Origin of Mangetotransport Properties in APCVD Deposited Tin Oxide Thin Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Thin Film Deposition by APCVD
2.2. Structural Characterization
2.2.1. Scanning Electron Microscopy (SEM)
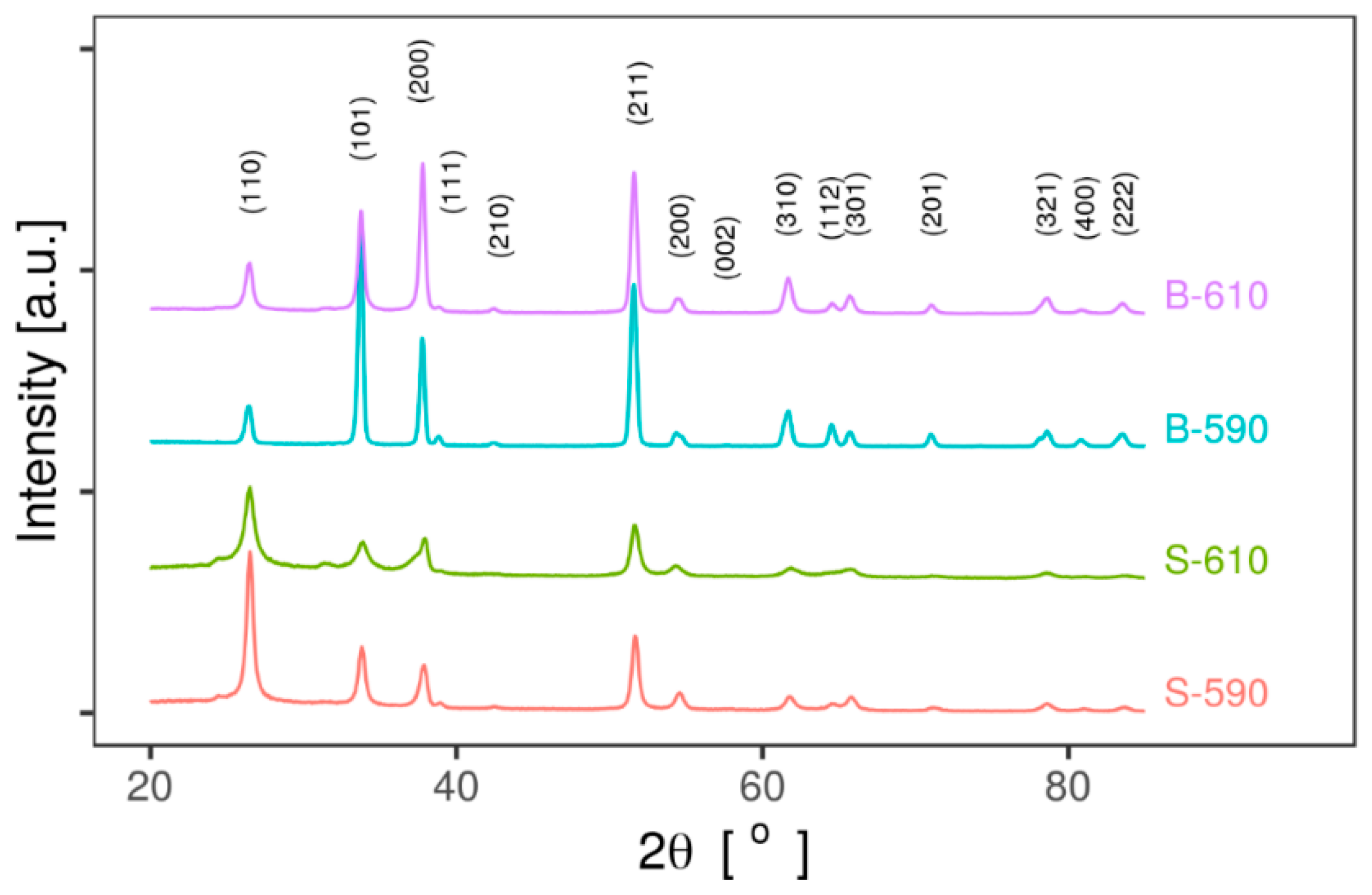
2.2.2. Grazing Incidence X-Ray Diffraction (GIXRD)
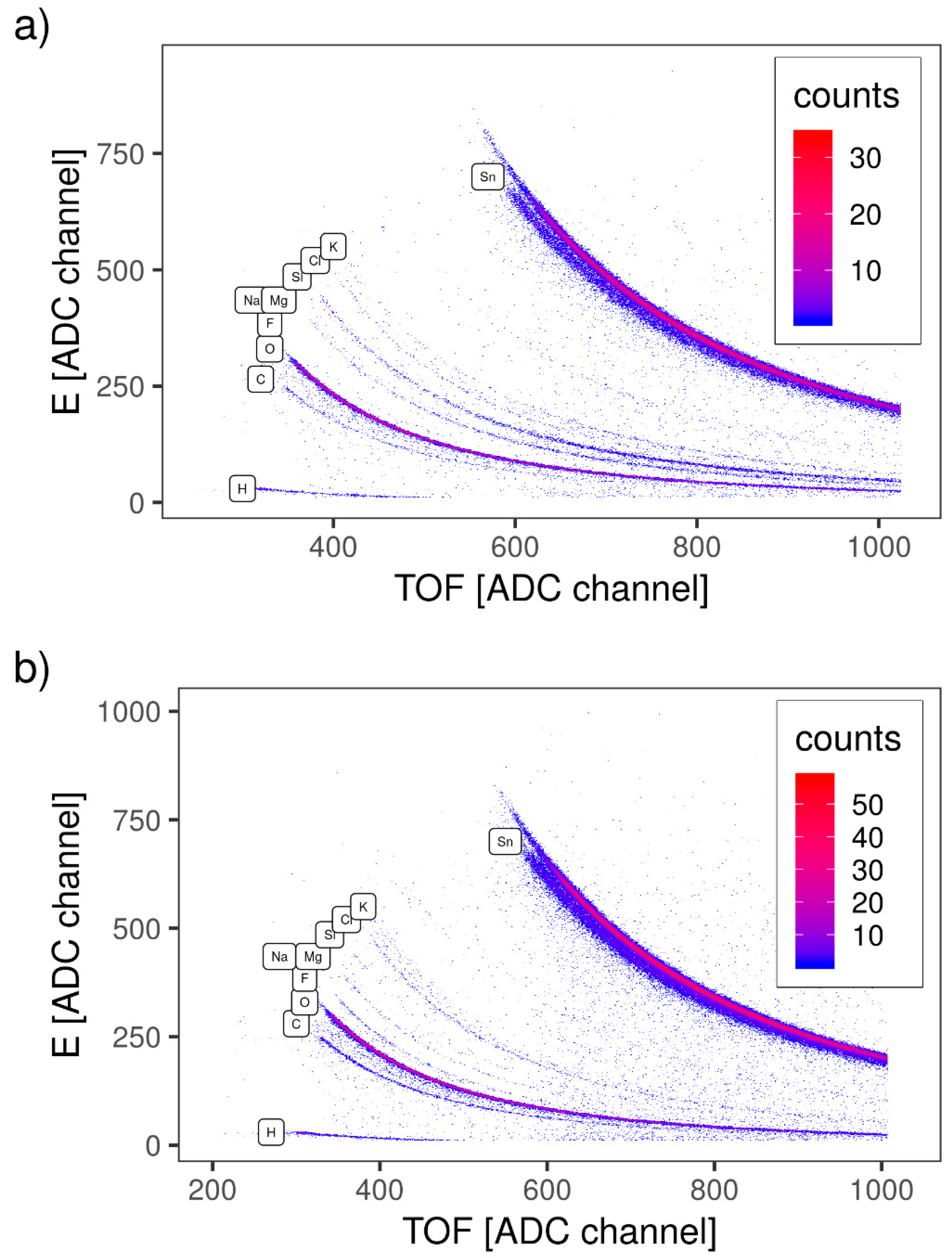
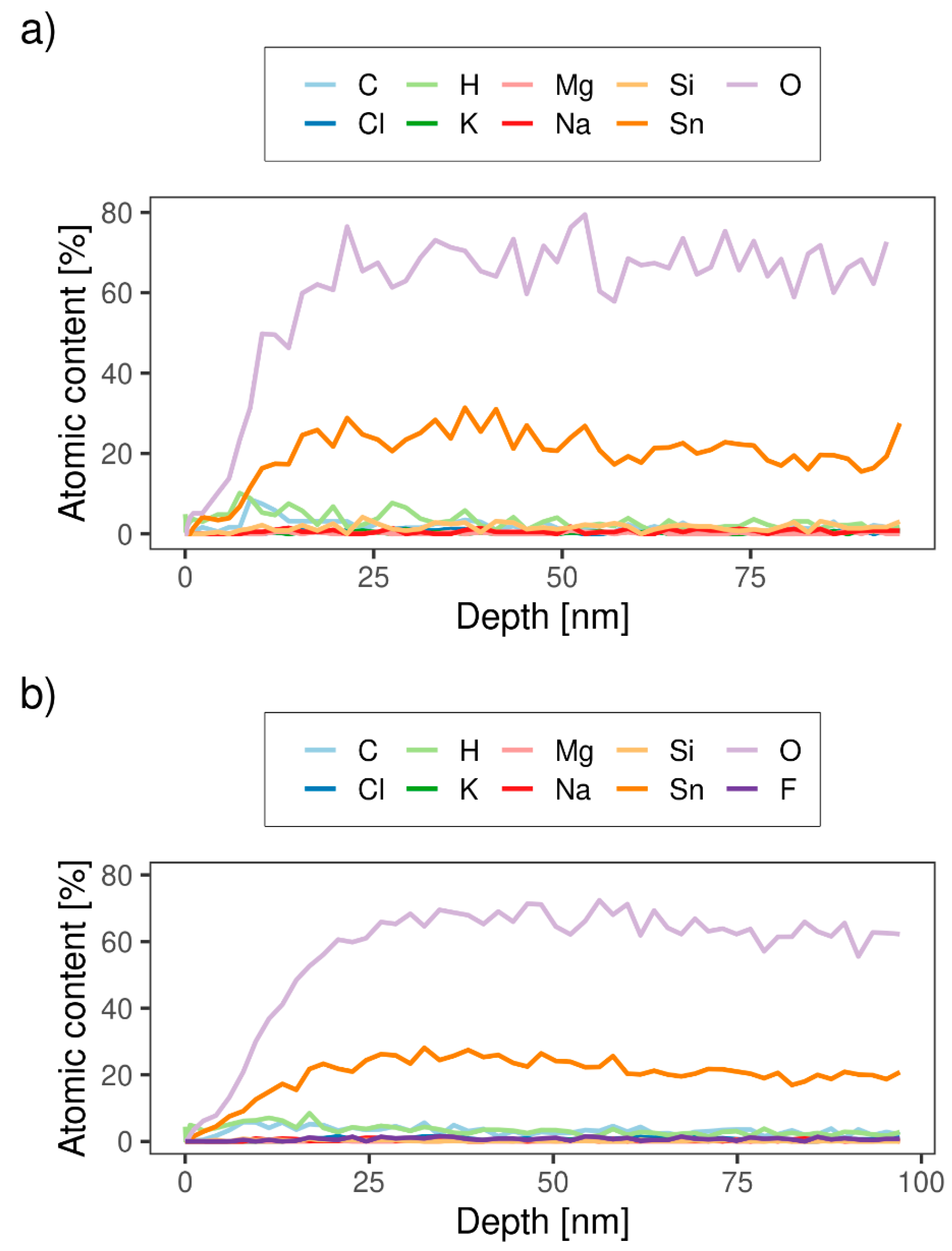
2.2.3. Time-of-Flight Elastic Recoil Detection Analysis (TOF-ERDA)
2.3. Transport Characterization
2.3.1. Impedance Spectroscopy
2.3.2. Magnetotransport
3. Results and Discussion
3.1. Structural Properties
3.1.1. Scanning Electron Microscopy
3.1.2. Grazing Incidence X-Ray Diffraction
3.1.3. TOF-ERDA
3.2. Transport Properties
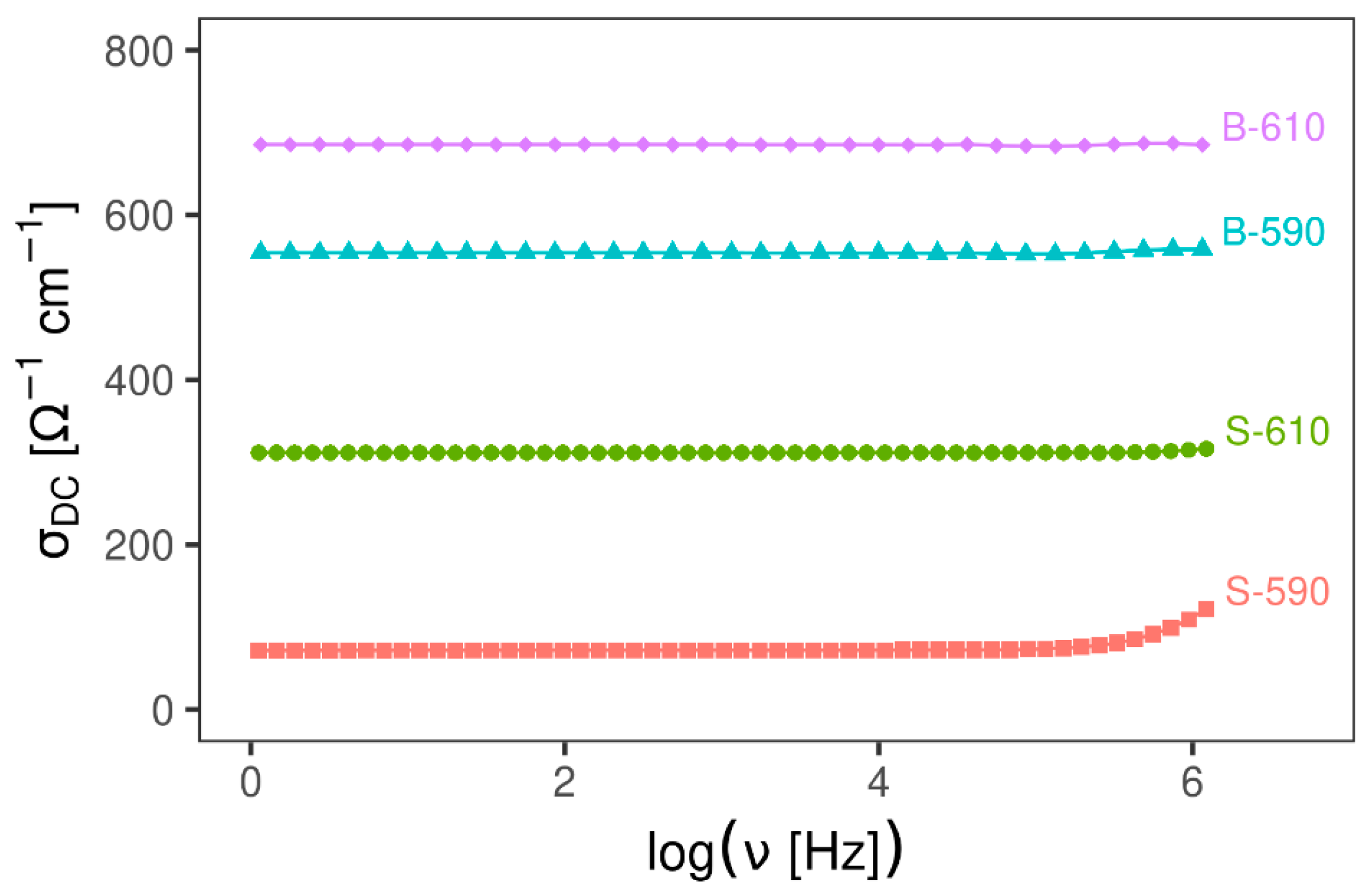
3.2.1. Impedance Spectroscopy
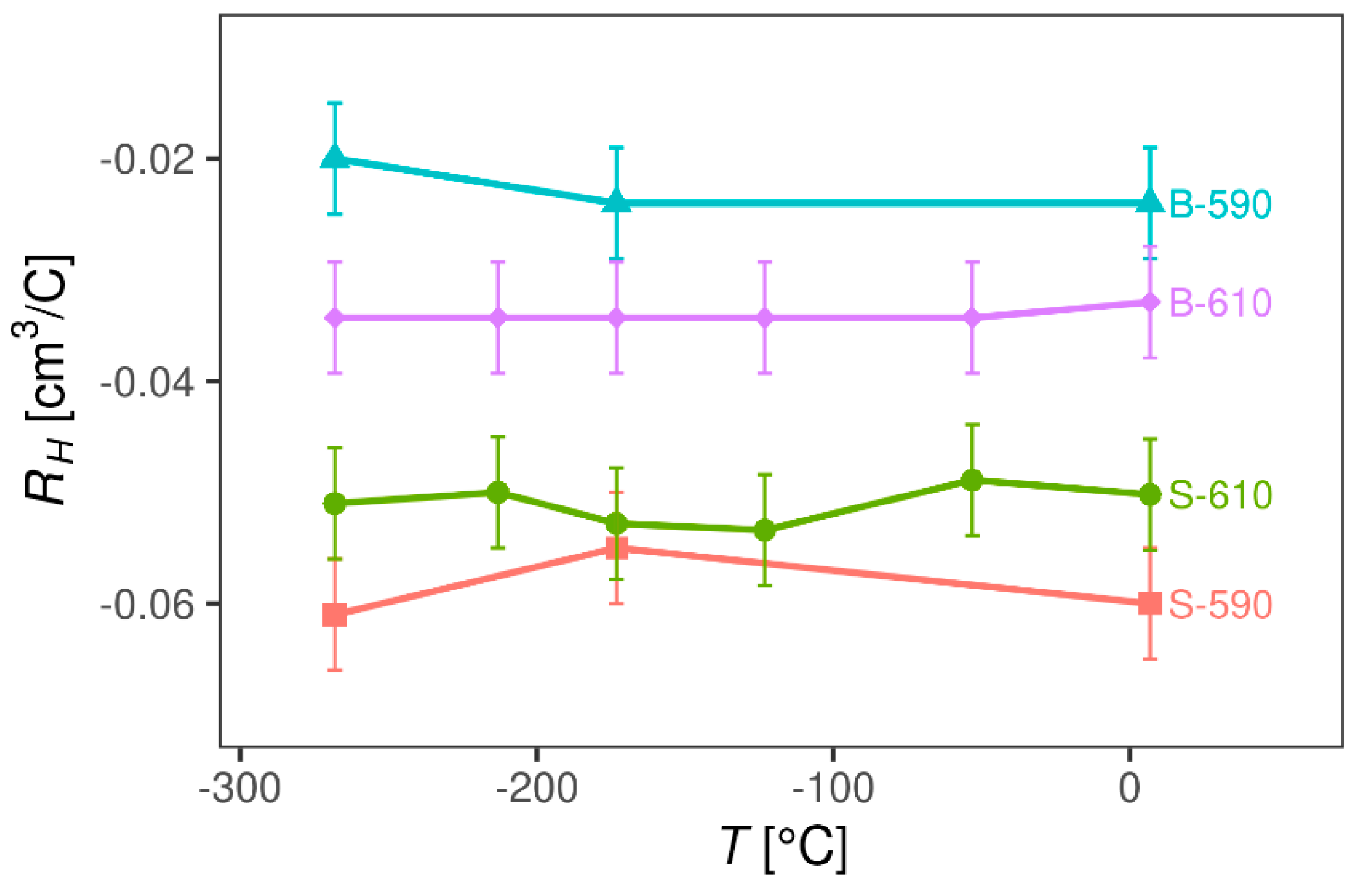
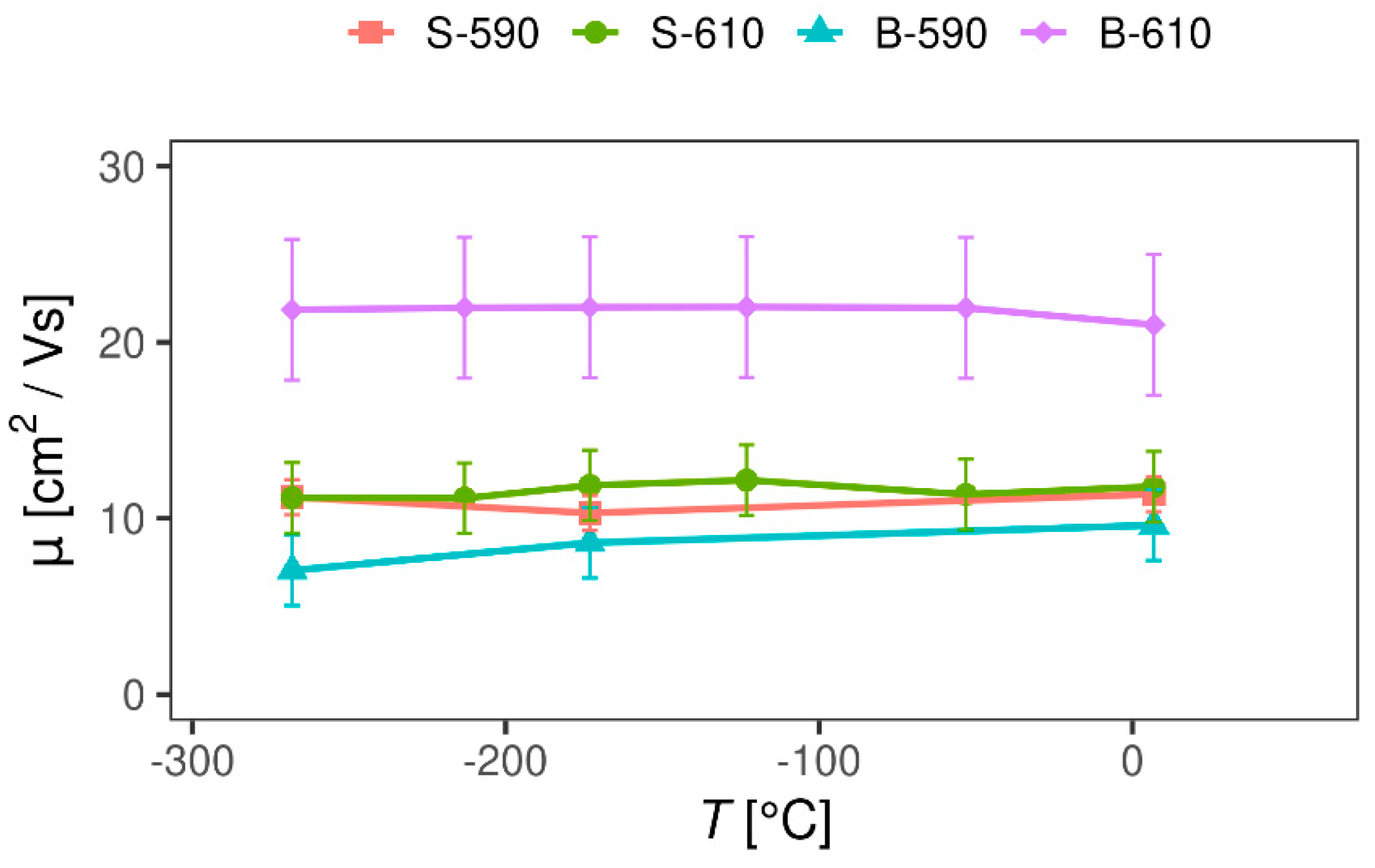
3.2.2. Magnetotransport Probe
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Afre, R.A.; Sharma, N.; Sharon, M.; Sharon, M. Transparent conducting oxide films for various applications: A review. Rev. Adv. Mater. Sci. 2018, 53, 79–89. [Google Scholar] [CrossRef]
- Exarhos, G.J.; Zhou, X.-D. Discovery-based design of transparent conducting oxide films. Thin Solid Films 2007, 515, 7025–7052. [Google Scholar] [CrossRef]
- Hosono, H.; Ueda, K. Transparent Conductive Oxides. In Springer Handbook of Electronic and Photonic Materials; Kasap, S., Capper, P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; p. 1. ISBN 978-3-319-48931-5. [Google Scholar]
- Anthony, J.W. (Ed.) Handbook of Mineralogy; Mineral Data Pub: Tucson, AZ, USA, 1990; ISBN 978-0-9622097-0-3. [Google Scholar]
- Chen, Z.; Pan, D.; Li, Z.; Jiao, Z.; Wu, M.; Shek, C.-H.; Wu, C.M.L.; Lai, J.K.L. Recent Advances in Tin Dioxide Materials: Some Developments in Thin Films, Nanowires, and Nanorods. Chem. Rev. 2014, 114, 7442–7486. [Google Scholar] [CrossRef] [PubMed]
- Aliano, A.; Cicero, G.; Nili, H.; Green, N.G.; García-Sánchez, P.; Ramos, A.; Lenshof, A.; Laurell, T.; Qi, A.; Chan, P.; et al. Atmospheric Pressure Chemical Vapor Deposition (APCVD). In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer Netherlands: Dordrecht, Germany, 2012; p. 146. ISBN 978-90-481-9750-7. [Google Scholar]
- Calnan, S.; Tiwari, A.N. High mobility transparent conducting oxides for thin film solar cells. Thin Solid Film. 2010, 518, 1839–1849. [Google Scholar] [CrossRef]
- Djerdj, I.; Gracin, D.; Juraić, K.; Meljanac, D.; Bogdanović-Radović, I.; Pletikapić, G. Structural analysis of monolayered and bilayered SnO2 thin films. Surface Coat. Technol. 2012, 211, 24–28. [Google Scholar] [CrossRef]
- Gordon, R. Chemical vapor deposition of coatings on glass. J. Non Cryst. Solids 1997, 218, 81–91. [Google Scholar] [CrossRef]
- Volintiru, I.; de Graaf, A.; van Deelen, J.; Poodt, P. The influence of methanol addition during the film growth of SnO2 by atmospheric pressure chemical vapor deposition. Thin Solid Film. 2011, 519, 6258–6263. [Google Scholar] [CrossRef]
- Rebuffi, L.; Plaisier, J.R.; Abdellatief, M.; Lausi, A.; Scardi, P. MCX: A synchrotron radiation beamline for X-ray diffraction line profile analysis. Z. Für Anorg. Und Allg. Chem. 2014, 640, 3100–3106. [Google Scholar] [CrossRef]
- Henke, B.L.; Gullikson, E.M.; Davis, J.C. X-Ray Interactions: Photoabsorption, scattering, transmission, and reflection at E = 50–30,000 eV, Z = 1–92. At. Data Nucl. Data Tables 1993, 54, 181–342. [Google Scholar] [CrossRef]
- Siketić, Z.; Radović, I.B.; Jakšić, M. Development of a time-of-flight spectrometer at the Ruder Bošković Institute in Zagreb. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2008, 266, 1328–1332. [Google Scholar] [CrossRef]
- Wurst, J.C.; Nelson, J.A. Lineal intercept technique for measuring grain size in two-phase polycrystalline ceramics. J. Am. Ceram. Soc. 1972, 55, 109. [Google Scholar] [CrossRef]
- Matsui, Y.; Mitsuhashi, M.; Yamamoto, Y.; Higashi, S. Influence of alcohol on grain growth of tin oxide in chemical vapor deposition. Thin Solid Film. 2007, 515, 2854–2859. [Google Scholar] [CrossRef]
- Matsui, Y.; Mitsuhashi, M.; Goto, Y. Early stage of tin oxide film growth in chemical vapor deposition. Surf. Coat. Technol. 2003, 169–170, 549–552. [Google Scholar] [CrossRef]
- Ghoshtagore, R.N. Mechanism of CVD Thin Film SnO2 Formation. J. Electrochem. Soc. 1978, 125, 110. [Google Scholar] [CrossRef]
- Gražulis, S.; Daškevič, A.; Merkys, A.; Chateigner, D.; Lutterotti, L.; Quirós, M.; Serebryanaya, N.R.; Moeck, P.; Downs, R.T.; Le Bail, A. Crystallography open database (COD): An open-access collection of crystal structures and platform for world-wide collaboration. Nucleic Acids Res. 2012, 40, D420–D427. [Google Scholar] [CrossRef] [PubMed]
- Shanthi, S.; Anuratha, H.; Subramanian, C.; Ramasamy, P. Effect of fluorine doping on structural, electrical and optical properties of sprayed SnO2 thin films. J. Cryst. Growth 1998, 194, 369–373. [Google Scholar] [CrossRef]
- Barrett, C. Structure of Metals; Horney Press: New York, NY, USA, 2007; ISBN 978-1-4437-3140-9. [Google Scholar]
- Patterson, A.L. The scherrer formula for X-Ray particle size determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Kaiser, N. Review of the fundamentals of thin-film growth. Appl. Opt. 2002, 41, 3053–3060. [Google Scholar] [CrossRef]
- Arstila, K.; Julin, J.; Laitinen, M.I.; Aalto, J.; Konu, T.; Kärkkäinen, S.; Rahkonen, S.; Raunio, M.; Itkonen, J.; Santanen, J.-P.; et al. Potku—New analysis software for heavy ion elastic recoil detection analysis. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2014, 331, 34–41. [Google Scholar] [CrossRef]
- Schiettekatte, F. Fast Monte Carlo for ion beam analysis simulations. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2008, 266, 1880–1885. [Google Scholar] [CrossRef]
- Kasap, S.O.; Capper, P. (Eds.) Springer Handbook of Electronic and Photonic Materials; Springer: New York, NY, USA, 2006; ISBN 978-0-387-26059-4. [Google Scholar]
- Prins, M.W.J.; Grosse-Holz, K.-O.; Cillessen, J.F.M.; Feiner, L.F. Grain-boundary-limited transport in semiconducting SnO2 thin films: Model and experiments. J. Appl. Phys. 1998, 83, 888–893. [Google Scholar] [CrossRef]
- Wang, J.T.; Shi, X.L.; Liu, W.W.; Zhong, X.H.; Wang, J.N.; Pyrah, L.; Sanderson, K.D.; Ramsey, P.M.; Hirata, M.; Tsuri, K. Influence of preferred orientation on the electrical conductivity of fluorine-doped tin oxide films. Sci. Rep. 2015, 4, 3679. [Google Scholar] [CrossRef] [PubMed]
- Fritzsche, H.; Lark-Horovitz, K. Electrical properties of p-type indium antimonide at low temperatures. Phys. Rev. 1955, 99, 400–405. [Google Scholar] [CrossRef]
- Woods, J.F.; Chen, C.Y. Negative magnetoresistance in impurity conduction. Phys. Rev. 1964, 135, A1462–A1466. [Google Scholar] [CrossRef]
- Sasaki, W. Negative magnetoresistance in the metallic impurity conduction of n-type germanium. J. Phys. Soc. Jpn. 1965, 20, 825–833. [Google Scholar] [CrossRef]
- Dauzhenka, T.A.; Ksenevich, V.K.; Bashmakov, I.A.; Galibert, J. Origin of negative magnetoresistance in polycrystalline SnO2 films. Phys. Rev. B 2011, 83, 165309. [Google Scholar] [CrossRef]
- Giovannini, B.; Hedgcock, F.T. Influence of temperature on the two band model for negative magnetoresistance in heavily doped semiconductors. Solid State Commun. 1972, 11, 367–370. [Google Scholar] [CrossRef]
- Boon, M.R. Negative Magnetoresistance in Doped Semiconductors. Phys. Rev. B 1973, 7, 761–762. [Google Scholar] [CrossRef]
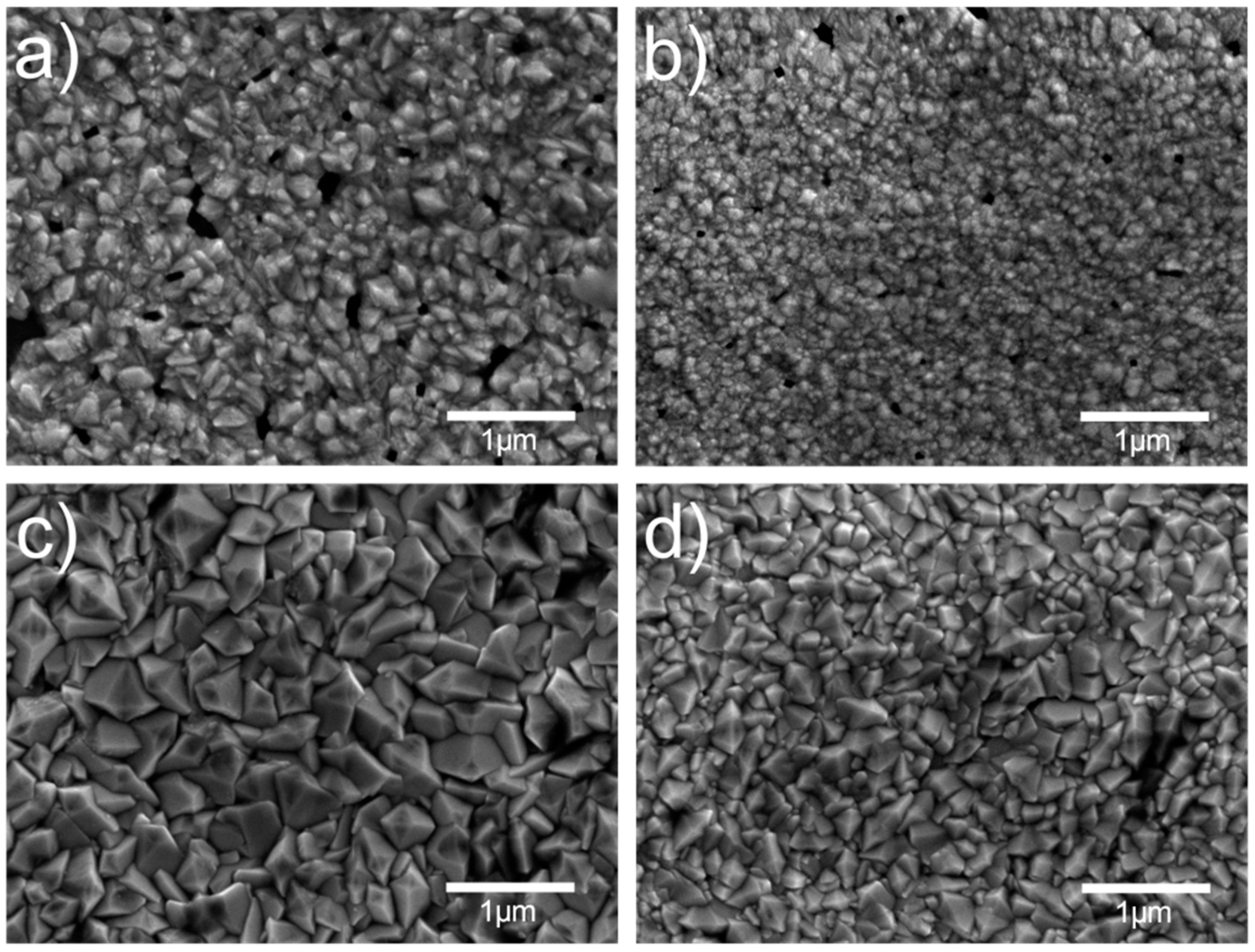


| Sample Label | Deposition Process Type | Deposition Temperature (°C) | Thickness (nm) |
|---|---|---|---|
| S-590 | one-step | 590 | 390 |
| S-610 | one-step | 610 | 300 |
| B-590 | two-step | 590 | 920 |
| B-610 | two-step | 610 | 710 |
| Sample | Lattice Parameters | Preferred Orientation Texture Coefficient | Average Crystallite Size (nm) | ||||
|---|---|---|---|---|---|---|---|
| a (nm) | c (nm) | (110) | (101) | (200) | (211) | ||
| S-590 | 0.474(9) | 0.318(4) | 0.144 | 0.090 | 0.104 | 0.151 | 17 ± 1 |
| S-610 | 0.475(7) | 0.318(8) | 0.143 | 0.101 | 0.140 | 0.136 | 9 ± 3 |
| B-590 | 0.475(9) | 0.318(7) | 0.019 | 0.141 | 0.102 | 0.166 | 22 ± 3 |
| B-610 | 0.475(7) | 0.318(5) | 0.031 | 0.087 | 0.173 | 0.159 | 20 ± 3 |
| Sample | H (at.%) | C (at.%) | O (at.%) | F (at.%) | Na (at.%) | Mg (at.%) | Si (at.%) | Cl (at.%) | K (at.%) | Sn (at.%) |
|---|---|---|---|---|---|---|---|---|---|---|
| S-590 | 2.8 ± 0.3 | 1.7 ± 0.2 | 62 ± 4 | - | 0.6 ± 0.1 | 0.18 ± 0.06 | 1.8 ± 0.2 | 0.7 ± 0.1 | 0.25 ± 0.06 | 29 ± 2 |
| B-590 | 2.9 ± 0.3 | 3.2 ± 0.3 | 62 ± 4 | 0.9 ± 0.1 | 0.26 ± 0.06 | 0.03 ± 0.02 | 0.11 ± 0.03 | 0.9 ± 0.1 | 0.3 ± 0.1 | 29 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juraić, K.; Gracin, D.; Čulo, M.; Rapljenović, Ž.; Plaisier, J.R.; Hodzic, A.; Siketić, Z.; Pavić, L.; Bohač, M. Origin of Mangetotransport Properties in APCVD Deposited Tin Oxide Thin Films. Materials 2020, 13, 5182. https://doi.org/10.3390/ma13225182
Juraić K, Gracin D, Čulo M, Rapljenović Ž, Plaisier JR, Hodzic A, Siketić Z, Pavić L, Bohač M. Origin of Mangetotransport Properties in APCVD Deposited Tin Oxide Thin Films. Materials. 2020; 13(22):5182. https://doi.org/10.3390/ma13225182
Chicago/Turabian StyleJuraić, Krunoslav, Davor Gracin, Matija Čulo, Željko Rapljenović, Jasper Rikkert Plaisier, Aden Hodzic, Zdravko Siketić, Luka Pavić, and Mario Bohač. 2020. "Origin of Mangetotransport Properties in APCVD Deposited Tin Oxide Thin Films" Materials 13, no. 22: 5182. https://doi.org/10.3390/ma13225182
APA StyleJuraić, K., Gracin, D., Čulo, M., Rapljenović, Ž., Plaisier, J. R., Hodzic, A., Siketić, Z., Pavić, L., & Bohač, M. (2020). Origin of Mangetotransport Properties in APCVD Deposited Tin Oxide Thin Films. Materials, 13(22), 5182. https://doi.org/10.3390/ma13225182









