Magneto-Rheological Fluid Assisted Abrasive Nanofinishing of β-Phase Ti-Nb-Ta-Zr Alloy: Parametric Appraisal and Corrosion Analysis
Abstract
1. Introduction
2. Materials and Methods
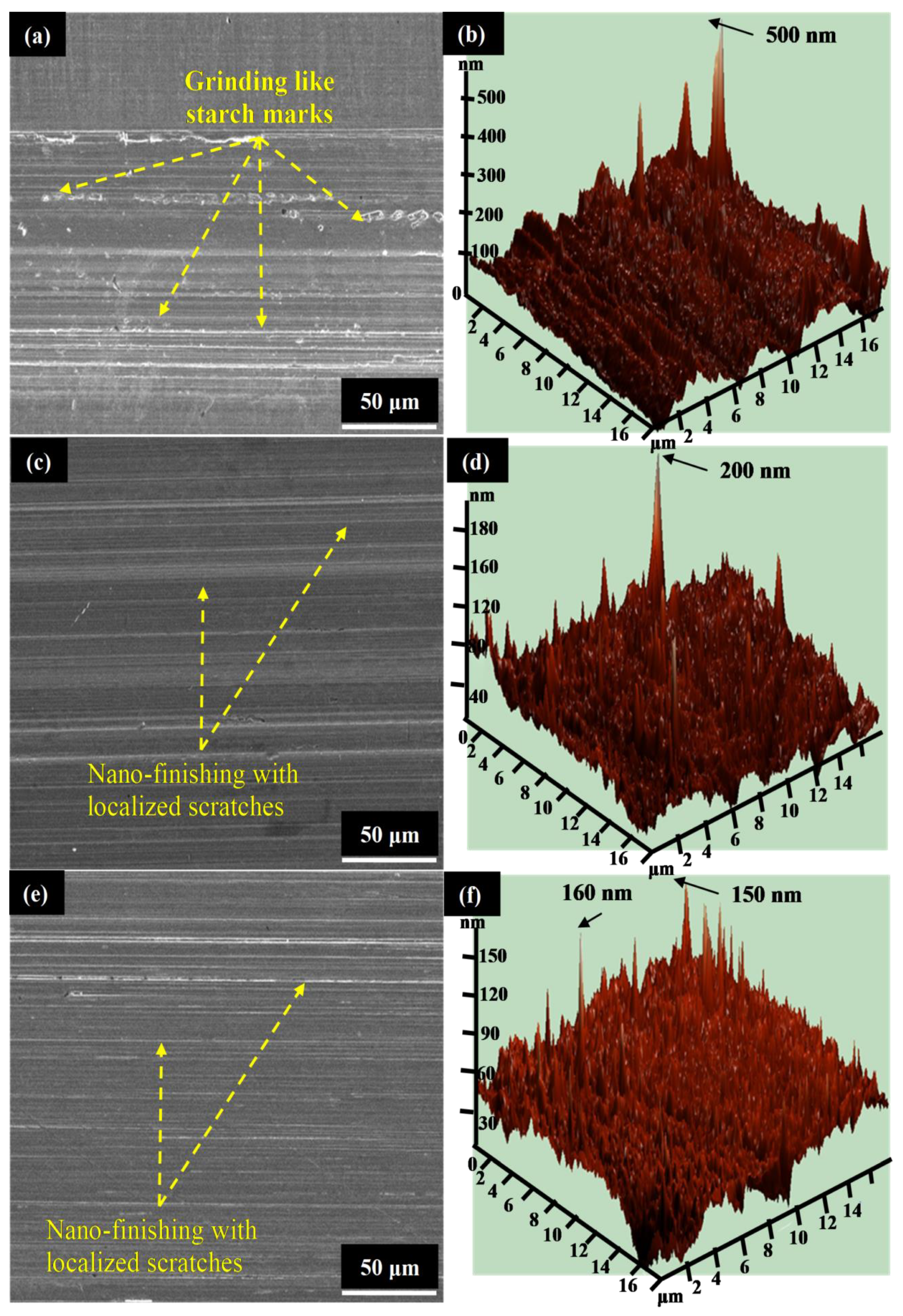
3. Results and Discussion
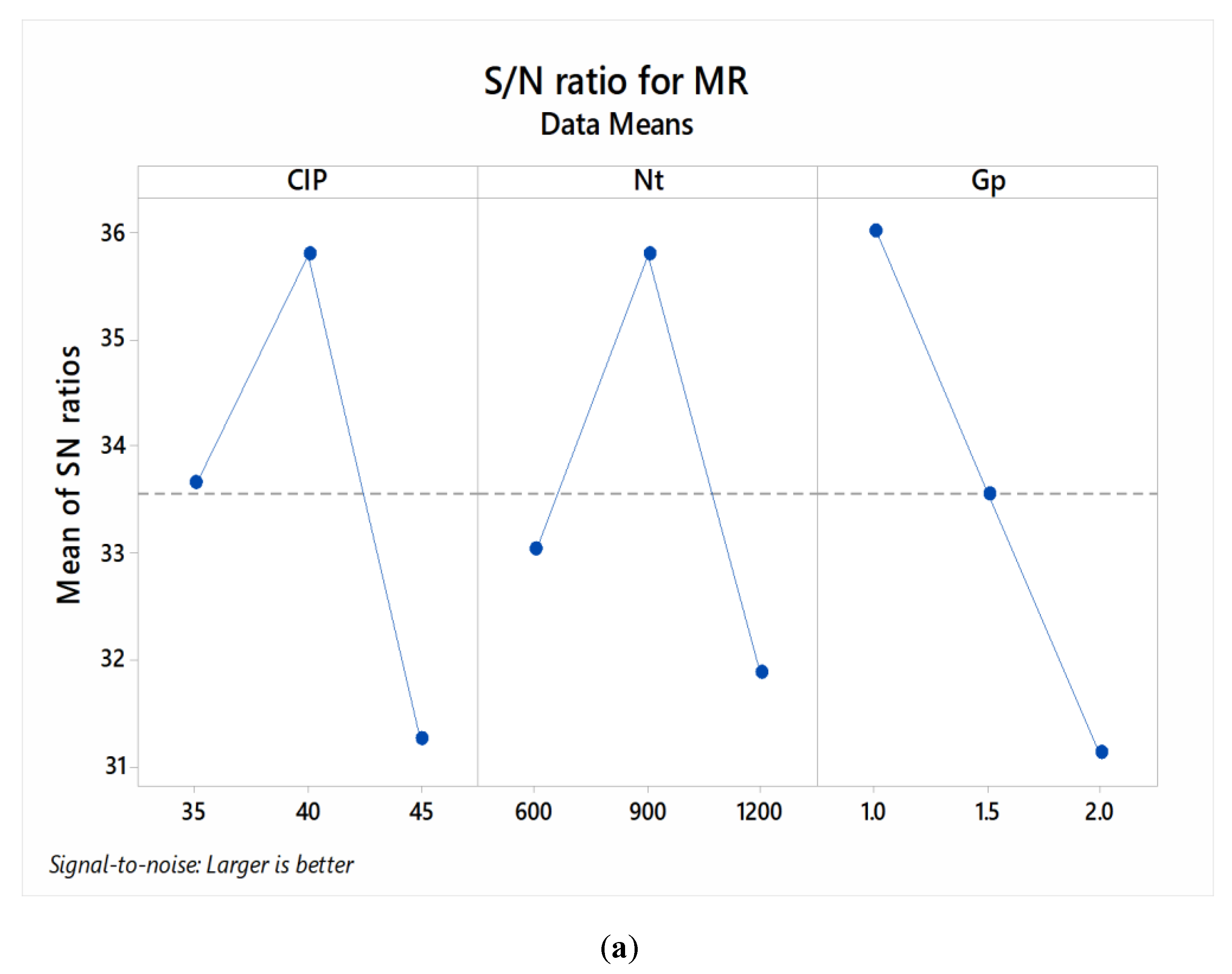
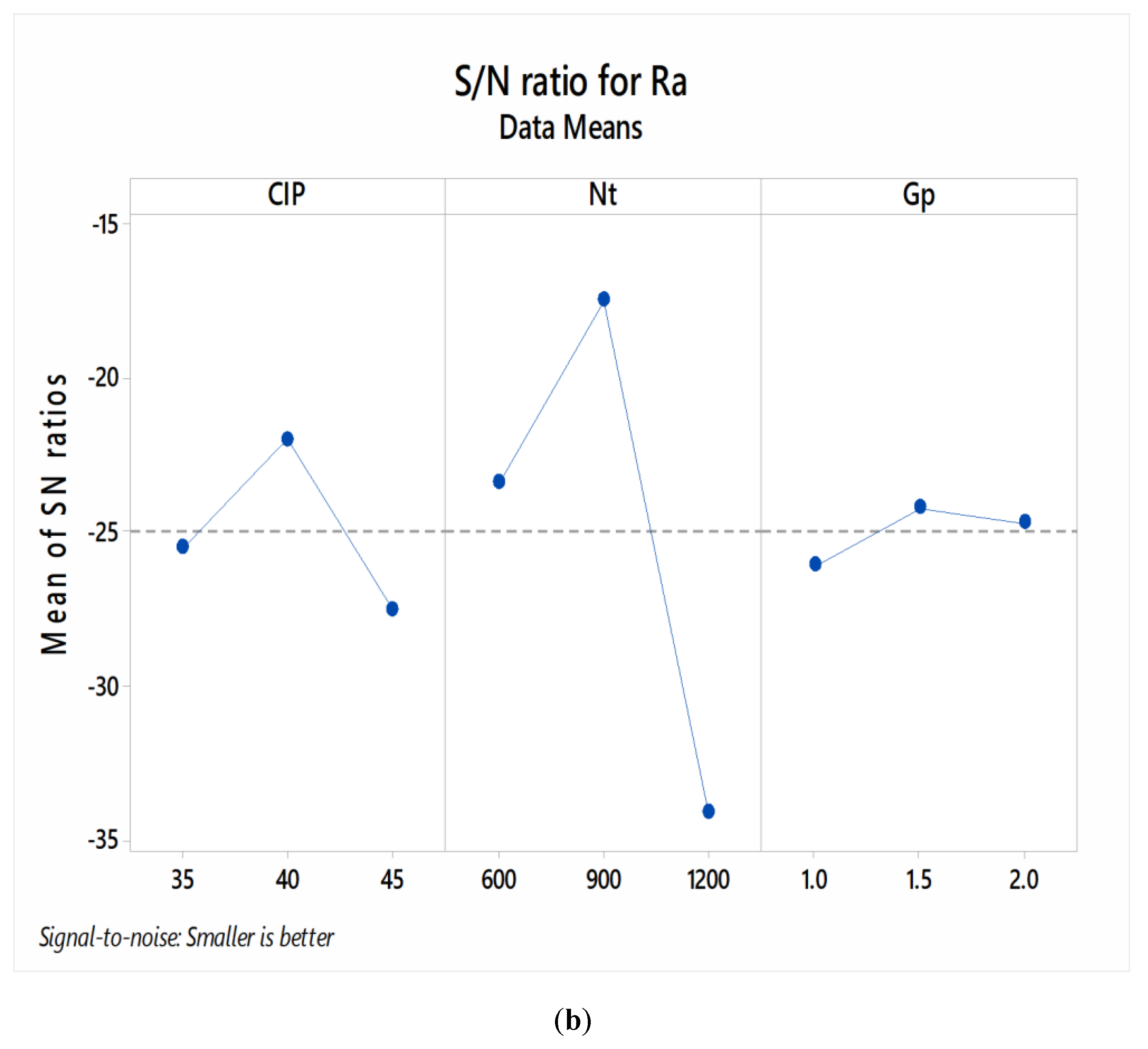
3.1. Parametric Optimization
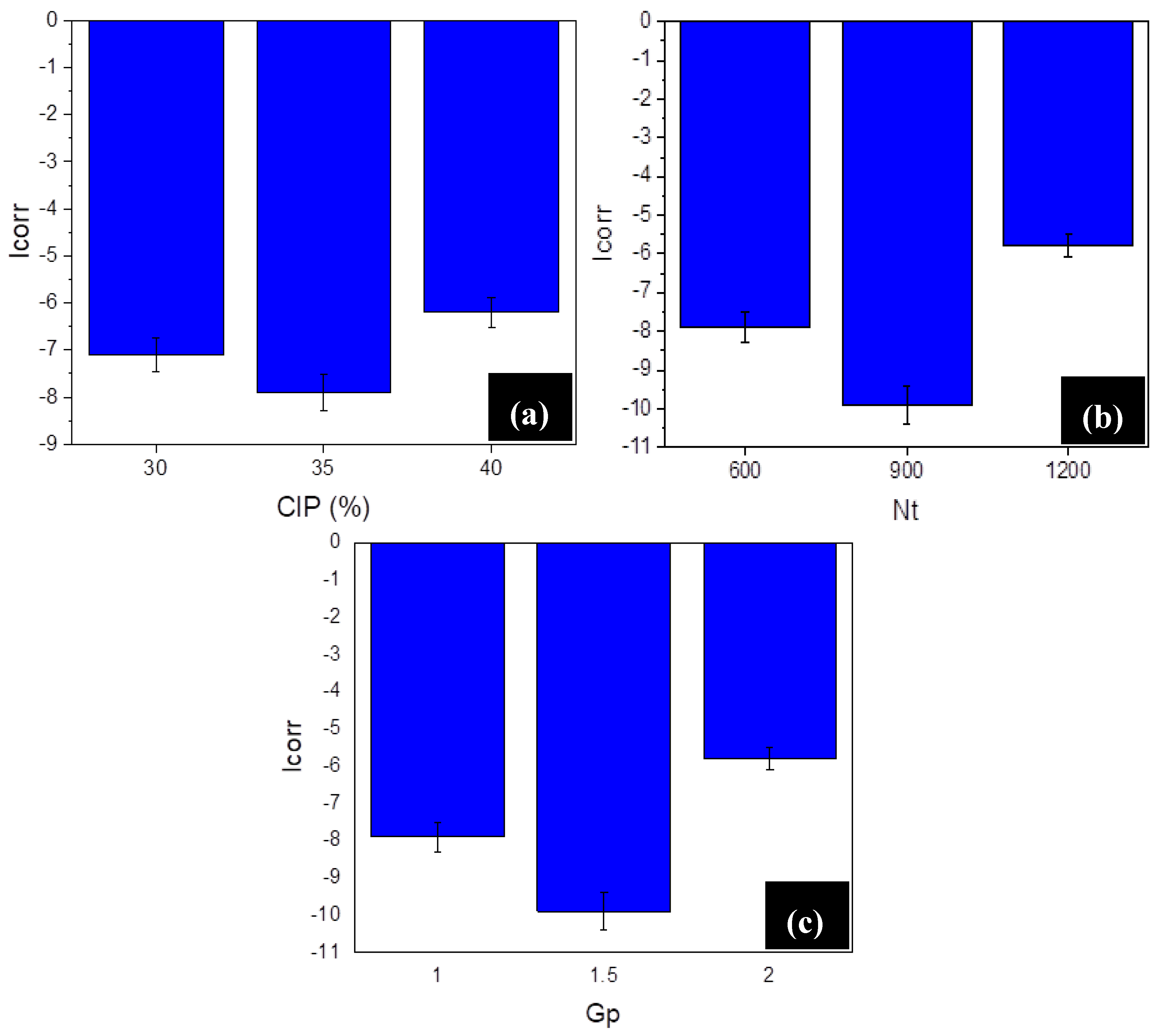
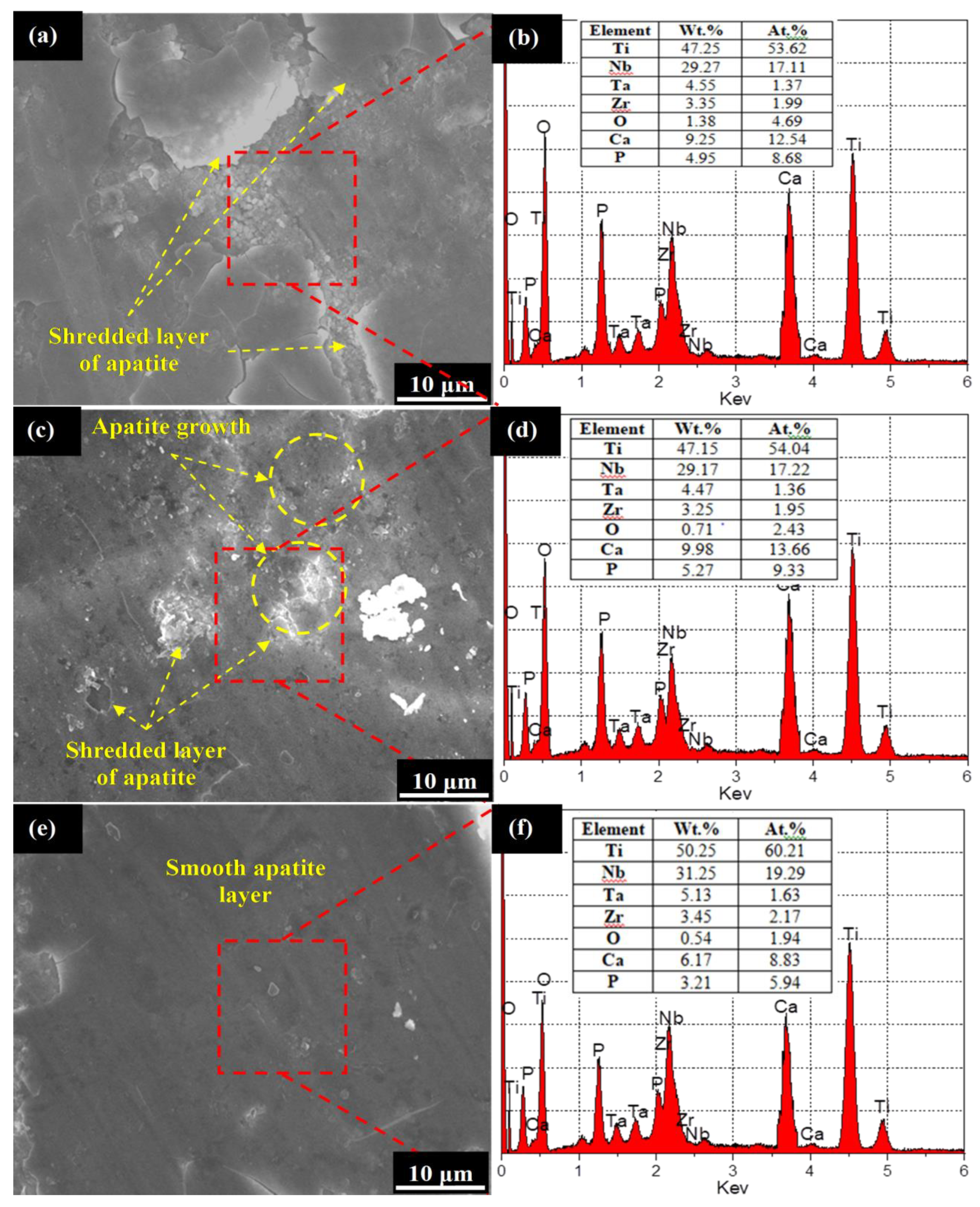
3.2. Corrosion Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Nakai, M. Titanium-based biomaterials for preventing stress shielding between implant devices and bone. Int. J. Biomater. 2011, 2011, 836587. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Hu, L.; Ding, Q. Effective solution for the tribological problems of Ti-6Al-4V: Combination of laser surface texturing and solid lubricant film. Surf. Coat. Technol. 2012, 206, 5060–5066. [Google Scholar] [CrossRef]
- Collipriest, J.E., Jr. An experimentalist’s view of the surface flaw problem. In The Surface Crack—Physical Problems and Computational Solutions; ASME: New York, NY, USA, 1972; pp. 43–61. [Google Scholar]
- Lee, T.M.; Chang, E.; Yang, C.Y. A comparison of the surface characteristics and ion release of Ti6Al4V and heat-treated Ti6Al4V. J. Biomed. Mater. Res. 2000, 50, 499–511. [Google Scholar] [CrossRef]
- Zaffe, D.; Bertoldi, C.; Consolo, U. Accumulation of aluminium in lamellar bone after implantation of titanium plates, Ti–6Al–4V screws, hydroxyapatite granules. Biomaterials 2004, 25, 3837–3844. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, L.; Fu, Y.; Qin, J.; Lu, W.; Zhang, D. Influence of oxygen content on microstructure and mechanical properties of Ti–Nb–Ta–Zr alloy. Mater. Des. 2011, 32, 2934–2939. [Google Scholar] [CrossRef]
- Stráský, J.; Harcuba, P.; Václavová, K.; Horváth, K.; Landa, M.; Srba, O.; Janeček, M. Increasing strength of a biomedical Ti-Nb-Ta-Zr alloy by alloying with Fe, Si and O. J. Mech. Behav. Biomed. Mater. 2017, 71, 329–336. [Google Scholar] [CrossRef]
- Prakash, C.; Kansal, H.K.; Pabla, B.S.; Puri, S. Experimental investigations in powder mixed electric discharge machining of Ti–35Nb–7Ta–5Zrβ-titanium alloy. Mater. Manuf. Process. 2017, 32, 274–285. [Google Scholar] [CrossRef]
- Prakash, C.; Kansal, H.K.; Pabla, B.S.; Puri, S. Processing and characterization of novel biomimetic nanoporous bioceramic surface on β-Ti implant by powder mixed electric discharge machining. J. Mater. Eng. Perform. 2015, 24, 3622–3633. [Google Scholar] [CrossRef]
- Prakash, C.; Kansal, H.K.; Pabla, B.S.; Puri, S. Multi-objective optimization of powder mixed electric discharge machining parameters for fabrication of biocompatible layer on β-Ti alloy using NSGA-II coupled with Taguchi based response surface methodology. J. Mech. Sci. Technol. 2016, 30, 4195–4204. [Google Scholar] [CrossRef]
- Prakash, C.; Uddin, M.S. Surface modification of β-phase Ti implant by hydroaxyapatite mixed electric discharge machining to enhance the corrosion resistance and in-vitro bioactivity. Surf. Coat. Technol. 2017, 326, 134–145. [Google Scholar] [CrossRef]
- Prakash, C.; Kansal, H.K.; Pabla, B.S.; Puri, S. Powder mixed electric discharge machining: An innovative surface modification technique to enhance fatigue performance and bioactivity of β-Ti implant for orthopedics application. J. Comput. Inf. Sci. Eng. 2016, 16, 041006. [Google Scholar] [CrossRef]
- Prakash, C.; Kansal, H.K.; Pabla, B.S.; Puri, S. Effect of surface nano-porosities fabricated by powder mixed electric discharge machining on bone-implant interface: An experimental and finite element study. Nanosci. Nanotechnol. Lett. 2016, 8, 815–826. [Google Scholar] [CrossRef]
- Singh, S.; Prakash, C.; Singh, H. Deposition of HA-TiO2 by plasma spray on β-phase Ti-35Nb-7Ta-5Zr alloy for hip stem: Characterization, mechanical properties, corrosion, and in-vitro bioactivity. Surf. Coat. Technol. 2020, 398, 126072. [Google Scholar] [CrossRef]
- Singh, H.; Prakash, C.; Singh, S. Plasma Spray Deposition of HA-TiO2 on β-phase Ti-35Nb-7Ta-5Zr Alloy for Hip Stem: Characterization of Bio-mechanical Properties, Wettability, and Wear Resistance. J. Bionic Eng. 2020, 17, 1029–1044. [Google Scholar] [CrossRef]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implant. Res. 2006, 17, 68–81. [Google Scholar] [CrossRef]
- Bain, C.A.; Moy, P.K. The association between the failure of dental implants and cigarette smoking. Int. J. Oral Maxillofac. Implant. 1993, 8, 609–615. [Google Scholar]
- Brocard, D.; Barthet, P.; Baysse, E.; Duffort, J.F.; Eller, P.; Justumus, P. A multicenter report on 1.022 consecutively placed ITI implants: A 7-year longitudinal study. Int. J. Oral Maxillofac. Implant. 2000, 15, 691–700. [Google Scholar]
- Karoussis, I.K.; Kotsovilis, S.; Fourmousis, I. A comprehensive and critical review of dental implant prognosis in periodontally compromised partially edentulous patients. Clin. Oral Implant. Res. 2007, 18, 669–679. [Google Scholar] [CrossRef]
- Balshe, A.A.; Eckert, S.E.; Koka, S.; Assad, D.A.; Weaver, A.L. The effects of smoking on the survival of smooth- and rough-surface dental implants. Int. J. Oral Maxillofac. Implant. 2008, 23, 1117–1122. [Google Scholar]
- Aaraj Khodaii, S.J.; Barazandeh, F.; Adibi, H.; Sarhan, A. Optimization of Grinding partially stabilized zirconia (PSZ) for dental Implant application. Modares Mech. Eng. 2018, 18, 187–194. [Google Scholar]
- Rao, P.S.; Jain, P.K.; Dwivedi, D.K. Optimization of key process parameters on electro chemical honing (ECH) of external cylindrical surfaces of titanium alloy Ti 6Al 4V. Mater. Today Proc. 2017, 4, 2279–2289. [Google Scholar] [CrossRef]
- Affatato, S.; Ruggiero, A. Surface analysis on revised hip implants with stem taper for wear and failure incidence evaluation: A first investigation. Measurement 2019, 145, 38–44. [Google Scholar] [CrossRef]
- Tian, Y.; Shi, C.; Fan, Z.; Zhou, Q. Experimental investigations on magnetic abrasive finishing of Ti-6Al-4V using a multiple pole-tip finishing tool. Int. J. Adv. Manuf. Technol. 2020, 106, 3071–3080. [Google Scholar] [CrossRef]
- Singh, H.; Singh, S.; Prakash, C. Current Trends in Biomaterials and Bio-manufacturing; Springer: Berlin, Germany, 2019. [Google Scholar]
- Basim, G.B.; Ozdemir, Z.; Mutlu, O. Biomaterials applications of chemical mechanical polishing. In Proceedings of the Planarization/CMP Technology (ICPT 2012), International Conference VDE 2012, Grenoble, France, 15–17 October 2012; pp. 1–5. [Google Scholar]
- Okawa, S.; Watanabe, K. Chemical mechanical polishing of titanium with colloidal silica containing hydrogen peroxide—Mirror polishing and surface properties. Dent. Mater. J. 2009, 28, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Zur Rahman Pompa, L.; Haider, W. Influence of electropolishing and magnetoelectropolishing on corrosion and biocompatibility of titanium implants. J. Mater. Eng. Perform. 2014, 23, 3907–3915. [Google Scholar]
- Okada, A.; Uno, Y.; Yabushita, N.; Uemura, K.; Raharjo, P. High efficient surface finishing of bio-titanium alloy by large-area electron beam irradiation. J. Mater. Process. Technol. 2004, 149, 506–511. [Google Scholar] [CrossRef]
- Sidpara, A.; Jain, V.K. Analysis of forces on the freeform surface in magnetorheological fluid based finishing process. Int. J. Mach. Tools Manuf. 2013, 69, 1–10. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, V.K.; Sidpara, A. Nanofinishing of freeform surfaces (knee joint implant) by rotational-magnetorheological abrasive flow finishing (R-MRAFF) process. Precis. Eng. 2015, 42, 165–178. [Google Scholar] [CrossRef]
- Barman, A.; Das, M. Force analysis during spot finishing of titanium alloy using novel tool in magnetic field assisted finishing process. Int. J. Precis. Technol. 2019, 8, 190–200. [Google Scholar]
- Barman, A.; Das, M. Nano-finishing of bio-titanium alloy to generate different surface morphologies by changing magnetorheological polishing fluid compositions. Precis. Eng. 2018, 51, 145–152. [Google Scholar] [CrossRef]
- Barman, A.; Das, M. Toolpath generation and finishing of bio-titanium alloy using novel polishing tool in MFAF process. Int. J. Adv. Manuf. Technol. 2019, 100, 1123–1135. [Google Scholar] [CrossRef]
- Parameswari, G.; Jain, V.K.; Ramkumar, J.; Nagdeve, L. Experimental investigations into nanofinishing of Ti6Al4V flat disc using magnetorheological finishing process. Int. J. Adv. Manuf. Technol. 2019, 100, 1055–1065. [Google Scholar] [CrossRef]
- Nagdeve, L.; Jain, V.K.; Ramkumar, J. Preliminary investigations into nano-finishing of freeform surface (femoral) using inverse replica fixture. Int. J. Adv. Manuf. Technol. 2019, 100, 1081–1092. [Google Scholar] [CrossRef]
- Barman, A.; Das, M. Magnetic field assisted finishing process for super-finished Ti alloy implant and its 3D surface characterization. J. Micromanufacturing 2018, 1, 154–169. [Google Scholar] [CrossRef]
- Singh, S.; Prakash, C. Effect of cryogenic treatment on the microstructure, mechanical properties and finishability of β-TNTZ alloy for orthopedic applications. Mater. Lett. 2020, 278, 128461. [Google Scholar] [CrossRef]
- Málek, J.; Hnilica, F.; Veselý, J.; Smola, B.; Bartáková, S.; Vaněk, J. The influence of chemical composition and thermo-mechanical treatment on Ti–Nb–Ta–Zr alloys. Mater. Des. 2012, 35, 731–740. [Google Scholar] [CrossRef]
- Majumdar, P. Microstructural Evaluation of Boron Free and Boron Containing Heat-Treated Ti-35Nb-7.2 Zr-5.7 Ta Alloy. Microsc. Microanal. 2012, 18, 295. [Google Scholar] [CrossRef]
- Tang, X.; Ahmed, T.; Rack, H.J. Phase transformations in Ti-Nb-Ta and Ti-Nb-Ta-Zr alloys. J. Mater. Sci. 2000, 35, 1805–1811. [Google Scholar] [CrossRef]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data aAnalysis for Biologists; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Rosalbino, F.; De Negri, S.; Scavino, G.; Saccone, A. Electrochemical corrosion behaviour of binary magnesium-heavy rare earth alloys. Metall. Ital. 2018, 2, 34–43. [Google Scholar]
- Babbar, A.; Prakash, C.; Singh, S.; Gupta, M.K.; Mia, M.; Pruncu, C.I. Application of hybrid nature-inspired algorithm: Single and bi-objective constrained optimization of magnetic abrasive finishing process parameters. J. Mater. Res. Technol. 2020, 4, 7961–7974. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Dao, T.P.; Prakash, C.; Singh, S.; Pramanik, A.; Krolczyk, G.; Pruncu, C.I. Machining parameter optimization in shear thickening polishing of gear surfaces. J. Mater. Res. Technol. 2020, 9, 5112–5126. [Google Scholar] [CrossRef]
- Abron, A.; Hopfensperger, M.; Thompson, J.; Cooper, L.F. Evaluation of a predictive model for implant surface topography effects on early osseointegration in the rat tibia model. J. Prosthet. Dent. 2001, 85, 40–46. [Google Scholar] [CrossRef]
- Ogawa, T.; Nishimura, I. Genes differentially expressed in titanium implant healing. J. Dent. Res. 2006, 85, 566–570. [Google Scholar] [CrossRef]
- Wang, G.; Wan, Y.; Wang, T.; Liu, Z. Corrosion behavior of titanium implant with different surface morphologies. Procedia Manuf. 2017, 10, 363–370. [Google Scholar] [CrossRef]
- Kubo, K.; Tsukimura, N.; Iwasa, F.; Ueno, T.; Saruwatari, L.; Aita, H.; Chiou, W.A.; Ogawa, T. Cellular behavior on TiO2 nanonodular structures in a micro-to-nanoscale hierarchy model. Biomaterials 2009, 30, 5319–5329. [Google Scholar] [CrossRef] [PubMed]


| Symbol | Process Parameters | Unit | Range |
|---|---|---|---|
| CIP | Iron (Fe); size~25 µm | % by vol. | 30, 35, 40 |
| Nt | Rotational speed of tool | rpm | 600, 900, 1200 |
| Gp | Work-gap | Mm | 1, 1.5, 2 |
| Experiment No. | CIP | Nt | Gp |
|---|---|---|---|
| 1 | 35 | 600 | 1.0 |
| 2 | 35 | 900 | 1.5 |
| 3 | 35 | 1200 | 2.0 |
| 4 | 40 | 600 | 1.5 |
| 5 | 40 | 900 | 2.0 |
| 6 | 40 | 1200 | 1.0 |
| 7 | 45 | 600 | 2.0 |
| 8 | 45 | 900 | 1.0 |
| 9 | 45 | 1200 | 1.5 |
| Experiment No. | MR | Ra | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Raw Value (g) | Mean | S/N | Raw Value (nm) | Mean | S/N | |||||
| 1 | 60 | 50 | 70 | 60 | 35.56 | 16 | 15 | 11 | 14 | −22.92 |
| 2 | 50 | 55 | 75 | 60 | 35.56 | 8 | 10 | 6 | 8 | −18.06 |
| 3 | 28 | 35 | 30 | 31 | 29.83 | 60 | 55 | 65 | 60 | −35.56 |
| 4 | 68 | 55 | 57 | 60 | 35.56 | 10 | 12 | 8 | 10 | −20.00 |
| 5 | 65 | 50 | 65 | 60 | 35.56 | 4 | 2 | 6 | 4 | −12.04 |
| 6 | 68 | 67 | 60 | 65 | 36.26 | 45 | 50 | 40 | 45 | −33.06 |
| 7 | 23 | 27 | 25 | 25 | 27.96 | 21 | 20 | 22 | 21 | −26.44 |
| 8 | 66 | 62 | 67 | 65 | 36.26 | 14 | 15 | 10 | 13 | −22.28 |
| 9 | 30 | 28 | 32 | 30 | 29.54 | 75 | 50 | 55 | 60 | −35.56 |
| Overall mean S/N ratio (mo) | 33.56 | 25.10 | ||||||||
| Source | Degree of Freedom | Sum of Square | Variance | F-Test | p-Value | Contribution (%) |
|---|---|---|---|---|---|---|
| MR | ||||||
| CIP | 2 | 30.9716 | 15.4858 | 53.19 | 0.018 * | 33.64 |
| Nt | 2 | 24.3382 | 12.1691 | 41.80 | 0.023 * | 26.44 |
| Gp | 2 | 36.1651 | 18.0825 | 62.11 | 0.016 * | 39.28 |
| Residual Error | 2 | 0.5823 | 0.2912 | 0.632 | ||
| Total | 8 | 92.0573 | 100 | |||
| Ra | ||||||
| CIP | 2 | 47.086 | 23.543 | 2.40 | 0.294 | 9.39 |
| Nt | 2 | 429.047 | 214.523 | 21.85 | 0.044 * | 85.57 |
| Gp | 2 | 5.630 | 2.815 | 0.29 | 0.777 | 1.12 |
| Residual Error | 2 | 19.639 | 9.819 | 3.91 | ||
| Total | 8 | 501.401 | 100 | |||
| Level | CIP | Nt | Gp |
|---|---|---|---|
| MR | |||
| 1 | 33.65 | 33.03 | 36.03 * |
| 2 | 35.79 * | 35.79 * | 33.56 |
| 3 | 31.25 | 31.88 | 31.12 |
| Delta | 4.54 | 3.92 | 4.91 |
| Rank | 2 | 3 | 1 |
| Ra | |||
| 1 | −25.52 | −23.40 | −26.09 |
| 2 | −21.98 * | −17.46 * | −24.23 * |
| 3 | −27.51 | −34.14 | −24.68 |
| Delta | 5.53 | 16.68 | 1.86 |
| Rank | 2 | 1 | 3 |
| Responses | Predicted Values | Confirmatory Values | Difference (±) |
|---|---|---|---|
| MR (g) | 105.8 | 103.7 | 2.1 |
| Ra (nm) | 4.63 | 4.67 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.; Prakash, C.; Pramanik, A.; Basak, A.; Shabadi, R.; Królczyk, G.; Bogdan-Chudy, M.; Babbar, A. Magneto-Rheological Fluid Assisted Abrasive Nanofinishing of β-Phase Ti-Nb-Ta-Zr Alloy: Parametric Appraisal and Corrosion Analysis. Materials 2020, 13, 5156. https://doi.org/10.3390/ma13225156
Singh S, Prakash C, Pramanik A, Basak A, Shabadi R, Królczyk G, Bogdan-Chudy M, Babbar A. Magneto-Rheological Fluid Assisted Abrasive Nanofinishing of β-Phase Ti-Nb-Ta-Zr Alloy: Parametric Appraisal and Corrosion Analysis. Materials. 2020; 13(22):5156. https://doi.org/10.3390/ma13225156
Chicago/Turabian StyleSingh, Sunpreet, Chander Prakash, Alokesh Pramanik, Animesh Basak, Rajasekhara Shabadi, Grzegorz Królczyk, Marta Bogdan-Chudy, and Atul Babbar. 2020. "Magneto-Rheological Fluid Assisted Abrasive Nanofinishing of β-Phase Ti-Nb-Ta-Zr Alloy: Parametric Appraisal and Corrosion Analysis" Materials 13, no. 22: 5156. https://doi.org/10.3390/ma13225156
APA StyleSingh, S., Prakash, C., Pramanik, A., Basak, A., Shabadi, R., Królczyk, G., Bogdan-Chudy, M., & Babbar, A. (2020). Magneto-Rheological Fluid Assisted Abrasive Nanofinishing of β-Phase Ti-Nb-Ta-Zr Alloy: Parametric Appraisal and Corrosion Analysis. Materials, 13(22), 5156. https://doi.org/10.3390/ma13225156











