Deformation Behavior of Nanocrystalline Body-Centered Cubic Iron with Segregated, Foreign Interstitial: A Molecular Dynamics Study
Abstract
1. Introduction
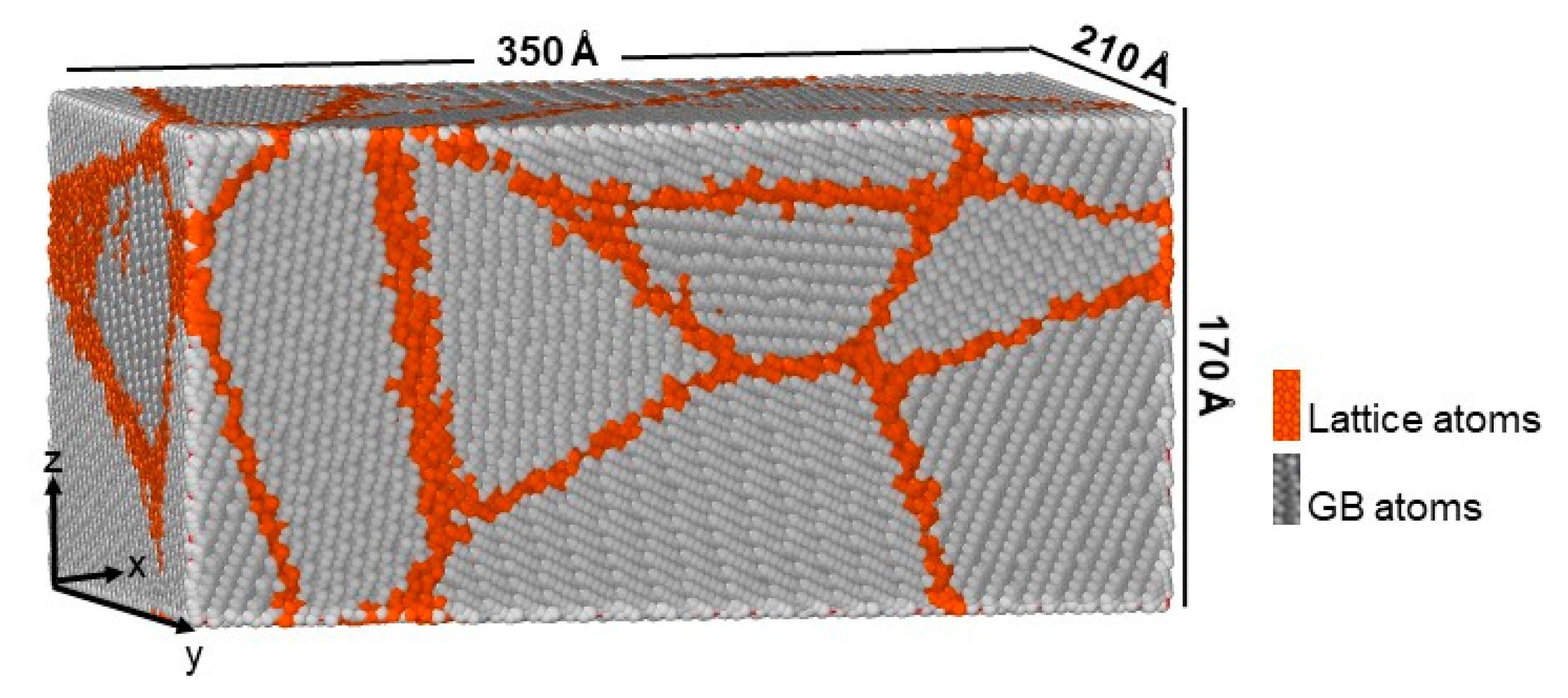
2. Simulation Details
3. Results and Discussion
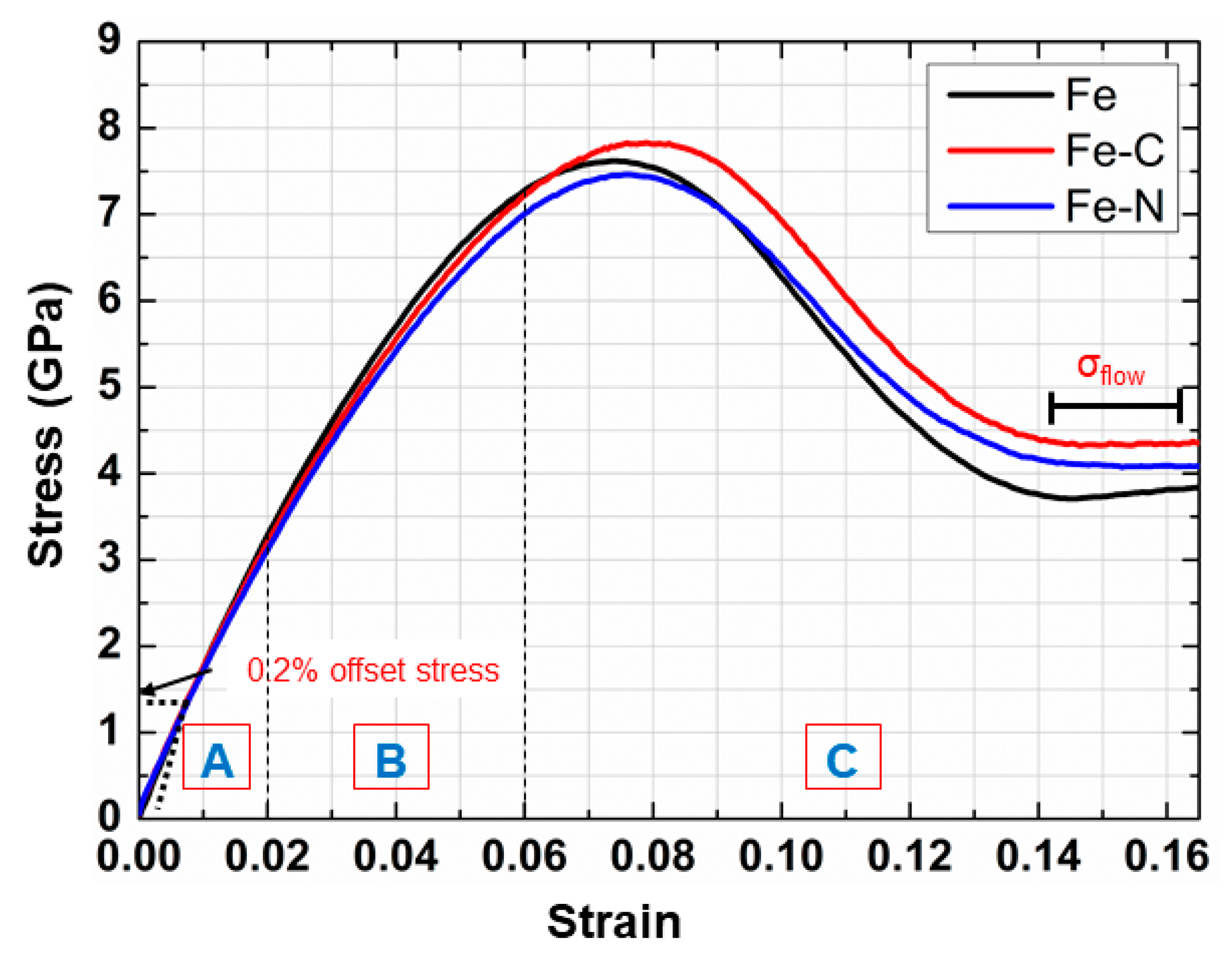
3.1. Stress–Strain Curves of nc Samples
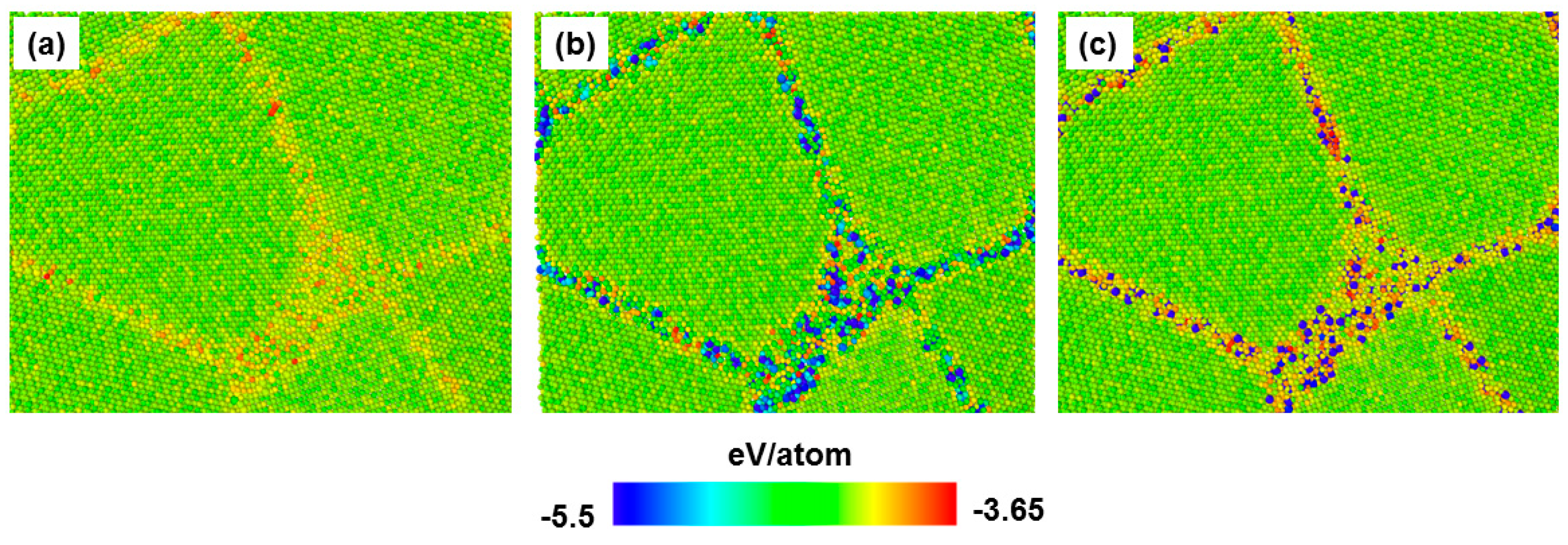
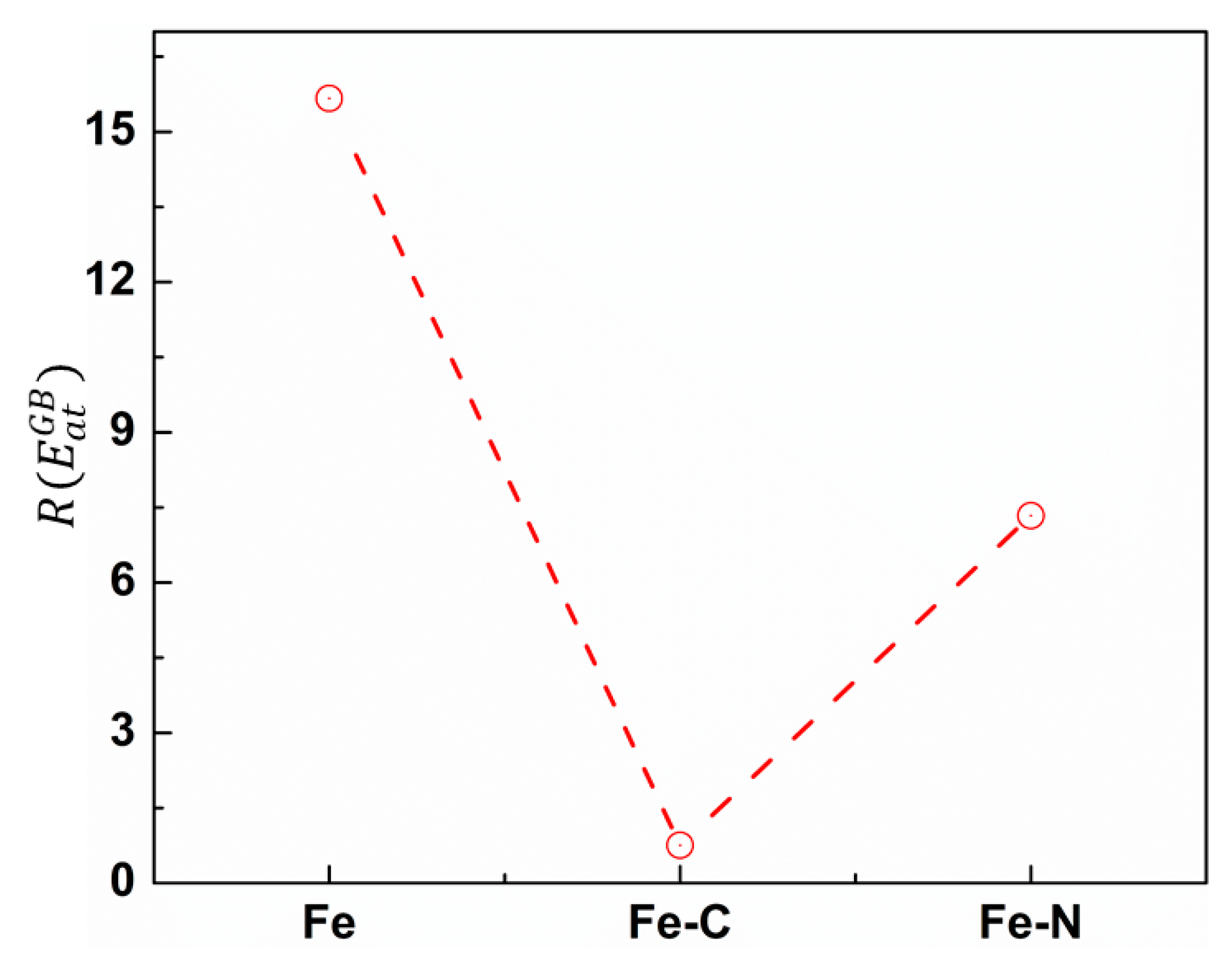
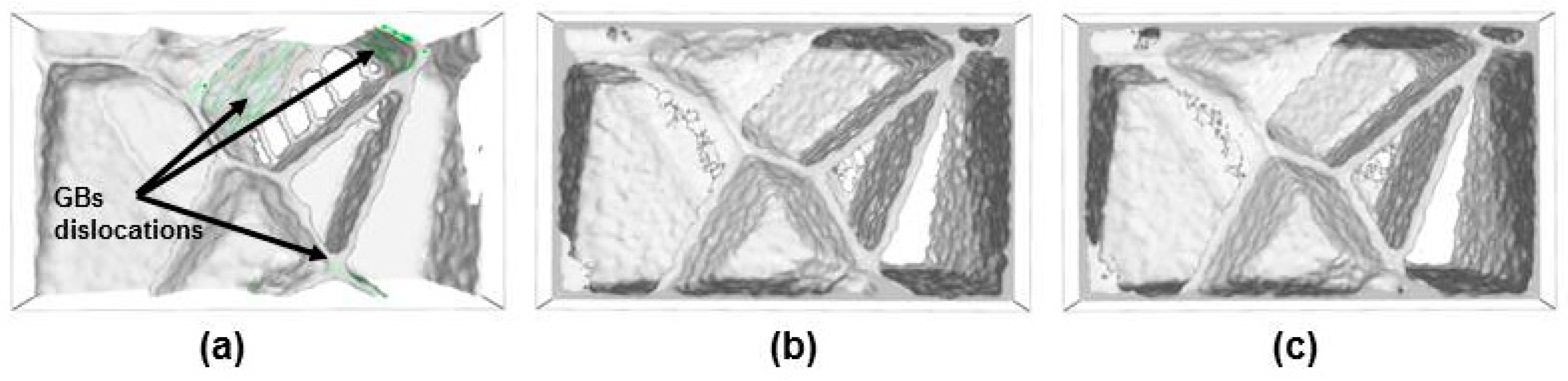
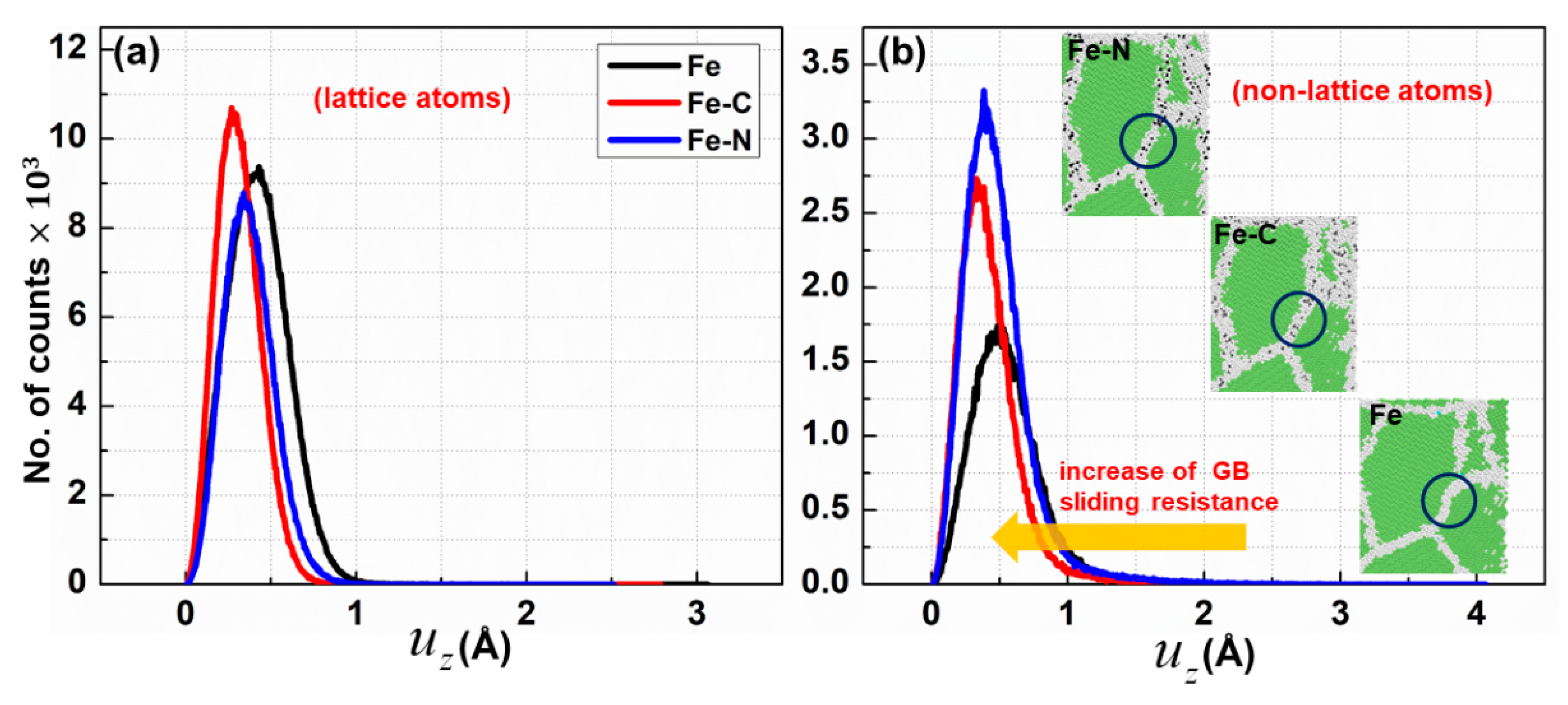
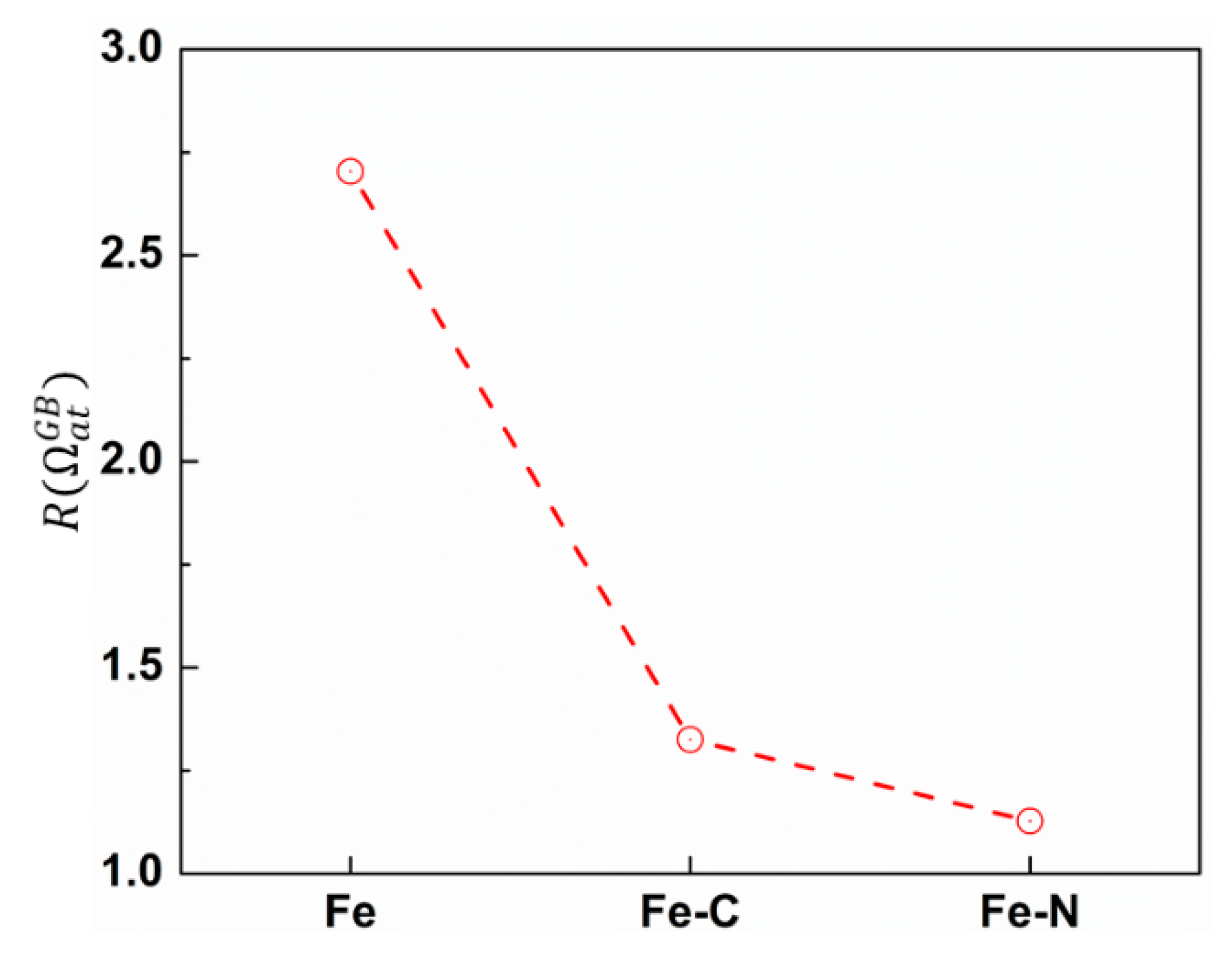
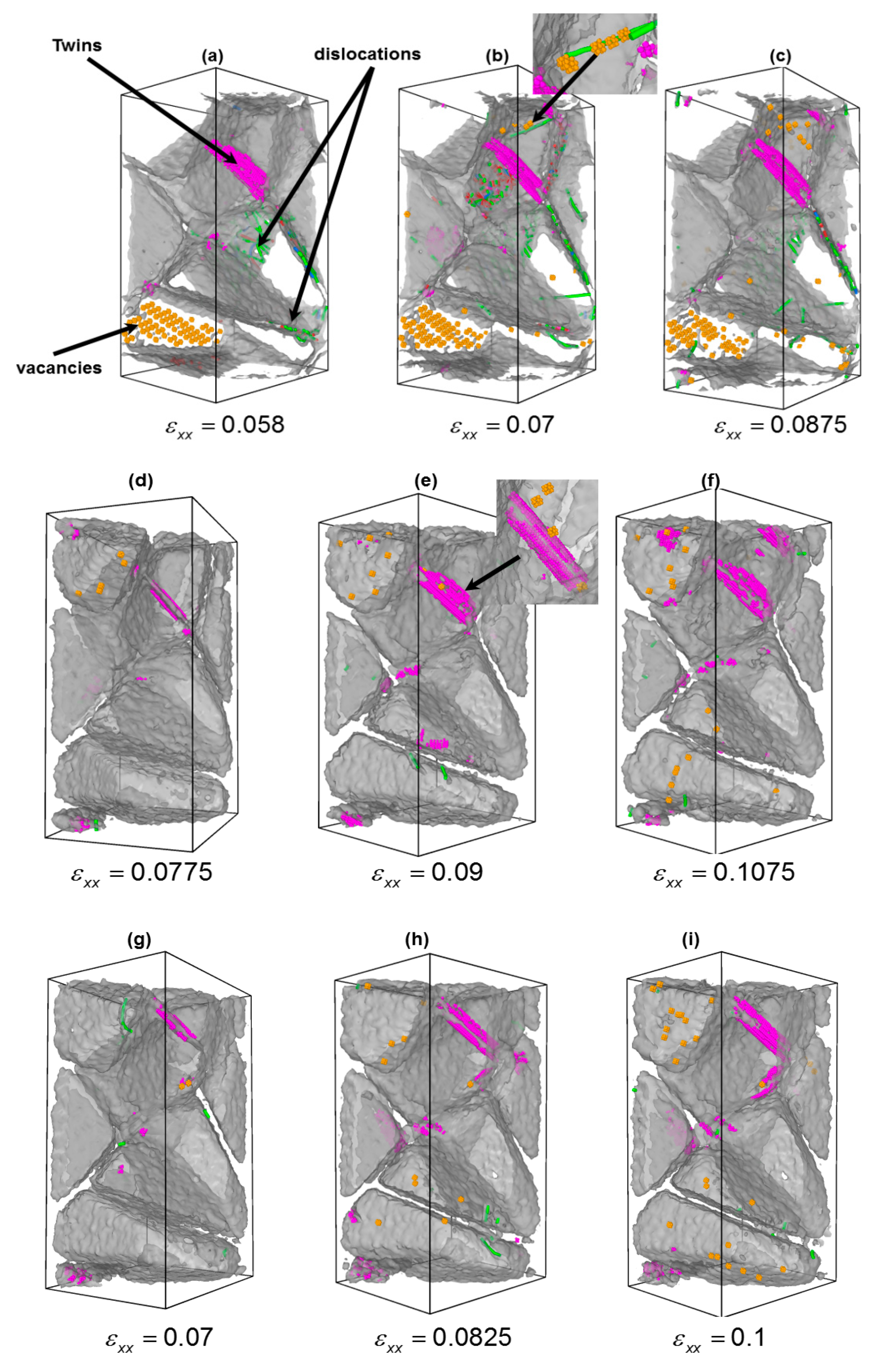
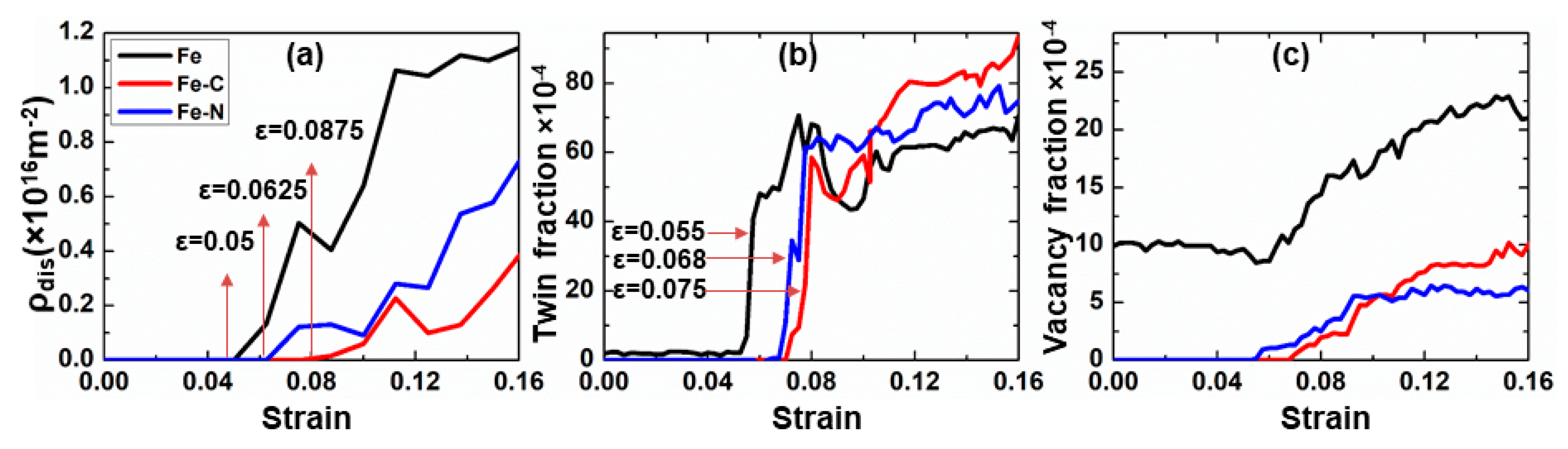
3.2. Effect of Carbon/Nitrogen Segregation on the Plastic Deformation Processes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A Calculation of generalized planer fault energy (GPFE) curve.
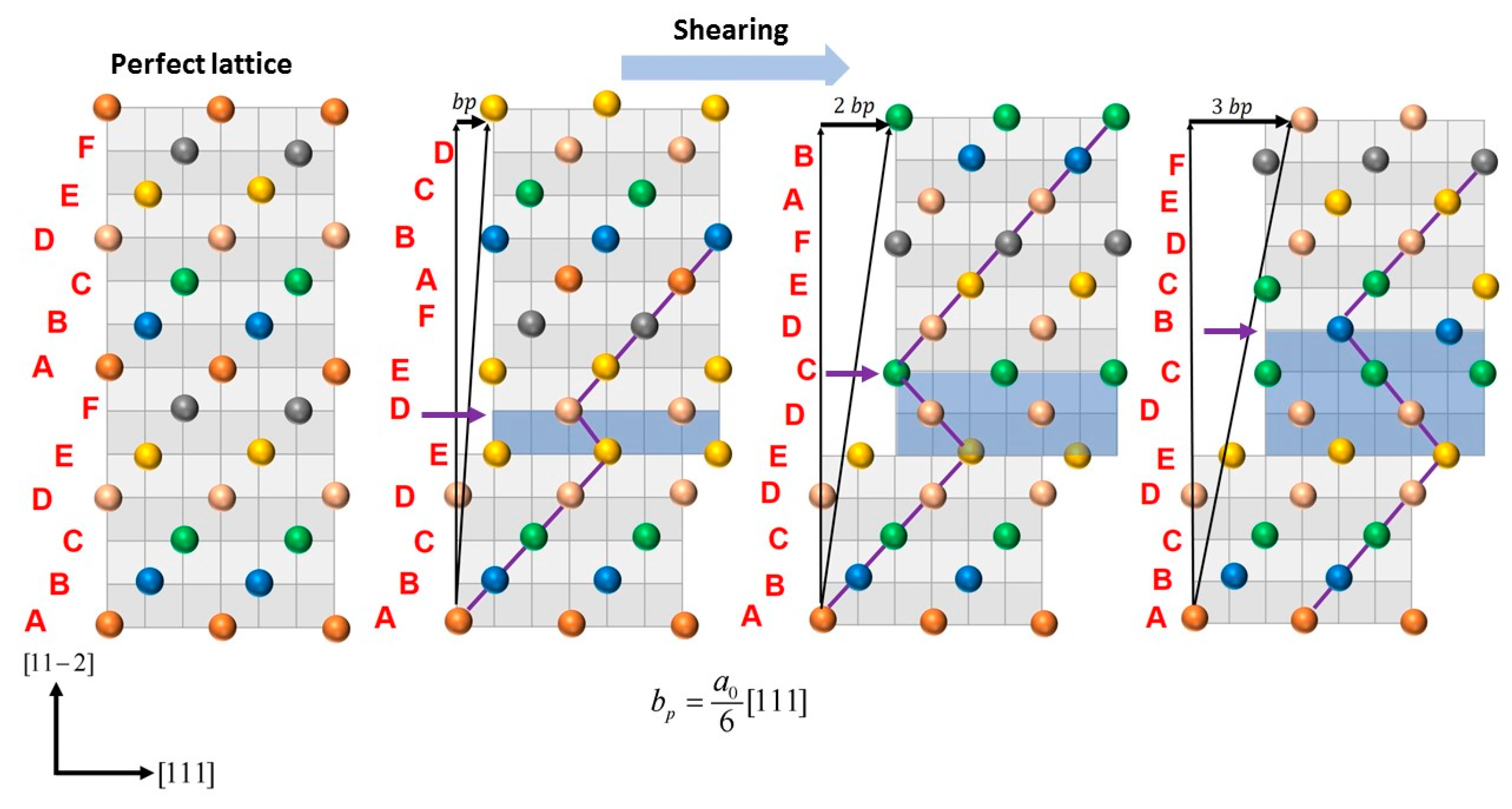
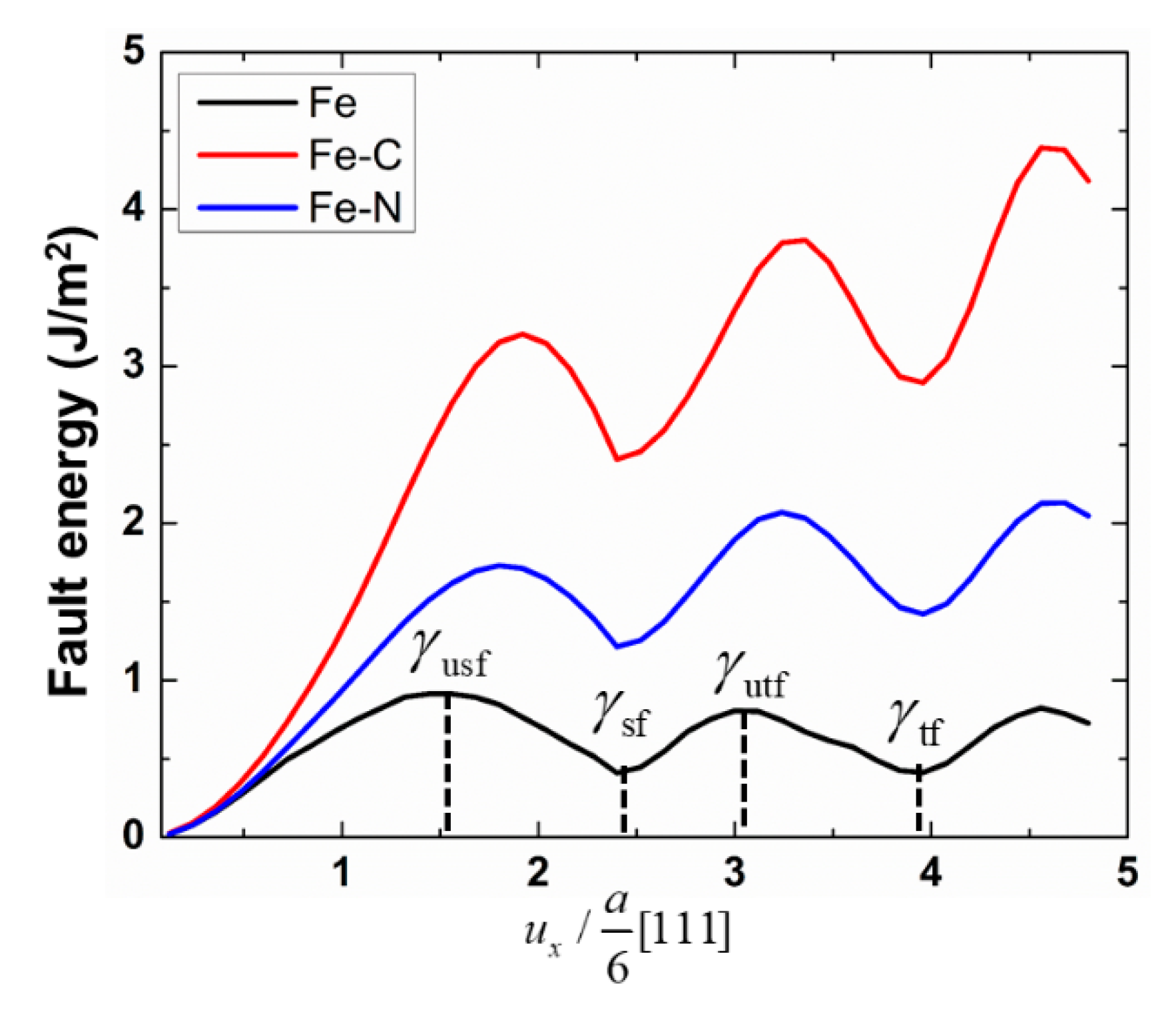
| Sample | mJ/m2 | mJ/m2 | mJ/m2 | ||
|---|---|---|---|---|---|
| Fe | 912 | 410 | 805 | 0.44 | 0.88 |
| Fe-N | 1807 | 1213 | 2070 | 0.67 | 1.145 |
| Fe-C | 3073 | 2408 | 3660 | 0.78 | 1.191 |
References
- Wang, Y.M.; Cheng, S.; Wei, Q.M.; Ma, E.; Nieh, T.G.; Hamza, A. Effects of annealing and impurities on tensile properties of electrodeposited nanocrystalline Ni. Scr. Mater. 2004, 51, 1023–1028. [Google Scholar] [CrossRef]
- Detor, A.J.; Schuh, C.A. Microstructural evolution during the heat treatment of nanocrystalline alloys. J. Mater. Res. 2007, 22, 3233–3248. [Google Scholar] [CrossRef]
- Vo, N.Q.; Averback, R.S.; Bellon, P.; Caro, A. Limits of hardness at the nanoscale: Molecular dynamics simulations. Phys. Rev. B 2008, 78, 241402. [Google Scholar] [CrossRef]
- Vo, N.Q.; Averback, R.S.; Bellon, P.; Caro, A. Yield strength in nanocrystalline Cu during high strain rate deformation. Scr. Mater. 2009, 61, 76–79. [Google Scholar] [CrossRef]
- Yin, W.M.; Whang, S.H.; Mirshams, R.A. Effect of interstitials on tensile strength and creep in nanostructured Ni. Acta Mater. 2005, 53, 383–392. [Google Scholar] [CrossRef]
- Yin, W.M.; Whang, S.H. Creep in boron-doped nanocrystalline nickel. Scr. Mater. 2001, 44, 569–574. [Google Scholar] [CrossRef]
- Rajgarhia, R.K.; Spearot, D.E.; Saxena, A. Plastic deformation of nanocrystalline copper-antimony alloys. J. Mater. Res. 2010, 25, 411–421. [Google Scholar] [CrossRef]
- Wolf, D. Structure-energy correlation for grain boundaries in F.C.C. metals—I. Boundaries on the (111) and (100) planes. Acta Metall. 1989, 37, 1983–1993. [Google Scholar] [CrossRef]
- Gleiter, H. On the structure of grain boundaries in metals. Mater. Sci. Eng. 1982, 52, 91–131. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Li, H. Grain growth in electrodeposited nanocrystalline fcc Ni–Fe alloys. Scr. Mater. 2006, 55, 263–266. [Google Scholar] [CrossRef]
- Hibbard, G.D.; Aust, K.T.; Erb, U. Thermal stability of electrodeposited nanocrystalline Ni–Co alloys. Mater. Sci. Eng. A 2006, 433, 195–202. [Google Scholar] [CrossRef]
- Li, H.Q.; Ebrahimi, F. An investigation of thermal stability and microhardness of electrodeposited nanocrystalline nickel-21% iron alloys. Acta Mater. 2003, 51, 3905–3913. [Google Scholar] [CrossRef]
- Detor, A.J.; Schuh, C.A. Tailoring and patterning the grain size of nanocrystalline alloys. Acta Mater. 2007, 55, 371–379. [Google Scholar] [CrossRef]
- Günther, B.; Kumpmann, A.; Kunze, H.-D. Secondary recrystallization effects in nanostructured elemental metals. Scr. Metall. Mater. 1992, 27, 833–838. [Google Scholar] [CrossRef]
- Weissmüller, J.; Löffler, J.; Kleber, M. Atomic structure of nanocrystalline metals studied by diffraction techniques and EXAFS. Nanostructured Mater. 1995, 6, 105–114. [Google Scholar] [CrossRef]
- Vo, N.Q.; Schäfer, J.; Averback, R.S.; Albe, K.; Ashkenazy, Y.; Bellon, P. Reaching theoretical strengths in nanocrystalline Cu by grain boundary doping. Scr. Mater. 2011, 65, 660–663. [Google Scholar] [CrossRef]
- Takeda, K.; Nakada, N.; Tsuchiyama, T.; Takaki, S. Effect of interstitial elements on Hall-Petch coefficient of ferritic iron. ISIJ Int. 2008, 48, 1122–1125. [Google Scholar] [CrossRef]
- Takaki, S. Effect of Carbon and Nitrogen on the Hall-Petch Coefficient of Ferritic Iron (Review on the Hall-Petch Relation in Ferritic Iron). In Materials Science Forum; Trans Tech Publications Ltd.: Kapellweg, Sweden, 2010; Volume 638–642, pp. 168–173. [Google Scholar] [CrossRef]
- Takaki, S.; Akama, D.; Nakada, N.; Tsuchiyama, T. Effect of Grain Boundary Segregation of Interstitial Elements on Hall–Petch Coefficient in Steels. Mater. Trans. 2014, 55, 28–34. [Google Scholar] [CrossRef]
- Takahashi, J.; Kawakami, K.; Ushioda, K.; Takaki, S.; Nakata, N.; Tsuchiyama, T. Quantitative analysis of grain boundaries in carbon- and nitrogen-added ferritic steels by atom probe tomography. Scr. Mater. 2012, 66, 207–210. [Google Scholar] [CrossRef]
- Wu, R.; Freeman, A.J.; Olson, G.B. Effects of carbon on Fe-grain-boundary cohesion: First-principles determination. Phys. Rev. B 1996, 53, 7504–7509. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, R.; Riku, M.; Taketomi, S.; Miyazaki, N. Hydrogen–Grain Boundary Interaction in Fe, Fe–C, and Fe–N Systems. Prog. Nucl. Sci. Technol. 2011, 2, 9–15. [Google Scholar] [CrossRef]
- Masumura, T.; Nakada, N.; Tsuchiyama, T.; Takaki, S.; Koyano, T.; Adachi, K. The difference in thermal and mechanical stabilities of austenite between carbon- and nitrogen-added metastable austenitic stainless steels. Acta Mater. 2015, 84, 330–338. [Google Scholar] [CrossRef]
- Voronoi, G. Nouvelles applications des paramètres continus à la théorie des formes quadratiques. Deuxième mémoire. Recherches sur les parallélloèdres primitifs. Crelles J. 2009, 1908, 198–287. [Google Scholar] [CrossRef]
- Hirel, P. Atomsk: A tool for manipulating and converting atomic data files. Comput. Phys. Commun. 2015, 197, 212–219. [Google Scholar] [CrossRef]
- Honeycutt, J.D.; Andersen, H.C. Molecular dynamics study of melting and freezing of small Lennard-Jones clusters. J. Phys. Chem. 1987, 91, 4950–4963. [Google Scholar] [CrossRef]
- Goncalves, M.C.; Margarido, F. (Eds.) Materials for Construction and Civil Engineering: Science, Processing, and Design; Springer International Publishing: Basel, Switzerland, 2015; ISBN 978-3-319-08235-6. [Google Scholar]
- Lee, B.-J. A modified embedded-atom method interatomic potential for the Fe–C system. Acta Mater. 2006, 54, 701–711. [Google Scholar] [CrossRef]
- Lee, B.-J.; Lee, T.-H.; Kim, S.-J. A modified embedded-atom method interatomic potential for the Fe–N system: A comparative study with the Fe–C system. Acta Mater. 2006, 54, 4597–4607. [Google Scholar] [CrossRef]
- Stukowski, A.; Albe, K. Extracting dislocations and non-dislocation crystal defects from atomistic simulation data. Model. Simul. Mater. Sci. Eng. 2010, 18, 085001. [Google Scholar] [CrossRef]
- Stukowski, A.; Arsenlis, A. On the elastic–plastic decomposition of crystal deformation at the atomic scale. Model. Simul. Mater. Sci. Eng. 2012, 20, 035012. [Google Scholar] [CrossRef]
- Stukowski, A. Structure identification methods for atomistic simulations of crystalline materials. Model. Simul. Mater. Sci. Eng. 2012, 20, 045021. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO–the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2010, 18, 015012. [Google Scholar] [CrossRef]
- Begau, C.; Hartmaier, A.; George, E.P.; Pharr, G.M. Atomistic processes of dislocation generation and plastic deformation during nanoindentation. Acta Mater. 2011, 59, 934–942. [Google Scholar] [CrossRef]
- Begau, C.; Hua, J.; Hartmaier, A. A novel approach to study dislocation density tensors and lattice rotation patterns in atomistic simulations. J. Mech. Phys. Solids 2012, 60, 711–722. [Google Scholar] [CrossRef]
- Millett, P.C.; Selvam, R.P.; Saxena, A. Molecular dynamics simulations of grain size stabilization in nanocrystalline materials by addition of dopants. Acta Mater. 2006, 54, 297–303. [Google Scholar] [CrossRef]
- Fen, Y.-Q.; Wang, C.-Y. Electronic effects of nitrogen and phosphorus on iron grain boundary cohesion. Comput. Mater. Sci. 2001, 20, 48–56. [Google Scholar] [CrossRef]
- Cottrell, A.H. The Mechanical Properties of Matter; Wiley: New York, NY, USA, 1964; pp. 272–283. ISBN 978-0-471-17676-3. [Google Scholar]
- Van Swygenhoven, H.; Derlet, P.M. Grain-boundary sliding in nanocrystalline fcc metals. Phys. Rev. B 2001, 64, 224105. [Google Scholar] [CrossRef]
- Rycroft, C.H. VORO++: A Three-Dimensional Voronoi Cell Library in C++; Lawrence Berkeley National Lab. (LBNL): Berkeley, CA, USA, 2009; Volume 19. [CrossRef]
- Kelly, A.; Knowles, K.M. Crystallography and Crystal Defect, 2nd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; ISBN 978-0-470-75014-8. [Google Scholar]
- Cottrell, A.H.; Bilby, B.A. LX. A mechanism for the growth of deformation twins in crystals. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1951, 42, 573–581. [Google Scholar] [CrossRef]
- Meyers, M.A.; Vöhringer, O.; Lubarda, V.A. The onset of twinning in metals: A constitutive description. Acta Mater. 2001, 49, 4025–4039. [Google Scholar] [CrossRef]
- De Rességuier, T.; Hallouin, M. Stress relaxation and precursor decay in laser shock-loaded iron. J. Appl. Phys. 1998, 84, 1932–1938. [Google Scholar] [CrossRef]
- Magee, C.L.; Hoffman, D.W.; Davies, R.G. The effect of interstitial solutes on the twinning stress of b.c.c. metals. Philos. Mag. 1971, 23, 1531–1540. [Google Scholar] [CrossRef]
- Marian, J.; Cai, W.; Bulatov, V.V. Dynamic transitions from smooth to rough to twinning in dislocation motion. Nat. Mater. 2004, 3, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Seeger, A. Production of lattice vacancies in metals by deformation twinning. Philos. Mag. Lett. 2007, 87, 95–102. [Google Scholar] [CrossRef]
- Vítek, V. Multilayer stacking faults and twins on {211} planes in B.C.C. metals. Scr. Metall. 1970, 4, 725–732. [Google Scholar] [CrossRef]
- Saunders, N.; Miodownik, A.P. CALPHAD (Calculation of Phase Diagrams): A Comprehensive Guide; Elsevier: Amsterdam, The Netherlands, 1998; ISBN 978-0-08-052843-4. [Google Scholar]
- Kaufman, L.; Bernstein, H. Computer Calculation of Phase Diagrams with Special Reference to Refractory Metals; Academic Press: Cambridge, MA, USA, 1970. [Google Scholar]
- Porter, D.A.; Easterling, K.E. Phase Transformations in Metals and Alloys, 3rd ed.; Revised Reprint; CRC Press: Boca Raton, FL, USA, 1992; ISBN 978-0-7487-5741-1. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlMotasem, A.T.; Posselt, M.; Polcar, T. Deformation Behavior of Nanocrystalline Body-Centered Cubic Iron with Segregated, Foreign Interstitial: A Molecular Dynamics Study. Materials 2020, 13, 5351. https://doi.org/10.3390/ma13235351
AlMotasem AT, Posselt M, Polcar T. Deformation Behavior of Nanocrystalline Body-Centered Cubic Iron with Segregated, Foreign Interstitial: A Molecular Dynamics Study. Materials. 2020; 13(23):5351. https://doi.org/10.3390/ma13235351
Chicago/Turabian StyleAlMotasem, Ahmed Tamer, Matthias Posselt, and Tomas Polcar. 2020. "Deformation Behavior of Nanocrystalline Body-Centered Cubic Iron with Segregated, Foreign Interstitial: A Molecular Dynamics Study" Materials 13, no. 23: 5351. https://doi.org/10.3390/ma13235351
APA StyleAlMotasem, A. T., Posselt, M., & Polcar, T. (2020). Deformation Behavior of Nanocrystalline Body-Centered Cubic Iron with Segregated, Foreign Interstitial: A Molecular Dynamics Study. Materials, 13(23), 5351. https://doi.org/10.3390/ma13235351







