Influence of Reaction Parameters on the Gelation of Silanised Linseed Oil
Abstract
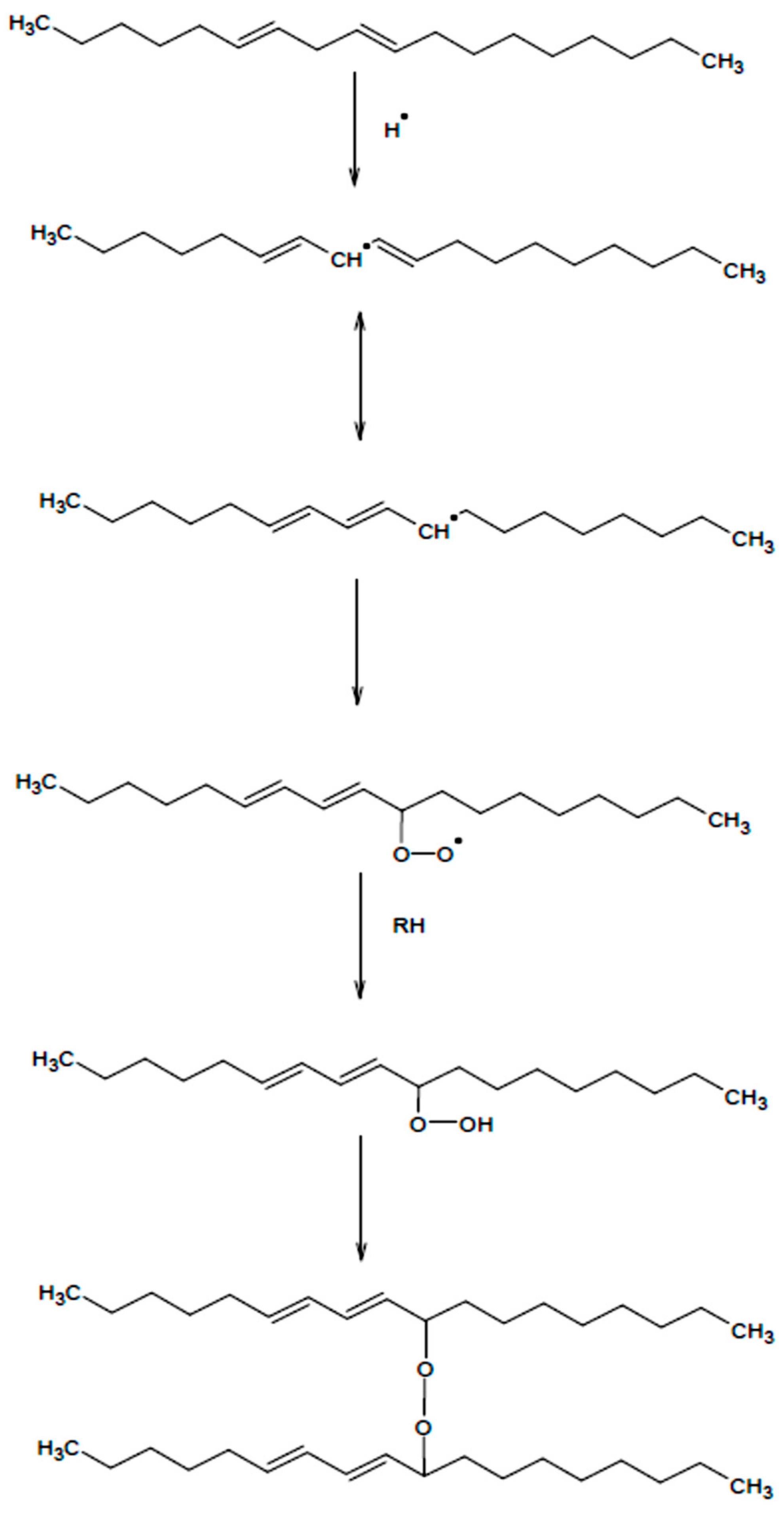
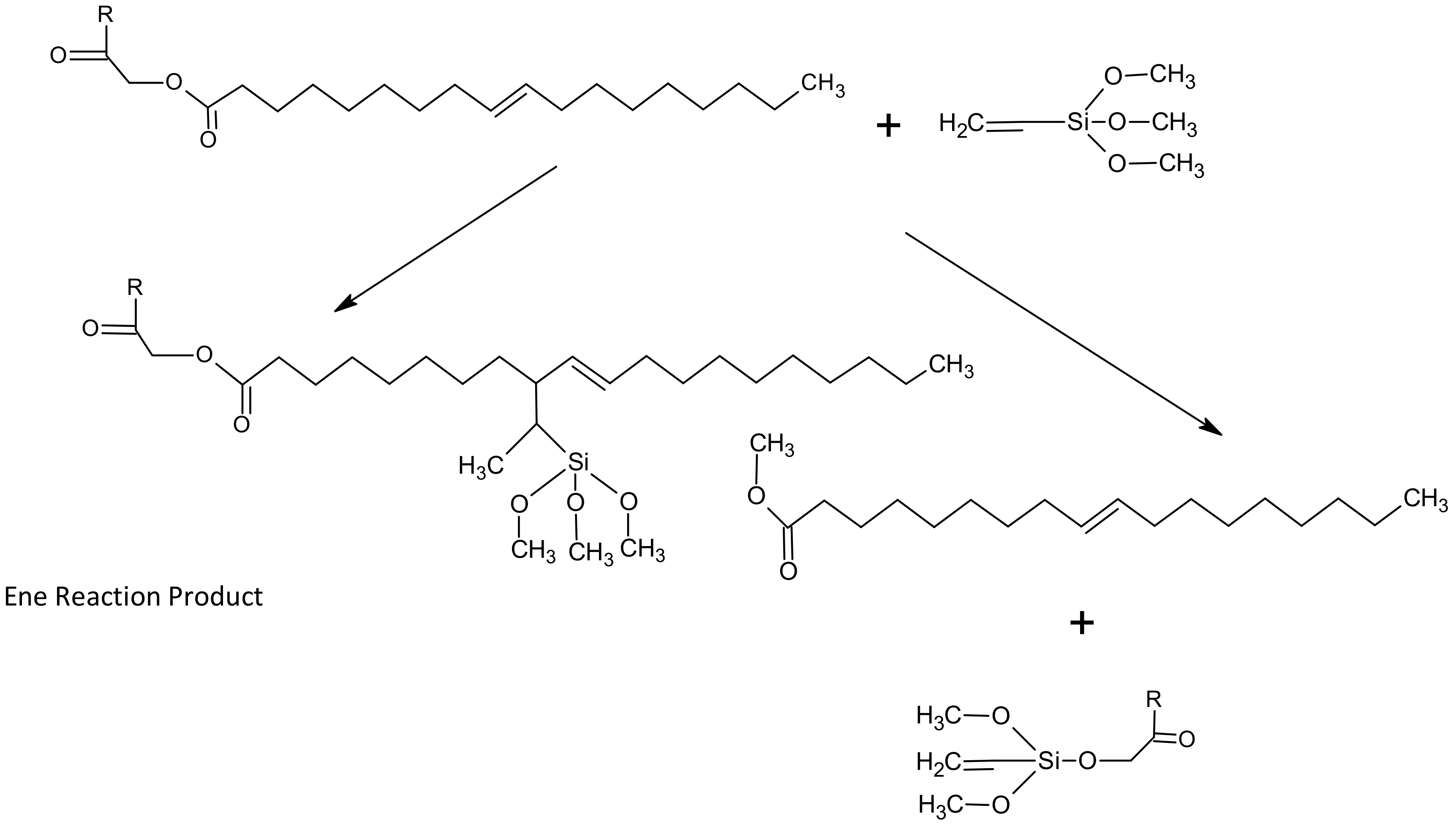
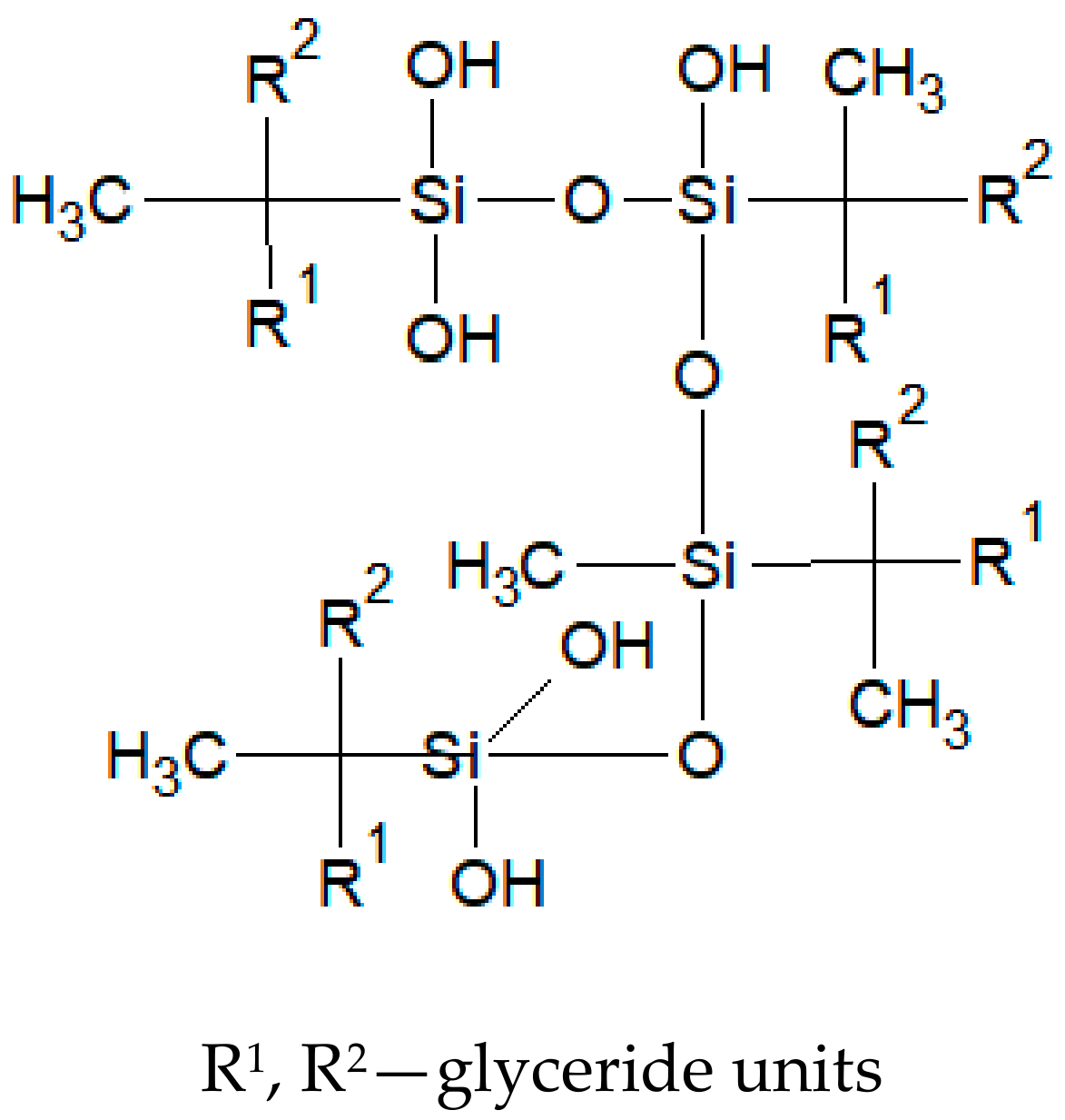
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Silylation Reaction
2.3. Characterisation of Silylated Oil
2.3.1. Acidic Number of Silylated Oil
- LK—acid number (mg KOH/g),
- 56.1—constant value (molar mass KOH) (g/mol),
- c—concentration of potassium hydroxide solution (mol/l),
- V1—volume of titrant consumed in the blank test (ml),
- V2—volume of titrant consumed in the determination (ml),
- m—mass of the analytical sample.
2.3.2. GC-FID Analysis
2.3.3. Polydispersity Index
- Mw > Mn
- Mn—average molar mass (by number) (g/mol)
- Mw—average molar mass (mass) (g/mol)
| PDI | Polymer Description |
|---|---|
| 1.0 | Początek formularza hypothetical monodisperse polymer, living polymers (almost monodispersive) Dół formularza |
| 1.5–2.0 | addition polymers |
| <5.0 | polymers with a low molecular weight distribution |
| 5.0–20.0 | polymers with an average molecular weight distribution |
| >20 | polymers with a high molecular weight distribution |
| 8.0–30.0 | coordination polymers |
| 20.0–50.0 | branched polymers |
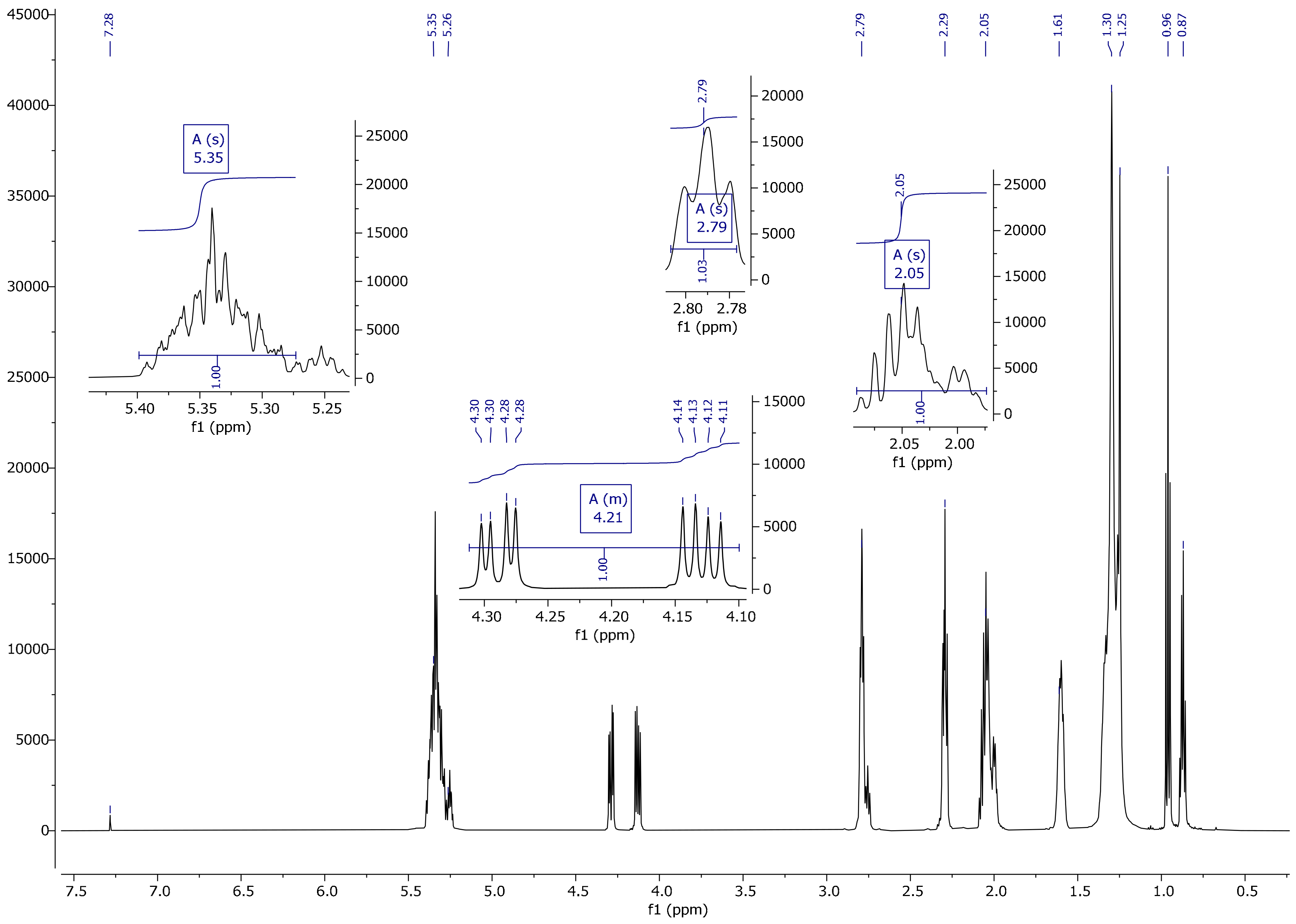
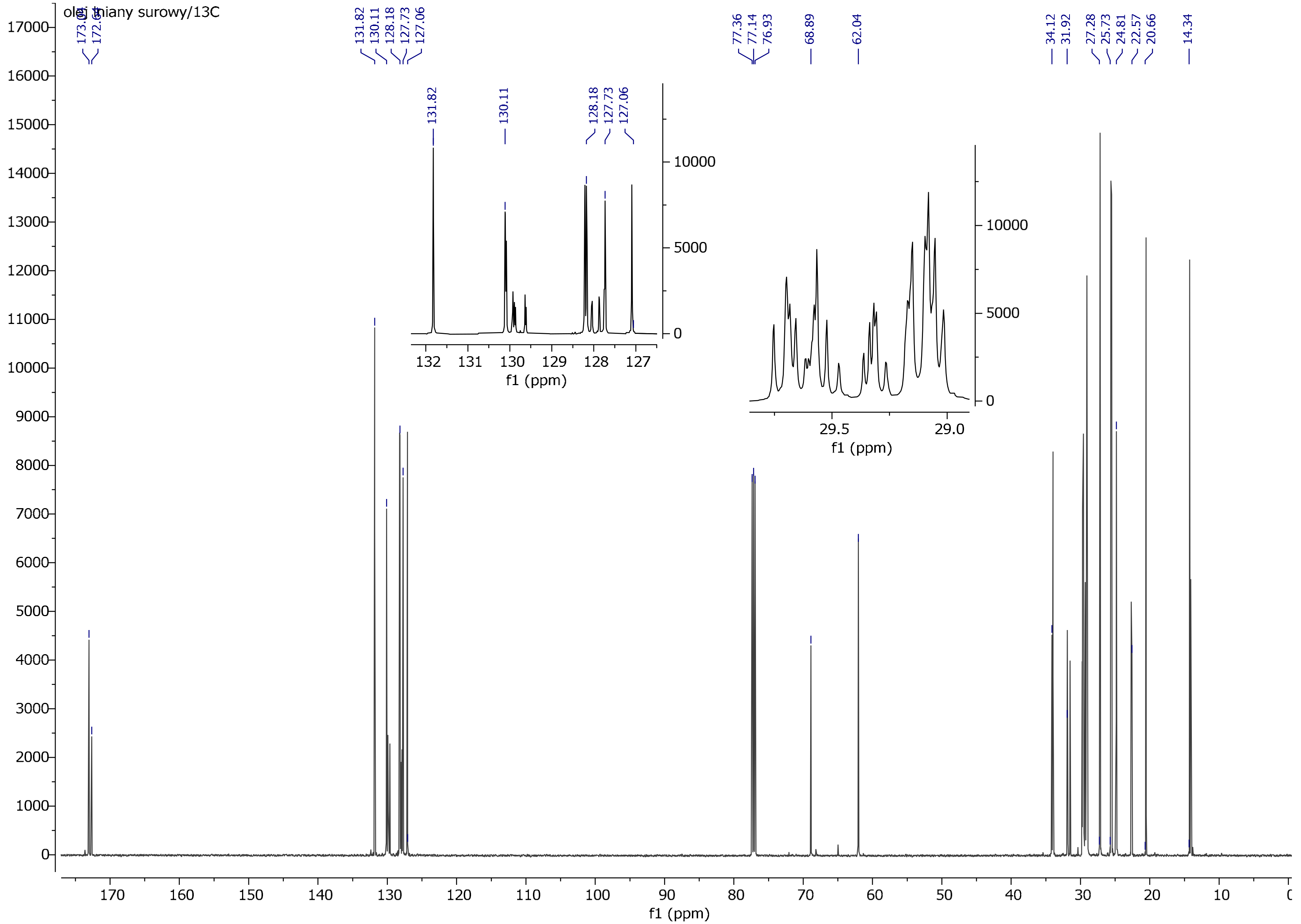
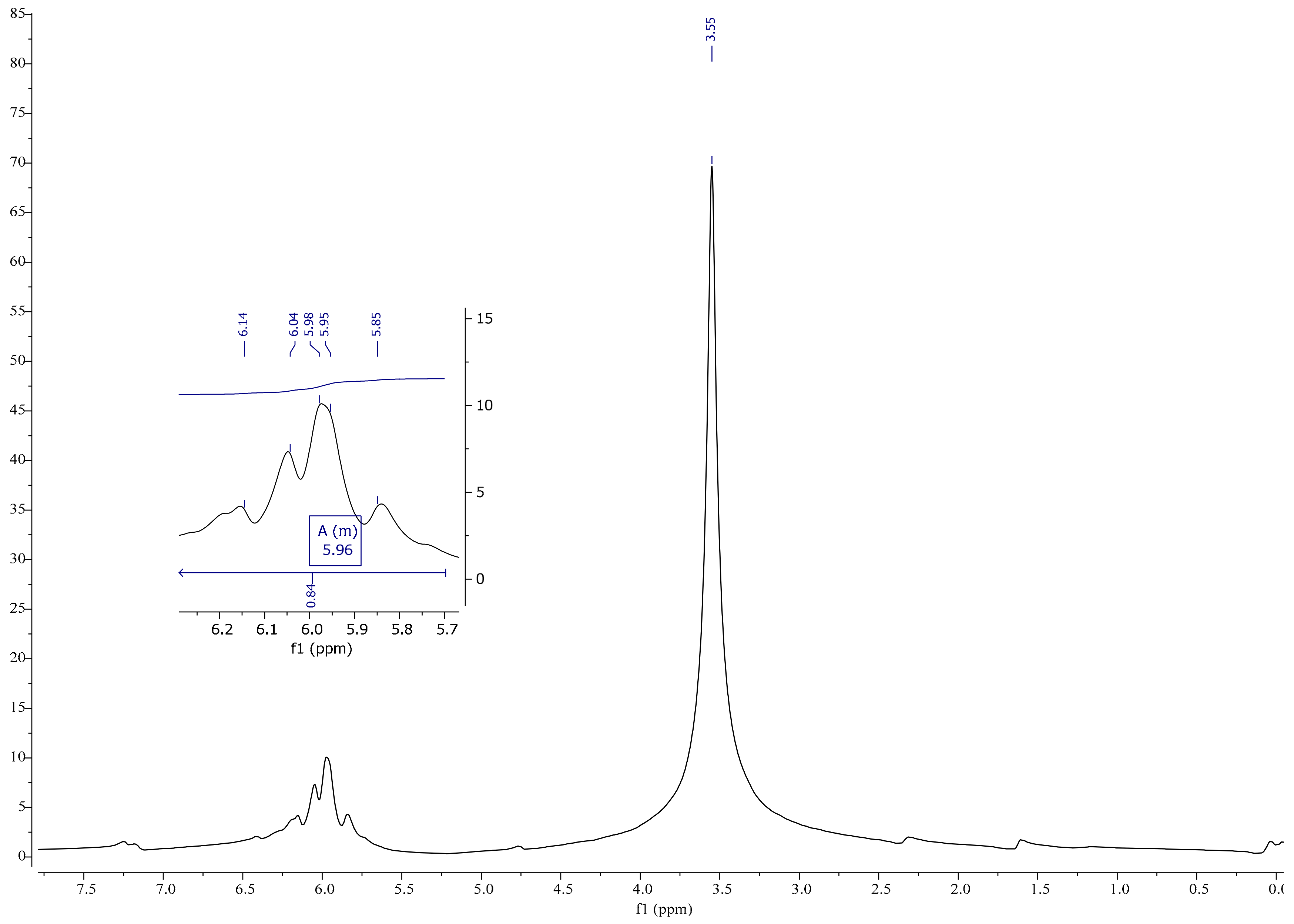
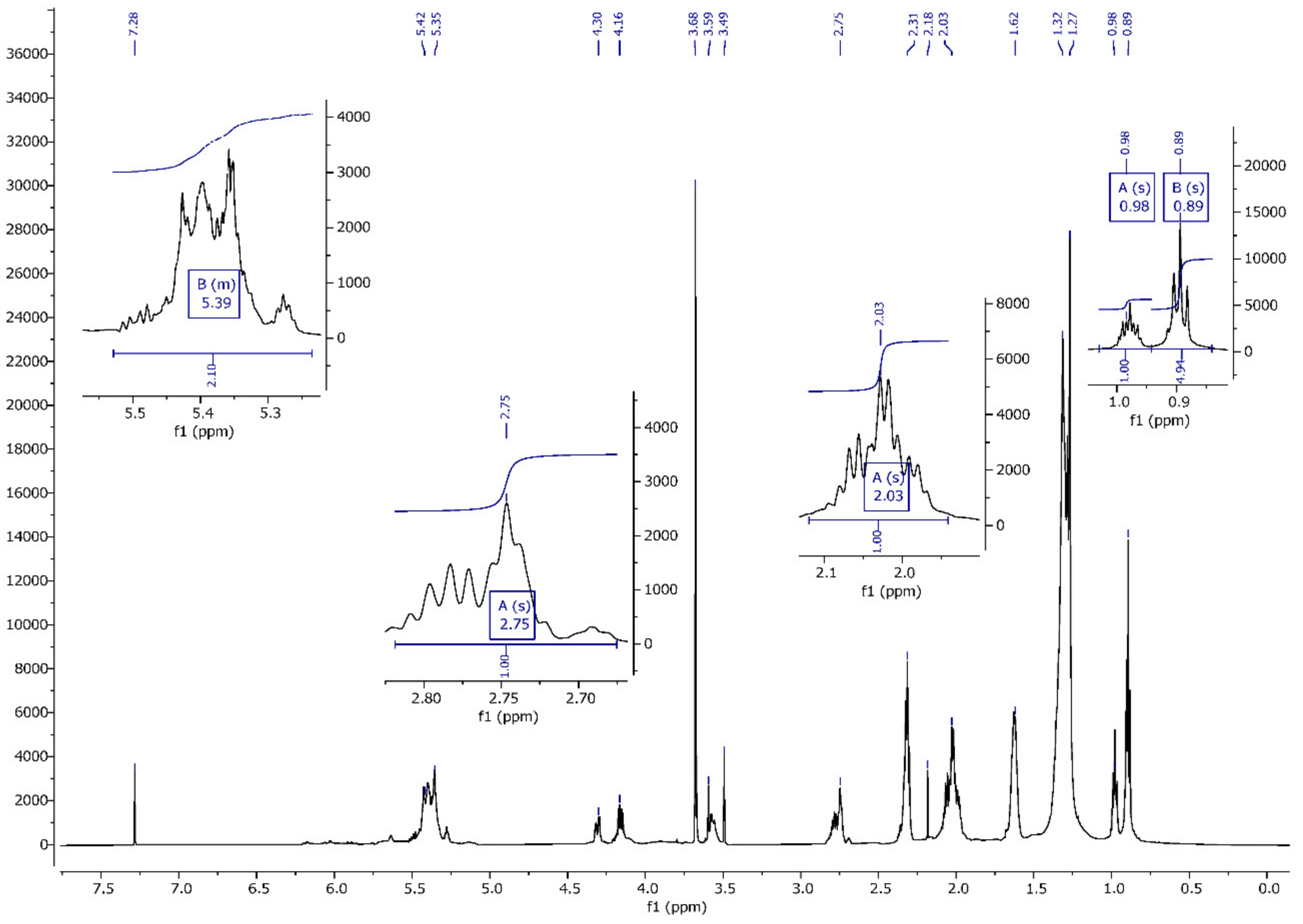
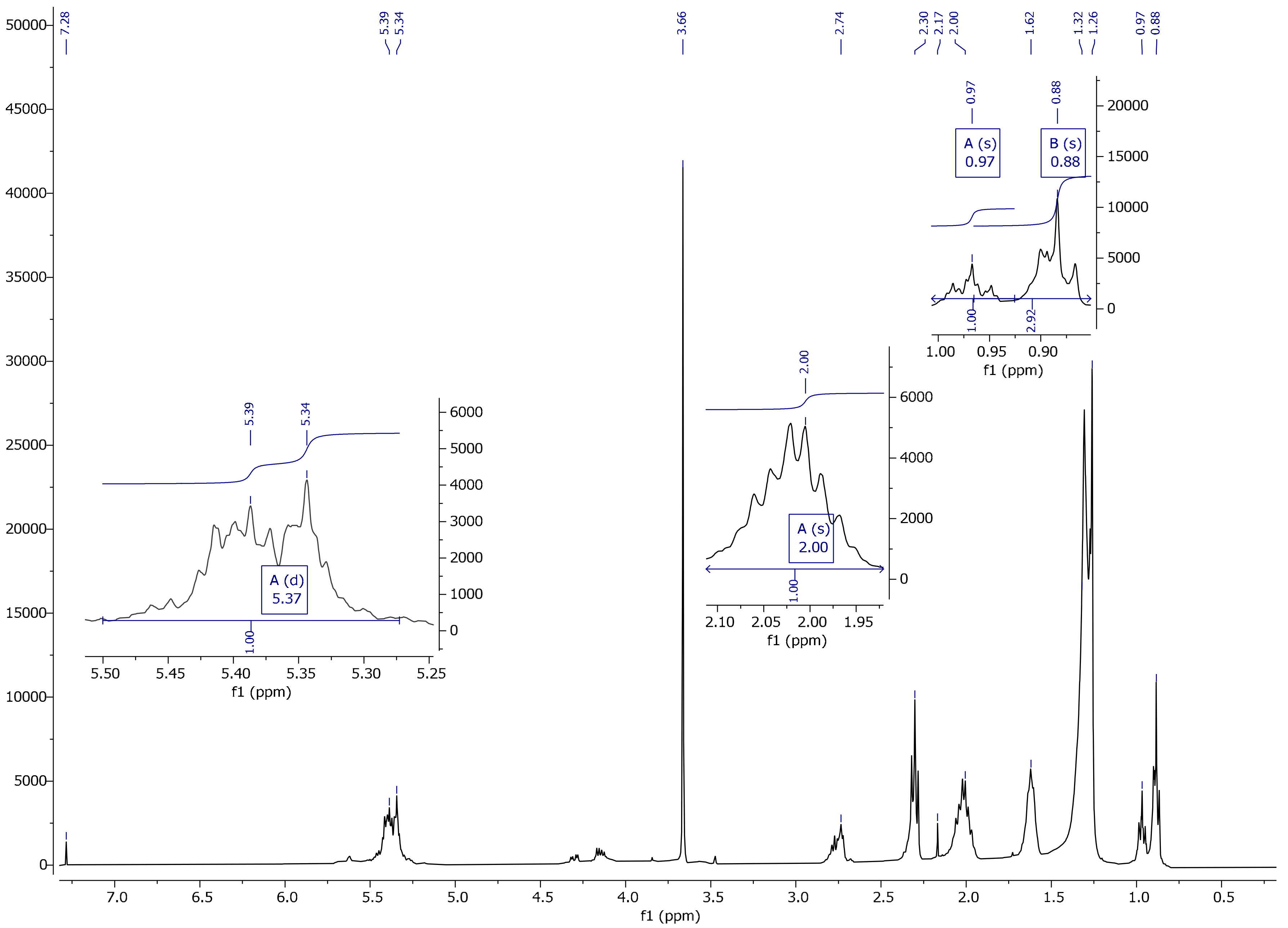
2.3.4. Nuclear Magnetic Resonance (1H, 13C)
3. Results
3.1. The Acid Number Determination
3.2. GC-FID Analysis
3.3. GC-FID Analysis
3.4. NMR Spectrum Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Güner, F.S.; Yağci, Y.; Erciyes, A.T. Polymers from Triglyceride Oils. Prog. Polym. Sci. 2006, 31, 633–670. [Google Scholar] [CrossRef]
- Depczynska, E.; Mazela, B. Modern bio-based wood coatings-potential for the future. Surf. Coat. Int. 2019, 102, 78–83. [Google Scholar]
- Frias, C.F.; Serra, A.C.; Ramalho, A.; Coelho, J.F.; Fonseca, A.C. Preparation of fully biobased epoxy resins from soybean oil based amine hardeners. Ind. Crop. Prod. 2017, 109, 434–444. [Google Scholar] [CrossRef]
- Patton, T.C. Alkyd Resin Technology: Formulating Techniques and Allied CalCulations; Interscience Publishers: New York, NY, USA, 1962. [Google Scholar]
- Solomon, D.H.; Swift, J.D. The Influence of catalyst on the glycerolysis of linseed oil. OCCA J. 1966, 49, 915–927. [Google Scholar]
- Wholf, R.H. Coconut Oil Modified Alkyd Resins and Copolymers Thereof with an Alkyl Acrylate. U.S. Patent US3374194A, 19 March 1968. [Google Scholar]
- Athawale, V.D.; Nimbalkar, R.V. Waterborne Coatings Based on Renewable Oil Resources: An Overview. J. Am. Oil Chem. Soc. 2010, 88, 159–185. [Google Scholar] [CrossRef]
- Patel, C.J.; Mannari, V. Air-drying bio-based polyurethane dispersion from cardanol: Synthesis and characterization of coatings. Prog. Org. Coat. 2014, 77, 997–1006. [Google Scholar] [CrossRef]
- Datta, J.; Głowińska, E. Effect of hydroxylated soybean oil and bio-based propanediol on the structure and thermal properties of synthesized bio-polyurethanes. Ind. Crop. Prod. 2014, 61, 84–91. [Google Scholar] [CrossRef]
- Bora, M.M.; Gogoi, P.; Deka, D.C.; Kakati, D.K. Synthesis and characterization of yellow oleander (Thevetia peruviana) seed oil-based alkyd resin. Ind. Crop. Prod. 2014, 52, 721–728. [Google Scholar] [CrossRef]
- Islam, M.R.; Beg, M.D.H.; Jamari, S.S. Alkyd Based Resin from Non-drying Oil. Procedia Eng. 2014, 90, 78–88. [Google Scholar] [CrossRef]
- Assanvo, E.F.; Gogoi, P.; Dolui, S.K.; Baruah, S.D. Synthesis, characterization, and performance characteristics of alkyd resins based on Ricinodendron heudelotii oil and their blending with epoxy resins. Ind. Crop. Prod. 2015, 65, 293–302. [Google Scholar] [CrossRef]
- Liu, C.; Li, J.; Lei, W.; Zhou, Y. Development of biobased unsaturated polyester resin containing highly functionalized castor oil. Ind. Crop. Prod. 2014, 52, 329–337. [Google Scholar] [CrossRef]
- Dutta, N.; Karak, N.; Dolui, S. Synthesis and characterization of polyester resins based on Nahar seed oil. Prog. Org. Coat. 2004, 49, 146–152. [Google Scholar] [CrossRef]
- Rabek, J.F. Współczesna Wiedza o Polimerach; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2016. [Google Scholar]
- Becher, P. Fatty Acids in Industry: Processes, Properties, Derivatives, Applications. Robert W. Johnson and Earle Fritz (eds.). Marcel Dekker, New York and Basel, 1989, pp. xvi + 667. J. Dispers. Sci. Technol. 1990, 11, 433–434. [Google Scholar] [CrossRef]
- Hess, P.S.; O’Hare, G.A. Oxidation of Linseed Oil. Ind. Eng. Chem. 1950, 42, 1424–1431. [Google Scholar] [CrossRef]
- Mallégol, J.; Lemaire, J.; Gardette, J.-L. Drier influence on the curing of linseed oil. Prog. Org. Coat. 2000, 39, 107–113. [Google Scholar] [CrossRef]
- Blayo, A.; Gandini, A.; Le Nest, J.-F. Chemical and rheological characterizations of some vegetable oils derivatives commonly used in printing inks. Ind. Crop. Prod. 2001, 14, 155–167. [Google Scholar] [CrossRef]
- Taylor, W.L. Blowing drying oils. J. Am. Oil Chem. Soc. 1950, 27, 472–476. [Google Scholar] [CrossRef]
- Veigel, S.; Lems, E.-M.; Grüll, G.; Hansmann, C.; Rosenau, T.; Zimmermann, T.; Gindl, W. Simple Green Route to Performance Improvement of Fully Bio-Based Linseed Oil Coating Using Nanofibrillated Cellulose. Polymers 2017, 9, 425. [Google Scholar] [CrossRef]
- Chang, C.-W.; Chang, J.-P.; Lu, K.-T. Synthesis of Linseed Oil-Based Waterborne Urethane Oil Wood Coatings. Polymers 2018, 10, 1235. [Google Scholar] [CrossRef]
- Lu, K.-T.; Chang, J.-P. Synthesis and Antimicrobial Activity of Metal-Containing Linseed Oil-Based Waterborne Urethane Oil Wood Coatings. Polymers 2020, 12, 663. [Google Scholar] [CrossRef]
- Jebrane, M.; Cai, S.; Sandstrom, C.; Terziev, N. The reactivity of linseed and soybean oil with different epoxidation degree towards vinyl acetate and impact of the resulting copolymer on the wood durability. Express Polym. Lett. 2017, 11, 383–395. [Google Scholar] [CrossRef]
- Srinivasan, M. Synthesis, Properties and Applications of Bio-Based Materials; Michigan State University, Materials Science and Engineering: East Langing, MI, USA, 2010; ISBN 1-124-39739-6. [Google Scholar]
- Zhuang, Y. Novel Synthetic Route to Biobased Silylated Soybean Oil for Use as Coating Material; Michigan State University, Chemical Engineering: East Langing, MI, USA, 2011; ISBN 1-124-86366-4. [Google Scholar]
- Tambe, C.; Dewasthale, S.; Shi, X.; Graiver, D.; Narayan, R. Silylation of Non-Terminal Double Bonds of Natural Oils. Silicon 2015, 8, 87–98. [Google Scholar] [CrossRef]
- Han, C.; Bian, J.; Liu, H.; Han, L.; Wang, S.; Dong, L.; Chen, S. An investigation of the effect of silane water-crosslinking on the properties of poly(L-lactide). Polym. Int. 2010, 59, 695–703. [Google Scholar] [CrossRef]
- Schneider, J.; Bourque, K.; Narayan, R. Moisture curable toughened poly(lactide) utilizing vinyltrimethoxysilane based crosslinks. Express Polym. Lett. 2016, 10, 799–809. [Google Scholar] [CrossRef]
- Rahmat, M. Silane crosslinking of poly(lactic acid): The effect of simultaneous hydrolytic degradation. Express Polym. Lett. 2015, 9, 1133–1141. [Google Scholar] [CrossRef]
- Depczynska, E.; Furgal, M.; Mazela, B.; Perdoch, W. Silylated Linseed Oil-Invisible Wood Protection? Surf. Coat. Int. 2019, 102, 22–26. [Google Scholar]
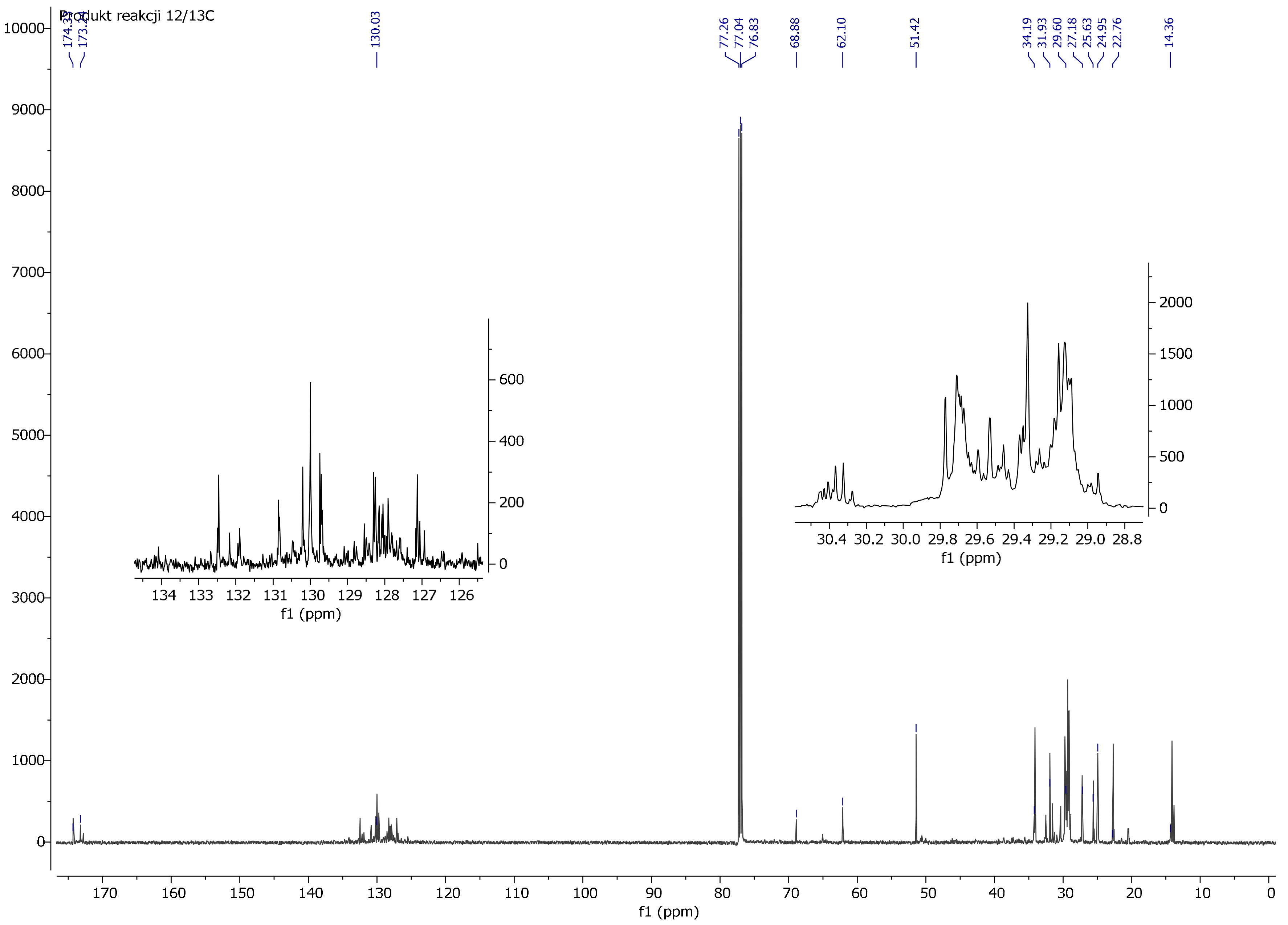
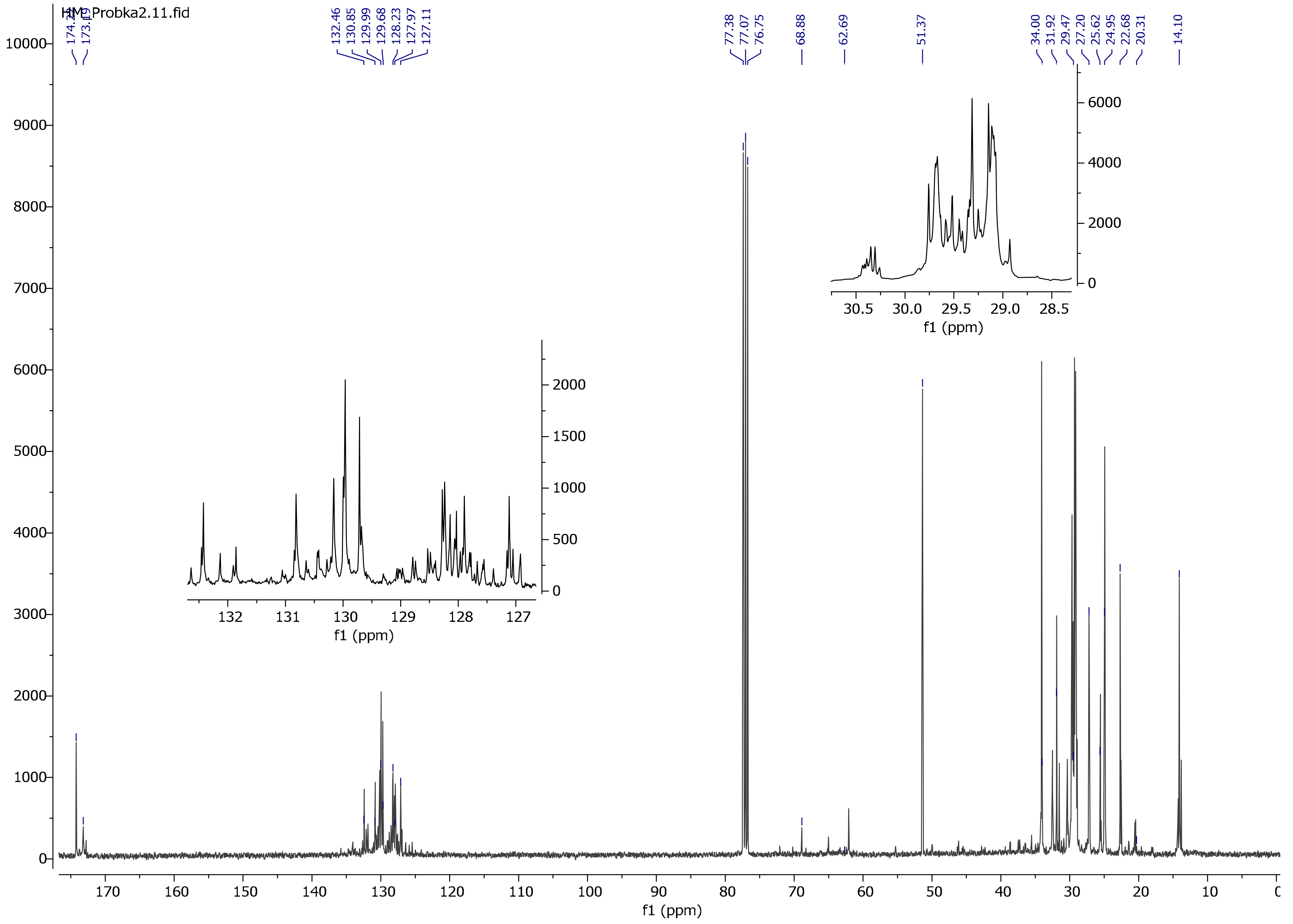
| Reaction ID | Luperox 101 for 1 Mol of Oil [mol] | VTMOS for 1 Mol of Oil [mol] | Moment of VTMOS Addition | Reaction Time at 280 °C [h] |
|---|---|---|---|---|
| 1 | 0 | 0 | No addition | 12 |
| 2 | 0.04 | 0 | No addition | 12 |
| 3 | 0.04 | 0.6 | At the beginning of reaction | 6 |
| 4 | 0.04 | 0.6 | 3rd h of reaction | 6 |
| 5 | 0.04 | 1.2 | At the beginning of reaction | 3 |
| 6 | 0 | 1.2 | At the beginning of reaction | 12 |
| Reaction ID | Acid Number [mg KOH/g] |
|---|---|
| 1 | 4.59 |
| 2 | 6.14 |
| 3 | 0.47 |
| 4 | 0.23 |
| 5 | 0.47 |
| Raw linseed oil | 0.30 |
| Sample | VTMOS Content [%] | Product Consistency | |||
|---|---|---|---|---|---|
| Reaction ID | 0 h | 3 h | 6 h | 12 h | |
| 1 | <0.02 | <0.02 | <0.02 | <0.02 | liquid |
| 2 | <0.02 | <0.02 | <0.02 | <0.02 | liquid |
| 3 | 8 | 0.15 | <0.02 | – | gel |
| 4 | <0.02 | 8.1 | 0.3 | – | liquid |
| 5 | 15 | 3.2 | – | – | liquid |
| 6 | 15 | 2.8 | 0.09 | <0.02 | gel |
| Sample | 0 h | 3 h | 6 h | 12 h | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reaction ID | Mn | Mw | PDI | Mn | Mw | PDI | Mn | Mw | PDI | Mn | Mw | PDI |
| 1 | 1313 | 1413 | 1.08 | 1577 | 2203 | 1.45 | 1876 | 3320 | 1.77 | 2357 | 7036 | 3.90 |
| 2 | 1319 | 1561 | 1.18 | 1625 | 2653 | 1.63 | 1858 | 3315 | 1.79 | 2187 | 6064 | 2.77 |
| 3 | 1310 | 1677 | 1.28 | 1242 | 4804 | 3.86 | 1576 | 24459 | 15.52 | – | – | – |
| 4 | 1563 | 2744 | 1.75 | 1586 | 2579 | 1.62 | 1340 | 6186 | 4.61 | – | – | – |
| 5 | 1311 | 1611 | 1.23 | 1440 | 3879 | 2.69 | – | – | – | – | – | – |
| 6 | 1322 | 1421 | 1.07 | 1478 | 3711 | 3.62 | 1021 | 9269 | 9.08 | 714 | 2166 | 3.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Depczyńska, E.; Perdoch, W.; Mazela, B. Influence of Reaction Parameters on the Gelation of Silanised Linseed Oil. Materials 2020, 13, 5376. https://doi.org/10.3390/ma13235376
Depczyńska E, Perdoch W, Mazela B. Influence of Reaction Parameters on the Gelation of Silanised Linseed Oil. Materials. 2020; 13(23):5376. https://doi.org/10.3390/ma13235376
Chicago/Turabian StyleDepczyńska, Ewelina, Waldemar Perdoch, and Bartłomiej Mazela. 2020. "Influence of Reaction Parameters on the Gelation of Silanised Linseed Oil" Materials 13, no. 23: 5376. https://doi.org/10.3390/ma13235376
APA StyleDepczyńska, E., Perdoch, W., & Mazela, B. (2020). Influence of Reaction Parameters on the Gelation of Silanised Linseed Oil. Materials, 13(23), 5376. https://doi.org/10.3390/ma13235376






