.3 | Na2O | 0.3 | Mo | 0.8 | P2O5 | 0.3 |
| Sr | 0.1 | SrO | 0.1 | Ba | 0.7 | BaO | 0.3 |
| Ba | 0.1 | BaO | 0.1 | Nb | 0.5 | SrO | 0.3 |
| Zr | 0.09 | ZrO2 | 0.1 | P | 0.3 | Tb4O7 | 0.1 |
| V | 0.03 | V2O5 | 0.0 | Mn | 0.2 | MnO | 0.08 |
| Y | 0.02 | P2O5 | 0.0 | Cl | 0.06 | SO3 | 0.06 |
| P | 0.01 | Y2O3 | 0.0 | S | 0.03 | Cl | 0.03 |
| Nb | 0.00 | Cr2O3 | 0.0 | Cu | 0.03 | V2O5 | 0.03 |
| LOI (wt %) | 0.41% | LOI (wt %) | 0.68% | ||||
| Label | Binder (b) | Bottom Ash (B) | Binder Fraction in Mixture (Fr) (= S/(S + B)) | Water (w) | |||
|---|---|---|---|---|---|---|---|
| GGBFS | CaO | CaCl2 | Total (S) | ||||
| B/S0.0 | 94 | 4 | 2 | 100 | 0 | 1.00 | 40 (w/b = 0.4) |
| B/S0.2 | 20 | 0.83 | |||||
| B/S0.4 | 40 | 0.71 | |||||
| B/S0.6 | 60 | 0.63 | |||||
| B/S0.8 | 80 | 0.56 | |||||
| B/S1.0 | 100 | 0.50 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, D.; Yum, W.S.; Song, H.; Yoon, S.; Bae, Y.; Oh, J.E. Use of Coal Bottom Ash and CaO-CaCl2-Activated GGBFS Binder in the Manufacturing of Artificial Fine Aggregates through Cold-Bonded Pelletization. Materials 2020, 13, 5598. https://doi.org/10.3390/ma13245598
Jeon D, Yum WS, Song H, Yoon S, Bae Y, Oh JE. Use of Coal Bottom Ash and CaO-CaCl2-Activated GGBFS Binder in the Manufacturing of Artificial Fine Aggregates through Cold-Bonded Pelletization. Materials. 2020; 13(24):5598. https://doi.org/10.3390/ma13245598
Chicago/Turabian StyleJeon, Dongho, Woo Sung Yum, Haemin Song, Seyoon Yoon, Younghoon Bae, and Jae Eun Oh. 2020. "Use of Coal Bottom Ash and CaO-CaCl2-Activated GGBFS Binder in the Manufacturing of Artificial Fine Aggregates through Cold-Bonded Pelletization" Materials 13, no. 24: 5598. https://doi.org/10.3390/ma13245598
APA StyleJeon, D., Yum, W. S., Song, H., Yoon, S., Bae, Y., & Oh, J. E. (2020). Use of Coal Bottom Ash and CaO-CaCl2-Activated GGBFS Binder in the Manufacturing of Artificial Fine Aggregates through Cold-Bonded Pelletization. Materials, 13(24), 5598. https://doi.org/10.3390/ma13245598








