Permeabilities and Mechanical Properties of Hardened Cement Pastes Modified with Sodium Laurate and Nano Silica
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Preparation of Cement Paste Modified with SL and NS
2.3. Characterization
2.3.1. FE-SEM, XPS, and FTIR Measurement
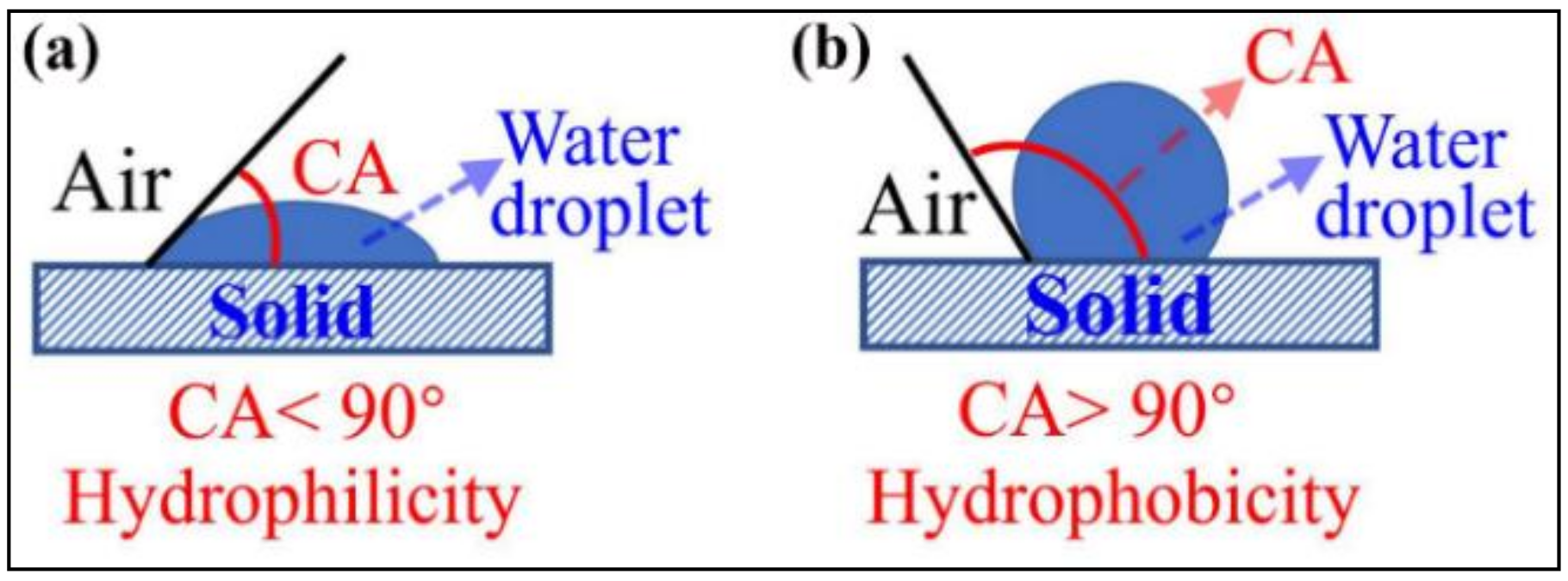
2.3.2. Surface Wettability Measurement
2.3.3. Measurement of Flexural Strength and Compressive Strength
2.3.4. Measurement of Water Absorption
3. Results and Discussion
3.1. Wettability
3.2. SEM
3.3. XPS and FT-IR
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, P.; Liu, Z.; Wang, Y.; Yang, J.; Han, S.; Zhao, T. 3D neutron tomography of steel reinforcement corrosion in cement-based composites. Constr. Build. Mater. 2018, 162, 561–565. [Google Scholar] [CrossRef]
- Xu, B.; Yuan, B.; Wang, Y. Anti-corrosion cement for sour gas (H2S-CO2) storage and production of HTHP deep wells. Appl. Geochem. 2018, 96, 155–163. [Google Scholar]
- Beglarigale, A.; Yazıcı, H. Electrochemical corrosion monitoring of steel fiber embedded in cement based composites. Cem. Concr. Comp. 2017, 83, 427–446. [Google Scholar] [CrossRef]
- Song, J.; Zhao, D.; Han, Z.; Xu, W.; Lu, Y.; Liu, X.; Liu, B.; Carmalt, C.; Deng, X.; Parkin, I. Super-robust superhydrophobic concrete. J. Mater. Chem. A 2017, 5, 14542–14550. [Google Scholar] [CrossRef]
- Song, J.; Li, Y.; Xu, W.; Liu, H.; Lu, Y. Inexpensive and non-fluorinated superhydrophobic concrete coating for anti-icing and anti-corrosion. J. Colloid Interface Sci. 2019, 541, 86–92. [Google Scholar] [CrossRef]
- Xue, X.; Li, Y.; Yang, Z.; He, Z.; Dai, J.; Xu, L.; Zhang, W. A systematic investigation of the waterproofing performance and chloride resistance of a self-developed waterborne silane-based hydrophobic agent for mortar and concrete. Constr. Build. Mater. 2018, 155, 939–946. [Google Scholar] [CrossRef]
- Li, G.; Yue, J.; Guo, C.; Ji, Y. Influences of modified nanoparticles on hydrophobicity of concrete with organic film coating. Constr. Build. Mater. 2018, 169, 1–7. [Google Scholar] [CrossRef]
- Corcione, C.; Striani, R.; Capone, C.; Molfetta, M.; Vendetta, S.; Frigione, M. Preliminary study of the application of a novel hydrophobic photopolymerizable nano-structured coating on concrete substrates. Prog. Org. Coat. 2018, 121, 182–189. [Google Scholar] [CrossRef]
- Chen, H.; Feng, P.; Du, Y.; Jiang, J.; Sun, W. The effect of superhydrophobic nano-silica particles on the transport and mechanical properties of hardened cement pastes. Constr. Build. Mater. 2018, 182, 620–628. [Google Scholar] [CrossRef]
- Xu, S.; Ma, Q.; Wang, J. Combined effect of isobutyltriethoxysilane and silica fume on the performance of natural hydraulic lime-based mortars. Constr. Build. Mater. 2018, 162, 181–191. [Google Scholar] [CrossRef]
- Li, Y.; Gou, L.; Wang, H.; Wang, Y.; Zhang, J.; Li, N.; Hu, S.; Yang, J. Fluorine-free superhydrophobic carbon-based coatings on the concrete. Mater. Lett. 2018, 244, 31–34. [Google Scholar] [CrossRef]
- Li, Q.; Yang, K.; Yang, C. An alternative admixture to reduce sorptivity of alkali-activated slag cement by optimising pore structure and introducing hydrophobic film. Cem. Concr. Comp. 2018, 95, 183–192. [Google Scholar] [CrossRef]
- Subbiah, K.; Park, D.; Lee, Y.; Velu, S.; Lee, H.; Jang, H.; Choi, H. Development of water-repellent cement mortar using silane enriched with nanomaterials. Prog. Org. Coat. 2018, 125, 48–60. [Google Scholar]
- Wong, H.; Barakat, R.; Alhilali, A.; Saleh, M.; Cheeseman, C. Hydrophobic concrete using waste paper sludge ash. Cem. Concr. Res. 2015, 70, 9–20. [Google Scholar] [CrossRef]
- Atla, S.; Huang, Y.; Yang, J.; Chen, H.; Kuo, Y.; Hsu, C.; Lee, W.; Chen, C.; Hsu, D.; Chen, C. Hydrophobic Calcium Carbonate for Cement Surface. Crystals 2017, 7, 371. [Google Scholar] [CrossRef] [Green Version]
- Jeong, Y.; Kang, S.; Kim, M.; Moon, J. Acceleration of cement hydration from supplementary cementitious materials: Performance comparison between silica fume and hydrophobic silica. Cem. Concr. Res. 2020, 112, 103688. [Google Scholar] [CrossRef]
- Liu, X.; Ma, B.; Tan, H.; Zhang, T.; Mei, J.; Qi, H.; Chen, P.; Wang, J. Effects of colloidal nano-SiO2 on the immobilization of chloride ions in cement-fly ash system. Cem. Concr. Res. 2020, 110, 103596. [Google Scholar] [CrossRef]
- Qu, Z.; Yu, Q. Synthesizing super-hydrophobic ground granulated blast furnace slag to enhance the transport property of lightweight aggregate concrete. Constr. Build. Mater. 2018, 191, 176–186. [Google Scholar] [CrossRef]
- Liu, P.; Feng, C.; Wang, F.; Gao, Y.; Yang, J.; Zhang, W.; Yang, L. Hydrophobic and water-resisting behavior of Portland cement incorporated by oleic acid modified fly ash. Mater. Struct. 2018, 51, 38. [Google Scholar] [CrossRef]
- Zhu, Y.; Kou, S.; Poon, C.; Dai, J.; Li, Q. Influence of silane-based water repellent on the durability properties of recycled aggregate concrete. Cem. Concr. Res. 2018, 35, 32–38. [Google Scholar] [CrossRef]
- Li, R.; Gao, Q.; Dong, Q.; Luo, C.; Sheng, L.; Liang, J. Template-free electrodeposition of ultra-high adhesive superhydrophobic Zn/Zn stearate coating with ordered hierarchical structure from deep eutectic solvent. Surf. Coat. Technol. 2020, 403, 126267. [Google Scholar] [CrossRef]
- Parsaie, A.; Khanaposhtani, M.; Riazi, M.; Tamsilian, Y. Magnesium stearate-coated superhydrophobic sponge for oil/water separation: Synthesis, properties, application. Sep. Purif. Technol. 2018, 251, 117105. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; Zhao, X.; Hu, X.; Yang, L.; Wang, N.; Li, Y.; Hou, B. Biomimetic one step fabrication of manganese stearate superhydrophobic surface as an efficient barrier against marine corrosion and Chlorella vulgaris-induced biofouling. Chem. Eng. J. 2016, 306, 441–451. [Google Scholar] [CrossRef]
- Liu, P.; Cao, L.; Zhao, W.; Xia, Y.; Huang, W.; Li, Z. Insights into the superhydrophobicity of metallic surfaces prepared by electrodeposition involving spontaneous adsorption of airborne hydrocarbons. Appl. Surf. Sci. 2015, 324, 576–583. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, Z.; Gao, Y.; Liu, C. Mechanical strength enhancement and mechanism of hardened cement paste incorporating ZIF-8. Mater. Lett. 2020, 268, 127582. [Google Scholar] [CrossRef]
- Nazer, A.; Payá, J.; Borrachero, M.; Monzó, J. Use of ancient copper slags in Portland cement and alkali activated cement matrices. J. Environ. Manag. 2016, 167, 115–123. [Google Scholar] [CrossRef]
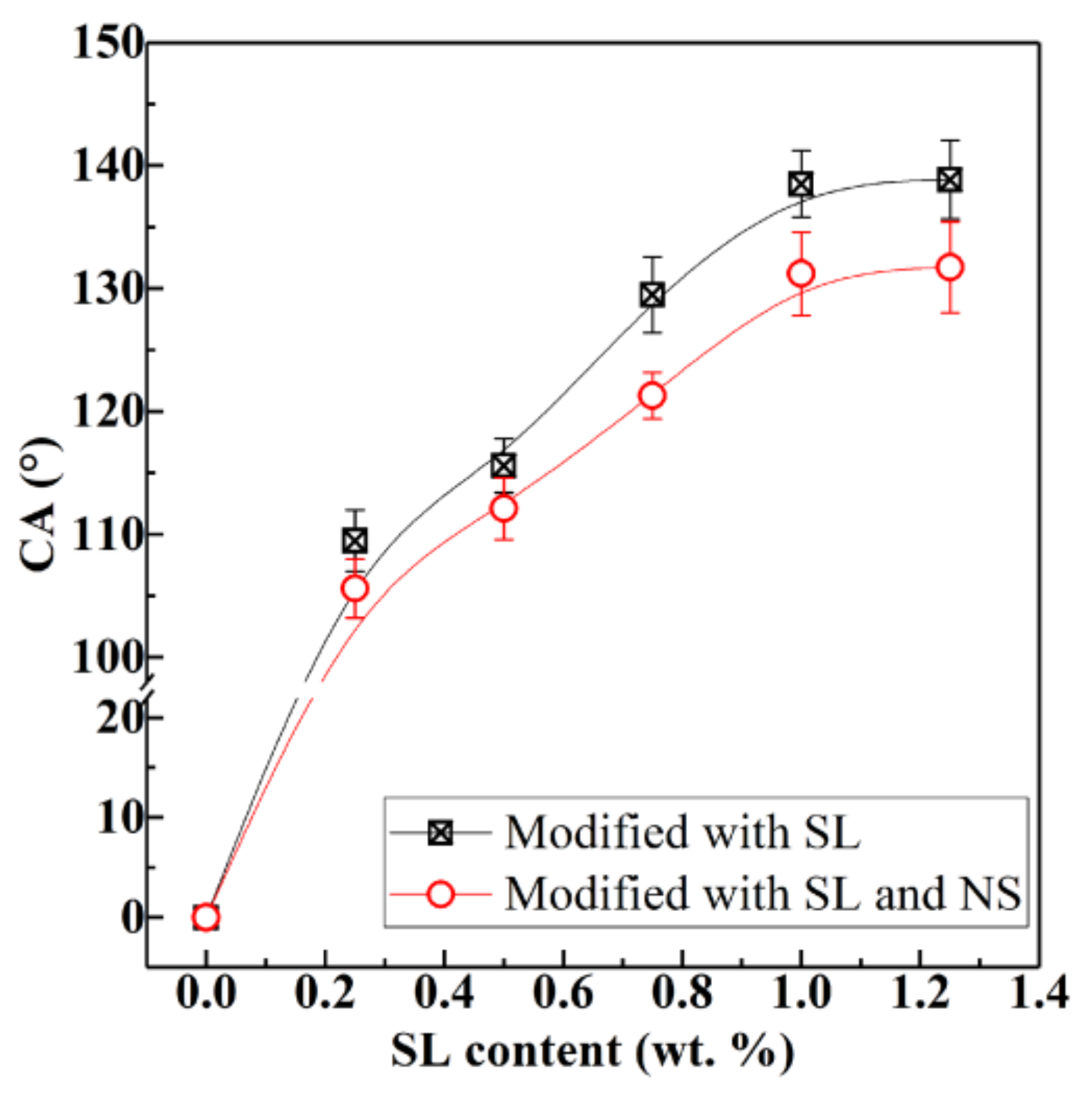
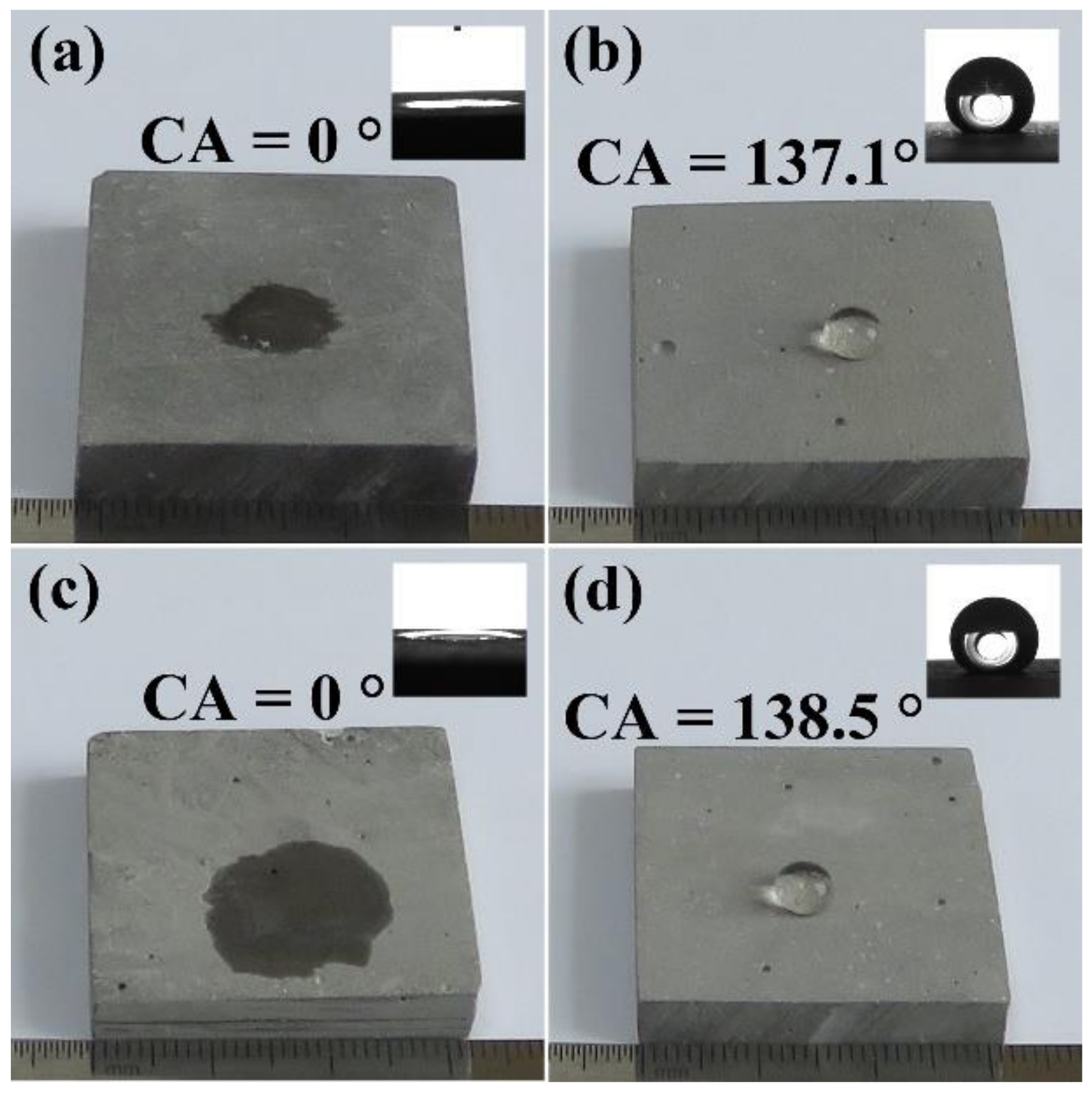

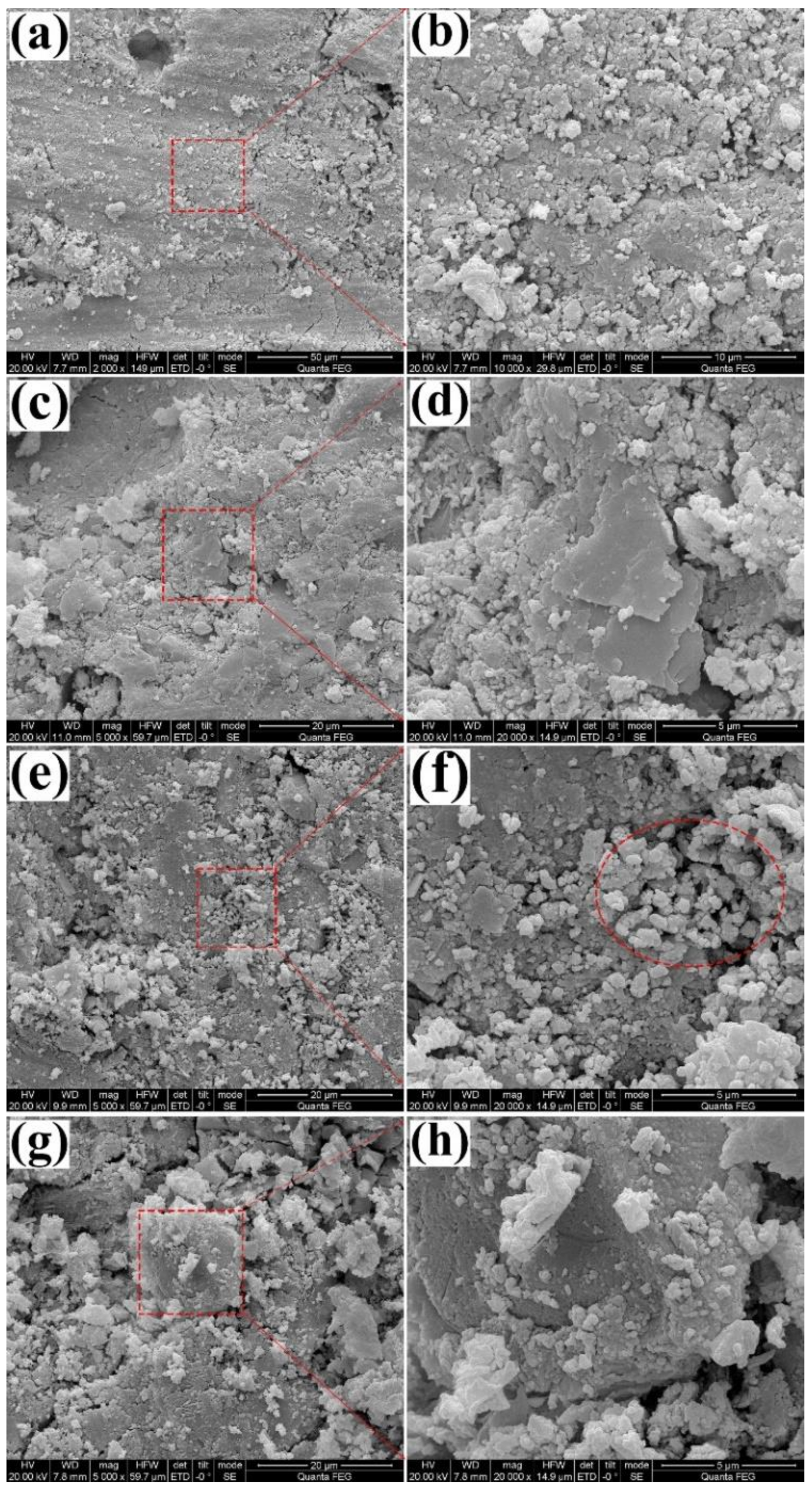


| Specimen | Mass (g) | Content (%) | Content (%) | CA (°) | |||
|---|---|---|---|---|---|---|---|
| Cement | Water | SL | NS | SL | NS | ||
| HCP | 450 | 180 | 0 | 0 | 0 | 0% | 0 |
| HCPSL0.25 | 448.875 | 180 | 1.125 | 0 | 0.25 | 0% | 109.5 |
| HCPSL0.5 | 447.75 | 180 | 2.25 | 0 | 0.5% | 0% | 115.6 |
| HCPSL0.75 | 446.625 | 180 | 3.375 | 0 | 0.75% | 0% | 129.5 |
| HCPSL1.0 | 445.5 | 180 | 4.5 | 0 | 1.0% | 0% | 138.5 |
| HCPSL1.25 | 444.375 | 180 | 5.625 | 0 | 1.25% | 0% | 141.7 |
| HCPNS0.5 | 447.75 | 180 | 0 | 2.25 | 0% | 0.5% | 0 |
| HCPNS1.0 | 445.5 | 180 | 0 | 4.5 | 0% | 1.0% | 0 |
| HCPNS1.5 | 443.25 | 180 | 0 | 6.75 | 0% | 1.5% | 0 |
| HCPSL0.25NS | 444.375 | 180 | 1.125 | 4.5 | 0.25% | 1.0% | 105.6 |
| HCPSL0.5NS | 443.25 | 180 | 2.25 | 4.5 | 0.5% | 1.0% | 112.1 |
| HCPSL0.75NS | 442.125 | 180 | 3.375 | 4.5 | 0.75% | 1.0% | 121.3 |
| HCPSL1.0 NS | 441 | 180 | 4.5 | 4.5 | 1.0% | 1.0% | 131.2 |
| HCPSL1.25NS | 439.875 | 180 | 5.625 | 4.5 | 1.25% | 1.0% | 136.7 |
| Samples | HCP | HCPSL1.0 | HCPNS1.0 | HCPSL1.0NS1.0 |
|---|---|---|---|---|
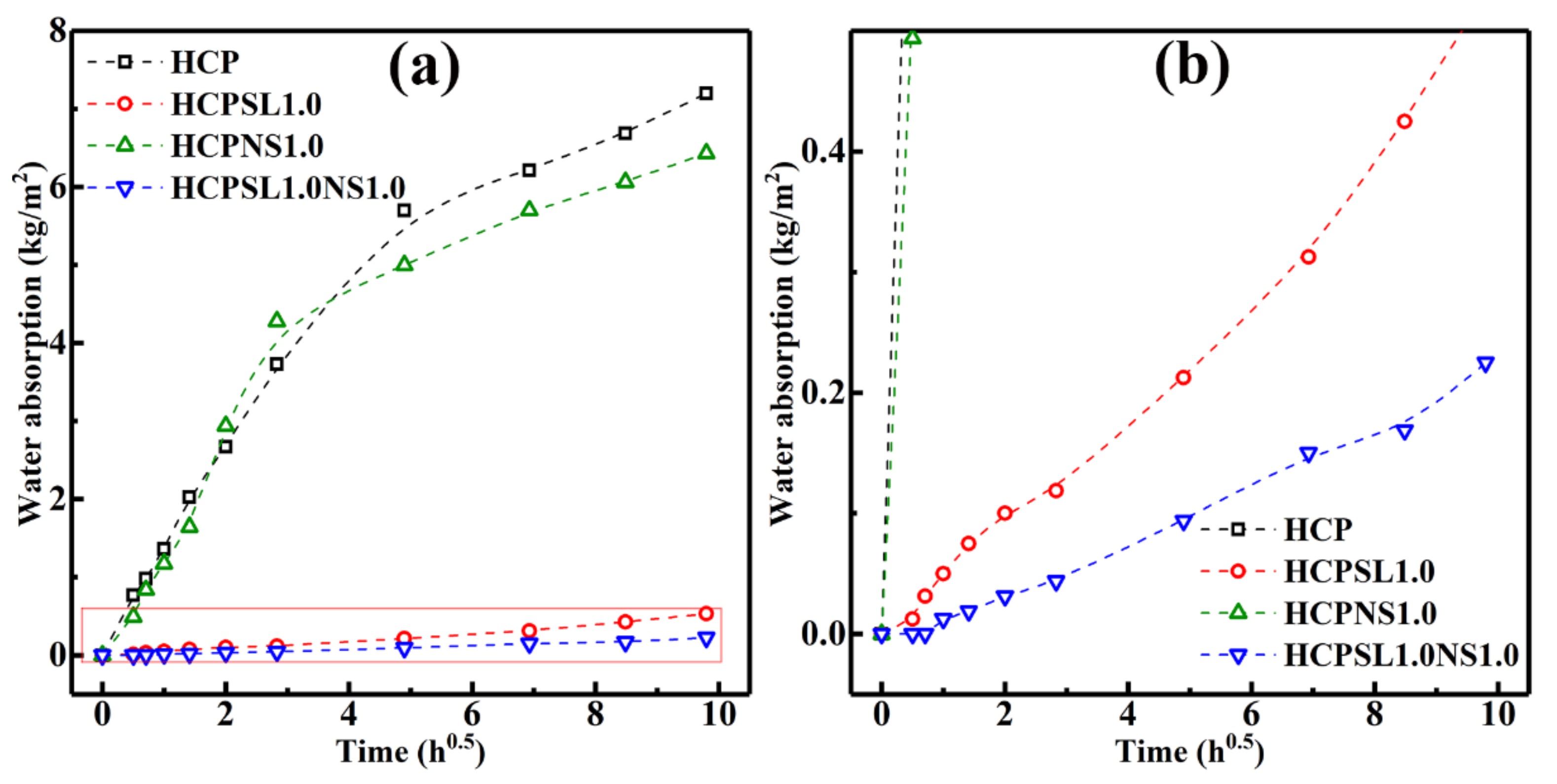
| Water absorption (%) | 9.93 | 0.75 | 8.60 | 0.36 |
| Capillary water adsorption coefficient (kg/m2·h0.5) | 0.6602 | 0.0513 | 0.7345 | 0.0228 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Lei, S. Permeabilities and Mechanical Properties of Hardened Cement Pastes Modified with Sodium Laurate and Nano Silica. Materials 2020, 13, 4867. https://doi.org/10.3390/ma13214867
Wang F, Lei S. Permeabilities and Mechanical Properties of Hardened Cement Pastes Modified with Sodium Laurate and Nano Silica. Materials. 2020; 13(21):4867. https://doi.org/10.3390/ma13214867
Chicago/Turabian StyleWang, Fajun, and Sheng Lei. 2020. "Permeabilities and Mechanical Properties of Hardened Cement Pastes Modified with Sodium Laurate and Nano Silica" Materials 13, no. 21: 4867. https://doi.org/10.3390/ma13214867
APA StyleWang, F., & Lei, S. (2020). Permeabilities and Mechanical Properties of Hardened Cement Pastes Modified with Sodium Laurate and Nano Silica. Materials, 13(21), 4867. https://doi.org/10.3390/ma13214867




