Environmentally-Benign Phytic Acid-Based Multilayer Coating for Flame Retardant Cotton
Abstract
1. Introduction
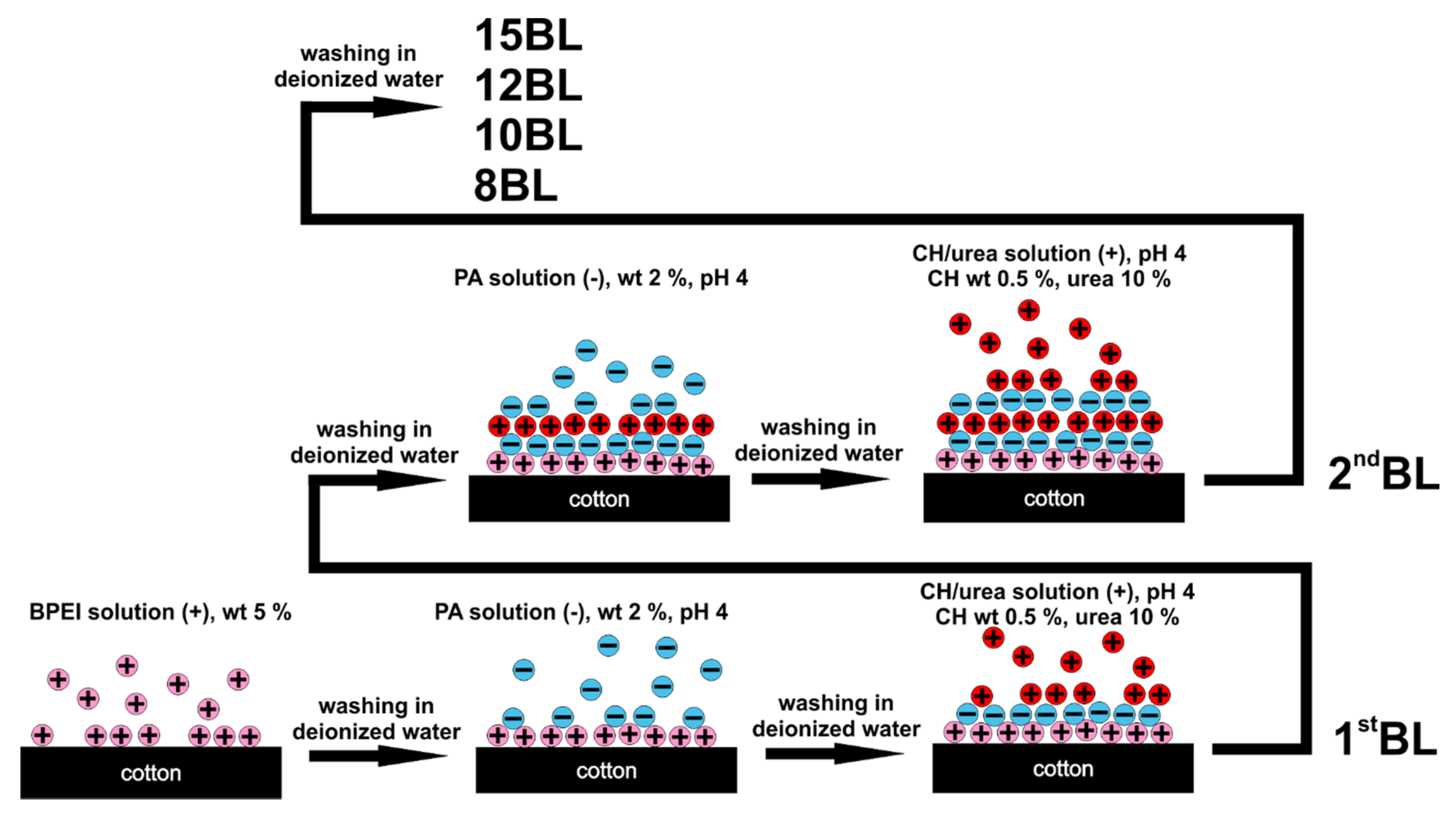
2. Materials and Methods
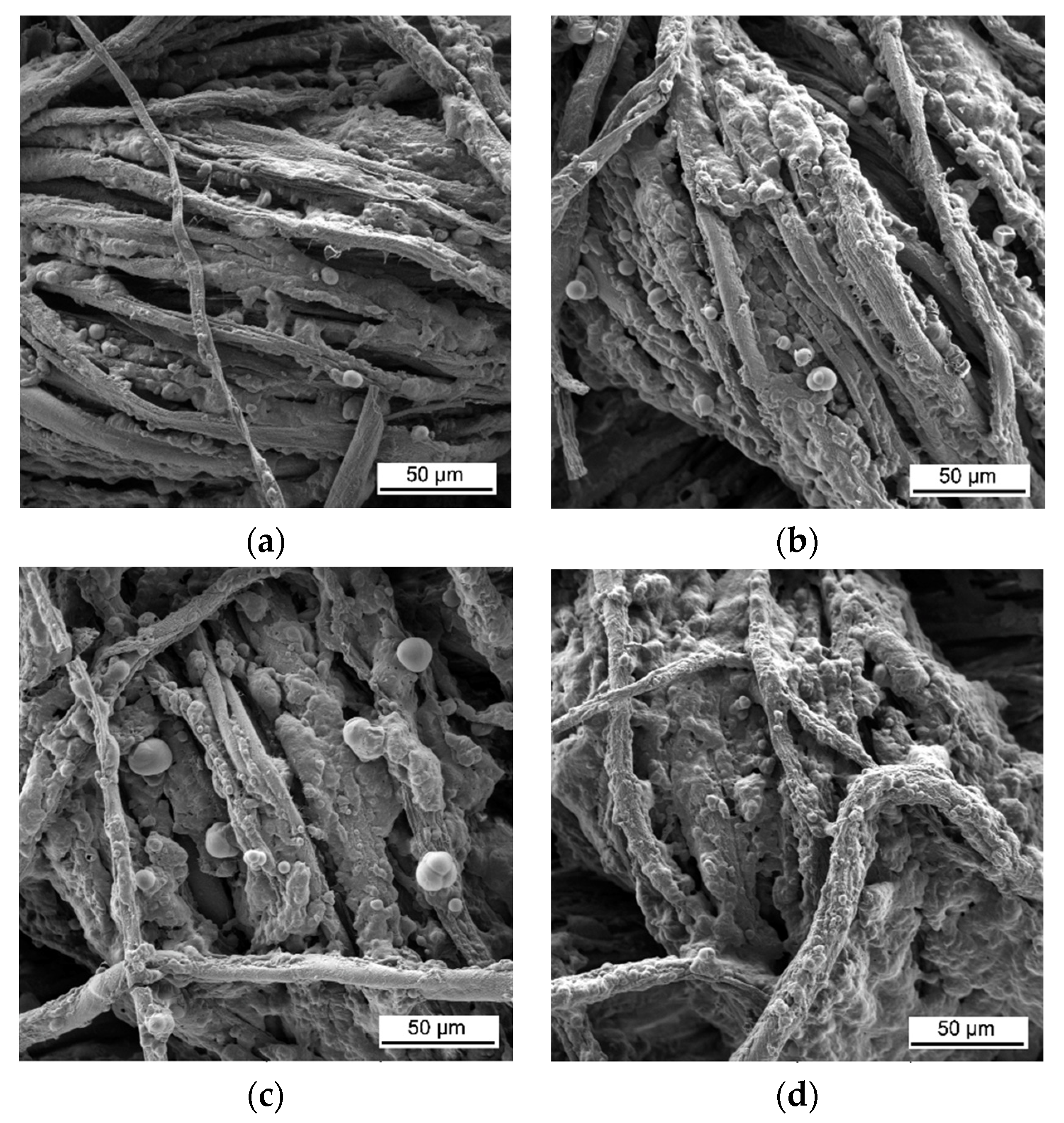
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Magovac, E.; Bischof, S. Non-halogen FR treatment of cellulosic textiles. Tekstil 2015, 64, 298–309. [Google Scholar]
- Hull, T.R.; Law, R.J.; Bergman, Å. Environmental drivers for replacement of halogenated flame retardants. In Polymer Green Flame Retardants, 1st ed.; Papaspyrides, C.D., Kiliaris, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 119–179. [Google Scholar]
- Yang, J.; Zhao, Y.; Li, M.; Du, M.; Li, X.; Li, Y. A review of a class of emerging contaminants: The classification, distribution, intensity of consumption, synthesis routes, environmental effects and expectation of pollution abatement to organophosphate flame retardants (opfrs). Int. J. Mol. Sci. 2019, 20, 2874. [Google Scholar] [CrossRef]
- Mohamed, A.L.; Hassabo, A.G. Flame retardant of cellulosic materials and their composites. In Flame Retardants; Visakh, P.M., Arao, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 247–314. [Google Scholar]
- Yang, C.Q. Flame resistant cotton. In Handbook of Fire Resistant Textiles; Kilinc, F.S., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 208–2012. [Google Scholar]
- Schramm, C.; Bischof-Vukusic, S.; Katovic, D. Non-formaldehyde durable press finishing of dyed fabrics: Evaluation of cotton-bound polycarboxylic acids. Color. Technol. 2002, 118, 244–249. [Google Scholar] [CrossRef]
- Holder, K.M.; Smith, R.J.; Grunlan, J.C. A review of flame retardant nanocoatings prepared using layer-by-layer assembly of polyelectrolytes. J. Mater. Sci. 2017, 52, 12923–12959. [Google Scholar] [CrossRef]
- Malucelli, G. Surface-engineered fire protective coatings for fabrics through sol-gel and layer-by-layer methods: An overview. Coatings 2016, 6, 33. [Google Scholar] [CrossRef]
- Richardson, J.J.; Cui, J.; Björnmalm, M.; Braunger, J.A.; Ejima, H.; Caruso, F. Innovation in layer-by-layer assembly. Chem. Rev. 2016, 116, 14828–14867. [Google Scholar] [CrossRef]
- Richardson, J.J.; Bjornmalm, M.; Caruso, F. Technology-driven layer-by-layer assembly of nanofilms. Science 2015, 348, aaa2491. [Google Scholar] [CrossRef]
- Stana-Kleinschek, K.; Ribitsch, V. Electrokinetic properties of processed cellulose fibers. Colloids Surf. A Physicochem. Eng. Asp. 1998, 140, 127–138. [Google Scholar] [CrossRef]
- Tarbuk, A.; Grancaric, A.M.; Leskovac, M. Novel cotton cellulose by cationisation during the mercerisation process-part 1: Chemical and morphological changes. Cellulose 2014, 21, 2167–2179. [Google Scholar] [CrossRef]
- Wågberg, L. Cellulose fibers and fibrils as templates for the layer-by-layer (LbL) technology. In Multilayer Thin Films: Sequential Assembly of Nanocomposite Materials, 2nd ed.; Decher, G., Schlenoff, J.B., Eds.; Wiley-VCH: New York, NY, USA, 2012; Volume 1, pp. 171–187. [Google Scholar]
- Alongi, J.; Carosio, F.; Frache, A.; Malucelli, G. Layer-by-layer coatings assembled through dipping, vertical or horizontal spray for cotton flame retardancy. Carbohydr. Polym. 2013, 92, 114–119. [Google Scholar] [CrossRef]
- Xue, C.H.; Wu, Y.; Guo, X.J.; Liu, B.Y.; Wang, H.D.; Jia, S.T. Superhydrophobic, flame-retardant and conductive cotton fabrics via layer-by-layer assembly of carbon nanotubes for flexible sensing electronics. Cellulose 2020, 27, 3455–3468. [Google Scholar] [CrossRef]
- Lin, D.; Zeng, X.; Li, H.; Lai, X. Facile fabrication of superhydrophobic and flame-retardant coatings on cotton fabrics via layer-by-layer assembly. Cellulose 2018, 25, 3135–3149. [Google Scholar] [CrossRef]
- Liu, L.; Huang, Z.; Pan, Y.; Wang, X.; Song, L.; Hu, Y. Finishing of cotton fabrics by multi-layered coatings to improve their flame retardancy and water repellency. Cellulose 2018, 25, 4791–4803. [Google Scholar] [CrossRef]
- Li, S.; Lin, X.; Liu, Y.; Li, R.; Ren, X.; Huang, T.-S.T.S. Phosphorus-nitrogen-silicon-based assembly multilayer coating for the preparation of flame retardant and antimicrobial cotton fabric. Cellulose 2019, 26, 4213–4223. [Google Scholar] [CrossRef]
- Li, P.; Wang, B.; Liu, Y.Y.; Xu, Y.J.; Jiang, Z.M.; Dong, C.H.; Zhang, L.; Liu, Y.; Zhu, P. Fully bio-based coating from chitosan and phytate for fire-safety and antibacterial cotton fabrics. Carbohydr. Polym. 2020, 237, 116173. [Google Scholar] [CrossRef]
- Laufer, G.; Kirkland, C.; Morgan, A.B.; Grunlan, J.C. Intumescent multilayer nanocoating, made with renewable polyelectrolytes, for flame-retardant cotton. Biomacromolecules 2012, 13, 2843–2848. [Google Scholar] [CrossRef]
- Skoglund, E.; Carlsson, N.G.; Sandberg, A.S. Phytate. In Healthgrain Methods: Analysis of Bioactive Components in Small Grain Cereals; Shewry, P.R., Ward, J.L., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2009; pp. 129–139. [Google Scholar]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and chitosan: Structure, properties and applications in biomedical engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and chitosan: Production and application of versatile biomedical nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Schmidt-Nielsen, B. Urea excretion in mammals. Physiol. Rev. 1958, 38, 139–168. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Z.; Leng, Q.; Wang, Y. Eco-friendly flame retardant coating deposited on cotton fabrics from bio-based chitosan, phytic acid and divalent metal ions. Int. J. Biol. Macromol. 2019, 140, 303–310. [Google Scholar] [CrossRef]
- International Organization for Standardization. Plastics—Determination of Burning Behaviour by Oxygen Index—Part 2: Ambient-Temperature Test; ISO 4589-2:2017; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- American Society for Testing Materials. Standard Test Method for Flame Resistance of Textiles (Vertical Test); ASTM D6413/D6413M-15; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- American Society for Testing Materials. Standard Test Method for Determining Flammability Characteristics of Plastics and Other Solid Materials Using Microscale Combustion Calorimetry; ASTM D7309-19a; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Horrocks, A.R. Textiles. In Fire Retardant Materials; Horrocks, A.R., Price, D., Eds.; Woodhead Publishing: Cambridge, UK, 2001; pp. 128–181. [Google Scholar]
- Zhuge, J.; Chen, X.; KS, A.; Manica, D.P. Microscale combustion calorimeter-application and limitation. Fire Mater. 2016, 40, 987–998. [Google Scholar] [CrossRef]
- Yang, C.Q.; He, Q.; Lyon, R.E.; Hu, Y. Investigation of the flammability of different textile fabrics using micro-scale combustion calorimetry. Polym. Degrad. Stab. 2010, 95, 108–115. [Google Scholar] [CrossRef]
- Shafizadeh, F.; Fu, Y.L. Pyrolysis of cellulose. Carbohydr. Res. 1973, 29, 113–122. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhang, Q.Y.; Cheng, B.W.; Ren, Y.L.; Zhang, Y.G.; Ding, C. Durable flame retardant cellulosic fibers modified with novel, facile and efficient phytic acid-based finishing agent. Cellulose 2018, 25, 799–811. [Google Scholar] [CrossRef]
- Nam, S.; Condon, B.D.; White, R.H.; Zhao, Q.; Yao, F.; Cintrón, M.S. Effect of urea additive on the thermal decomposition kinetics of flame retardant greige cotton nonwoven fabric. Polym. Degrad. Stab. 2012, 97, 738–746. [Google Scholar] [CrossRef]
- Camino, G. Flame retardants: Intumescent systems. In Plastics Additives; Pritchard, G., Ed.; Springer: Dordrecht, The Netherlands, 1998; pp. 297–306. [Google Scholar]


| Number of BL | Weight Gain (%) | LOI (%) | Time (s) |
|---|---|---|---|
| Control | - | 18 | 25 |
| 8 | 12.36 | 26 | 26 |
| 10 | 17.29 | 28 | 40 |
| 12 | 18.19 | 29 | 34 |
| 15 | 20.12 | 31 | 30 |
| Number of BL | 8 | 10 | 12 | 15 |
|---|---|---|---|---|
 |  |  |  | |
| Char length (cm) | n/a | 13.0 | 12.0 | 12.5 |
| After flame time (s) | n/a | 0 | 0 | 0 |
| After glow time (s) | n/a | 0 | 0 | 0 |
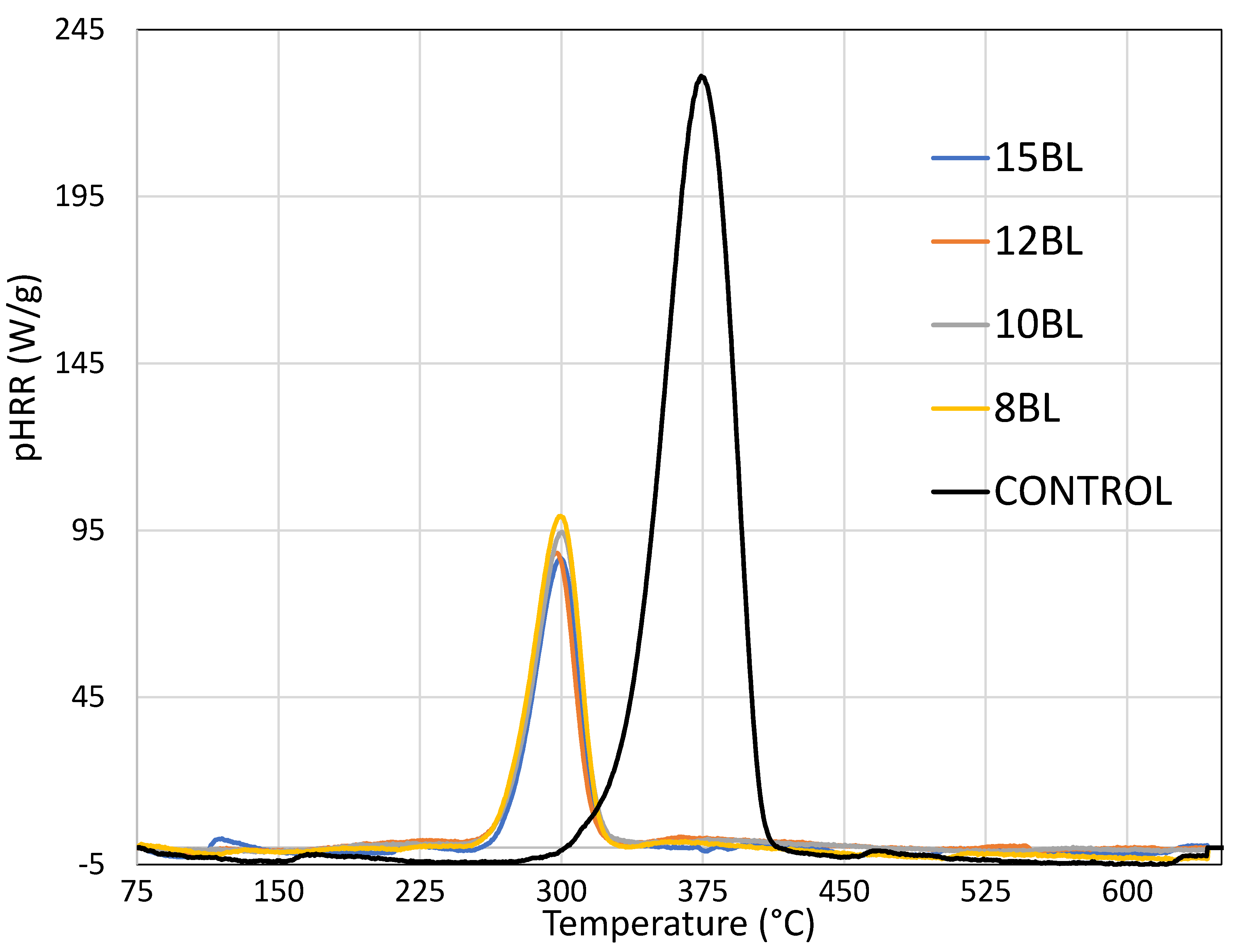
| Number of BL | pHRR (W/g) | ∆HRR (%) | THR (kJ/g) | ∆THR (%) | TpHRR (°C) |
|---|---|---|---|---|---|
| Control | 234.8 (5.7) | - | 11.1 (0.9) | - | 380 (1.7) |
| 8 BL | 101.0 (4.8) | 57.0 | 3.6 (0.8) | 67.6 | 302 (2.3) |
| 10 BL | 95.1 (6.3) | 59.5 | 3.3 (0.7) | 70.3 | 303 (3.0) |
| 12 BL | 88.6 (4.5) | 62.3 | 3.0 (0.5) | 73.0 | 299 (1.8) |
| 15 BL | 86.2 (6.1) | 63.3 | 2.2 (0.8) | 80.2 | 303 (2.1) |
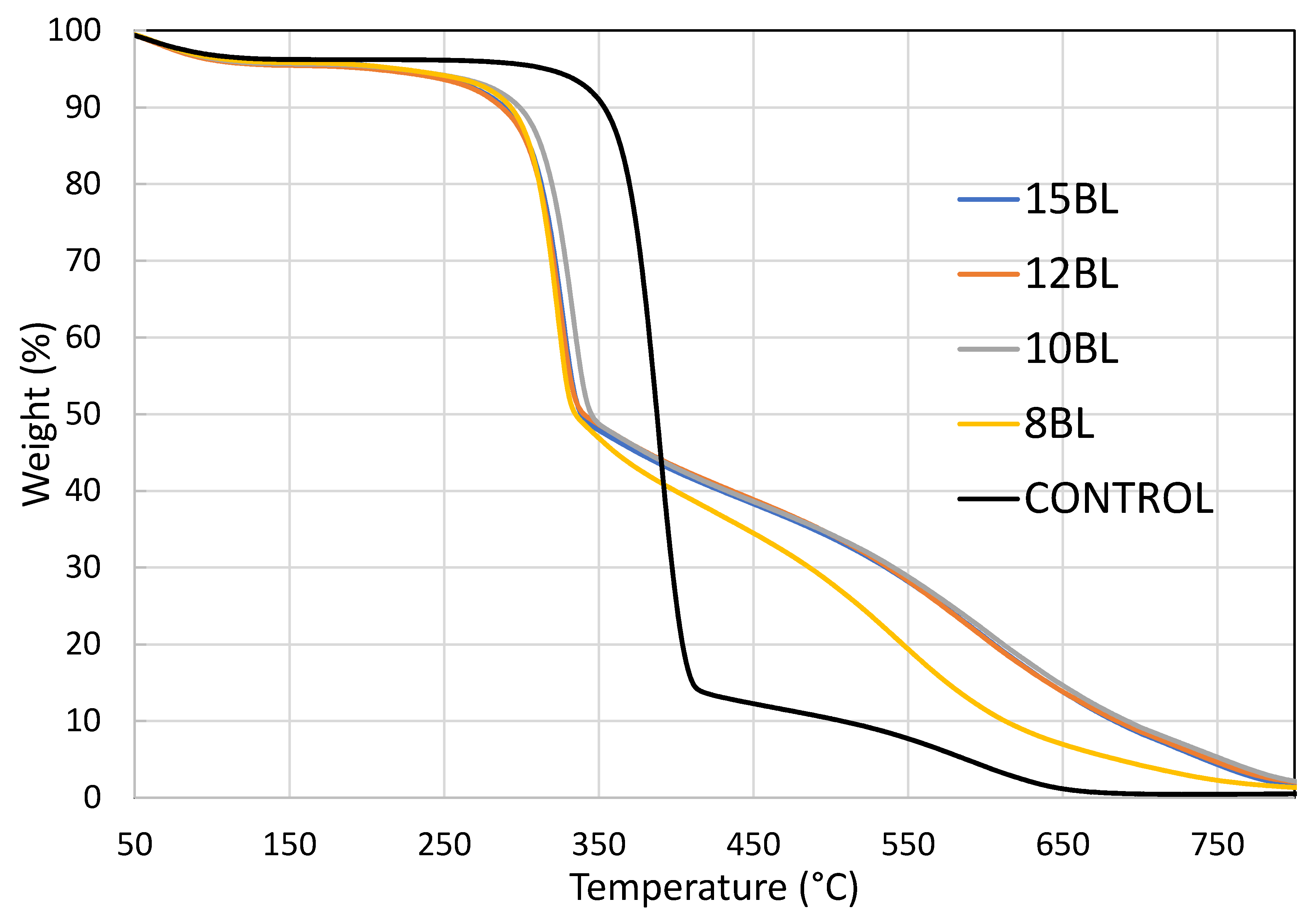
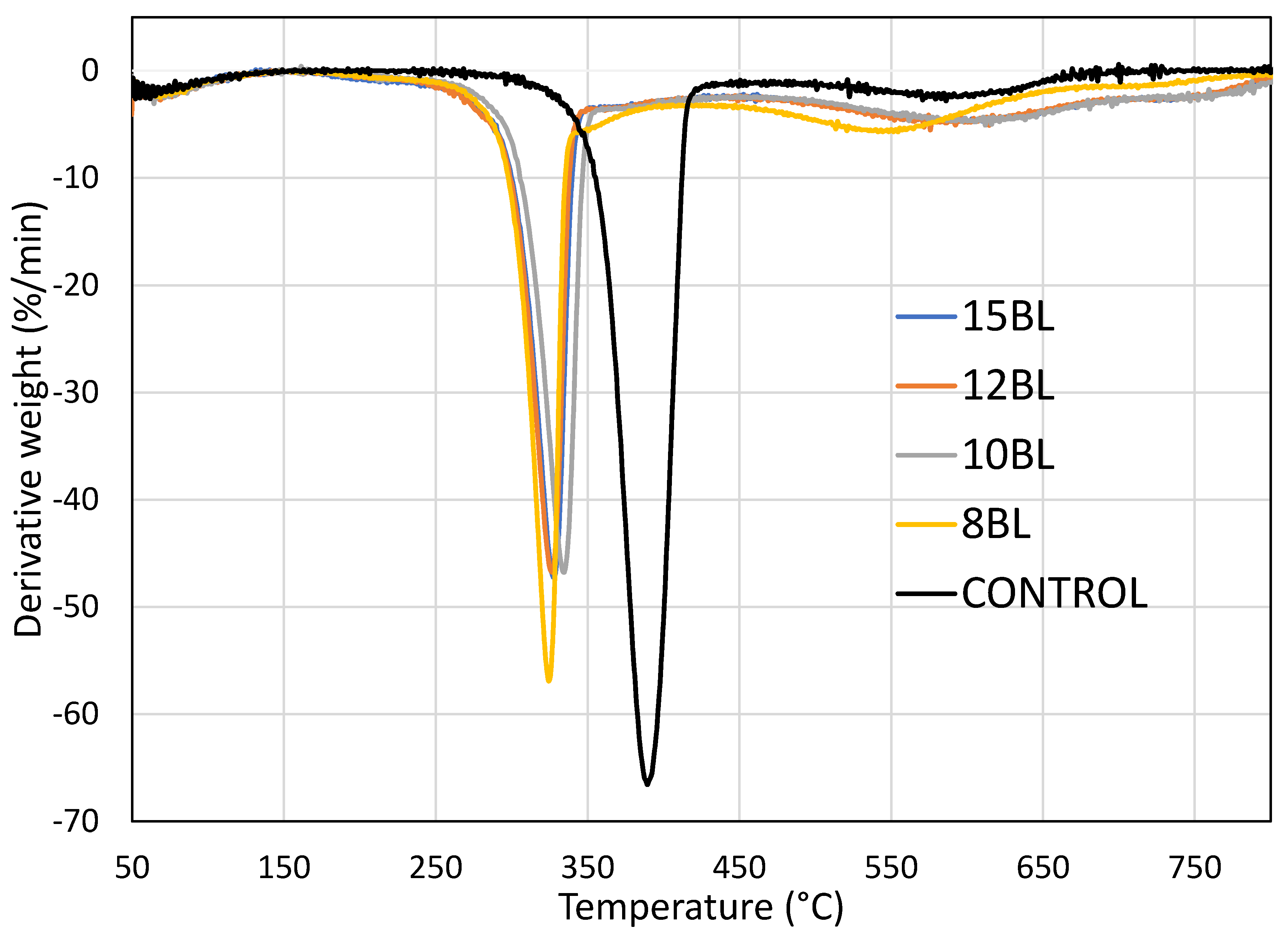
| Number of BL | T1max (°C) | Char Yield at T1max (%) | T2max (°C) | Char Yield at T2max (%) | Char Yield at 650 °C (%) |
|---|---|---|---|---|---|
| Control | 389 | 46.28 | 585 | 5.20 | 1.17 |
| 8 | 323 | 63.53 | 552 | 19.10 | 6.96 |
| 10 | 334 | 62.50 | 604 | 21.13 | 14.63 |
| 12 | 326 | 62.63 | 592 | 21.93 | 13.82 |
| 15 | 327 | 63.00 | 607 | 19.76 | 13.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magovac, E.; Jordanov, I.; Grunlan, J.C.; Bischof, S. Environmentally-Benign Phytic Acid-Based Multilayer Coating for Flame Retardant Cotton. Materials 2020, 13, 5492. https://doi.org/10.3390/ma13235492
Magovac E, Jordanov I, Grunlan JC, Bischof S. Environmentally-Benign Phytic Acid-Based Multilayer Coating for Flame Retardant Cotton. Materials. 2020; 13(23):5492. https://doi.org/10.3390/ma13235492
Chicago/Turabian StyleMagovac, Eva, Igor Jordanov, Jaime C. Grunlan, and Sandra Bischof. 2020. "Environmentally-Benign Phytic Acid-Based Multilayer Coating for Flame Retardant Cotton" Materials 13, no. 23: 5492. https://doi.org/10.3390/ma13235492
APA StyleMagovac, E., Jordanov, I., Grunlan, J. C., & Bischof, S. (2020). Environmentally-Benign Phytic Acid-Based Multilayer Coating for Flame Retardant Cotton. Materials, 13(23), 5492. https://doi.org/10.3390/ma13235492







