The Dynamic Viscoelasticity of Dental Soft Polymer Material Containing Citrate Ester-Based Plasticizers
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
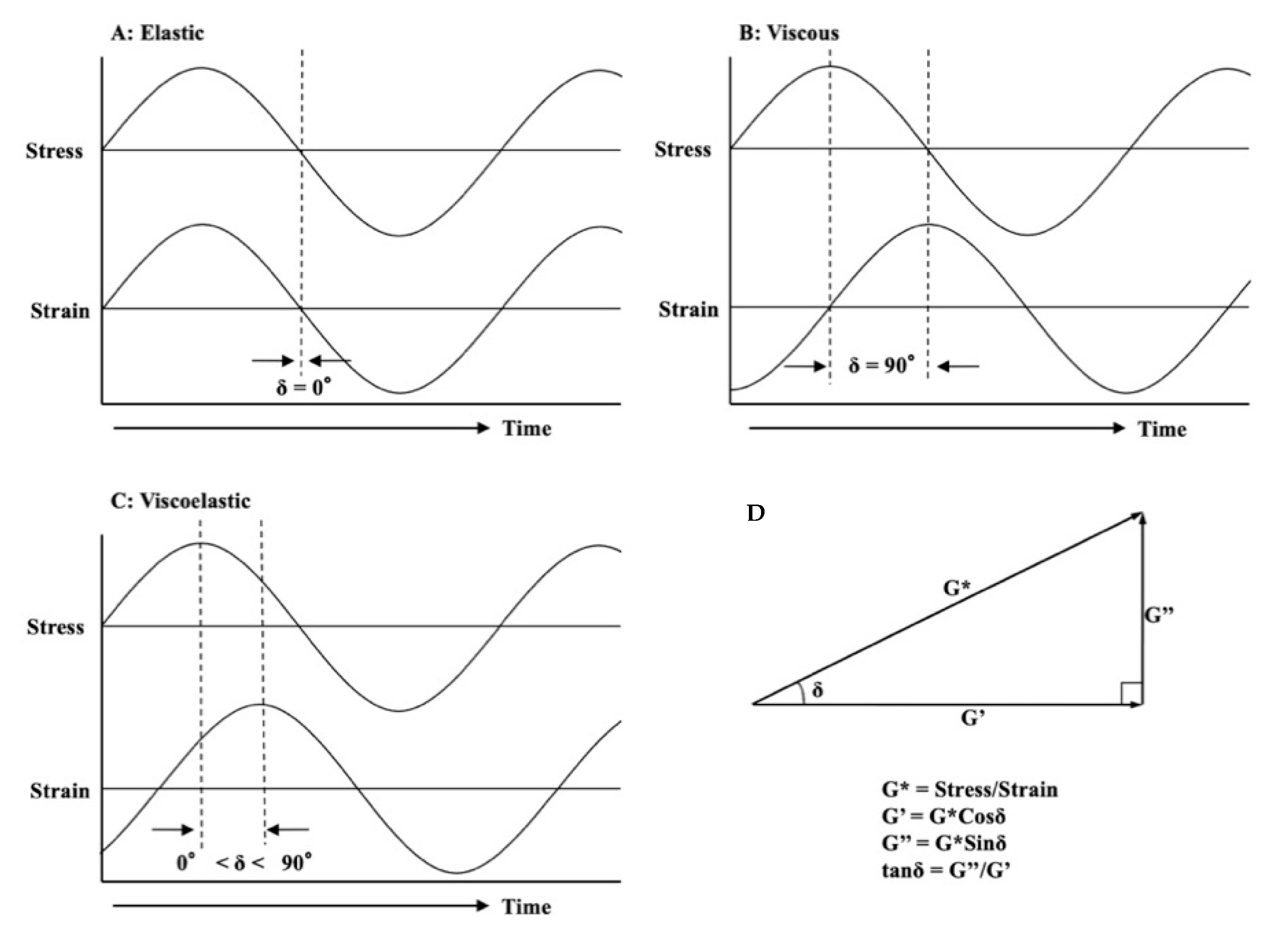
2.2. Dynamic Viscoelasticity Measurement
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hong, G.; Maeda, T.; Li, Y.A.; Sadamori, S.; Hamada, T.; Murata, H. Influence of PMMA polymer on the dynamic viscoelasticity and plasticizer leachability of tissue conditioner. Dent. Mater. J. 2010, 29, 374–380. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murata, H.; Shigeto, N.; Hamada, T. Viscoelastic properties of tissue conditioners stress relaxation test using Maxwell model analogy. J. Oral. Rehabil. 1990, 17, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Maeda, T.; Murata, H.; Sasaki, K. The dynamic viscoelasticity and plasticizer leachability of tissue conditioners. Gerodontology. 2012, 29, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Li, Y.A.; Maeda, T.; Mizumachi, W.; Sadamori, S.; Hamada, T.; Murata, H. Influence of storage methods on the surface roughness of tissue conditioner. Dent. Mater. J. 2008, 27, 153–158. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hong, G.; Tsuka, H.; Maeda, T.; Akagawa, Y.; Sasaki, K. The dynamic viscoelasticity and water absorption characteristics of soft acrylic resin materials containing adipates and maleate plasticizer. Dent. Mater. J. 2012, 31, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Narasaki, Y.; Hamada, T.; McCabe, J.F. An alcohol-free tissue conditioner a laboratory evaluation. J. Dent. 2006, 34, 307–315. [Google Scholar] [CrossRef]
- Jones, D.W.; Hall, G.C.; Sutow, E.J.; Langman, M.F.; Robertson, K.N. Chemical and molecular weight analyses of prosthodontic soft polymers. J. Dent. Res. 1991, 70, 874–879. [Google Scholar] [CrossRef]
- Jones, D.W.; Sutow, E.J.; Hall, G.C.; Tobin, W.M.; Graham, B.S. Dental soft polymers: Plasticizer composition and leachability. Dent. Mater. 1998, 4, 1–7. [Google Scholar] [CrossRef]
- Park, S.K.; Lee, Y.K.; Lim, B.S.; Kim, C.W. Changes in properties of short-term-use soft liners after thermocycling. J. Oral. Rehabil. 2004, 31, 717–724. [Google Scholar] [CrossRef]
- Munksgaard, E.C. Plasticizers in denture soft-lining materials: Leaching and biodegradation. Eur. J. Oral. Sci. 2005, 113, 166–169. [Google Scholar] [CrossRef]
- Lima, J.F.M.; Maciel, J.G.; Arrais, C.A.G.; Porto, V.C.; Urban, V.M.; Neppelenbroek, K.H. Effect of incorporating antifungals in the water sorption and solubility of interim resilient liners for denture base relining. J. Prosthet. Dent. 2016, 115, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Maciel, J.G.; Sugio, C.Y.C.; Chaves, G.C.; Procópio, A.L.F.; Urban, V.M.; Neppelenbroek, K.H. Determining acceptable limits for water sorption and solubility of interim denture resilient liners. J. Prodthet. Dent. 2019, 121, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Toki, K.; Hong, G.; Hamada, T. Effect of tissue conditioners on the dynamic viscoelastic properties of a heat-polymerized denture base. J. Prosthet. Dent. 2002, 88, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.C.; Cheng, K.C.; Huang, C.C.; Lee, B.S. Development of new tissue conditioner using acetyl tributyl citrate and novel hyperbranched polyester to improve viscoelastic stability. Dent. Mater. 2015, 31, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.A.; Henttu, P.; Parker, M.G.; Sumpter, J.P. The estrogenic activity of phthalate esters in vitro. Environ. Health Perspect. 1997, 105, 802–811. [Google Scholar] [CrossRef]
- Vom Saal, F.S.; Cooke, P.S.; Buchanan, D.L.; Palanza, P.; Thayer, K.A.; Nagel, S.C.; Parmigiani, S.; Welshons, W.V. A physiologically based approach to the study of bisphenol and other estrogenic chemicals on the size of reproductive organs, Daily sperm production and behavior. Toxicol. Ind. Health 1998, 14, 239–260. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Kawaguchi, M.; Miyazaki, K.; Nakamura, M. Estrogenic activity of tissue conditioners in vitro. Dent. Mater. 2003, 19, 341–346. [Google Scholar] [CrossRef]
- Dahl, J.E.; Frangou-Polyzois, M.J.; Polyzois, G.L. In vitro biocompatibility of denture relining materials. Gerodontology 2006, 23, 17–22. [Google Scholar] [CrossRef]
- Cederberg, I. Information from KEMI; Swedish Chemicals Inspectorate: Sundbyberg, Sweden, 2005; p. 13. [Google Scholar]
- Takeshita, A.; Igarashi-Migitaka, J.; Nishiyama, K. Acetyl tributyl citrate, the most widely used phthalate substitute plasticizer, Induces cytochrome P450 3A through steroid and xenobiotic receptor. Toxicol. Sci. 2011, 123, 460–470. [Google Scholar] [CrossRef]
- Chiellini, F.; Ferri, M.; Morelli, A.; Dipaola, L.; Latini, G. Perspectives on alternatives to phthalate plasticized poly (vinyl chloride) in medical devices applications. Prog. Polym. Sci. 2013, 38, 1067–1088. [Google Scholar] [CrossRef]
- Bonini, M.; Errani, E.; Zerbinati, G.; Ferri, E.; Girotti, S. Extraction and gas chromatographic evaluation of plasticizers content in food packing films. Microchem. J. 2008, 90, 31–36. [Google Scholar] [CrossRef]
- Fiume, M.M.; Heldreth, B.A.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; et al. Safety assessment of citric acid, inorganic citrate salts, and alkyl citrate esters as used in cosmetics. Int. J. Toxicol. 2014, 33, 16s–46s. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, Y.; Yoshida, K.; Takase, K.; Valanezhad, A.; Watanave, I.; Kojio, K.; Murata, H. Evaluation of viscoelastic properties, hardness, and glass transition temperature of soft denture liners and tissue conditioner. Odontology 2020, 108, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Chimori, H.; Hamada, T.; McCabe, J.F. Viscoelasticity of dental tissue conditioners during the sol-gel transition. J. Dent. Res. 2005, 84, 376–381. [Google Scholar] [CrossRef]
- Scribante, A.; Poggio, C.; Gallo, S.; Riva, P.; Cuocci, A.; Carbone, M.; Arciola, C.R.; Colombo, M. In vitro re-hardening of bleached enamel using mineralizing pastes: Toward preventing bacterial colonization. Materials 2020, 13, 818. [Google Scholar] [CrossRef]
- Naoe, T.; Hasebe, A.; Horiuchi, R.; Makita, Y.; Okazaki, Y.; Yasuda, K.; Matsuo, K.; Yoshida, Y.; Tsuga, K.; Abe, Y.; et al. Development of tissue conditioner containing cetylpyridinium chloride montmorillonite as new antimicrobaial agent: Pilot study on antimicrobial activity and biocompatibility. J. Prosthodont. Res. 2020, 64, 435–443. [Google Scholar] [CrossRef]
- De Foggi, C.C.; Ayres, M.S.B.; Feltrin, G.P.; Jorge, J.H.; Machado, A.L. Effect of surface characteristics of soft liners and tissue conditioners and saliva on the adhesion and biofilm formation. Am. J. Dent. 2018, 31, 45–52. [Google Scholar]
- Takakusaki, K.; Fueki, K.; Tsutsumi, C.; Tsutsumi, Y.; Iwasaki, N.; Hanawa, T.; Takahashi, H.; Takakuda, K.; Wakabayashi, N. Effect of incorporation of surface pre-reacted glass ionomer filler in tissue conditioner on the inhibition of Candida albicans adhesion. Dent. Mater. J. 2018, 37, 453–459. [Google Scholar] [CrossRef]
- Naito, Y.; Yumoto, H.; Kumar, K.; Matsuo, T.; Hirota, K.; Miyake, Y.; Nagao, K.; Tomotake, Y.; Jimbo, R.; Ichikawa, T. Antifungal and mechanical properties of tissue conditioner containing plant-derived component; An in vitro study. J. Proshtodont. 2018, 27, 665–669. [Google Scholar] [CrossRef]
- Lee, H.L.; Wang, R.S.; Hsu, Y.C.; Chuang, C.C.; Chan, H.R.; Chiu, H.C.; Wang, Y.B.; Chen, K.Y.; Fu, E. Antifungal effect of tissue conditioners containing poly (acryloyloxyethyltrimethyl ammonium chloride)-grafted chitosan on Candida albicans growth in vitro. J. Dent. Sci. 2018, 13, 160–166. [Google Scholar] [CrossRef]
- Maior, L.F.S.; Maciel, P.P.; Ferreira, V.Y.N.; Dantas, C.L.G.; Lima, J.M.; Castellano, L.R.C.; Batista, A.U.D.; Bonan, P.R.F. Antifungal activity and Share A hardness of a tissue conditioner incorporated with terpinene-4-ol and cinnamaldehyde. Clin. Oral. Investig. 2019, 23, 2837–2848. [Google Scholar] [CrossRef] [PubMed]






| Code | Polymer | Weight-Average Molecular Weight | Particle Size (μm) | Manufacturer |
|---|---|---|---|---|
| PEMA-A | SDP-32A | 1.08 × 105 | 23.3 | Negami Chemical Industrial Co., LTD., Ishikawa, Japan |
| PEMA-B | SDP-32B | 2.39 × 105 | 18.6 | |
| PEMA-C | SDP-32C | 3.75 × 105 | 19.1 | |
| PEMA-D | SDP-32D | 5.30 × 105 | 18.6 | |
| PEMA-E | SDP-31E | 5.15 × 105 | 221.3 | |
| PEMA-F | SDP-31F | 5.14 × 105 | 176.5 | |
| PEMA-G | SDP-31G | 5.34 × 105 | 100.2 | |
| PEMA-H | SDP-31H | 5.36 × 105 | 63.1 | |
| PEMA-I | D-250E | 5.00 × 105 | 37.5 |
| Plasticizer | Manufacturer | Lot. No. |
|---|---|---|
| Citroflex C-2 [Triethyl Citrate (TEC)] | Tokyo Chemical Industry Co., LTD., Tokyo, Japan | A0822 |
| Citroflex A-2 [Acetyl Triethyl Citrate (ATEC)] | Tokyo Chemical Industry Co., LTD., Tokyo, Japan | A0068 |
| Citroflex A-4 [Acetyl Tributyl Citrate (ATBC)] | Tokyo Chemical Industry Co., LTD., Tokyo, Japan | C0367 |
| Ethyl alcohol (EtOH) | Wako Pure Chemical Industries Ltd., Osaka, Japan | KWP4183 |
| Code | Powders | Liquids | P/L by Weight |
|---|---|---|---|
| PEC-A | PEMA-A 100wt% | TEC or ATEC or ATBC 95wt% + EtOH 5wt% | 1.35 |
| PEC-B | PEMA-B 100wt% | ||
| PEC-C | PEMA-C 100wt% | ||
| PEC-D | PEMA-D 100wt% | ||
| PEC-E | PEMA-E 100wt% | ||
| PEC-F | PEMA-F 100wt% | ||
| PEC-G | PEMA-G 100wt% | ||
| PEC-H | PEMA-H 100wt% | ||
| PEC-I | PEMA-I 100wt% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, G.; Wang, W.-q.; Sun, L.; Han, J.-m.; Sasaki, K. The Dynamic Viscoelasticity of Dental Soft Polymer Material Containing Citrate Ester-Based Plasticizers. Materials 2020, 13, 5078. https://doi.org/10.3390/ma13225078
Hong G, Wang W-q, Sun L, Han J-m, Sasaki K. The Dynamic Viscoelasticity of Dental Soft Polymer Material Containing Citrate Ester-Based Plasticizers. Materials. 2020; 13(22):5078. https://doi.org/10.3390/ma13225078
Chicago/Turabian StyleHong, Guang, Wei-qi Wang, Lu Sun, Jian-min Han, and Keiichi Sasaki. 2020. "The Dynamic Viscoelasticity of Dental Soft Polymer Material Containing Citrate Ester-Based Plasticizers" Materials 13, no. 22: 5078. https://doi.org/10.3390/ma13225078
APA StyleHong, G., Wang, W.-q., Sun, L., Han, J.-m., & Sasaki, K. (2020). The Dynamic Viscoelasticity of Dental Soft Polymer Material Containing Citrate Ester-Based Plasticizers. Materials, 13(22), 5078. https://doi.org/10.3390/ma13225078







