The Optical and Electrical Performance of CuO Synthesized by Anodic Oxidation Based on Copper Foam
Abstract
:1. Introduction
2. Experimental
Synthesis and Characterization of CuO Nanostructure
3. Result and Discussion
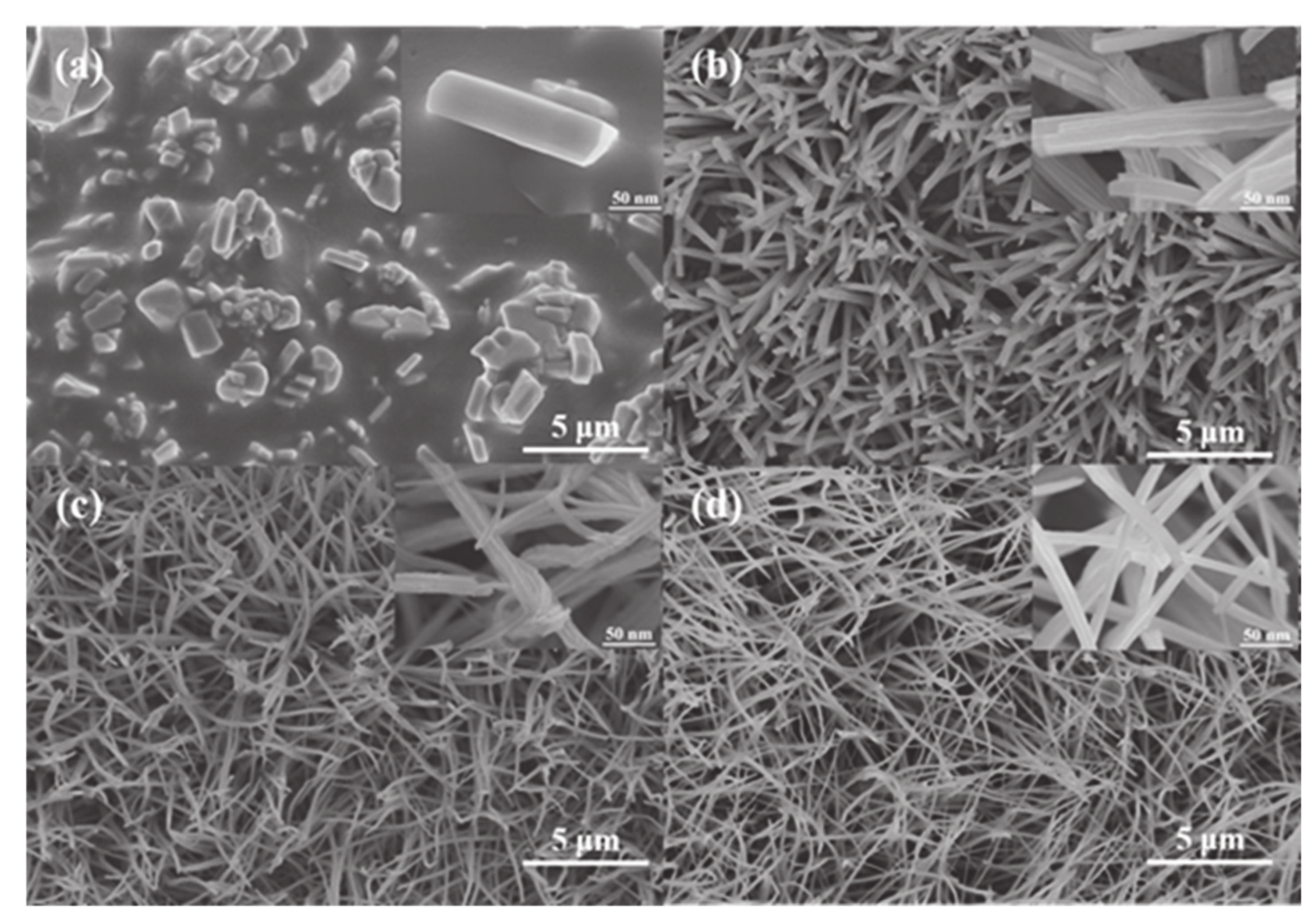
3.1. The Effect of Current Density on CuO Morphology
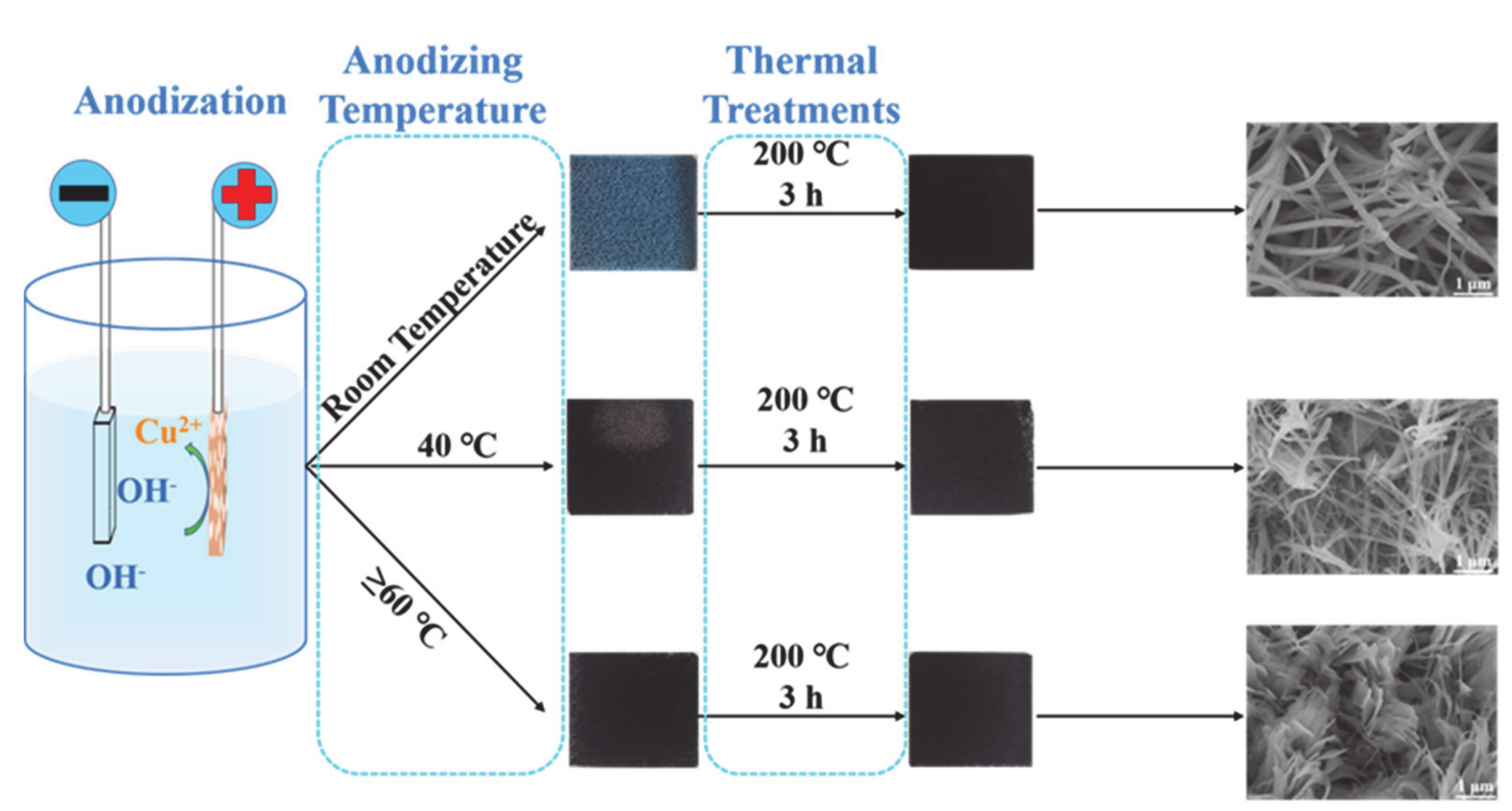
3.2. The Effect of Anodizing Temperature on CuO Morphology
3.3. Phase Structure Analysis of CuO
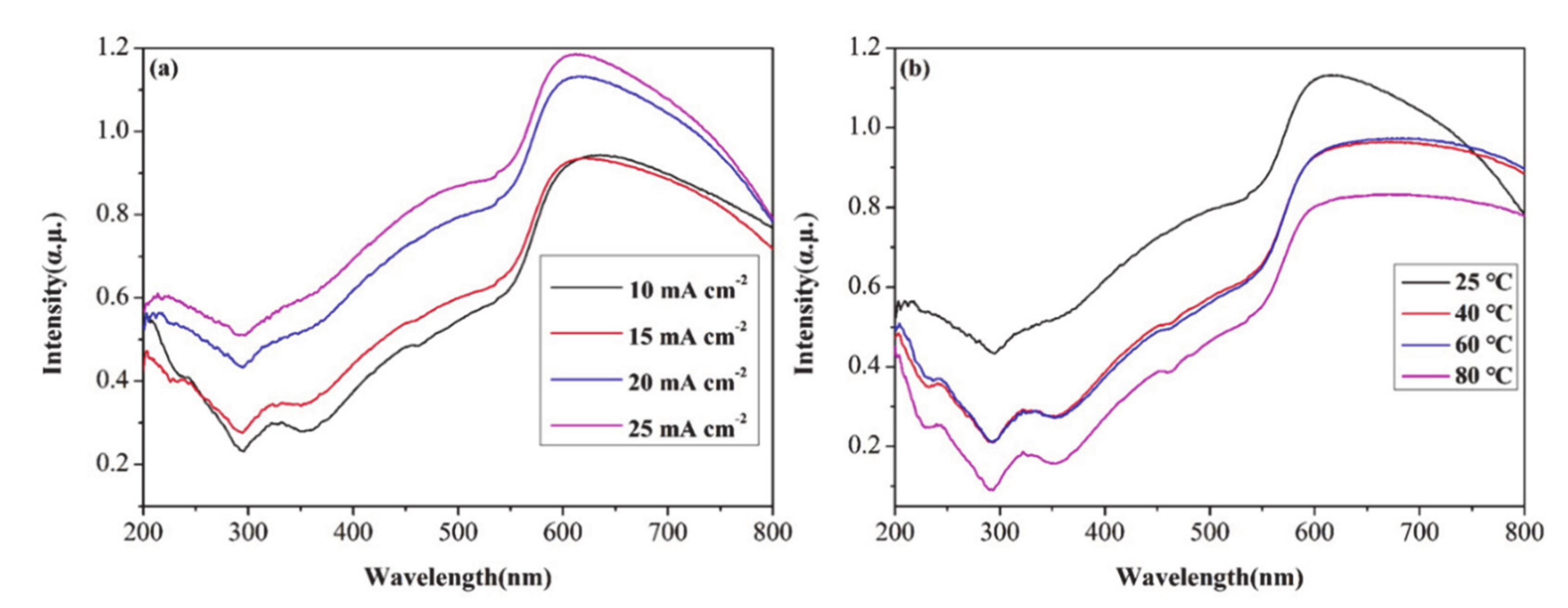
3.4. UV-Vis Absorption Spectra of CuO
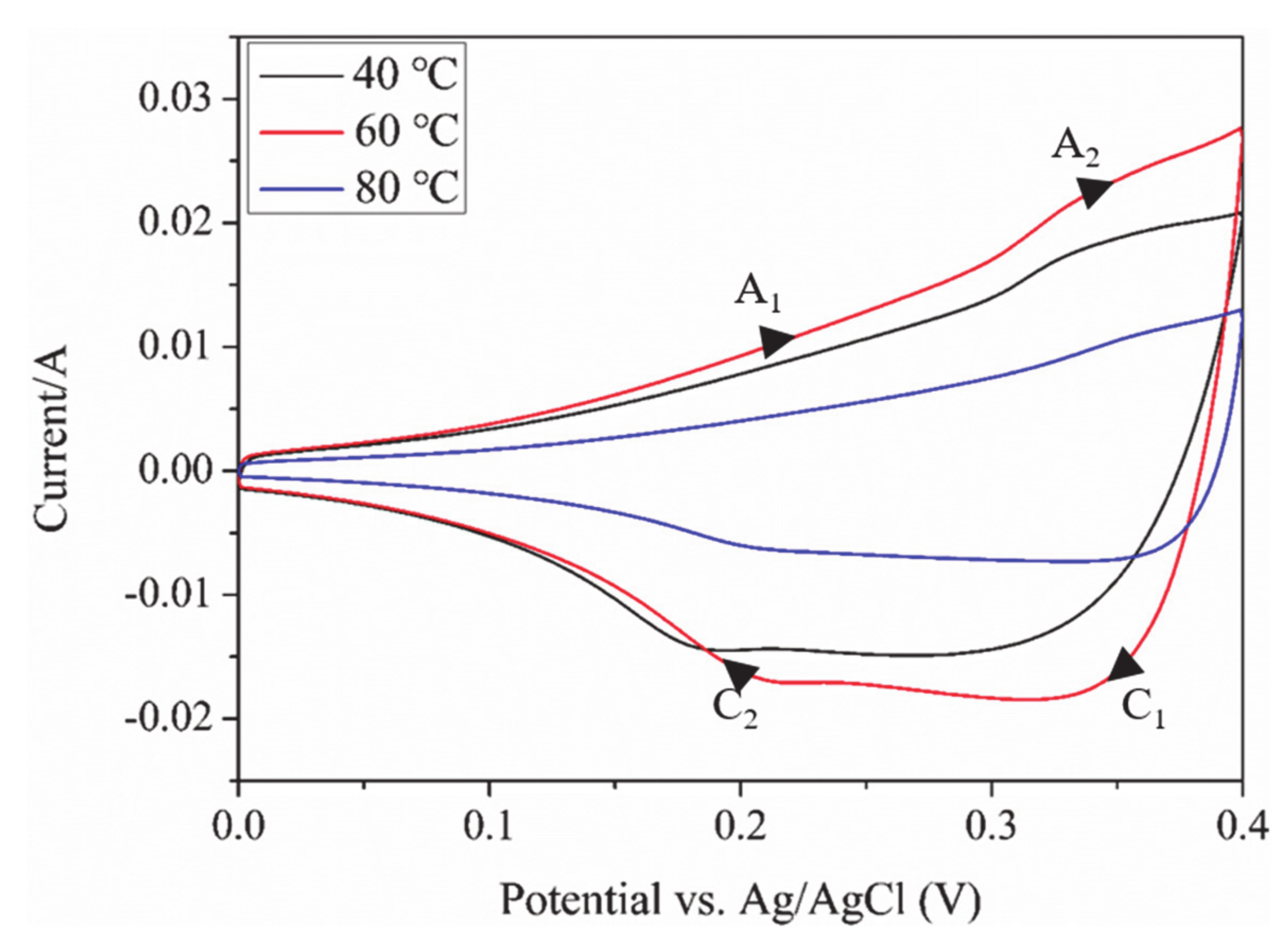
3.5. Properties of Electrochemical
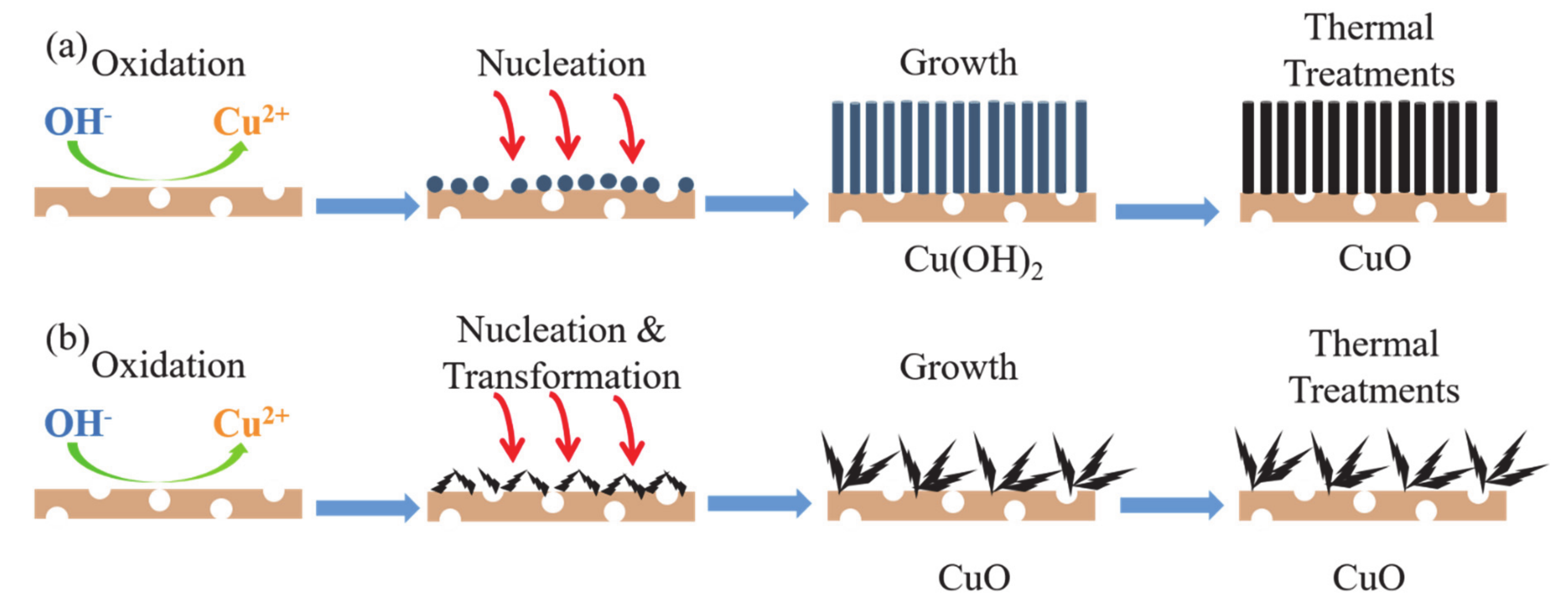
3.6. Growth Mechanism of CuO/Cu Foam
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Terence, K.S.W.; Siarhei, Z.; Saeid, M.P.; Goutam, K.D. Current status and future prospects of copper oxide heterojunction solar cells. Materials 2016, 9, 271–291. [Google Scholar]
- Senobari, S.; Alireza, N.E. A comprehensive study on the photocatalytic activity of coupled copper oxide-cadmium sulfide nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 196, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jiangfang, W.; Guojuan, J.; Hao, Z.; Shanghong, Z.; Xiaopeng, T.; Xinyue, Z.; Jing, W.; Haiquan, S.; Chuanjian, Z.; et al. Structural origin of high catalytic activity for preferential CO oxidation over CuO/CeO2 nanocatalysts with different shapes. Appl. Catal. B Environ. 2018, 239, 665–676. [Google Scholar]
- Masudy Panah, S.; Kong, E.; Dasineh Khiavi, N.; Katal, R.; Gong, X. Aluminum-incorporated p-CuO/n-ZnO photocathode coated with nanocrystal-engineered TiO2 protective layer for photo-electrochemical water splitting and hydrogen generation. J. Mater. Chem. A 2018, 6, 11951–11965. [Google Scholar] [CrossRef]
- Masudy Panah, S.; Siavash Moalhar, R.; Chin Sheng, C.; Huiru, T.; Ten It, W.; Dongzhi, C.; Goutam Kumar, D. Nanocrystal engineering of sputter-grown CuO photocathode for visible-light-driven electrochemical water splitting. ACS Appl. Mater. Interfaces 2015, 8, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Leonard, N.; Eugene, T.; Teck, G.; Xin, Z.; Zhong, C.; Chien, S.; Han, S. Mesoporous SiO2/BiVO4/CuO x nanospheres for Z-scheme, visible light aerobic C–N coupling and dehydrogenation. Appl. Mater. Today 2019, 15, 192–202. [Google Scholar]
- Fernandes, D.M.; Silva, R.; Winkler, A.A.; Radovanovic, E.; Melo, M.A.; Pineda, E.A. Synthesis and characterization of ZnO/CuO and a mixed Zn and Cu oxide. Mater. Chem. Phys. 2009, 115, 110–115. [Google Scholar] [CrossRef]
- Malka, E.; Perelshtein, I.; Lipovsky, A.; Shalom, Y.; Naparstek, L.; Perkas, N.; Patick, T.; Lubart, R.; Nitzan, Y.; Banin, E.; et al. Eradication of multi-drug resistant bacteria by a novel Zn-doped CuO nanocomposite. Small 2013, 9, 4069–4076. [Google Scholar] [CrossRef]
- Cuiping, Y.; Jiewu, C.; Yan, W.; Hongmei, Z.; Jianfang, Z.; Xia, S.; Jiaqin, L.; Yong, Z.; Yucheng, W. Porous HKUST-1 derived CuO/Cu2O shell wrapped Cu(OH)2 derived CuO/Cu2O core nanowire arrays for electrochemical nonenzymatic glucose sensors with ultrahigh sensitivity. Appl. Surf. Sci. 2018, 439, 11–17. [Google Scholar]
- Bao, W.; Xinglong, W.; Chunying, S.; Yugao, G.; Chunru, W. Synthesis of CuO/graphene nanocomposite as a high-performance anode material for lithium-ion batteries. J. Mater. Chem. 2010, 20, 10661–10664. [Google Scholar]
- Dong, H.; Guanda, W.; Guolong, L.; Jihao, B.; Hui, S.; Chun, Z. Facile route to achieve mesoporous Cu(OH)2 nanorods on copper foam for high-performance super-capacitor electrode. J. Alloy. Compd. 2017, 699, 706–712. [Google Scholar]
- Abraham, N.; Rufus, A.; Unni, C.; Philip, D. Dye sensitized solar cells using catalytically active CuO/ZnO nanocomposite synthesized by single step method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 200, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Aggeliki, P.; Nikos, B.; Antonella, G.; Jerry, L.; Yong, W.; Constantine, J.P.; Evangelos, P.; Fotis, K.K.; Vera, M.; et al. Preparation of CuO/SBA-15 catalyst by the modified ammonia driven deposition precipitation method with a high thermal stability and an efficient automotive CO and hydrocarbons conversion. Appl. Catal. B Environ. 2018, 223, 103–115. [Google Scholar]
- Hongxia, Z.; Milin, Z. Synthesis of CuO nanocrystalline and their application as electrode materials for capacitors. Mater. Chem. Phys. 2008, 108, 184–187. [Google Scholar]
- Kaviyarasu, K.; Maria, M.M.; Anand, K.; Manikaandan, E.; Maaza, M. Synthesis and characterization studies of MgO/CuO nanocrystals by wet-chemical method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 142, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Banhart, J. Manufacture, characterization and application of cellular metals and metal foams. Prog. Mater. Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- Ashby, M.F.; Evans, A.G.; Fleck, N.A.; Gibson, L.J.; Hultchinson, J.W.; Wadley, H.N.G. Metal foams, a design guide. Appl. Mech. Rev. 2012, 23, 119–120. [Google Scholar]
- Pestryakov, A.N.; Fyodoroy, A.A.; Shurov, V.A. Foam metal catalysts with intermediate support for deep oxidation of hydrocarbons. React. Kinet. Catal. Lett. 1994, 53, 347–352. [Google Scholar] [CrossRef]
- Jiaqi, C.; Liying, D.; Xinghui, W.; Wangyang, L.; Yonghui, X.; Jie, Z.; Shuying, C. Stable lithium metal anode achieved by in-situ grown CuO nanowire arrays on Cu foam. Energy Fuels 2020, 34, 7684–7691. [Google Scholar]
- Carrera Crespo, J.; Huerta Flores, A.; Torres Martinez, L.; Juarez Ranmirez, I. Effect of the Cu foam pretreatment in the growth and inhibition of copper oxide nanoneedles obtained by thermal oxidation and their evaluation as photocathodes. Mater. Sci. Semicond. Process. 2019, 102, 104604–104611. [Google Scholar] [CrossRef]
- DucDuong, L.; SungYeol, P.; Young-Wook, C.; Yong-Shin, K. Wire-like bundle arrays of copper hydroxide prepared by the electrochemical anodization of Cu foil. Bull. Korean Chem. Soc. 2010, 31, 2283–2288. [Google Scholar]
- Yunhu, L.; Sha, C.; Xiuling, L.; Jichuan, H.; Jinling, Y.; Guiling, W.; Dianxue, C. Nanostructured CuO directly grown on copper foam and their supercapacitance performance. Electrochim. Acta 2012, 85, 393–398. [Google Scholar]
- Barbieri, O.; Hahn, M.; Herzog, A.; Kotz, R. Capacitance limits of high surface area activated carbons for double layer capacitors. Carbon 2005, 43, 1303–1310. [Google Scholar] [CrossRef]
- Katakabe, T.; Kaneko, T.; Watanabe, M.; Fukushimma, T.; Aida, T. Electric double-layer capacitors using ‘Bucky Gels’ consisting of an ionic liquid and carbon nanotubes. J. Electrochem. Soc. 2005, 152, A1913–A1916. [Google Scholar] [CrossRef]
- Kun, W.; Xiaomao, D.; Chongjun, Z.; Xiuzhen, Q.; Yunlong, X. Facile synthesis of Cu2O/CuO/RGO nanocomposite and its superior cyclability in super-capacitor. Electrochim. Acta 2015, 152, 433–442. [Google Scholar]
- Jizhang, C.; Junling, X.; Shuang, Z.; Ni, Z.; Chingping, W. Facile and scalable fabrication of three-dimensional Cu(OH)2 nanoporous nanorods for solid-state super-capacitor. J. Mater. Chem. A 2015, 3, 17385–17391. [Google Scholar]
- Gao, X.P.; Bao, J.L.; Pan, G.L. Preparation and electrochemical performance of polycrystalline and single crystalline CuO nanorods as anode materials for Li ion battery. J. Phys. Chem. B 2004, 108, 5547–5551. [Google Scholar] [CrossRef]
- Shinde, S.K.; Dubal, D.P.; Ghodake, G.S.; Kim, D.Y.; Fulari, V.J. Nanoflower-like CuO/Cu(OH)2 hybrid thin films: synthesis and electrochemical super-capacitive properties. J. Electroanal. Chem. 2014, 732, 80–85. [Google Scholar] [CrossRef]
- Dong, H.; Shuangxi, X.; Bangning, S.; Hao, C.; Hui, S.; Chun, Z. Design and construction of three-dimensional flower-like CuO hierarchical nanostructures on copper foam for high performance super-capacitor. Electrochim. Acta 2016, 210, 639–645. [Google Scholar]
- Deepak, P.D.; Girish, S.G.; Rudolf, H.; Chandrakant, D.L. Mild chemical strategy to grow micro-roses and micro-woolen like arranged CuO nanosheets for high performance super-capacitors. J. Power Sour. 2013, 242, 687–698. [Google Scholar]
- Akhavan, O.; Azimirad, R.; Safa, S.; Hasani, E. CuO/Cu(OH)2 hierarchical nanostructures as bactericidal as bactericidal photo-catalysts. J. Mater. Chem. 2011, 21, 9634–9640. [Google Scholar] [CrossRef]
- Xufeng, W.; Hua, B.; Jiaxin, Z.; Fengen, C.; Gaoquan, S. Copper hydroxide nanoneedle and nanotube arrays fabricated by anodization of copper. J. Phys. Chem. 2005, 109, 22836–22842. [Google Scholar]
- Moosavifard, S.E.; El-Kay, M.F.; Rahmanifar, M.S.; Kaner, R.B.; Mousavi, M.F. Designing 3D highly ordered nanoporous CuO electrodes for high-performance asymmetric super-capacitors. ACS Appl. Mater. Interfaces 2015, 4, 4851–4860. [Google Scholar] [CrossRef] [PubMed]
- Yuxin, Z.; Ming, H.; Fei, L.; Zhongquan, W. Controlled synthesis of hierarchical CuO nanostructures for electrochemical capacitor electrodes. Int. J. Electrochem. Sci. 2013, 8, 8645–8661. [Google Scholar]




| Scan Rates (mV s‒1) | Temperature (°C) | ||
|---|---|---|---|
| 40 | 60 | 80 | |
| 2 | 0.093263 | 0.103937 | 0.049606 |
| 5 | 0.069931 | 0.082675 | 0.037224 |
| 10 | 0.058045 | 0.072527 | 0.030095 |
| 15 | 0.052582 | 0.063057 | 0.027631 |
| 20 | 0.048352 | 0.059108 | 0.025253 |
| 30 | 0.04282 | 0.053433 | 0.022761 |
| 40 | 0.039119 | 0.04663 | 0.020696 |
| 50 | 0.03561 | 0.042637 | 0.019347 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Cao, B.; Wang, C.; Zhang, Y.; Yao, H.; Wang, Y. The Optical and Electrical Performance of CuO Synthesized by Anodic Oxidation Based on Copper Foam. Materials 2020, 13, 5411. https://doi.org/10.3390/ma13235411
Wang B, Cao B, Wang C, Zhang Y, Yao H, Wang Y. The Optical and Electrical Performance of CuO Synthesized by Anodic Oxidation Based on Copper Foam. Materials. 2020; 13(23):5411. https://doi.org/10.3390/ma13235411
Chicago/Turabian StyleWang, Boyou, Binhua Cao, Chen Wang, Yubo Zhang, Huifang Yao, and Yongqian Wang. 2020. "The Optical and Electrical Performance of CuO Synthesized by Anodic Oxidation Based on Copper Foam" Materials 13, no. 23: 5411. https://doi.org/10.3390/ma13235411
APA StyleWang, B., Cao, B., Wang, C., Zhang, Y., Yao, H., & Wang, Y. (2020). The Optical and Electrical Performance of CuO Synthesized by Anodic Oxidation Based on Copper Foam. Materials, 13(23), 5411. https://doi.org/10.3390/ma13235411





