Surface Properties of Poly(Hydroxyurethane)s Based on Five-Membered Bis-Cyclic Carbonate of Diglycidyl Ether of Bisphenol A
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
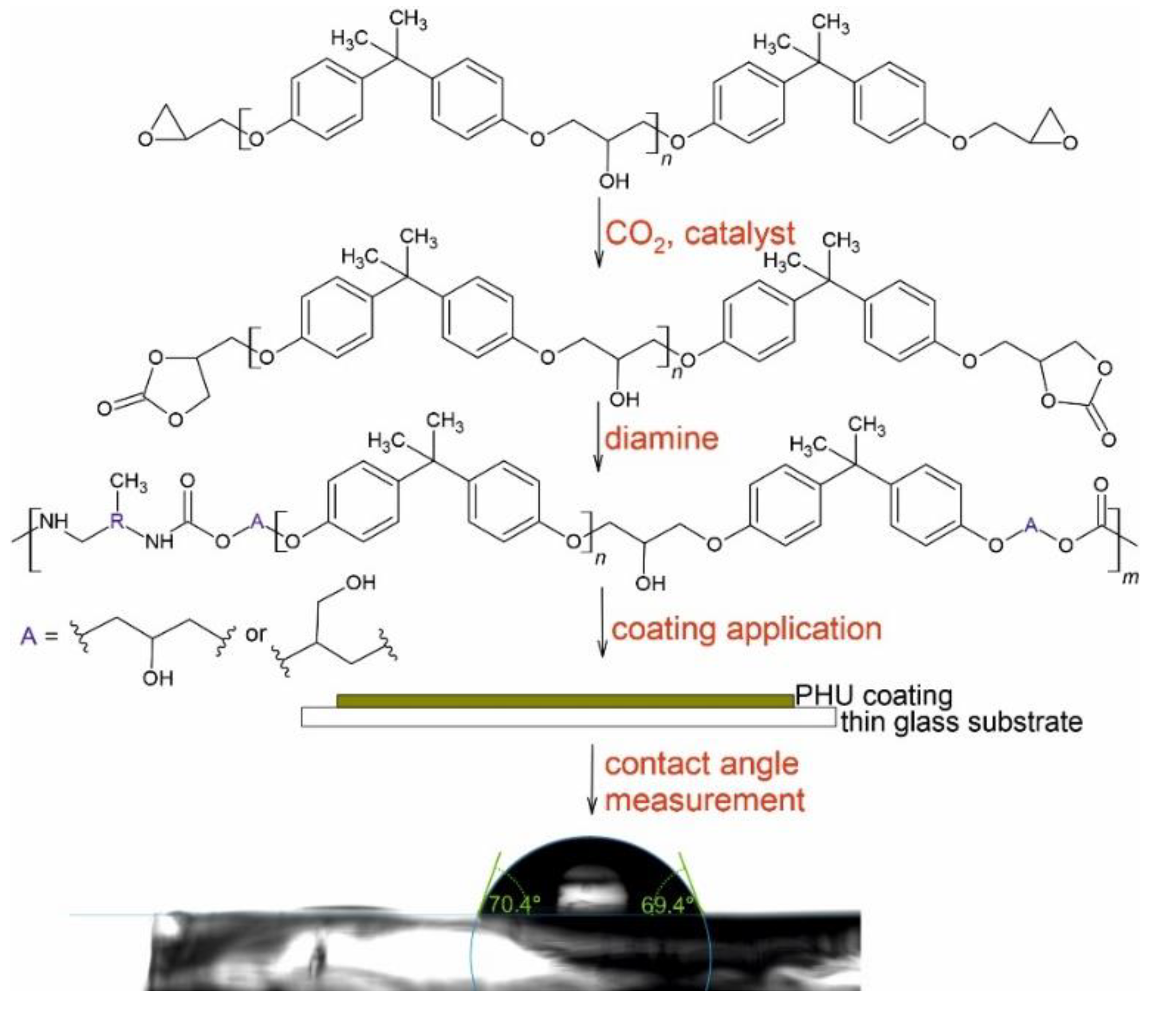
2.3. General Procedure for the Preparation of PHUs
3. Results and Discussion
3.1. PHUs Synthesis
3.2. Thermal Characterization
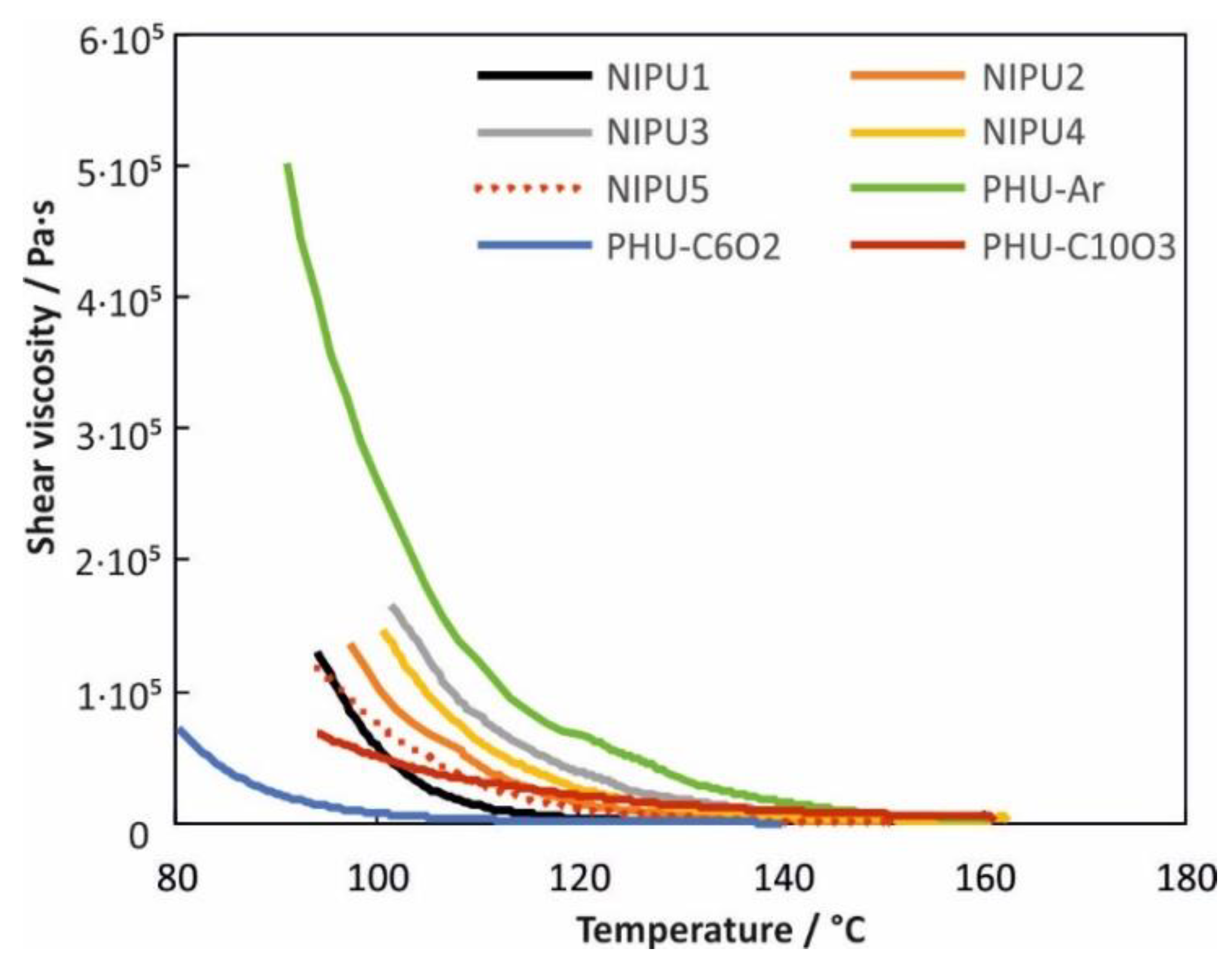
3.3. Rheological Properties
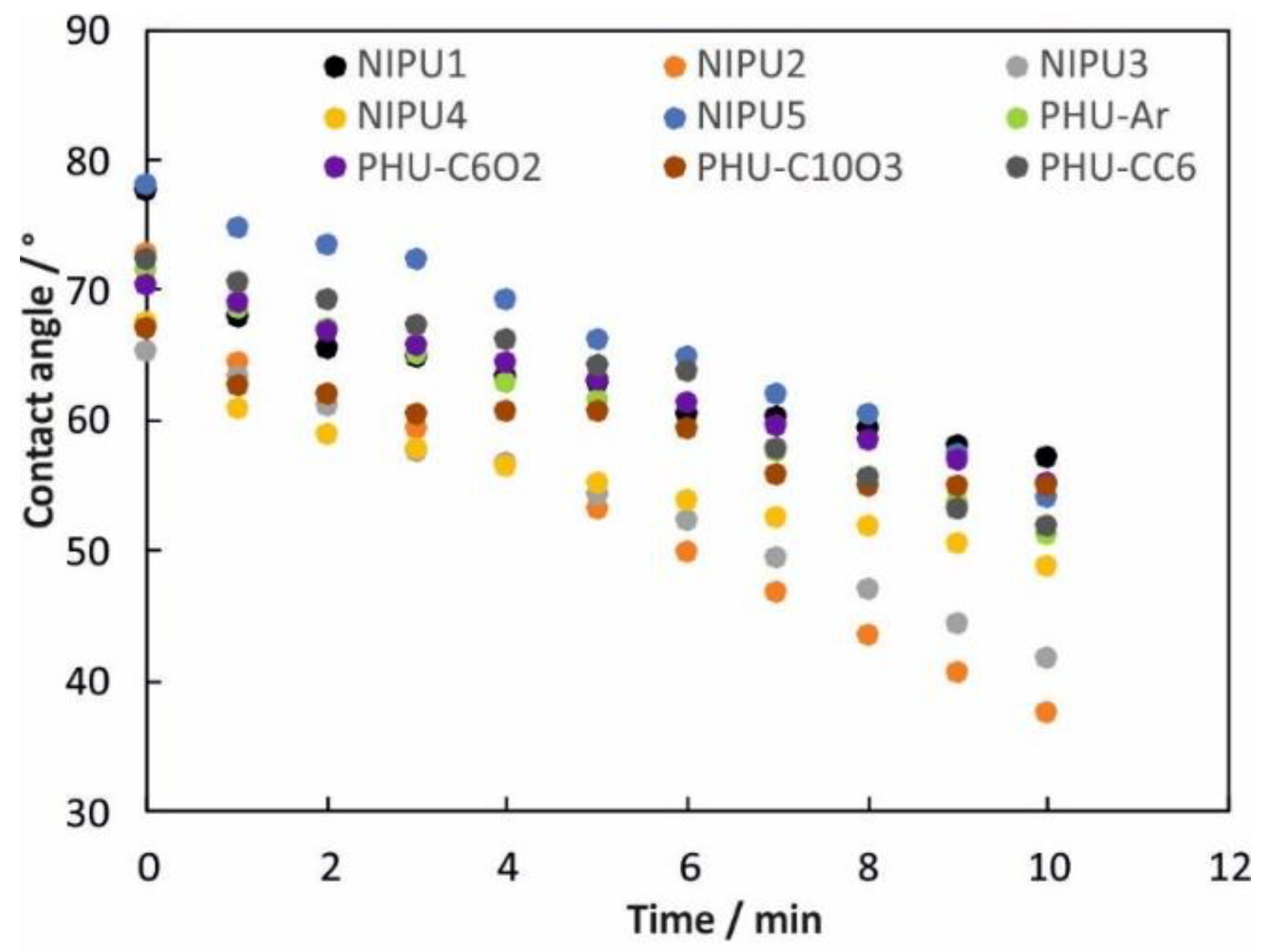
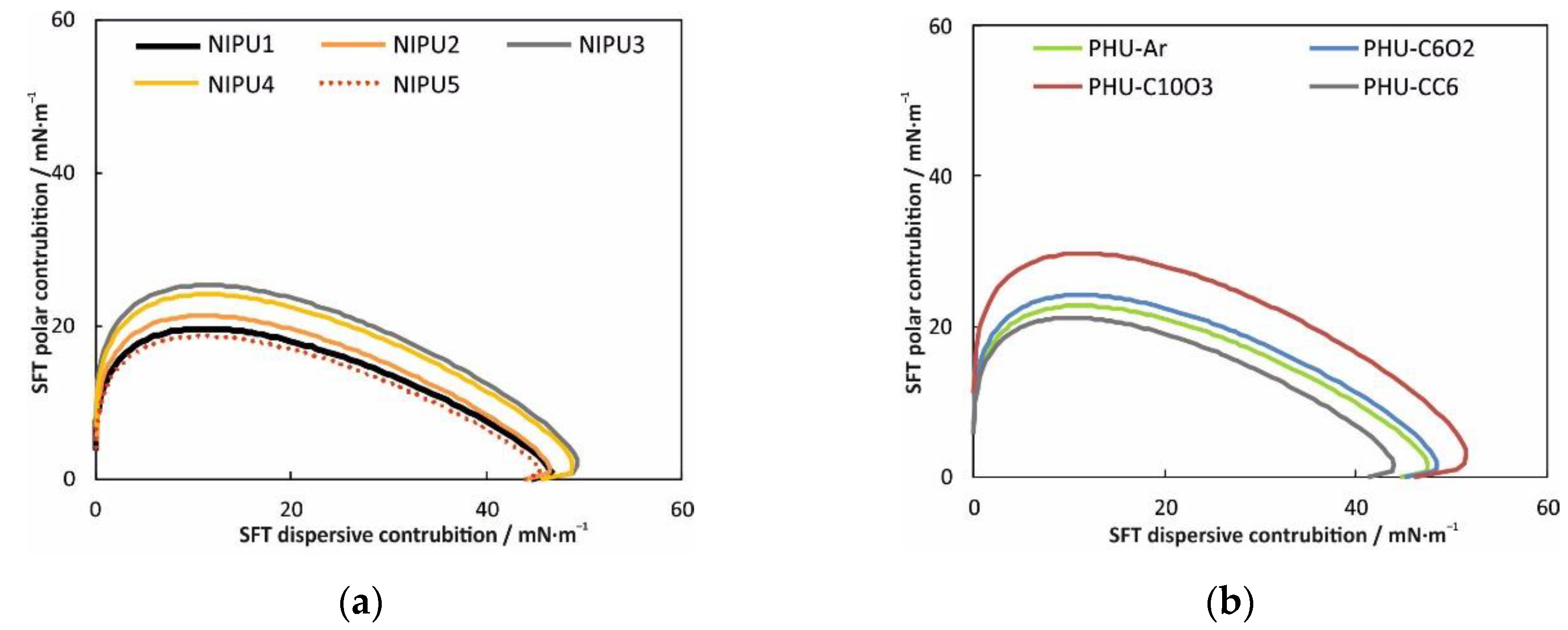
3.4. Wettability, Surface Free Energy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bismarck, A.; Kumru, M.E.; Springer, J. Influence of Oxygen Plasma Treatment of PAN-Based Carbon Fibers on Their Electrokinetic and Wetting Properties. J. Colloid Interface Sci. 1999, 210, 60–72. [Google Scholar] [CrossRef]
- Chmielewski, T.; Golański, D.; Hudycz, M.; Sałacinski, T.; Świercz, R. Właściwości powierzchniowe i strukturalne tytanowej powłoki osadzanej tarciowo na podłożu ceramicznym AlN. Przem. Chem. 2019, 98, 50–55. [Google Scholar] [CrossRef]
- Dwivedi, C.; Pandey, I.; Pandey, H.; Ramteke, P.W.; Pandey, A.C.; Mishra, S.B.; Patil, S. Electrospun Nanofibrous Scaffold as a Potential Carrier of Antimicrobial Therapeutics for Diabetic Wound Healing and Tissue Regeneration. Nano Microscale Drug Deliv. Syst. 2017, 147–164. [Google Scholar] [CrossRef]
- Chibowski, E.; Perea-Carpio, R. Problems of contact angle and solid surface free energy determination. Adv. Colloid Interface Sci. 2002, 98, 245–264. [Google Scholar] [CrossRef]
- Zettlemoyer, A.C. Chemistry and physics of interfaces II. J. Colloid Interface Sci. 1972, 40, 129. [Google Scholar] [CrossRef]
- Żenkiewicz, M. Comparative study on the surface free energy of a solid calculated by different methods. Polym. Test. 2007, 26, 14–19. [Google Scholar] [CrossRef]
- Żenkiewicz, M. Methods for the calculation of surface free energy of solids. J. Achiev. Mater. Manuf. Eng. 2007, 24, 137–145. [Google Scholar]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Chaudhury, M.K.; Good, R.J. Interfacial Lifshitz-van der Waals and polar interactions in macroscopic systems. Chem. Rev. 1988, 88, 927–941. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Good, R.J.; Chaudhury, M.K. The role of van der Waals forces and hydrogen bonds in “hydrophobic interactions” between biopolymers and low energy surfaces. J. Colloid Interface Sci. 1986, 111, 378–390. [Google Scholar] [CrossRef]
- Cornille, A.; Michaud, G.; Simon, F.; Fouquay, S.; Auvergne, R.; Boutevin, B.; Caillol, S. Promising mechanical and adhesive properties of isocyanate-free poly(hydroxyurethane). Eur. Polym. J. 2016, 84, 404–420. [Google Scholar] [CrossRef]
- Merenyi, S. REACH: Regulation (EC) No 1907/2006: Consolidated Version (June 2012) with an Introduction and Future Prospects Regarding the Area of Chemicals Legislation; GRIN Verlag: Münich, Germany, 2012; Volume 2, ISBN 3656293538. [Google Scholar]
- Carré, C.; Ecochard, Y.; Caillol, S.; Avérous, L. From the Synthesis of Biobased Cyclic Carbonate to Polyhydroxyurethanes: A Promising Route towards Renewable Non-Isocyanate Polyurethanes. ChemSusChem 2019, 12, 3410–3430. [Google Scholar] [CrossRef] [PubMed]
- Fuensanta, M.; Khoshnood, A.; Rodríguez-Llansola, F.; Martín-Martínez, J.M. New Waterborne Polyurethane-Urea Synthesized with Ether-Carbonate Copolymer and Amino-Alcohol Chain Extenders with Tailored Pressure-Sensitive Adhesion Properties. Materials 2020, 13, 627. [Google Scholar] [CrossRef] [PubMed]
- Tounici, A.; Martín-Martínez, J.M. Addition of Graphene Oxide in Different Stages of the Synthesis of Waterborne Polyurethane-Urea Adhesives and Its Influence on Their Structure, Thermal, Viscoelastic and Adhesion Properties. Materials 2020, 13, 2899. [Google Scholar] [CrossRef]
- Panchireddy, S.; Thomassin, J.-M.; Grignard, B.; Damblon, C.; Tatton, A.; Jerome, C.; Detrembleur, C. Reinforced poly(hydroxyurethane) thermosets as high performance adhesives for aluminum substrates. Polym. Chem. 2017, 8, 5897–5909. [Google Scholar] [CrossRef]
- Tomita, H.; Sanda, F.; Endo, T. Structural analysis of polyhydroxyurethane obtained by polyaddition of bifunctional five-membered cyclic carbonate and diamine based on the model reaction. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 851–859. [Google Scholar] [CrossRef]
- Cornille, A.; Serres, J.; Michaud, G.; Simon, F.; Fouquay, S.; Boutevin, B.; Caillol, S. Syntheses of epoxyurethane polymers from isocyanate free oligo-polyhydroxyurethane. Eur. Polym. J. 2016, 75, 175–189. [Google Scholar] [CrossRef]
- Cornille, A.; Dworakowska, S.; Bogdal, D.; Boutevin, B.; Caillol, S. A new way of creating cellular polyurethane materials: NIPU foams. Eur. Polym. J. 2015, 66, 129–138. [Google Scholar] [CrossRef]
- Xi, X.; Wu, Z.; Pizzi, A.; Gerardin, C.; Lei, H.; Zhang, B.; Du, G. Non-isocyanate polyurethane adhesive from sucrose used for particleboard. Wood Sci. Technol. 2019, 53, 393–405. [Google Scholar] [CrossRef]
- Błażek, K.; Kasprzyk, P.; Datta, J. Diamine derivatives of dimerized fatty acids and bio-based polyether polyol as sustainable platforms for the synthesis of non-isocyanate polyurethanes. Polymer 2020, 205, 122768. [Google Scholar] [CrossRef]
- Lambeth, R.H. Progress in hybrid non-isocyanate polyurethanes. Polym. Int. 2020. [Google Scholar] [CrossRef]
- Briou, B.; Vu, N.D.; Caillol, S.; Robin, J.-J.; Duguet, N.; Lemaire, M.; Etienne, P.; Bonnet, L.; Lapinte, V. Polyurethane Thermosets Using Lipidic Poly(α-Hydroxyketone). J. Am. Oil Chem. Soc. 2020, 97, 81–91. [Google Scholar] [CrossRef]
- Ecochard, Y.; Leroux, J.; Boutevin, B.; Auvergne, R.; Caillol, S. From multi-functional siloxane-based cyclic carbonates to hybrid polyhydroxyurethane thermosets. Eur. Polym. J. 2019, 120, 109280. [Google Scholar] [CrossRef]
- Tryznowski, M.; Świderska, A.; Gołofit, T.; Zołek-Tryznowska, Z. Wood adhesive application of poly(hydroxyurethane)s synthesized with a dimethyl succinate-based amide backbone. RSC Adv. 2017, 7. [Google Scholar] [CrossRef]
- Panchireddy, S.; Grignard, B.; Thomassin, J.-M.; Jerome, C.; Detrembleur, C. Bio-based poly(hydroxyurethane) glues for metal substrates. Polym. Chem. 2018, 9, 2650–2659. [Google Scholar] [CrossRef]
- Zhang, K.; Nelson, A.M.; Talley, S.J.; Chen, M.; Margaretta, E.; Hudson, A.G.; Moore, R.B.; Long, T.E. Non-isocyanate poly(amide-hydroxyurethane)s from sustainable resources. Green Chem. 2016, 18, 4667–4681. [Google Scholar] [CrossRef]
- Tryznowski, M.; Zołek-Tryznowska, Z.; Świderska, A.; Parzuchowski, P.G. Synthesis, characterization and reactivity of a six-membered cyclic glycerol carbonate bearing a free hydroxyl group. Green Chem. 2016, 18. [Google Scholar] [CrossRef]
- Tryznowski, M.; Żołek-Tryznowska, Z.; Izdebska-Podsiadły, J. The wettability effect of branched polyglycerols used as performance additives for water-based printing inks. J. Coat. Technol. Res. 2018, 15. [Google Scholar] [CrossRef]
- Leitsch, E.K.; Heath, W.H.; Torkelson, J.M. Polyurethane/polyhydroxyurethane hybrid polymers and their applications as adhesive bonding agents. Int. J. Adhes. Adhes. 2016, 64, 1–8. [Google Scholar] [CrossRef]
- Tryznowski, M.; Izdebska-Podsiadły, J.; Żołek-Tryznowska, Z. Wettability and surface free energy of NIPU coatings based on bis(2,3-dihydroxypropyl)ether dicarbonate. Prog. Org. Coat. 2017, 109. [Google Scholar] [CrossRef]
- Tryznowski, M.; Świderska, A.; Żołek-Tryznowska, Z.; Gołofit, T.; Parzuchowski, P.G. Facile route to multigram synthesis of environmentally friendly non-isocyanate polyurethanes. Polymer 2015, 80, 228–236. [Google Scholar] [CrossRef]
- Panchireddy, S.; Grignard, B.; Thomassin, J.-M.; Jerome, C.; Detrembleur, C. Catechol Containing Polyhydroxyurethanes as High-Performance Coatings and Adhesives. ACS Sustain. Chem. Eng. 2018, 6, 14936–14944. [Google Scholar] [CrossRef]
- Decostanzi, M.; Bonneaud, C.; Caillol, S. From hydroxyurethane methacrylates to hybrid nonisocyanate polyurethanes. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1224–1232. [Google Scholar] [CrossRef]
- Noriega, N.E.; Carrillo, A.; Castillo, S.J.; Mota, M.L. Production and Characterization of Non-Isocyanate Polyurethane/SiO2 Films Through a Sol-Gel Process for Thermal Insulation Applications. Polymers 2019, 11, 1596. [Google Scholar] [CrossRef]
- Green PolyurethaneTM. Available online: https://nanotechindustriesinc.com/GPU.php (accessed on 28 September 2020).
- Tomita, H.; Sanda, F.; Endo, T. Polyaddition behavior of bis(five- and six-membered cyclic carbonate)s with diamine. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 860–867. [Google Scholar] [CrossRef]
- Magliozzi, F.; Scali, A.; Chollet, G.; Montarnal, D.; Grau, E.; Cramail, H. Hydrolyzable Biobased Polyhydroxyurethane Networks with Shape Memory Behavior at Body Temperature. ACS Sustain. Chem. Eng. 2020, 8, 9125–9135. [Google Scholar] [CrossRef]
- Schimpf, V.; Heck, B.; Reiter, G.; Mülhaupt, R. Triple-Shape Memory Materials via Thermoresponsive Behavior of Nanocrystalline Non-Isocyanate Polyhydroxyurethanes. Macromolecules 2017, 50, 3598–3606. [Google Scholar] [CrossRef]
- Liu, G.; Wu, G.; Huo, S.; Jin, C.; Kong, Z. Synthesis and properties of non-isocyanate polyurethane coatings derived from cyclic carbonate-functionalized polysiloxanes. Prog. Org. Coat. 2017, 112, 169–175. [Google Scholar] [CrossRef]
- Capar, Ö.; Tabatabai, M.; Klee, J.E.; Worm, M.; Hartmann, L.; Ritter, H. Fast curing of polyhydroxyurethanes via ring opening polyaddition of low viscosity cyclic carbonates and amines. Polym. Chem. 2020. [Google Scholar] [CrossRef]
- Wu, Z.; Tang, L.; Dai, J.; Qu, J. Synthesis and properties of fluorinated non-isocyanate polyurethanes coatings with good hydrophobic and oleophobic properties. J. Coat. Technol. Res. 2019, 16, 1233–1241. [Google Scholar] [CrossRef]
- Ma, Z.; Li, C.; Fan, H.; Wan, J.; Luo, Y.; Li, B.-G. Polyhydroxyurethanes (PHUs) Derived from Diphenolic Acid and Carbon Dioxide and Their Application in Solvent- and Water-Borne PHU Coatings. Ind. Eng. Chem. Res. 2017, 56, 14089–14100. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Demirel, Y. A Novel Biodiesel and Glycerol Carbonate Production Plant. Int. J. Chem. React. Eng. 2011, 9, 9. [Google Scholar] [CrossRef]
- Ochoa-Gómez, J.R.; Gómez-Jiménez-Aberasturi, O.; Ramírez-López, C.; Belsué, M. A Brief Review on Industrial Alternatives for the Manufacturing of Glycerol Carbonate, a Green Chemical. Org. Process Res. Dev. 2012, 16, 389–399. [Google Scholar] [CrossRef]
- Mokhtari, C.; Malek, F.; Manseri, A.; Caillol, S.; Negrell, C. Reactive jojoba and castor oils-based cyclic carbonates for biobased polyhydroxyurethanes. Eur. Polym. J. 2019, 113, 18–28. [Google Scholar] [CrossRef]
- Ecochard, Y.; Caillol, S. Hybrid polyhydroxyurethanes: How to overcome limitations and reach cutting edge properties? Eur. Polym. J. 2020, 137, 109915. [Google Scholar] [CrossRef]
- Zabalov, M.V.; Levina, M.A.; Tiger, R.P. Polyurethanes without Isocyanates and Isocyanates without Phosgene as a New Field of Green Chemistry: Mechanism, Catalysis, and Control of Reactivity. Russ. J. Phys. Chem. B 2019, 13, 778–788. [Google Scholar] [CrossRef]
- Dabral, S.; Licht, U.; Rudolf, P.; Bollmann, G.; Hashmi, A.S.K.; Schaub, T. Synthesis and polymerisation of α-alkylidene cyclic carbonates from carbon dioxide, epoxides and the primary propargylic alcohol 1,4-butynediol. Green Chem. 2020, 22, 1553–1558. [Google Scholar] [CrossRef]
- Kotanen, S.; Laaksonen, T.; Sarlin, E. Feasibility of polyamines and cyclic carbonate terminated prepolymers in polyurethane/polyhydroxyurethane synthesis. Mater. Today Commun. 2020, 23, 100863. [Google Scholar] [CrossRef]
- Quienne, B.; Kasmi, N.; Dieden, R.; Caillol, S.; Habibi, Y. Isocyanate-Free Fully Biobased Star Polyester-Urethanes: Synthesis and Thermal Properties. Biomacromolecules 2020, 21, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Tryznowski, M.; Golofit, T.; Swiderska, A. Commercially available epoxy resin as a raw material for synthesis of 2, 2-bis [4-(2, 3-dihydroxypropoxy) phenyl] propane dicarbonate. Przem. Chem. 2017, 96, 1612–1616. [Google Scholar]
- Figovsky, O.L.; Shapovalov, L.; Axenov, O. Advanced coatings based upon non-isocyanate polyurethanes for industrial applications. Surf. Coat. Int. Part B Coat. Trans. 2004, 87, 83–90. [Google Scholar] [CrossRef]
- Figovsky, O.; Shapovalov, L.; Buslov, F. Ultraviolet and thermostable non-isocyanate polyurethane coatings. Surf. Coat. Int. Part B Coat. Trans. 2005, 88, 67–71. [Google Scholar] [CrossRef]
- Tenorio-Alfonso, A.; Sánchez, M.C.; Franco, J.M. A Review of the Sustainable Approaches in the Production of Bio-based Polyurethanes and Their Applications in the Adhesive Field. J. Polym. Environ. 2020, 28, 749–774. [Google Scholar] [CrossRef]
- Tryznowski, M.; Gołofit, T.; Świderska, A. Poly(hydroxyurethane)s with diethyl tartrate-based amide backbone by an isocyanate-free route: Use as adhesives. Polymer 2018, 144, 1–6. [Google Scholar] [CrossRef]
- Tryznowski, M.; Swiderska, A.; Zadykowicz, K.; Golofit, T. Carbonated epoxy resin Epidian 6 as a raw material for synthesis non-isocyanate poly (hydroxyurethanes). Przem. Chem. 2017, 96, 1992–1995. [Google Scholar] [CrossRef]
- Ochiai, B.; Inoue, S.; Endo, T. One-pot non-isocyanate synthesis of polyurethanes from bisepoxide, carbon dioxide, and diamine. J Polym. Sci A Polym. Chem. 2005, 43, 6613–6618. [Google Scholar] [CrossRef]
- Gindl, M.; Sinn, G.; Gindl, W.; Reiterer, A.; Tschegg, S. A comparison of different methods to calculate the surface free energy of wood using contact angle measurements. Colloids Surfaces A Physicochem. Eng. Asp. 2001, 181, 279–287. [Google Scholar] [CrossRef]
- Nanclares, J.; Petrović, Z.S.; Javni, I.; Ionescu, M.; Jaramillo, F. Segmented polyurethane elastomers by nonisocyanate route. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Cornille, A.; Blain, M.; Auvergne, R.; Andrioletti, B.; Boutevin, B.; Caillol, S. A study of cyclic carbonate aminolysis at room temperature: Effect of cyclic carbonate structures and solvents on polyhydroxyurethane synthesis. Polym. Chem. 2017, 8, 592–604. [Google Scholar] [CrossRef]
- Vanbiervliet, E.; Fouquay, S.; Michaud, G.; Simon, F.; Carpentier, J.-F.; Guillaume, S.M. Non-Isocyanate Polythiourethanes (NIPTUs) from Cyclodithiocarbonate Telechelic Polyethers. Macromolecules 2019, 52, 5838–5849. [Google Scholar] [CrossRef]
- Chen, Q.; Gao, K.; Peng, C.; Xie, H.; Zhao, Z.K.; Bao, M. Preparation of lignin/glycerol-based bis(cyclic carbonate) for the synthesis of polyurethanes. Green Chem. 2015, 17, 4546–4551. [Google Scholar] [CrossRef]
- Kim, M.-R.; Kim, H.-S.; Ha, C.-S.; Park, D.-W.; Lee, J.-K. Syntheses and thermal properties of poly(hydroxy)urethanes by polyaddition reaction of bis(cyclic carbonate) and diamines. J. Appl. Polym. Sci. 2001, 81, 2735–2743. [Google Scholar] [CrossRef]
| Name | Dicarbonate Mass (g) | Diamine | Diamine (mol) |
|---|---|---|---|
| NIPU1 1 | 27.2 | Ethylenediamine | 0.0704 |
| NIPU2 1 | 28.3 | Trimethylenediamine | 0.0738 |
| NIPU3 1 | 26.0 | Putrescine | 0.0678 |
| NIPU4 1 | 27.2 | Hexamethylenediamine | 0.0710 |
| NIPU5 1 | 26.9 | 2,2,4(2,4,4)-Trimethyl-1,6-hexanediamine | 0.0702 |
| PHU-Ar | 23.6 | m-Xylylenediamine | 0.0615 |
| PHU-C6O2 | 28.1 | 1,8-Diamino-3,6-dioxaoctane | 0.0732 |
| PHU-C10O3 | 27.9 | 4,7,10-Trioxa-1,13-tridecanediamine | 0.0729 |
| PHU-CC6 | 26.8 | Isophorone diamine | 0.0693 |
| Name | Tg (°C) | Td,5% (°C) | Td max (°C) | Weight Loss (%) |
|---|---|---|---|---|
| NIPU1 1 | 70 | 243 | 411 | 78 |
| NIPU2 1 | 68 | 249 | 374 | 53 |
| NIPU3 1 | 58 | 243 | 367 | 55 |
| NIPU4 1 | 58 | 273 | 360 | 48 |
| NIPU5 1 | 61 | 283 | 324 | 25 |
| PHU-Ar | 71 | 283 | 355 | 48 |
| PHU-C6O2 | 36 | 239 | 369 | 51 |
| PHU-C10O3 | 34 | 284 | 370 | 55 |
| PHU-CC6 | 115 | 269 | 371 | 49 |
| Name | γD | γP | γT | γLW | γAB | γ+ | γ− | γT |
|---|---|---|---|---|---|---|---|---|
| Water | 35.3 | 3.7 | 38.9 | 38.6 | 3.8 | 0.5 | 3.2 | 42.4 |
| Diiodomethane | 33.1 | 4.1 | 37.3 | 36.7 | 4.8 | 0.0 | 5.7 | 41.5 |
| Formamide | 31.1 | 3.0 | 34.1 | 33.8 | 3.1 | 2.3 | 0.1 | 36.9 |
| PHU | Contac Angle | Surface Free Energy (mJ∙m−2) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Owens-Wendt (dw) 1 | van Oss–Chaudhury–Good (dfw) 2 | ||||||||
| Water | γD | γP | γT | γLW | γAB | γ+ | γ− | γT | |
| NIPU1 | 77 | 45 | 4 | 48 | 45 | 4 | 2 | 2 | 48 |
| NIPU2 | 73 | 44 | 5 | 49 | 44 | 5 | 2 | 4 | 49 |
| NIPU3 | 66 | 46 | 8 | 53 | 46 | 5 | 1 | 10 | 51 |
| NIPU4 | 68 | 46 | 7 | 52 | 46 | 2 | 1 | 11 | 48 |
| NIPU5 | 79 | 44 | 3 | 47 | 44 | 1 | 0 | 6 | 45 |
| PHU-Ar | 71 | 45 | 6 | 50 | 45 | 4 | 1 | 8 | 48 |
| PHU-C6O2 | 68 | 45 | 7 | 52 | 45 | 1 | 0 | 15 | 46 |
| PHU-C10O3 | 58 | 46 | 11 | 57 | 46 | 10 | 1 | 31 | 55 |
| PHU-CC6 | 73 | 41 | 6 | 47 | 41 | 2 | 0 | 13 | 43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tryznowski, M.; Żołek-Tryznowska, Z. Surface Properties of Poly(Hydroxyurethane)s Based on Five-Membered Bis-Cyclic Carbonate of Diglycidyl Ether of Bisphenol A. Materials 2020, 13, 5184. https://doi.org/10.3390/ma13225184
Tryznowski M, Żołek-Tryznowska Z. Surface Properties of Poly(Hydroxyurethane)s Based on Five-Membered Bis-Cyclic Carbonate of Diglycidyl Ether of Bisphenol A. Materials. 2020; 13(22):5184. https://doi.org/10.3390/ma13225184
Chicago/Turabian StyleTryznowski, Mariusz, and Zuzanna Żołek-Tryznowska. 2020. "Surface Properties of Poly(Hydroxyurethane)s Based on Five-Membered Bis-Cyclic Carbonate of Diglycidyl Ether of Bisphenol A" Materials 13, no. 22: 5184. https://doi.org/10.3390/ma13225184
APA StyleTryznowski, M., & Żołek-Tryznowska, Z. (2020). Surface Properties of Poly(Hydroxyurethane)s Based on Five-Membered Bis-Cyclic Carbonate of Diglycidyl Ether of Bisphenol A. Materials, 13(22), 5184. https://doi.org/10.3390/ma13225184






