Evaluation of Preosteoblast MC3T3-E1 Cells Cultured on a Microporous Titanium Membrane Fabricated Using a Precise Mechanical Punching Process
Abstract
1. Introduction
2. Materials and methods
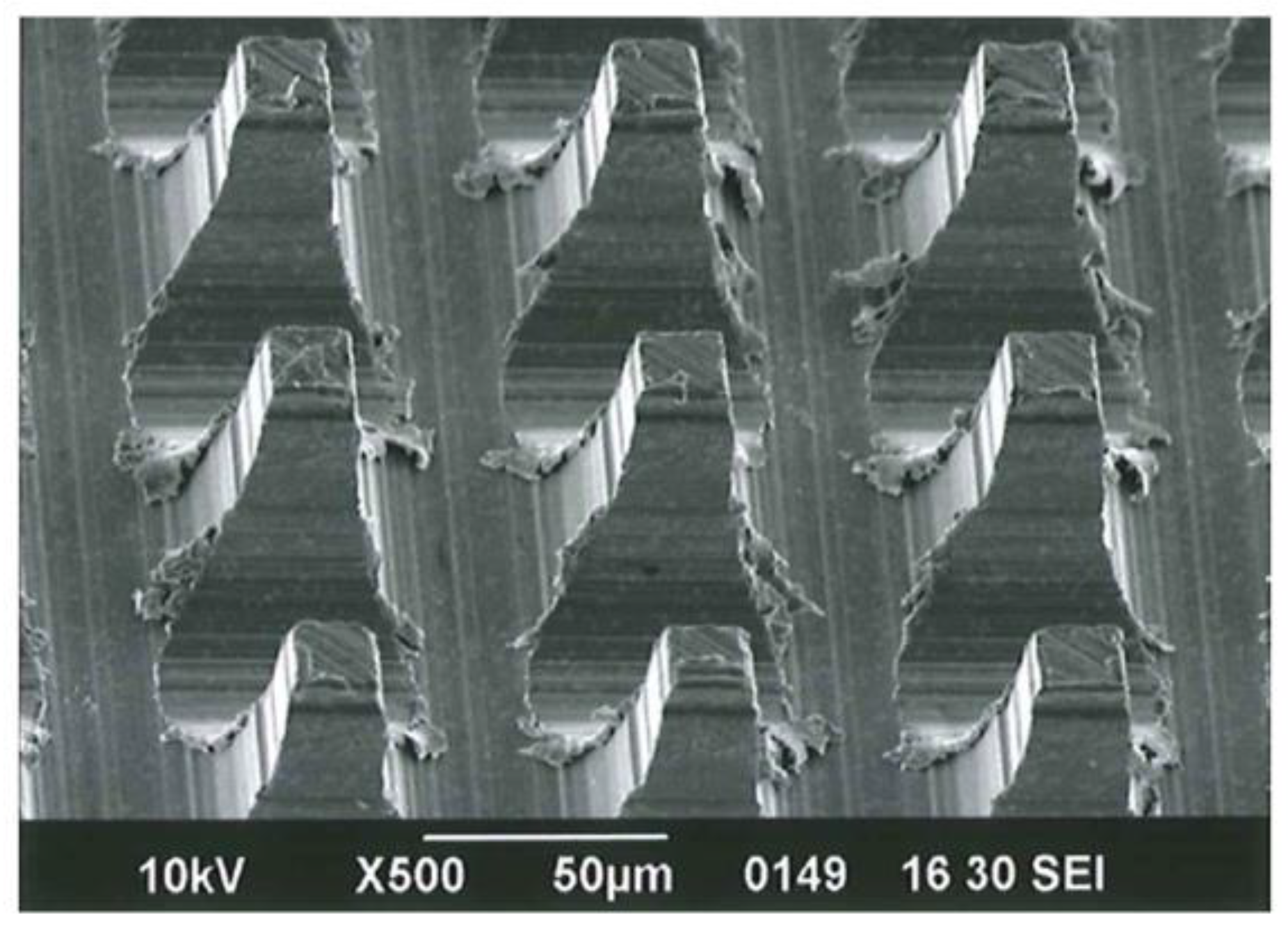
2.1. Cross-Cut Preparation of MPNP
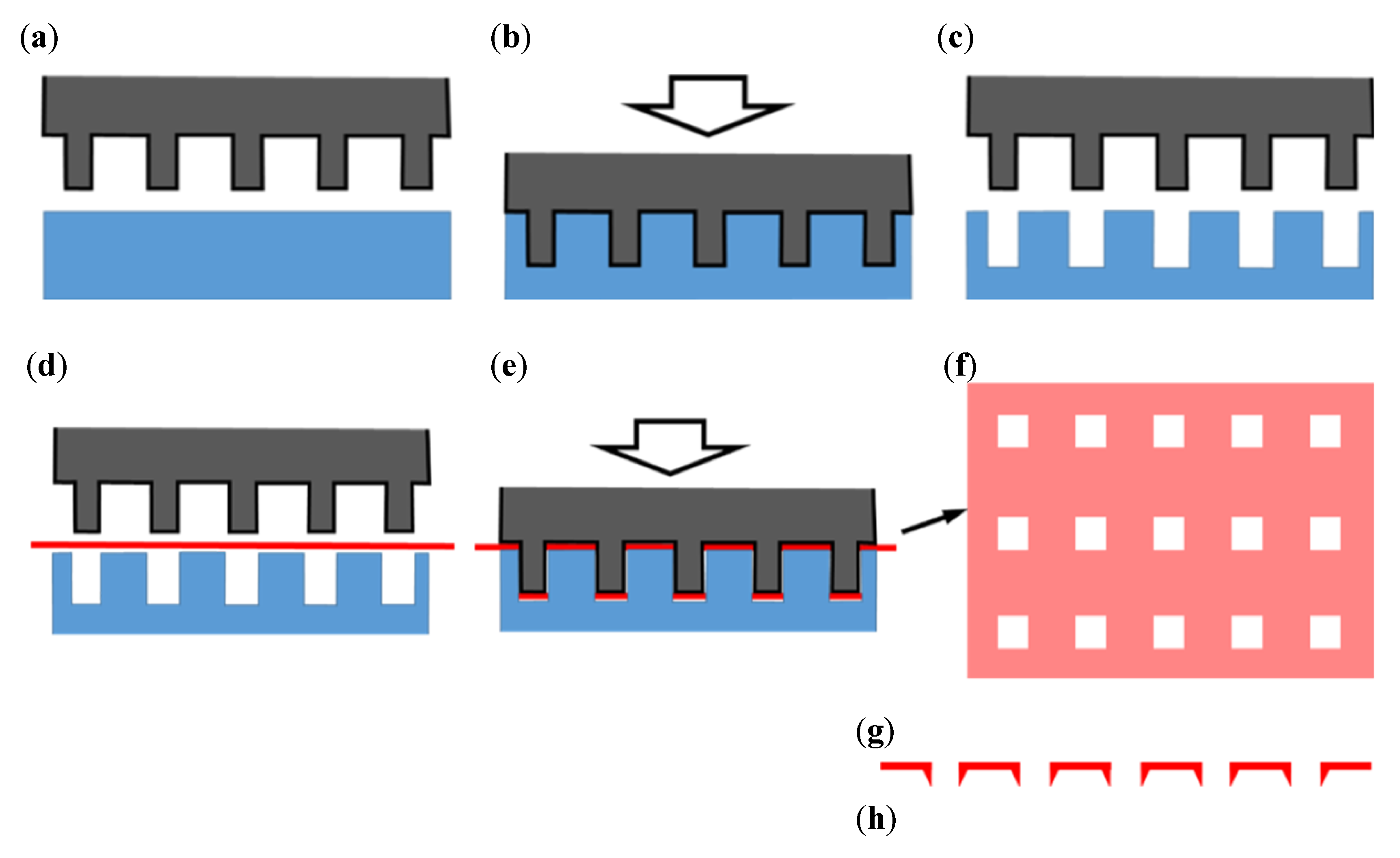
2.2. Fabrication of a Microperforated Ti Membrane
2.3. Preparation of Processed Ti Specimens for Cultivation
2.4. Cultivation of the Osteogenic Cell Line
2.5. Scanning Electron Microscope (SEM) Observations of Cell Morphology
2.6. Immunofluorescence Assay
2.7. Reverse Transcription and Real-Time Quantitative Polymerase Chain Reaction (RT-PCR)
2.8. Detection and Quantification of Mineralization
2.9. Statistical Analysis
3. Results
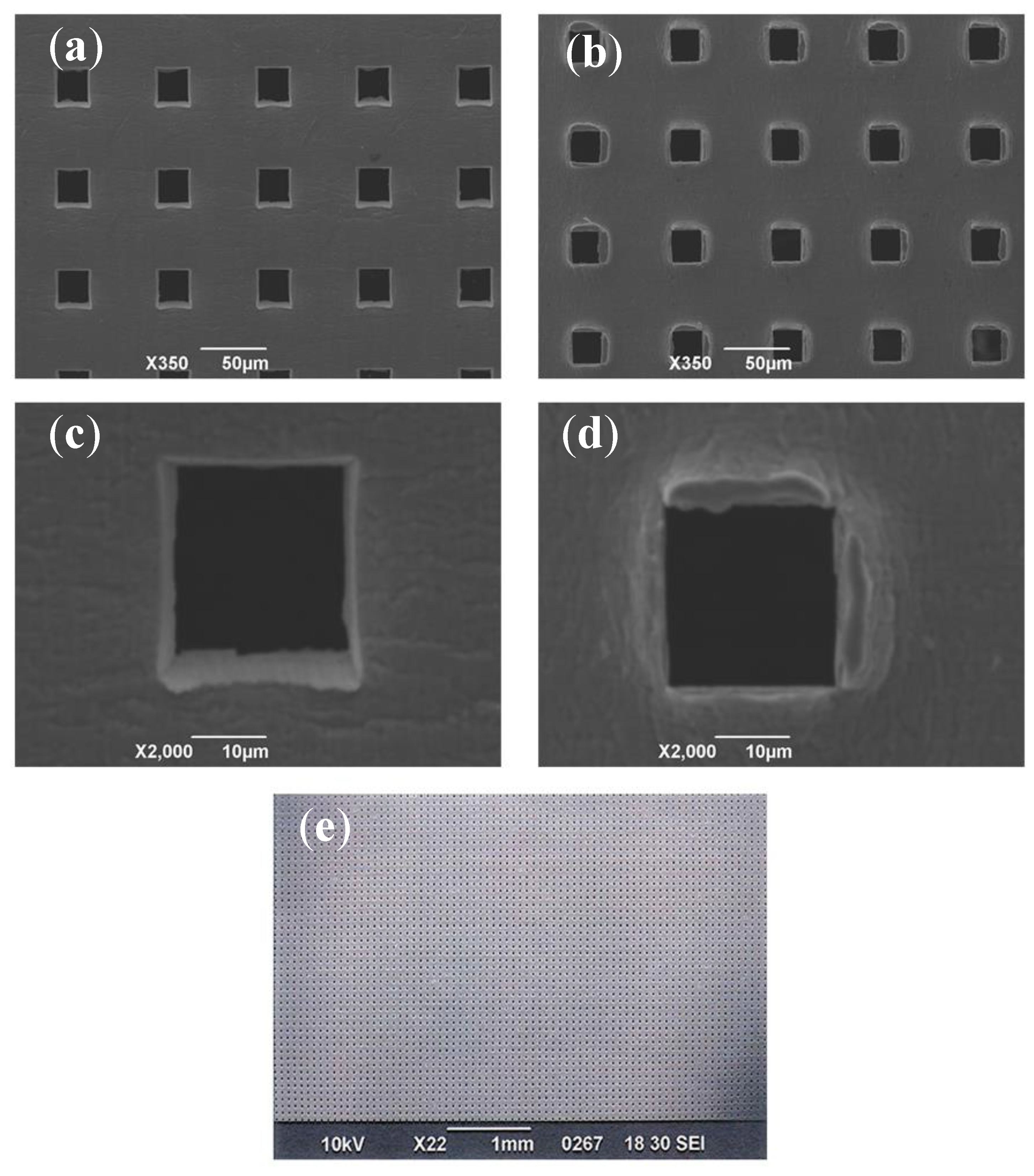
3.1. Microperforated Ti Membrane after MPNP Processing
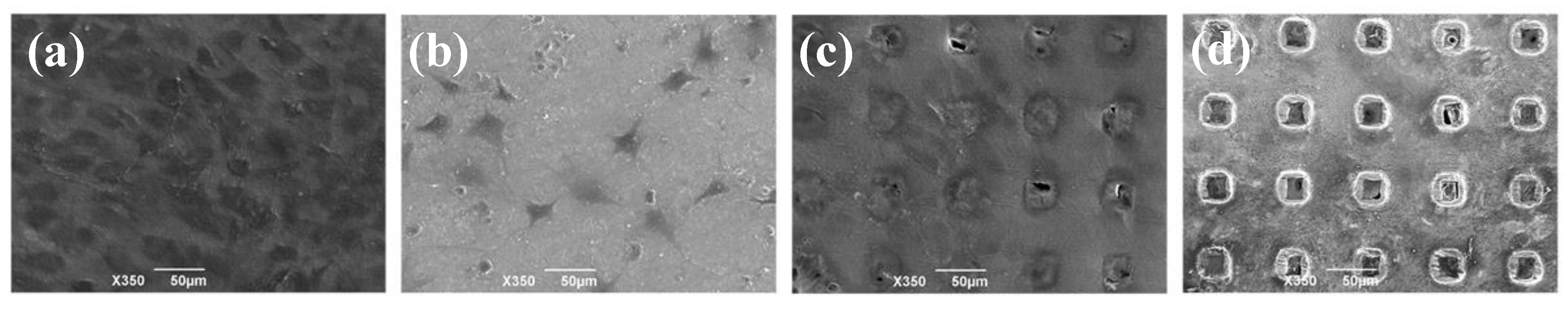
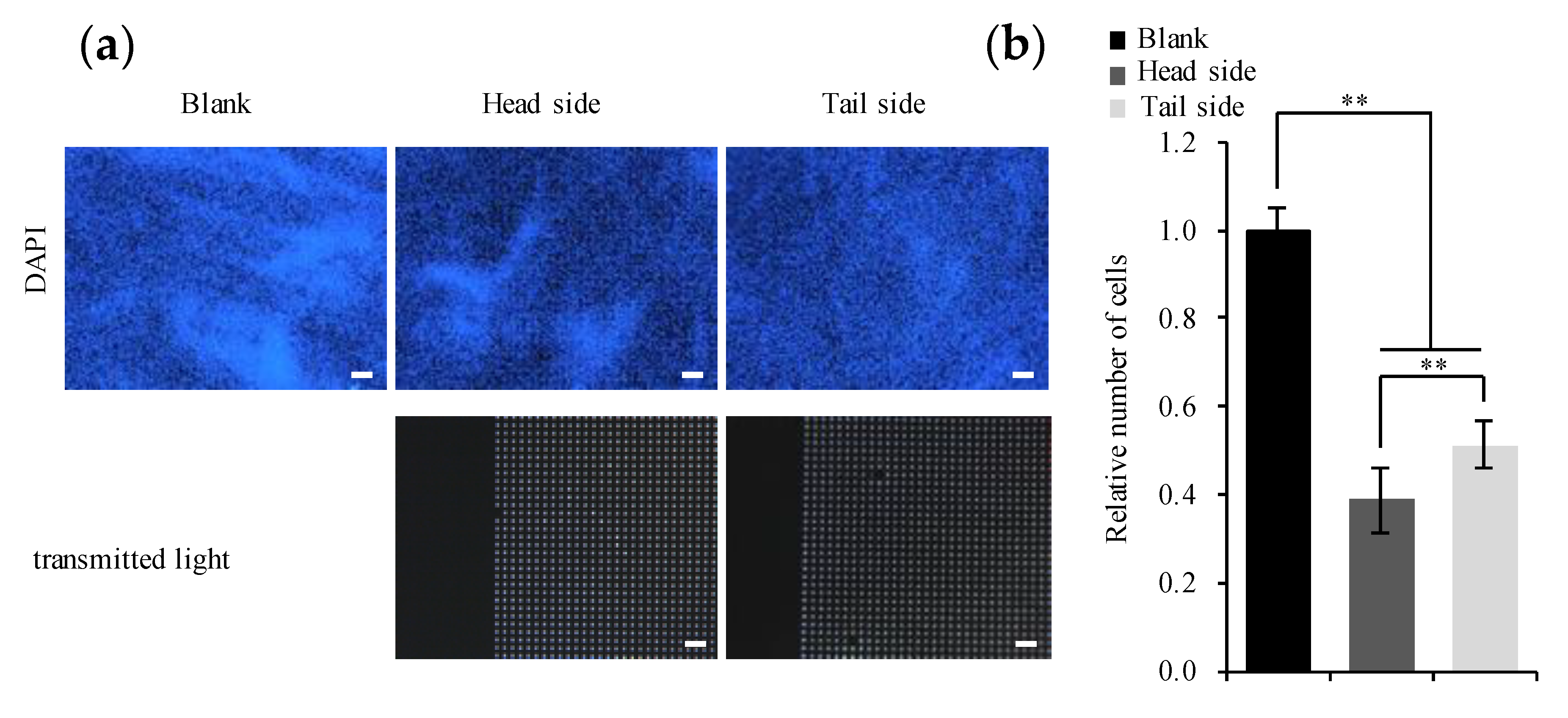
3.2. Cultivation of the Osteogenic Cell Line on the Microperforated Ti Membrane
3.3. Expression of Genes Involved in Osteogenic Differentiation
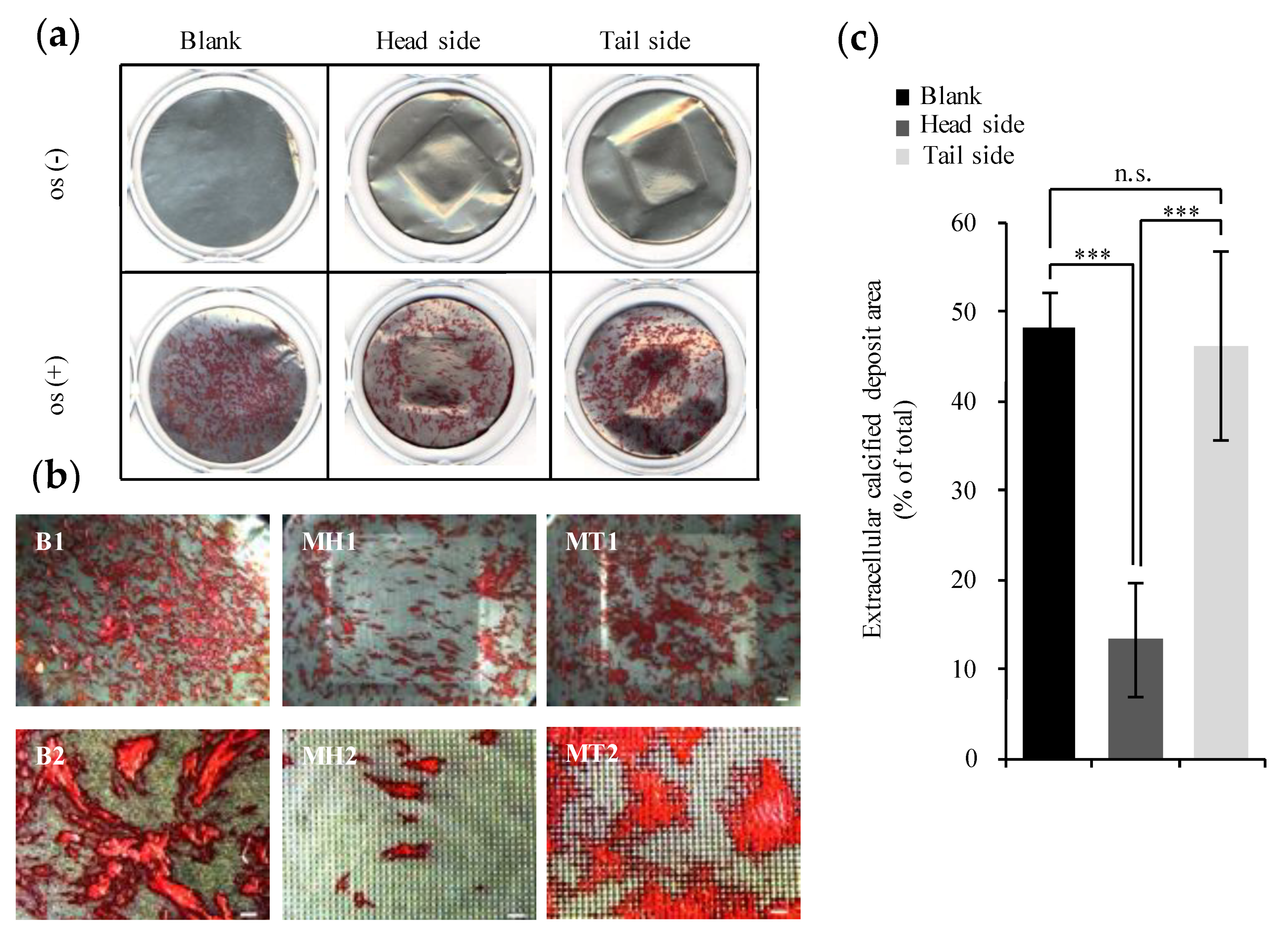
3.4. Evaluation of Calcification
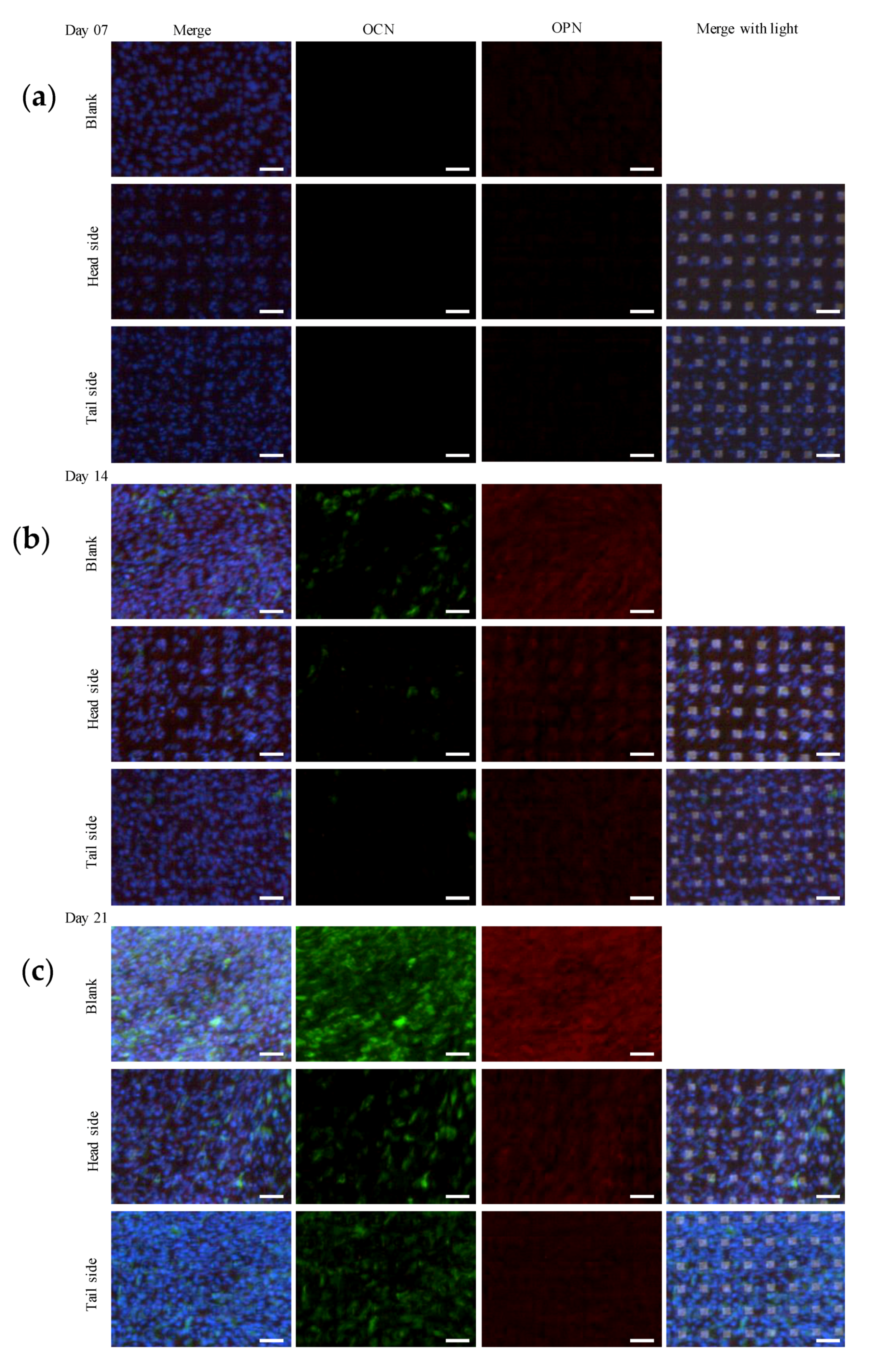
3.5. Protein Expression Involved in Osteogenic Differentiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lang, N. Oral Implants: The Paradigm Shift in Restorative Dentistry. J. Dent. Res. 2019, 98, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Li, X.; Wang, C.; Ji, P.; Wang, C. Mechanobiologically optimization of a 3D titanium-mesh implant for mandibular large defect: A simulated study. Mater. Sci. Eng. C 2019, 104, 109934. [Google Scholar] [CrossRef] [PubMed]
- Ilea, A.; Vrabie, O.-G.; Băbțan, A.-M.; Miclăuş, V.; Ruxanda, F.; Sárközi, M.; Barbu-Tudoran, L.; Mager, V.; Berce, C.; Boşca, A.B.; et al. Osseointegration of titanium scaffolds manufactured by selective laser melting in rabbit femur defect model. J. Mater. Sci. Mater. Med. 2019, 30, 26. [Google Scholar] [CrossRef] [PubMed]
- Gurzawska, K.; Svava, R.; Yihua, Y.; Haugshøj, K.B.; Dirscherl, K.; Levery, S.B.; Byg, I.; Damager, I.; Nielsen, M.W.; Jørgensen, B.; et al. Osteoblastic response to pectin nanocoating on titanium surfaces. Mater. Sci. Eng. C 2014, 43, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, T. Titanium-Tissue Interface Reaction and Its Control with Surface Treatment. Front. Bioeng. Biotechnol. 2019, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Aso, T.; Sasaki, R.; Ashida, M.; Tsutsumi, Y.; Doi, H.; Hanawa, T. Adhesion and differentiation behaviors of mesenchymal stem cells on titanium with micrometer and nanometer-scale grid patterns produced by femtosecond laser irradiation. J. Biomed. Mater. Res. Part A 2018, 106, 2735–2743. [Google Scholar] [CrossRef] [PubMed]
- Yeo, I.-S.L. Modifications of Dental Implant Surfaces at the Micro- and Nano-Level for Enhanced Osseointegration. Materials 2019, 13, 89. [Google Scholar] [CrossRef]
- Sfondrini, M.F.; Gandini, P.; Alcozer, R.; Vallittu, P.K.; Scribante, A. Failure load and stress analysis of orthodontic miniscrews with different transmucosal collar diameter. J. Mech. Behav. Biomed. Mater. 2018, 87, 132–137. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Pinholt, E.M. Brånemark and ITI dental implants in the human bone-grafted maxilla: A comparative evaluation. Clin. Oral Implant. Res. 2003, 14, 584–592. [Google Scholar] [CrossRef]
- Feller, L.; Jadwat, Y.; Khammissa, R.A.G.; Meyerov, R.; Schechter, I.; Lemmer, J. Cellular Responses Evoked by Different Surface Characteristics of Intraosseous Titanium Implants. BioMed Res. Int. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Yang, L.-Y.; Ou, K.-L.; Oreffo, R.O.C. Repositioning Titanium: An In Vitro Evaluation of Laser-Generated Microporous, Microrough Titanium Templates As a Potential Bridging Interface for Enhanced Osseointegration and Durability of Implants. Front. Bioeng. Biotechnol. 2017, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Mariscal-Muñoz, E.; Costa, C.A.; Tavares, H.S.; Bianchi, J.; Hebling, J.; Machado, J.P.; Lerner, U.H.; Souza, P.P.C. Osteoblast differentiation is enhanced by a nano-to-micro hybrid titanium surface created by Yb:YAG laser irradiation. Clin. Oral Investig. 2015, 20, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.-Y.; Yan, G.-B.; Bai, B.; Chen, Y.; Zhang, S.-J.; Yao, Y.-C.; Xia, H. Differential circRNA expression profiles during the BMP2-induced osteogenic differentiation of MC3T3-E1 cells. Biomed. Pharmacother. 2017, 90, 492–499. [Google Scholar] [CrossRef]
- Yan, X.; Yang, W.; Yang, F.; Kersten-Niessen, M.; Jansen, J.A.; Both, S.K. Effects of Continuous Passaging on Mineralization of MC3T3-E1 Cells with Improved Osteogenic Culture Protocol. Tissue Eng. Part C Methods 2014, 20, 198–204. [Google Scholar] [CrossRef]
- Nemoto, E.; Sakisaka, Y.; Tsuchiya, M.; Tamura, M.; Nakamura, T.; Kanaya, S.; Shimonishi, M.; Shimauchi, H. Wnt3a signaling induces murine dental follicle cells to differentiate into cementoblastic/osteoblastic cells via an osterix-dependent pathway. J. Periodontal Res. 2015, 51, 164–174. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, Z.; Gao, M.; Zou, C.; Che, Y.; Cody, B.; Zou, X.; Zhou, L. Bioadaptive Nanorod Topography of Titanium Surface to Control Cell Behaviors and Osteogenic Differentiation of Preosteoblast Cells. J. Mater. Sci. Technol. 2016, 32, 944–949. [Google Scholar] [CrossRef]
- Cao, S.; Li, H.; Li, K.; Lu, J.; Zhang, L. In vitromineralization of MC3T3-E1 osteoblast-like cells on collagen/nano-hydroxyapatite scaffolds coated carbon/carbon composites. J. Biomed. Mater. Res. Part A 2015, 104, 533–543. [Google Scholar] [CrossRef]
- Nelson, C.M.; Jean, R.P.; Tan, J.L.; Liu, W.F.; Sniadecki, N.J.; Spector, A.A.; Chen, C.S. Emergent patterns of growth controlled by multicellular form and mechanics. Proc. Natl. Acad. Sci. USA 2005, 102, 11594–11599. [Google Scholar] [CrossRef]
- Dalby, M.J.; Gadegaard, N.; Riehle, M.; Wilkinson, C.D.; Curtis, A.S. Investigating filopodia sensing using arrays of defined nano-pits down to 35 nm diameter in size. Int. J. Biochem. Cell Biol. 2004, 36, 2005–2015. [Google Scholar] [CrossRef]
- Dalby, M.J.; Riehle, M.; Johnstone, H.; Affrossman, S.; Curtis, A. Investigating the limits of filopodial sensing: A brief report using SEM to image the interaction between 10 nm high nano-topography and fibroblast filopodia. Cell Biol. Int. 2004, 28, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Ruprecht, V.; Monzo, P.; Ravasio, A.; Yue, Z.; Makhija, E.; Strale, P.O.; Gauthier, N.; Shivashankar, G.V.; Studer, V.; Albiges-Rizo, C.; et al. How cells respond to environmental cues—Insights from bio-functionalized substrates. J. Cell Sci. 2016, 130, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Khakbaznejad, A.; Chehroudi, B.; Brunette, D.M. Effects of titanium-coated micromachined grooved substrata on orienting layers of osteoblast-like cells and collagen fibers in culture. J. Biomed. Mater. Res. 2004, 70, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, M.; Meyer, U.; Bühner, M.; Joos, U.; Wiesmann, H.-P. Influence of titanium surfaces on attachment of osteoblast-like cells in vitro. Biomaterials 2004, 25, 625–631. [Google Scholar] [CrossRef]
- Dalby, M.J.; Di Silvio, L.; Gurav, N.; Annaz, B.; Kayser, M.V.; Bonfield, W. Optimizing HAPEX™ Topography Influences Osteoblast Response. Tissue Eng. 2002, 8, 453–467. [Google Scholar] [CrossRef]
- Jeon, H.; Koo, S.; Reese, W.M.; Loskill, P.; Grigoropoulos, C.P.; Healy, K.E. Directing cell migration and organization via nanocrater-patterned cell-repellent interfaces. Nat. Mater. 2015, 14, 918–923. [Google Scholar] [CrossRef]
- Hasegawa, H.; Masui, S.; Ishihata, H. New microperforated pure titanium membrane created by laser processing for guided regeneration of bone. Br. J. Oral Maxillofac. Surg. 2018, 56, 642–643. [Google Scholar] [CrossRef]
- Hasegawa, H.; Masui, S.; Ishihata, H.; Kaneko, T.; Ishida, D.; Endo, M.; Kanno, C.; Yamazaki, M.; Kitabatake, T.; Utsunomiya, S.; et al. Evaluation of a Newly Designed Microperforated Pure Titanium Membrane for Guided Bone Regeneration. Int. J. Oral Maxillofac. Implant. 2019, 34, 411–422. [Google Scholar] [CrossRef]
- Kochergin, S.; Morgunov, Y.; Saushkin, B. Particularities of Pulse Laser Cutting of Thin Plate Titanium Blanks. Procedia Eng. 2017, 206, 1161–1166. [Google Scholar] [CrossRef]
- Fukuyama, R.; Taniguchi, J.; Yoshikawa, K.; Yagishita, H. Micropiercing of titanium foil by a roll press method. Microelectron. Eng. 2015, 139, 53–59. [Google Scholar] [CrossRef]
- Fukuyama, R.; Taniguchi, J.; Yoshikawa, K.; Yagishita, H. Pierce characteristics of titanium foil with roll press method. Microelectron. Eng. 2016, 150, 74–83. [Google Scholar] [CrossRef]
- Ducheyne, P.; Healy, K.; Hutmacher, D. Comprehensive Biomaterials II, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Antanavičiūtė, I.; Šimatonis, L.; Ulčinas, O.; Gadeikytė, A.; Abakevičienė, B.; Tamulevicius, S.; Mikalayeva, V.; Skeberdis, V.A.; Stankevičius, E.; Tamulevičius, T. Femtosecond laser micro-machined polyimide films for cell scaffold applications. J. Tissue Eng. Regen. Med. 2017, 12, e760–e773. [Google Scholar] [CrossRef] [PubMed]
- Belair, D.G.; Wolf, C.J.; Wood, C.; Ren, H.; Grindstaff, R.; Padgett, W.; Swank, A.; Macmillan, D.; Fisher, A.; Winnik, W.; et al. Engineering human cell spheroids to model embryonic tissue fusion in vitro. PLoS ONE 2017, 12, e0184155. [Google Scholar] [CrossRef] [PubMed]
- Luttrell, L.M.; Dar, M.S.; Gesty-Palmer, D.; El-Shewy, H.M.; Robinson, K.M.; Haycraft, C.J.; Barth, J.L. Transcriptomic characterization of signaling pathways associated with osteoblastic differentiation of MC-3T3E1 cells. PLoS ONE 2019, 14, e0204197. [Google Scholar] [CrossRef] [PubMed]
- Gentile, F.; Tirinato, L.; Battista, E.; Causa, F.; Liberale, C.; Di Fabrizio, E.; Decuzzi, P. Cells preferentially grow on rough substrates. Biomaterials 2010, 31, 7205–7212. [Google Scholar] [CrossRef]
- Watari, S.; Hayashi, K.; Wood, J.A.; Russell, P.; Nealey, P.F.; Murphy, C.J.; Genetos, D.C. Modulation of osteogenic differentiation in hMSCs cells by submicron topographically-patterned ridges and grooves. Biomaterials 2012, 33, 128–136. [Google Scholar] [CrossRef]
- Gittens, R.A.; Olivares-Navarrete, R.; McLachlan, T.; Cai, Y.; Hyzy, S.L.; Schneider, J.M.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. Differential responses of osteoblast lineage cells to nanotopographically-modified, microroughened titanium–aluminum–vanadium alloy surfaces. Biomaterials 2012, 33, 8986–8994. [Google Scholar] [CrossRef]
- Hasegawa, H.; Kaneko, T.; Kanno, C.; Endo, M.; Akimoto, T.; Yamazaki, M.; Kitabatake, T.; Masui, S.; Ishihata, H.; Izumi, K. Evaluation of a Newly Designed Microperforated Titanium Membrane with Beta-Tricalcium Phosphate for Guided Bone Regeneration in Dog Mandibles. Int. J. Oral Maxillofac. Implant. 2019, 34, 1132–1142. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Ko, S.-Y.; Lee, W.; Chang, Y.-Y.; Kim, S.-H.; Yun, J.-H. Enhancement of Bone Ingrowth into a Porous Titanium Structure to Improve Osseointegration of Dental Implants: A Pilot Study in the Canine Model. Materials 2020, 13, 3061. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Sakisaka, Y.; Ishihata, H.; Maruyama, K.; Nemoto, E.; Chiba, S.; Nagamine, M.; Hasegawa, H.; Yamada, S. Evaluation of Preosteoblast MC3T3-E1 Cells Cultured on a Microporous Titanium Membrane Fabricated Using a Precise Mechanical Punching Process. Materials 2020, 13, 5288. https://doi.org/10.3390/ma13225288
Zhang J, Sakisaka Y, Ishihata H, Maruyama K, Nemoto E, Chiba S, Nagamine M, Hasegawa H, Yamada S. Evaluation of Preosteoblast MC3T3-E1 Cells Cultured on a Microporous Titanium Membrane Fabricated Using a Precise Mechanical Punching Process. Materials. 2020; 13(22):5288. https://doi.org/10.3390/ma13225288
Chicago/Turabian StyleZhang, Jingyu, Yukihiko Sakisaka, Hiroshi Ishihata, Kentaro Maruyama, Eiji Nemoto, Shigeki Chiba, Masaru Nagamine, Hiroshi Hasegawa, and Satoru Yamada. 2020. "Evaluation of Preosteoblast MC3T3-E1 Cells Cultured on a Microporous Titanium Membrane Fabricated Using a Precise Mechanical Punching Process" Materials 13, no. 22: 5288. https://doi.org/10.3390/ma13225288
APA StyleZhang, J., Sakisaka, Y., Ishihata, H., Maruyama, K., Nemoto, E., Chiba, S., Nagamine, M., Hasegawa, H., & Yamada, S. (2020). Evaluation of Preosteoblast MC3T3-E1 Cells Cultured on a Microporous Titanium Membrane Fabricated Using a Precise Mechanical Punching Process. Materials, 13(22), 5288. https://doi.org/10.3390/ma13225288



