Influence of Silver as a Catalyst on the Growth of β-Ga2O3 Nanowires on GaAs
Abstract
1. Introduction
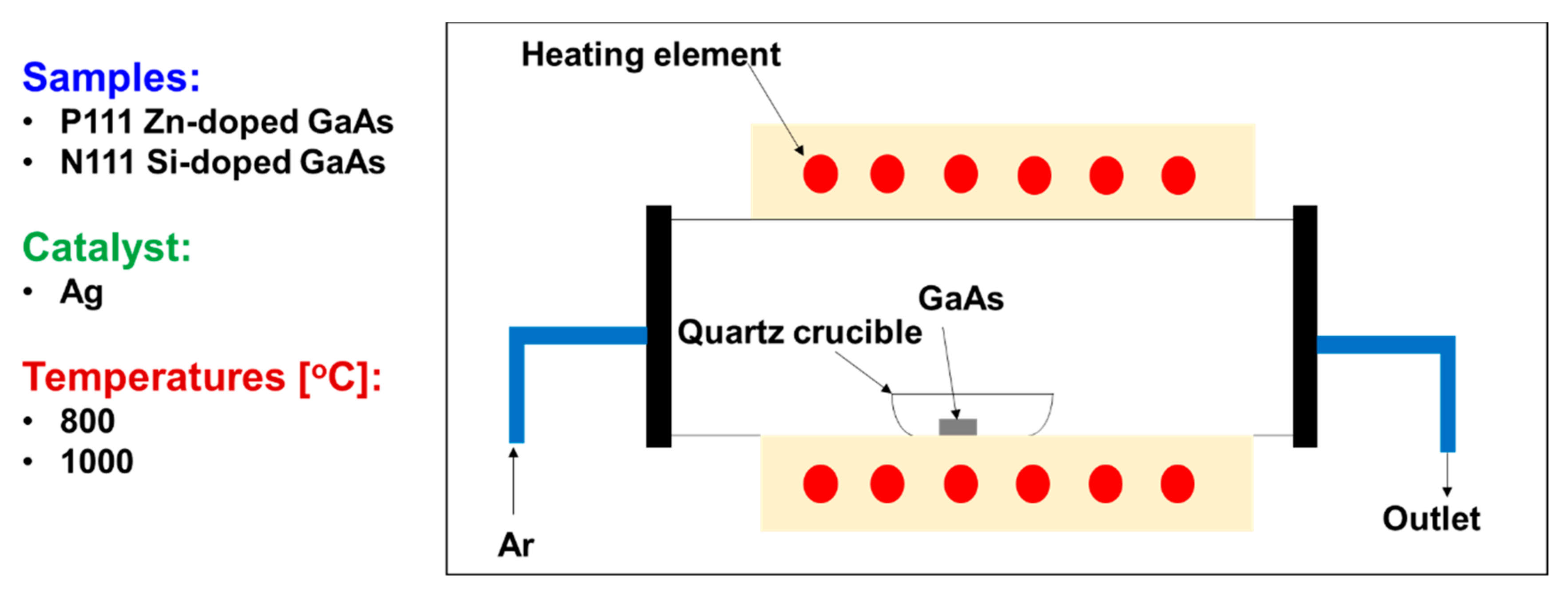
2. Materials and Methods
3. Results
3.1. Temperature Dependence of the Growth of Ga2O3 Nanowires
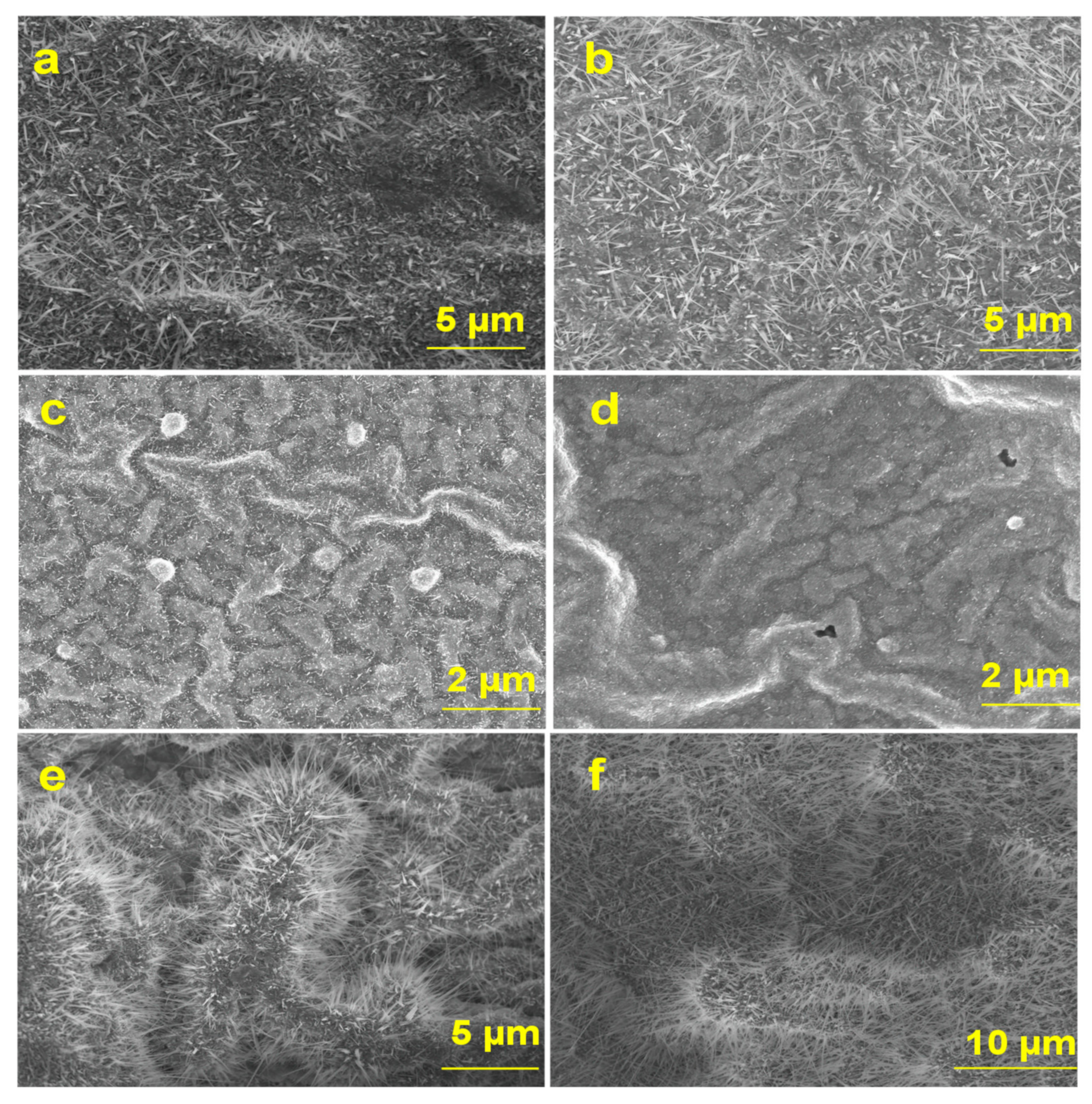
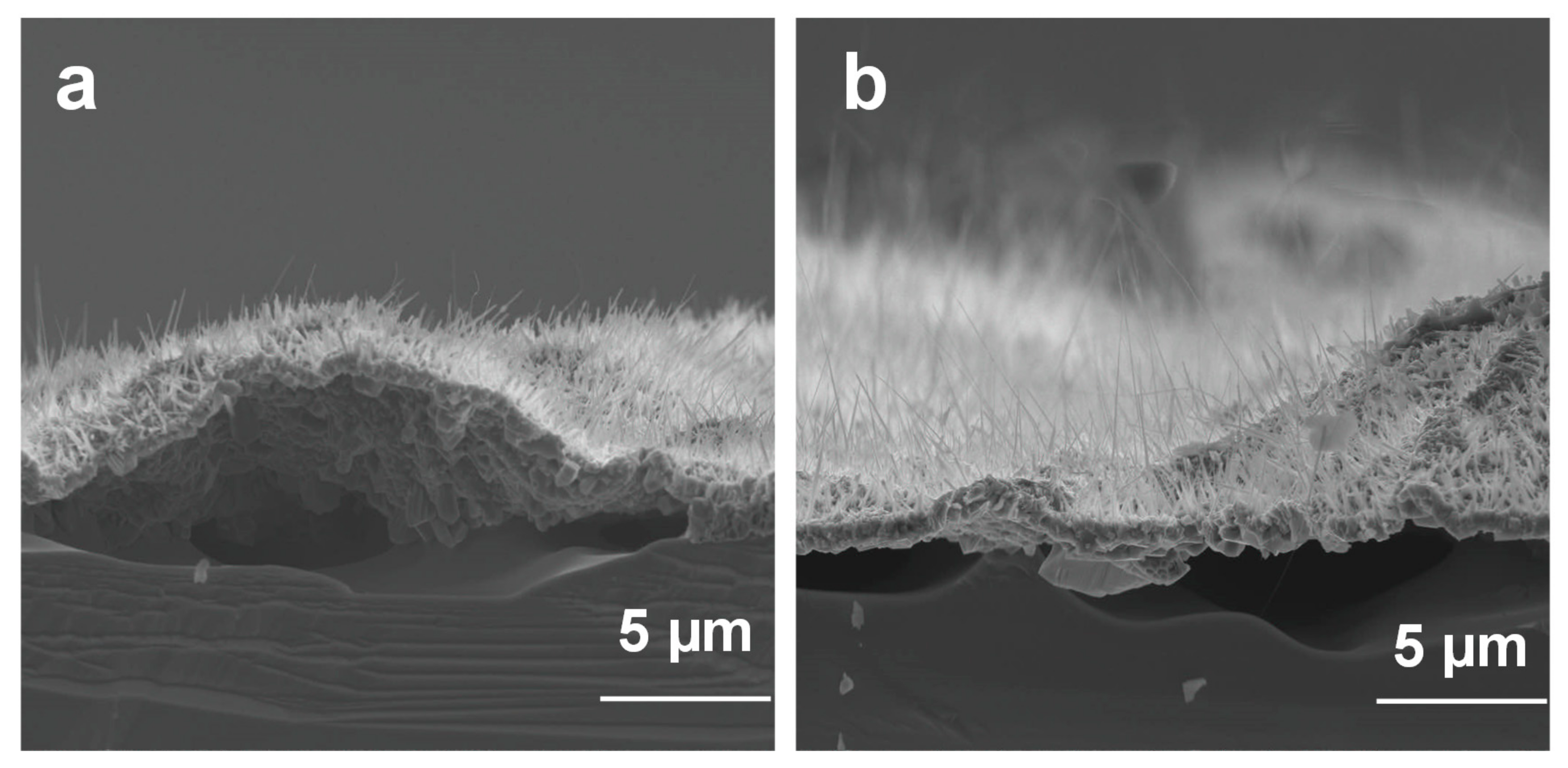
3.1.1. Ga2O3 Nanowires Morphologies
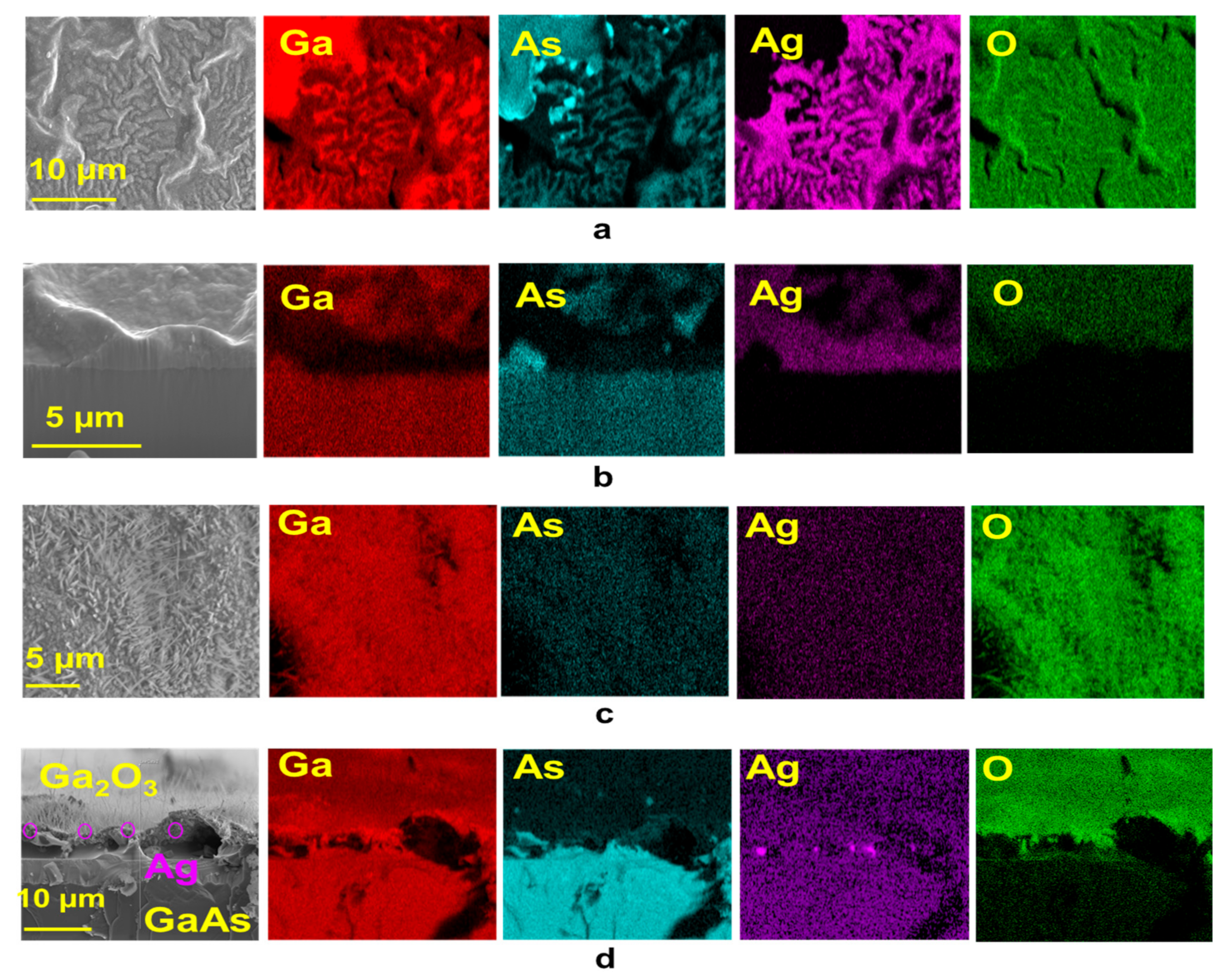
3.1.2. Ga2O3 Elemental Composition by EDS
3.1.3. XRD Characterization
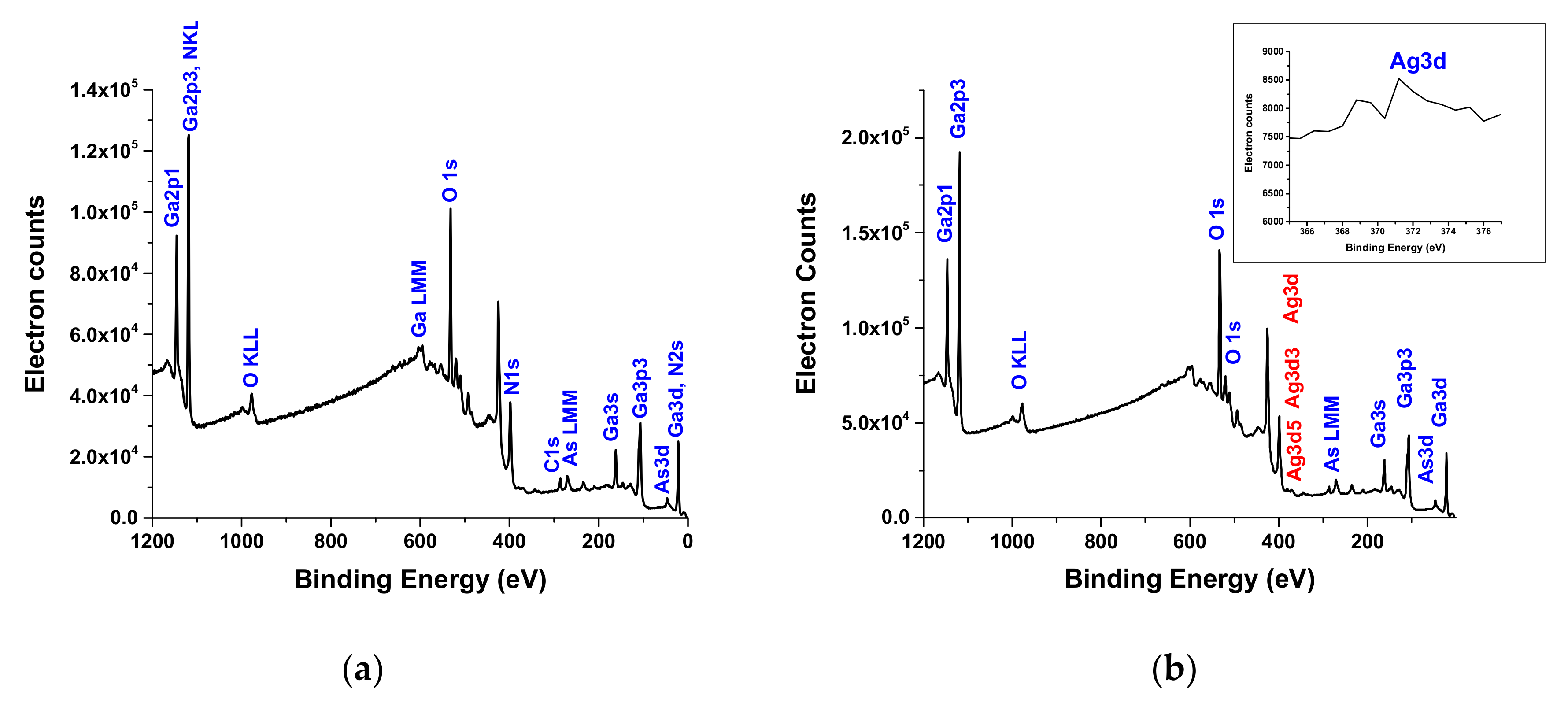
3.1.4. XPS Characterization
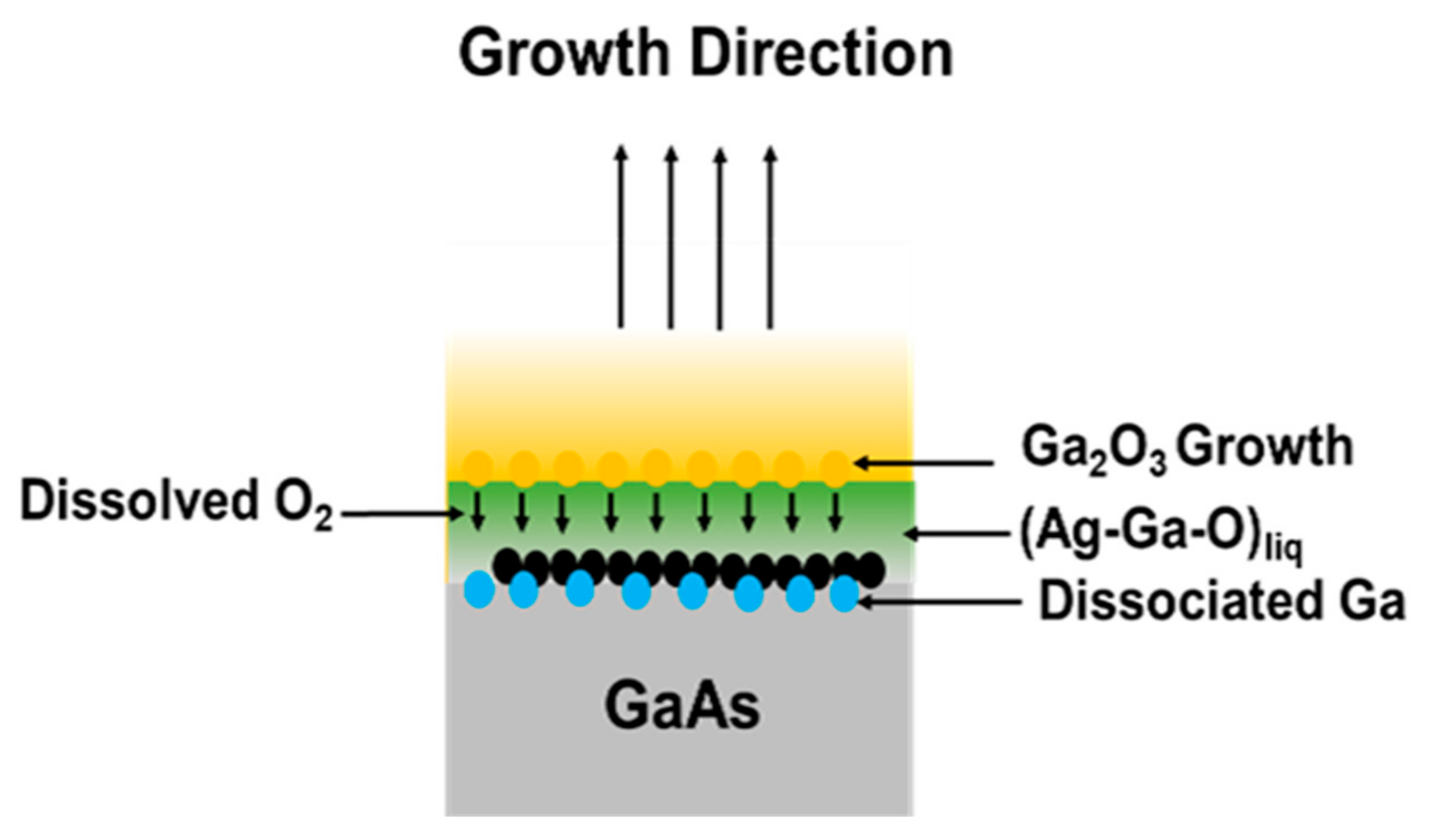
3.2. Growth Mechanism of β-Ga2O3 NWs on GaAs in the Presence of Ag
STEM
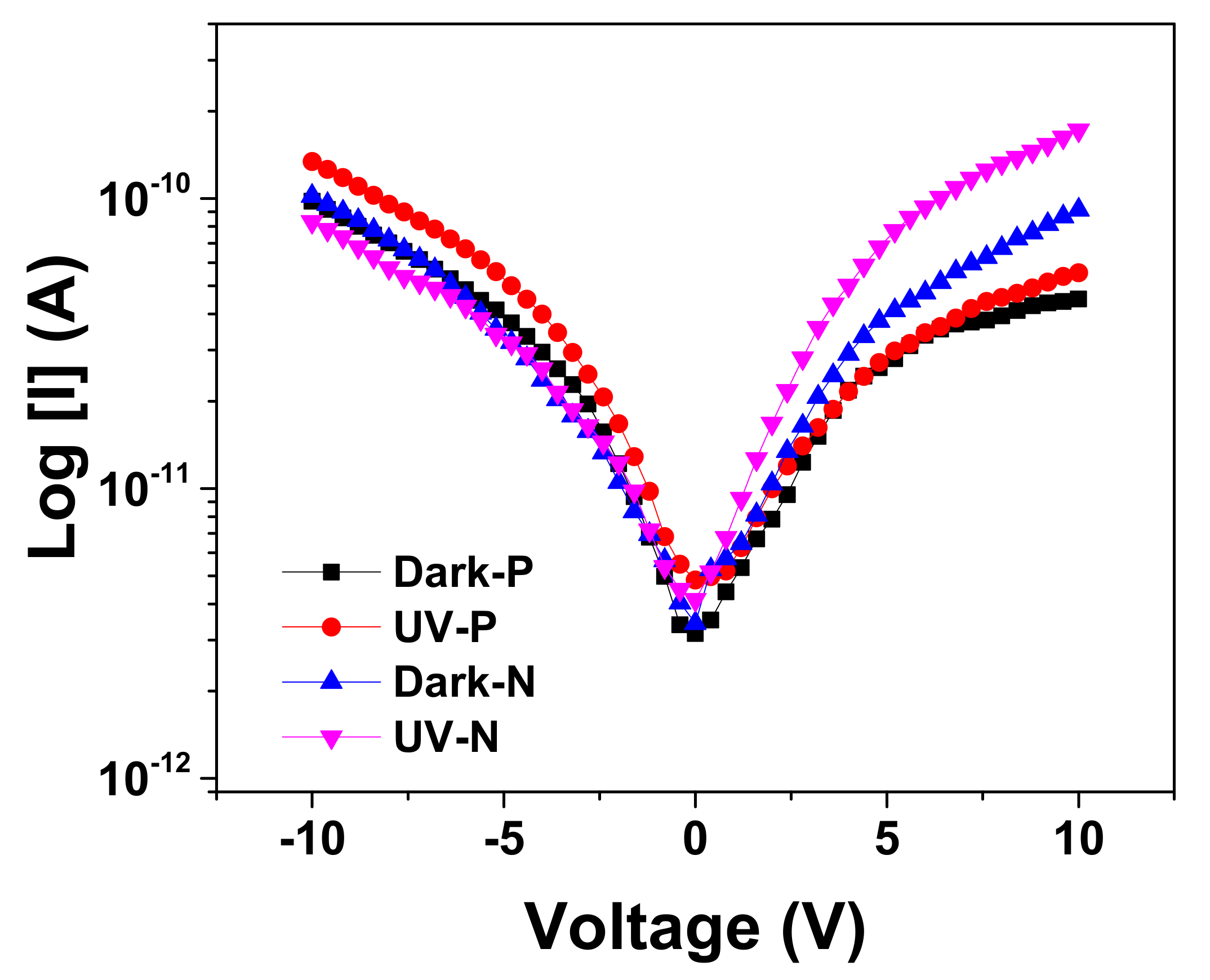
3.3. Electrical Measurements of β-Ga2O3 NWs on N(111) Si-Doped and P(111) Zn-Doped GaAs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lin, H.J.; Baltrus, J.P.; Gao, H.Y.; Ding, Y.; Nam, C.Y.; Ohodnicki, P.; Gao, P.X. Perovskite Nanoparticle-Sensitized Ga2O3 Nanorod Arrays for CO Detection at High Temperature. ACS Appl. Mater. Interfaces 2016, 8, 8880–8887. [Google Scholar] [CrossRef]
- Kaya, A.; Mao, H.; Gao, J.Y.; Chopdekar, R.V.; Takamura, Y.; Chowdhury, S.; Islam, M.S. An Investigation of Electrical and Dielectric Parameters of Sol-Gel Process Enabled beta-Ga2O3 as a Gate Dielectric Material. IEEE Trans. Electron. Devices 2017, 64, 2047–2053. [Google Scholar] [CrossRef]
- Pearton, S.J.; Yang, J.; Cary, P.H., IV; Ren, F.; Kim, J.; Tadjer, M.J.; Mastro, M.A. A review of Ga2O3 materials, processing, and devices. Appl. Phys. Rev. 2018, 5, 011301. [Google Scholar] [CrossRef]
- Kaya, A. β-Ga2O3 films grown via oxidation of GaAs substrates and their device demonstrations. In Proceedings of the Wide Bandgap Power Devices and Applications II, San Diego, CA, USA, 7–8 August 2017. [Google Scholar]
- Patil-Chaudhari, D.; Ombaba, M.; Oh, J.Y.; Mao, H.; Montgomery, K.H.; Lange, A.; Mahajan, S.; Woodall, J.M.; Islam, M.S. Solar Blind Photodetectors Enabled by Nanotextured beta-Ga2O3 Films Grown via Oxidation of GaAs Substrates. IEEE Photonics J. 2017, 9. [Google Scholar] [CrossRef]
- Bayam, Y.; Logeeswaran, V.J.; Katzenmeyer, A.M.; Sadeghian, R.B.; Chacon, R.J.; Wong, M.C.; Hunt, C.E.; Motomiya, K.; Jeyadevan, B.; Islam, M.S. Synthesis of Ga2O3 Nanorods with Ultra-Sharp Tips for High-Performance Field Emission Devices. Sci. Adv. Mater. 2015, 7, 211–218. [Google Scholar] [CrossRef][Green Version]
- Ghose, S.; Rahman, M.S.; Rojas-Ramirez, J.S.; Caro, M.; Droopad, R.; Arias, A.; Nedev, N. Structural and optical properties of beta-Ga2O3 thin films grown by plasma-assisted molecular beam epitaxy. J. Vac. Sci. Technol. B 2016, 34. [Google Scholar] [CrossRef]
- Ghose, S.; Rahman, S.; Hong, L.; Rojas-Ramirez, J.S.; Jin, H.; Park, K.; Klie, R.; Droopad, R. Growth and characterization of β-Ga2O3 thin films by molecular beam epitaxy for deep-UV photodetectors. J. Appl. Phys. 2017, 122, 095302. [Google Scholar] [CrossRef]
- Arias, A.; Nedev, N.; Ghose, S.; Rojas-Ramirez, J.S.; Mateos, D.; Curiel Alvarez, M.; Pérez, O.; Suárez, M.; Valdez-Salas, B.; Droopad, R. Structural, Optical, and Electrical Characterization of β-Ga2O3 Thin Films Grown by Plasma-Assisted Molecular Beam Epitaxy Suitable for UV Sensing. Adv. Mater. Sci. Eng. 2018, 2018, 9450157. [Google Scholar] [CrossRef]
- Feng, Q.; Li, F.G.; Dai, B.; Jia, Z.T.; Xie, W.L.; Xu, T.; Lu, X.L.; Tao, X.T.; Zhang, J.C.; Hao, Y. The properties of gallium oxide thin film grown by pulsed laser deposition. Appl. Surf. Sci. 2015, 359, 847–852. [Google Scholar] [CrossRef]
- Hu, J.Q.; Li, Q.; Meng, X.M.; Lee, C.S.; Lee, S.T. Synthesis of β-Ga2O3 Nanowires by Laser Ablation. J. Phys. Chem. B 2002, 106, 9536–9539. [Google Scholar] [CrossRef]
- Pallister, P.J.; Buttera, S.C.; Barry, S.T. Self-seeding gallium oxide nanowire growth by pulsed chemical vapor deposition. Phys. Status Solidi A 2015, 212, 1514–1518. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, V.; Singh, R. Diameter Tuning of β-Ga2O3 Nanowires Using Chemical Vapor Deposition Technique. Nanoscale Res. Lett. 2017, 12, 184. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Frost, R.L.; Yang, J.; Martens, W.N. Size and morphology control of gallium oxide hydroxide GaO(OH), nano- to micro-sized particles by soft-chemistry route without surfactant. J. Phys. Chem. C 2008, 112, 3568–3579. [Google Scholar] [CrossRef]
- Reddy, L.S.; Ko, Y.H.; Yu, J.S. Hydrothermal Synthesis and Photocatalytic Property of beta-Ga2O3 Nanorods. Nanoscale Res. Lett. 2015, 10. [Google Scholar] [CrossRef]
- Bae, H.J.; Yoo, T.H.; Yoon, Y.; Lee, I.G.; Kim, J.P.; Cho, B.J.; Hwang, W.S. High-Aspect Ratio β-Ga₂O₃ Nanorods via Hydrothermal Synthesis. Nanomaterials 2018, 8, 594. [Google Scholar] [CrossRef]
- Koshiga, F.; Sugano, T. Thermal-Oxidation of Gaas. Jpn. J. Appl. Phys. 1977, 16, 465–469. [Google Scholar] [CrossRef]
- Monteiro, O.R.; Evans, J.W. Thermal-Oxidation of Gallium-Arsenide. J. Vac. Sci. Technol. A 1989, 7, 49–54. [Google Scholar] [CrossRef]
- Kaya, A.; Dryden, D.M.; Woodall, J.M.; Islam, M.S. Spontaneous delamination via compressive buckling facilitates large-scale-Ga2O3 thin film transfer from reusable GaAs substrates. Phys. Status Solidi A 2017, 214. [Google Scholar] [CrossRef]
- Shorlin, K.; Zinke-Allmang, M. Shape cycle of Ga clusters on GaAs during coalescence growth. Surf. Sci. 2007, 601, 2438–2444. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Kim, E.T.; Dao, K.A. Ag nanoparticle catalyst based on Ga2O3/GaAs semiconductor nanowire growth by VLS method. J. Mater. Sci. Mater. Electron. 2015, 26, 8747–8752. [Google Scholar] [CrossRef]
- Alhalaili, B.; Vidu, R.; Islam, M.S. The Growth of Ga2O3 Nanowires on Silicon for Ultraviolet Photodetector. Sensors 2019, 19, 5301. [Google Scholar] [CrossRef]
- Sutter, E.; Idrobo, J.C.; Sutter, P. Synthesis and optoelectronic properties of ultrathin Ga2O3 nanowires. J. Mater. Chem. C 2020, 8, 11555–11562. [Google Scholar] [CrossRef]
- Alhalaili, B.; Bunk, R.; Vidu, R.; Islam, M.S. Dynamics Contributions to the Growth Mechanism of Ga2O3 Thin Film and NWs Enabled by Ag Catalyst. Nanomaterials 2019, 9, 1272. [Google Scholar] [CrossRef]
- Lindberg, C.; Whiticar, A.; Dick, K.A.; Skold, N.; Nygard, J.; Bolinsson, J. Silver as Seed-Particle Material for GaAs Nanowires-Dictating Crystal Phase and Growth Direction by Substrate Orientation. Nano Lett. 2016, 16, 2181–2188. [Google Scholar] [CrossRef]
- Huang, M.H.; Wu, Y.; Feick, H.; Tran, N.; Weber, E.; Yang, P. Catalytic Growth of Zinc Oxide Nanowires by Vapor Transport. Adv. Mater. 2001, 13, 113–116. [Google Scholar] [CrossRef]
- Choi, H.-J. Vapor–Liquid–Solid Growth of Semiconductor Nanowires. In Semiconductor Nanostructures for Optoelectronic Devices: Processing, Characterization and Applications; Yi, G.-C., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–36. [Google Scholar]
- Chun, H.J.; Choi, Y.S.; Bae, S.Y.; Seo, H.W.; Hong, S.J.; Park, J.; Yang, H. Controlled structure of gallium oxide nanowires. J. Phys. Chem. B 2003, 107, 9042–9046. [Google Scholar] [CrossRef]
- Chang, K.W.; Wu, J.J. Low-temperature catalytic growth of beta-Ga2O3 nanowires using single organometallic precursor. J. Phys. Chem. B 2004, 108, 1838–1843. [Google Scholar] [CrossRef]
- Song, P.Y.; Wu, Z.Y.; Shen, X.Y.; Kang, J.Y.; Fang, Z.L.; Zhang, T.Y. Self-consistent growth of single-crystalline ((2)over-bar01)beta-Ga2O3 nanowires using a flexible GaN seed nanocrystal. Crystengcomm 2017, 19, 625–631. [Google Scholar] [CrossRef]
- Levinsohn, N.; Beserman, R.; Cytermann, C.; Brener, R.; Khait, Y.L.; Regel, G.K.; Musolf, J.; Weyers, M.; Brauers, A.; Balk, P. Effect of Doping on the Thermal-Oxidation of Gaas. Appl. Phys. Lett. 1990, 56, 1131–1133. [Google Scholar] [CrossRef]
- Thurmond, C.D.; Schwartz, G.P.; Kammlott, G.W.; Schwartz, B. GaAs Oxidation and the Ga-As-O Equilibrium Phase Diagram. J. Electrochem. Soc. 1980, 127, 1366–1371. [Google Scholar] [CrossRef]
- Soci, C.; Zhang, A.; Xiang, B.; Dayeh, S.A.; Aplin, D.P.R.; Park, J.; Bao, X.Y.; Lo, Y.H.; Wang, D. ZnO nanowire UV photodetectors with high internal gain. Nano Lett. 2007, 7, 1003–1009. [Google Scholar] [CrossRef]
- Siciliano, T.; Tepore, M.; Genga, A.; Micocci, G.; Siciliano, M.; Tepore, A. Thermal oxidation of amorphous GaSe thin films. Vacuum 2013, 92, 65–69. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, N.H. Annealing effects on the properties of Ga2O3 thin films grown on sapphire by the metal organic chemical vapor deposition. Appl. Surf. Sci. 2004, 230, 301–306. [Google Scholar] [CrossRef]
- Siciliano, T.; Tepore, A.; Micocci, G.; Genga, A.; Siciliano, M.; Filippo, E. Formation of In2O3 Microrods in Thermal Treated InSe Single Crystal. Cryst. Growth Des. 2011, 11, 1924–1929. [Google Scholar] [CrossRef]
- Crist, V. Handbook of Monochromatic XPS Spectra, the Elements of Native Oxides; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar]
- Dong, C.Y.; Shang, D.S.; Shi, L.; Sun, J.R.; Shen, B.G.; Zhuge, F.; Li, R.W.; Chen, W. Roles of silver oxide in the bipolar resistance switching devices with silver electrode. Appl. Phys. Lett. 2011, 98. [Google Scholar] [CrossRef]
- Calderon, V.S.; Galindo, R.E.; Benito, N.; Palacio, C.; Cavaleiro, A.; Carvalho, S. Ag+release inhibition from ZrCN–Ag coatings by surface agglomeration mechanism: Structural characterization. J. Phys. D Appl. Phys. 2013, 46, 325303. [Google Scholar] [CrossRef]
- Graedel, T.E. Corrosion Mechanisms for Silver Exposed to the Atmosphere. J. Electrochem. Soc. 1992, 139, 1963–1970. [Google Scholar] [CrossRef]
- Stebounova, L.V.; Guio, E.; Grassian, V.H. Silver nanoparticles in simulated biological media: A study of aggregation, sedimentation, and dissolution. J. Nanoparticle Res. 2011, 13, 233–244. [Google Scholar] [CrossRef]
- Tersoff, J.; Jesson, D.E.; Tang, W.X. Decomposition Controlled by Surface Morphology during Langmuir Evaporation of GaAs. Phys. Rev. Lett. 2010, 105. [Google Scholar] [CrossRef]
- Wasilewski, Z.R.; Baribeau, J.M.; Beaulieu, M.; Wu, X.; Sproule, G.I. Studies of oxide desorption from GaAs substrates via Ga2O3 to Ga2O conversion by exposure to Ga flux. J. Vac. Sci. Technol. B 2004, 22, 1534–1538. [Google Scholar] [CrossRef]
- Assal, J.; Hallstedt, B.; Gauckler, L.J. Thermodynamic assessment of the silver-oxygen system. J. Am. Ceram. Soc. 1997, 81, 450–451. [Google Scholar] [CrossRef]
- Zinkevich, M.; Aldinger, F. Thermodynamic assessment of the gallium-oxygen system. J. Am. Ceram. Soc. 2004, 87, 683–691. [Google Scholar] [CrossRef]
- Waterhouse, G.I.N.; Bowmaker, G.A.; Metson, J.B. Oxygen chemisorption on an electrolytic silver catalyst: A combined TPD and Raman spectroscopic study. Appl. Surf. Sci. 2003, 214, 36–51. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, Y.Q.; Mema, R.; Zhou, G.W. Driving force and growth mechanism for spontaneous oxide nanowire formation during the thermal oxidation of metals. Acta Mater. 2011, 59, 2491–2500. [Google Scholar] [CrossRef]
- Yuan, L.; Zhou, A.G. The Growth of One-Dimensional Oxide Nanostructures by Thermal Oxidation of Metals. In International Journal of Nano Science, Nano Engineering and Nanotechnology; State University of New York at Binghamton: Binghamton, NY, USA, 2012; Volume 4. [Google Scholar]
- Yang, Q.; Yasuda, T.; Kukino, H.; Tanaka, M.; Tatsuoka, H. Growth of Nanowire and Nanobelt Based Oxides by Thermal Oxidation with Gallium. Nanowires Sci. Technol. 2010. [Google Scholar] [CrossRef]
- Varley, J.B.; Weber, J.R.; Janotti, A.; Van de Walle, C.G. Oxygen vacancies and donor impurities in beta-Ga2O3. Appl. Phys. Lett. 2010, 97. [Google Scholar] [CrossRef]
- Li, C.; Yan, J.L.; Zhang, L.Y.; Zhao, G. Electronic structures and optical properties of Zn-doped beta-Ga2O3 with different doping sites. Chin. Phys. B 2012, 21. [Google Scholar] [CrossRef]
- Higashiwaki, M.; Sasaki, K.; Kuramata, A.; Masui, T.; Yamakoshi, S. Gallium oxide (Ga2O3) metal-semiconductor field-effect transistors on single-crystal β-Ga2O3 (010) substrates. Appl. Phys. Lett. 2012, 100, 013504. [Google Scholar] [CrossRef]
- Ogita, M.; Higo, K.; Nakanishi, Y.; Hatanaka, Y. Ga2O3 thin film for oxygen sensor at high temperature. Appl. Surf. Sci. 2001, 175–176, 721–725. [Google Scholar] [CrossRef]
- Hoefer, U.; Frank, J.; Fleischer, M. High temperature Ga2O3-gas sensors and SnO2-gas sensors: A comparison. Sens. Actuat. B Chem. 2001, 78, 6–11. [Google Scholar] [CrossRef]
- Bartic, M. Mechanism of oxygen sensing on β-Ga2O3 single-crystal sensors for high temperatures. Phys. Status Solidi 2016, 213, 457–462. [Google Scholar] [CrossRef]


| Catalyst | Technique | Substrate | Avg. Nanowire | ||
|---|---|---|---|---|---|
| Diameter (nm) | Length | ||||
| Experimental Results obtained in this work | |||||
| No Catalyst | Oxidation | GaAs | 250–400 * | 800 nm–1.35 µm * | |
| Ag Thin Film | 25–40 * | Several tens of µm * | |||
| NPs | Ref. | Reported Results | |||
| Free Catalyst | [12] | CVD | Ga/Ga2O3 mixture and O2 | 40–70 | 10–20 µm |
| [28] | Si/SiO2 and Al2O3 | 50–75 | >10 µm | ||
| Ag NPs | [21] | Sol–gel and VLS | GaAs | 18–30 | Several tens of nm to a few hundred µms |
| Au NPs | [29] | Thermal VLS | Au-pretreated Si | 34 | 20–60 nm |
| Ni NPs | [28] | CVD | Ga/Ga2O3 mixture and O2 | 30–80 with an average value of 50 | up to 500 µm |
| Pt NPs | [30] | Thermal decomposition, oxidation and epitaxy | GaN films on sapphire | ∼80 | Several tens of µm |
| Composition (+/−) | 800 °C | 1000 °C | ||||||
|---|---|---|---|---|---|---|---|---|
| O | Ga | As | Ag | O | Ga | As | Ag | |
| N(111)-No Ag | 37.3 | 31.9 | 30.8 | 0.0 | 54.7 | 45.3 | 0.1 | 0.0 |
| N(111)-Ag | 47.1 | 32.4 | 12.9 | 7.6 | 54.8 | 45.2 | 0.0 | 0.0 |
| P(111)-No Ag | 38 | 31.0 | 29.8 | 1.2 | 54.8 | 45.1 | 0.1 | 0.0 |
| P(111)-Ag | 42.5 | 29.3 | 18.5 | 9.8 | 54.8 | 44.9 | 0.3 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhalaili, B.; Mao, H.; Dryden, D.M.; Cansizoglu, H.; Bunk, R.J.; Vidu, R.; Woodall, J.; Islam, M.S. Influence of Silver as a Catalyst on the Growth of β-Ga2O3 Nanowires on GaAs. Materials 2020, 13, 5377. https://doi.org/10.3390/ma13235377
Alhalaili B, Mao H, Dryden DM, Cansizoglu H, Bunk RJ, Vidu R, Woodall J, Islam MS. Influence of Silver as a Catalyst on the Growth of β-Ga2O3 Nanowires on GaAs. Materials. 2020; 13(23):5377. https://doi.org/10.3390/ma13235377
Chicago/Turabian StyleAlhalaili, Badriyah, Howard Mao, Daniel M. Dryden, Hilal Cansizoglu, Ryan James Bunk, Ruxandra Vidu, Jerry Woodall, and M. Saif Islam. 2020. "Influence of Silver as a Catalyst on the Growth of β-Ga2O3 Nanowires on GaAs" Materials 13, no. 23: 5377. https://doi.org/10.3390/ma13235377
APA StyleAlhalaili, B., Mao, H., Dryden, D. M., Cansizoglu, H., Bunk, R. J., Vidu, R., Woodall, J., & Islam, M. S. (2020). Influence of Silver as a Catalyst on the Growth of β-Ga2O3 Nanowires on GaAs. Materials, 13(23), 5377. https://doi.org/10.3390/ma13235377






