Magnesia (MgO) Production and Characterization, and Its Influence on the Performance of Cementitious Materials: A Review
Abstract
1. Introduction
2. Magnesia Production and Its Use in Cementitious Materials
2.1. Calcination of Magnesite
2.2. Calcination of Magnesium Hydroxide
2.3. Seawater and Brine
2.4. Extraction of Magnesia from Mg-Bearing Minerals
2.5. MgO in Cementitious Materials
3. Magnesia Characterization
3.1. Physical Properties
3.2. Reactivity
4. Characterization of Cementitious Materials with Additional MgO
4.1. Mechanical Properties
4.2. Durability Behaviour
4.3. Hydration Degree
4.4. Microscopic Analysis
5. Conclusions
- The compressive strength, flexural strength and tensile strength of cementitious materials decreased with the incorporation and increase in the MgO content, regardless of the material being added directly to the mix or to the cement clinker. This was mainly attributed to the porosity increment and lower hydration of the MgO mixes, when compared to conventional mixes without MgO. The reactivity of MgO showed insignificant influence on the strength of cementitious materials;
- The incorporation of MgO could lead to porosity decrease, when compared to that of conventional reference mixes, under accelerated carbonation. By contrast, porosity increased with the addition of MgO under ambient carbonation condition;
- The carbonation of concrete mixes produced with MgO tends to be higher than that of conventional concrete mixes. This trend becomes more evident with higher MgO incorporation levels;
- The chloride ion migration coefficient increased by incorporating MgO in mixes water-cured for 28 days and decreased in those water-cured for 360 days. This decrease was attributed to the reduced porosity with the addition of MgO in mixes cured for 360 days;
- The initial expansion of concrete mixes increased by increasing the MgO content;
- The shrinkage of cementitious materials decreased with the incorporation of MgO due to the compensation of the shrinkage by MgO hydration, during 1–5 days. The shrinkage of cementitious materials fell significantly by increasing the reactivity of MgO;
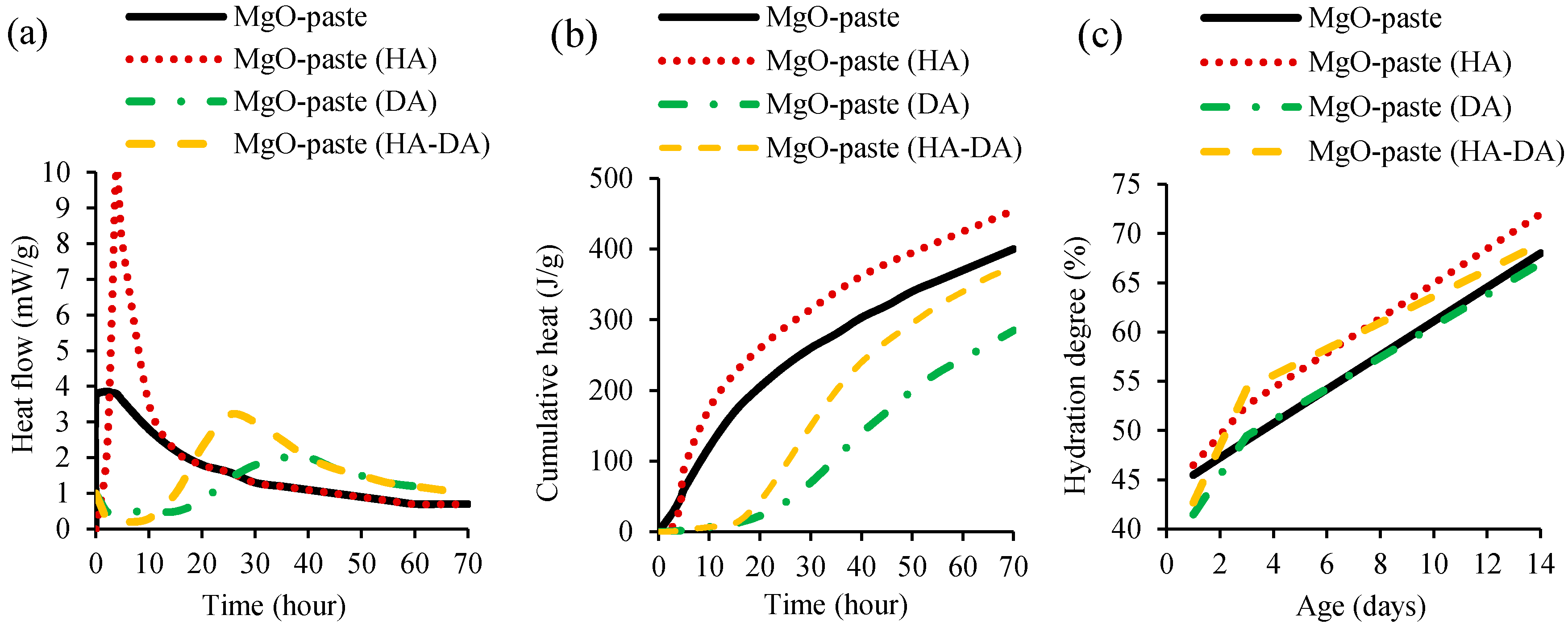
- The hydration degree of cementitious material mixes was not changed by the addition of MgO, during the first 7 h of mix production. This was attributed to the accelerated formation of C–S–H. However, the addition of MgO led to a decrease of maximum heat flow peak between 7–12 h and increase of heat flow between 12–48 h. The incorporation of hydration agent increased the MgO hydration, while the opposite effect was observed with the addition of dispersion agent, associated with the deflocculating effect of the latter;
- Microscopic analysis showed that cementitious materials produced with MgO may have had denser microstructure when compared to that of conventional reference mixes. This was attributed to MgO hydration products filling the pores and to the expansion effect of MgO.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Freedonia. World Construction Aggregates-Demand and Sales Forecasts, Market. Share, Market. Size, Market. Leaders; Industry study No. 3389 The Freedonia Group: Cleveland, OH, USA, 2016; p. 390. [Google Scholar]
- USGS. Commodity Statistics and Information Mineral. Yearbooks; USA Geological Survey: Washington, DC, USA, 2015.
- Marinković, S.; Radonjanin, V.; Malesev, M.; Ignjatovic, I. Comparative environmental assessment of natural and recycled aggregate concrete. Waste Manag. 2010, 30, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- de Schepper, M.; Heede, P.; Driessche, I.; de Belie, N. Life cycle assessment of completely recyclable concrete. Materials 2014, 7, 6010–6027. [Google Scholar] [CrossRef] [PubMed]
- Kurda, R.; de Brito, J.; Silvestre, J.D. Combined economic and mechanical performance optimization of recycled aggregate concrete with high volume of fly ash. Appl. Sci. 2018, 8, 1189. [Google Scholar] [CrossRef]
- Kurda, R.; de Brito, J.; Silvestre, J.D. Water absorption and electrical resistivity of concrete with recycled concrete aggregates and fly ash. Cem. Concr. Compos. 2019, 95, 169–182. [Google Scholar] [CrossRef]
- Berndt, M.L. Properties of sustainable concrete containing fly ash, slag and recycled concrete aggregate. Constr. Build. Mater. 2009, 23, 2606–2613. [Google Scholar] [CrossRef]
- Kou, S.C.; Poon, C.S.; Agrela, F. Comparisons of natural and recycled aggregate concretes prepared with the addition of different mineral admixtures. Cem. Concr. Compos. 2011, 33, 788–795. [Google Scholar] [CrossRef]
- Ferdous, W.; Manalo, A.; Wong, H.; Abousnina, R.; Ajarmeh, O.; Zhuge, Y.; Schubel, P. Optimal design for epoxy polymer concrete based on mechanical properties and durability aspects. Constr. Build. Mater. 2020, 232, 117–229. [Google Scholar] [CrossRef]
- Abousnina, R.; Manalo, A.; Ferdous, W.; Lokuge, W.; Benabed, B.; Al-Jabri, K. Characteristics, strength development and microstructure of cement mortar containing oil-contaminated sand. Constr. Build. Mater. 2020, 252, 119155. [Google Scholar] [CrossRef]
- Walling, S.; Provis, J. Magnesia-based cements: A journey of 150 years, and cements for the future? Chem. Rev. 2016, 116, 4170–4204. [Google Scholar] [CrossRef]
- Yuan, M.; Tang, M. Study on the mechanism of autogenous expansion of concrete used in Baishan Dam. J. Nanjing Inst. Chem. Technol. 1984, 2, 15–18. (In Chinese) [Google Scholar]
- Mehta, P. History and status of performance tests for evaluation of soundness of cements. In Cement Standards-Evolution and Trends, ASTM STP663-EB; American Society for Testing and Materials: Philadelphia, PA, USA, 1977; pp. 35–60. [Google Scholar]
- Du, C. A review of magnesium oxide in concrete. Concr. Int. 2005, 27, 45–50. [Google Scholar]
- Al-Tabbaa, A. Chapter 19: Reactive magnesia cement. In Eco-Efficient Concrete; Pacheco-Torgal, F., Jalali, S., Labrincha, J., John, V., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 523–543. [Google Scholar]
- Shand, M.A. The Chemistry Technology of Magnesia; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Taylor, F.H.W. Cement Chemistry; Academic Press: New York, NY, USA, 1990. [Google Scholar]
- Gregg, S.J.; Packer, R.K. The production of active solids by thermal decomposition. Part VI. The calcination of magnesium hydroxide. J. Chem. Soc. 1955, 51–55. [Google Scholar] [CrossRef]
- Ferrini, V.; De-Vito, C.; Mignardi, S. Synthesis of nesquehonite by reaction of gaseous CO2 with Mg chloride solution: Its potential role in the sequestration of carbon dioxide. J. Hazard. Mater. 2009, 168, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Hänchen, M.; Prigiobbe, V.B.R.; Mazzotti, M. Precipitation in the Mg-carbonate system-effects of temperature and CO2 pressure. Chem. Eng. Sci. 2008, 63, 1012–1028. [Google Scholar] [CrossRef]
- Back, M.; Bauer, M.; Stanjek, H.; Peiffer, S. Sequestration of CO2 after reaction with alkaline earth metal oxides CaO and MgO. Appl. Geochem. 2011, 26, 1097–1107. [Google Scholar] [CrossRef]
- Teir, S.; Eloneva, S.; Fogelholm, C.; Zevenhoven, R. Fixation of carbon dioxide by producing hydromagnesite from serpentinite. Appl. Energy 2009, 86, 214–218. [Google Scholar] [CrossRef]
- Zevenhoven, R.; Teir, S. Long term storage of CO2 as magnesium carbonate in Finland. In Proceedings of the 3rd Annual Conference on Carbon Capture and Sequestration, Alexandria, Egypt, 3–6 May 2004; pp. 1–11. [Google Scholar]
- Maroto-Valer, M.; Fauth, D.; Kuchta, M.; Zhang, Y.; Adresen, J. Activation of magnesium rich minerals as carbonation feedstock materials for CO2 sequestration. Fuel Process. Technol. 2005, 86, 1627–1645. [Google Scholar] [CrossRef]
- Mo, L.; Deng, M.; Tang, M.; Al-Tabbaa, A. MgO expansive cement and concrete in China: Past, present and future. Cem. Concr. Res. 2014, 57, 1–12. [Google Scholar] [CrossRef]
- Jin, F.; Al-Tabbaa, A. Characterisation of different commercial reactive magnesia. Adv. Cem. Res. 2013, 26, 101–113. [Google Scholar] [CrossRef]
- Francl, J.; Kingery, W.D. Thermal conductivity: IV, apparatus for determining thermal conductivity by a comparative method: Data for Pb, Al2O3, BeO, and MgO. J. Am. Ceram. Soc. 1954, 37, 80–84. [Google Scholar] [CrossRef]
- McQuarrie, M. Thermal conductivity: High-temperature method and results for alumina, magnesia, and beryllia from 1000 °C to 1800 °C. J. Am. Ceram. Soc. 1954, 37, 84–88. [Google Scholar] [CrossRef]
- Austin, J.B. The thermal expansion of some refractory oxides. J. Am. Ceram. Soc. 1931, 14, 795–810. [Google Scholar] [CrossRef]
- Ebert, H.; Tingwaldt, C. Expansion Measurements at temperatures up to 2000 °C. Physics 1936, 37, 471–474. [Google Scholar]
- Diepschlag, E.; Wulfesting, F. Electric conductivity of magnesia and some other refractory materials. Iron Steel Ind. 1929, 24, 32. [Google Scholar]
- Foëx, M. Electrical conductivity of beyllia and of magnesia at high temperatures. Comptes Rendus 1942, 214, 665–666. [Google Scholar]
- Liska, M.; Wilson, A.; Bensted, J. 13.4-MgO cements. In Lea’s Chemistry of Cement and Concrete, 5th ed.; Hewlett, P., Liska, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 609–620. [Google Scholar]
- Wenhua, S.; Chong, C.; Honghua, Z.; Kehao, C. Relationship between crystalline size and lattice distortion of MgO and its activity. J. Wuhan Univ. Technol. 1991, 13, 21–24. (In Chinese) [Google Scholar]
- Smithson, G.; Bakhshi, N. The kinetics and mechanism of the hydration of magnesium oxide in a batch reactor. Can. J. Chem. Eng. 1969, 47, 508–513. [Google Scholar] [CrossRef]
- Strydom, C.; Merwe, E.; Aphane, E. The effect of calcining conditions on the rehydration of dead burnt magnesium oxide using magnesium acetate as a hydrating agent. J. Therm. Anal. Calorim. 2005, 80, 659–662. [Google Scholar] [CrossRef]
- Mo, L.; Deng, M.; Tang, M. Effects of calcination condition on expansion property of MgO-type expansive agent used in cement-based materials. Cem. Concr. Res. 2010, 40, 437–446. [Google Scholar] [CrossRef]
- Wogelius, R.; Refson, K.; Fraser, D.; Grime, G.; Goff, J. Periclase surface. Geochim. Cosmochim. Acta 1995, 59, 1875–1881. [Google Scholar] [CrossRef]
- Liu, Z.; Cui, X.; Tang, M. MgO-type delayed expansive cement. Cem. Concr. Res. 1991, 21, 1049–1057. [Google Scholar]
- Abdalqader, A.F.; Al-Tabbaa, A. Mechanical and microstructural characterisation of multicomponent blended cements incorporating reactive magnesia. In Proceedings of the 1st Concrete Innovative Conference (CIC), Oslo, Norway, 11–13 June 2014; The Norwegian Concrete Association-Norsk Betongforening: Oslo, Norway, 2014; p. 93. Available online: https://www.researchgate.net/profile/Ahmed_Abdalqader/publication/269400461_MECHANICAL_AND_MICROSTRUCTURAL_CHARACTERISATION_OF_MULTICOMPONENT_BLENDED_CEMENTS_INCORPORATING_REACTIVE_MAGNESIA/links/558429dd08ae71f6ba8c3521/MECHANICAL-AND-MICROSTRUCTURAL-CHARACTERISATION-OF-MULTICOMPONENT-BLENDED-CEMENTS-INCORPORATING-REACTIVE-MAGNESIA.pdf (accessed on 21 January 2020).
- Mo, L.; Zhang, F.; Deng, M. Effects of carbonation treatment on the properties of hydrated fly ash-MgO-Portland cement blends. Constr. Build. Mater. 2015, 96, 147–154. [Google Scholar] [CrossRef]
- Mo, L.; Liu, M.; Al-Tabbaa, A.; Deng, M.; Lau, W.Y. Deformation and mechanical properties of quaternary blended cements containing ground granulated blast furnace slag, fly ash and magnesia. Cem. Concr. Res. 2015, 71, 7–13. [Google Scholar] [CrossRef]
- Mo, L.; Liu, M.; Al-Tabbaa, A.; Deng, M. Deformation and mechanical properties of the expansive cements produced by inter-grinding cement clinker and MgOs with various reactivities. Constr. Build. Mater. 2015, 80, 1–8. [Google Scholar] [CrossRef]
- Choi, S.; Jang, B.; Kim, J.; Lee, K. Durability characteristics of fly ash concrete containing lightly-burnt MgO. Constr. Build. Mater. 2014, 58, 77–84. [Google Scholar] [CrossRef]
- Mavroulidou, M.; Morrison, T.; Unsworth, C.; Gunn, M. Properties of concrete made of multicomponent mixes of low-energy demanding binders. Constr. Build. Mater. 2015, 101, 1122–1141. [Google Scholar] [CrossRef]
- Gao, P.-W.; Wu, S.-X.; Lu, X.-L.; Deng, M.; Lin, P.-H.; Wu, Z.-R.; Tang, M.-S. Soundness evaluation of concrete with MgO. Constr. Build. Mater. 2007, 21, 132–138. [Google Scholar] [CrossRef]
- Moradpour, R.; Taheri-Nassaj, E.; Parhizkar, T.; Ghodsian, M. The effects of nanoscale expansive agents on the mechanical properties of non-shrink cement-based composites: The influence of nano-MgO addition. Compos. Part. B 2013, 5, 193–202. [Google Scholar] [CrossRef]
- Pu, L.; Unluer, C. Investigation of carbonation depth and its influence on the performance and microstructure of MgO cement and PC mixes. Constr. Build. Mater. 2016, 120, 349–363. [Google Scholar] [CrossRef]
- Jin, F.; Gu, K.; Al-Tabbaa, A. Strength and drying shrinkage of reactive MgO modified alkali-activated slag paste. Constr. Build. Mater. 2014, 51, 395–404. [Google Scholar] [CrossRef]
- Wei, J.; Chen, Y.; Li, Y. The reaction mechanism between MgO and microsilica at room temperature. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2006, 21, 88–91. [Google Scholar]
- Dung, N.; Unluer, C. Improving the performance of reactive MgO cement-based concrete. Constr. Build. Mater. 2016, 126, 747–758. [Google Scholar] [CrossRef]
- Dung, N.; Unluer, C. Sequestration of CO2 in reactive MgO cement-based mixes with enhanced hydration mechanisms. Constr. Build. Mater. 2017, 143, 71–82. [Google Scholar] [CrossRef]
- Unluer, C.; Al-Tabbaa, A. Impact of hydrated magnesium carbonate additives on the carbonation of reactive MgO cements. Cem. Concr. Res. 2013, 54, 87–97. [Google Scholar] [CrossRef]
- Unluer, C.; Al-Tabbaa, A. Enhancing the carbonation of MgO cement porous blocks through improved curing conditions. Cem. Concr. Res. 2014, 59, 55–65. [Google Scholar] [CrossRef]
- Gonçalves, T.; Silva, R.V.; de Brito, J.; Fernández, J.; Esquinas, A. Mechanical and durability performance of mortars with fine recycled concrete aggregates and reactive magnesium oxide as partial cement replacement. Cem. Concr. Compos. 2020, 105, 103420. [Google Scholar] [CrossRef]
- Mo, L.; Panesar, D.K. Effects of accelerated carbonation on the microstructure of Portland cement pastes containing reactive MgO. Cem. Concr. Res. 2012, 42, 769–777. [Google Scholar] [CrossRef]
- Mo, L.; Deng, Y.; Lu, A.; Deng, M. Preparation of MgO- and CaO-bearing expansive agent used for cement-based materials. Key Eng. Mater. 2013, 539, 211–214. [Google Scholar] [CrossRef]
- Gao, P.; Xu, S.; Chen, X.; Li, J.; Lu, X. Research on autogenous volume deformation of concrete with MgO. Constr. Build. Mater. 2013, 40, 998–1001. [Google Scholar] [CrossRef]
- Kabir, H.; Hooton, R. Evaluating soundness of concrete containing shrinkage-compensating MgO admixtures. Constr. Build. Mater. 2020, 253, 119141. [Google Scholar] [CrossRef]
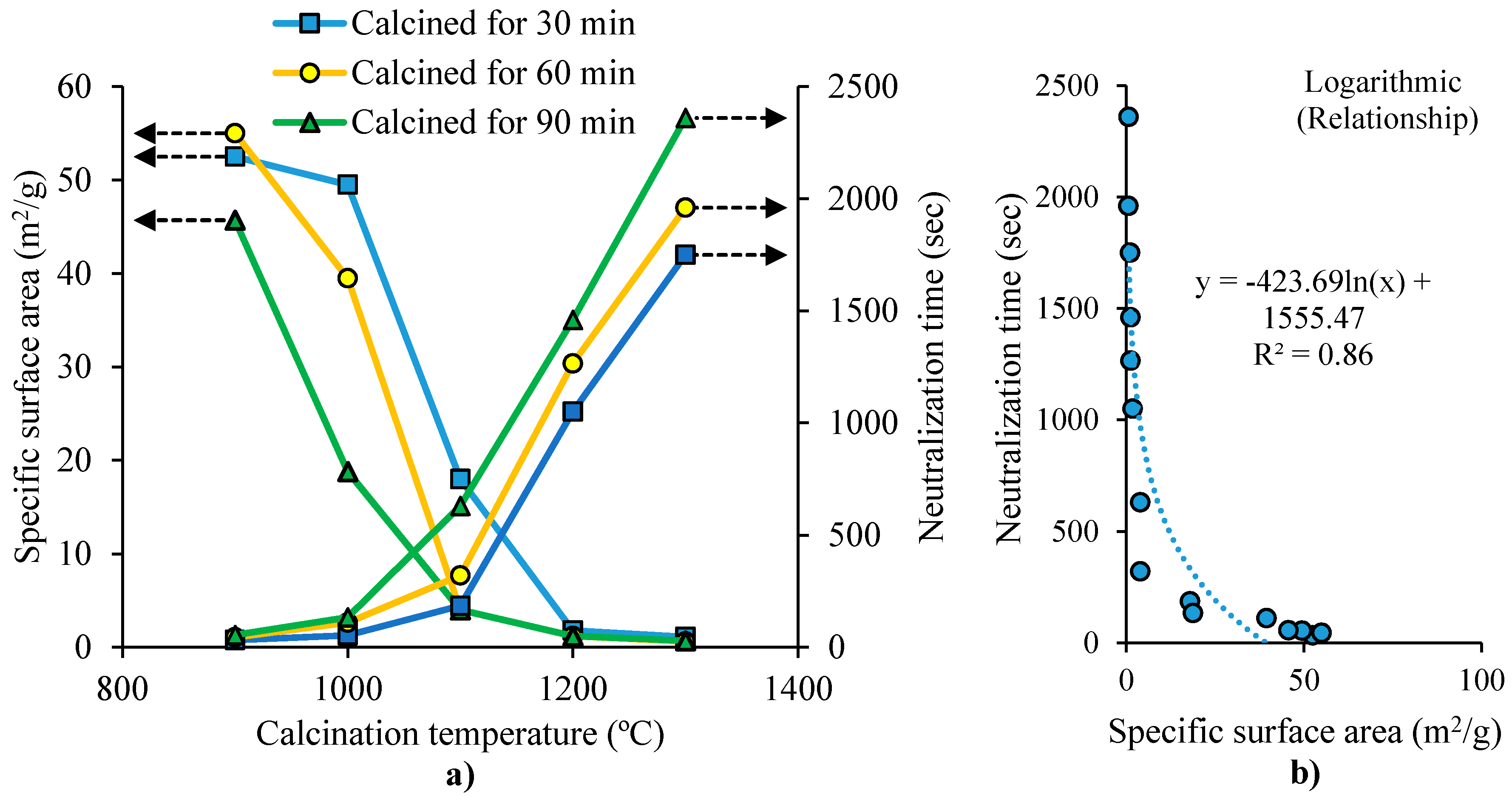
| Temperature (°C) | Thermal Conductivity of Polycrystalline Sintered Magnesia (cal s−1cm−2°C−1cm) [27] | Thermal Conductivity of Sintered Magnesia at High Temperatures (cal s−1cm−2°C−1cm) [28] | Average Thermal Expansion Coefficient (L) of Single-Crystal Periclase (×10−6/°C) [29] | Thermal Expansion Coefficient (L) of High-Purity Sintered Magnesia (×10−6/°C) [30] | Specific Heat Capacity (J·K−1 mol−1) [16] | Specific Electrical Resistance of Sintered Magnesia (×103 Ω) [16,31] | Specific Electric Resistance of High-Purity Magnesia (Ω) [32] |
|---|---|---|---|---|---|---|---|
| 0 | 0.1 | - | - | - | - | - | - |
| 25 | - | - | - | - | 37.1 | - | - |
| 50 | - | - | 6.7 | - | - | - | - |
| 100 | 0.083 | - | 9.1 | - | - | - | - |
| 300 | 0.067 | - | 11.6 | 12.0 | - | - | - |
| 327 | - | - | - | - | 47.4 | - | - |
| 500 | 0.031 | - | - | 12.6 | - | - | - |
| 600 | 0.026 | - | 13.0 | - | - | - | - |
| 700 | - | - | 13.2 | 13.2 | - | - | - |
| 727 | - | - | - | - | 51.2 | - | - |
| 800 | - | - | 13.5 | - | - | - | - |
| 900 | - | - | 13.7 | 13.7 | - | - | 9 × 107 |
| 950 | - | - | - | - | - | 120 | - |
| 1000 | - | 0.1 | 13.8 | - | - | 95 | - |
| 1100 | - | - | - | 14.7 | - | - | - |
| 1200 | - | 0.083 | - | - | - | - | - |
| 1300 | - | - | - | 14.5 | - | 9 | 2.5 × 105 |
| 1400 | - | 0.067 | - | - | - | - | - |
| 1500 | - | - | - | 15.0 | - | 1.5 | - |
| 1527 | - | - | - | - | 54.9 | - | - |
| 1600 | - | 0.031 | - | - | - | - | - |
| 1700 | - | 0.026 | - | 15.6 | - | - | 5.5 × 103 |
| 1800 | - | - | - | 16.0 | - | - | - |
| 2100 | - | - | - | - | - | - | 4.4 × 102 |
| 2527 | - | - | - | - | 58.5 | - | - |
| 3327 | - | - | - | - | 61.2 | - | - |
| Sample | Crystal Grain Size (nm) | Lattice Distortion (%) | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Width (nm) | Density (g/cm3) | Hydration Degree | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 2d | 7d | 15d | 30d | |||||||
| Calc. Temp. 900-Res. Time 30 | 21.2 | 0.1648 | 52.7 | 0.193 | 14.62 | 2.97 | - | - | - | - |
| Calc. Temp. 900-Res. Time 60 | 29.3 | 0.0560 | 55.2 | 0.223 | 16.10 | 3.02 | 97.1 | 100 | 100 | 100 |
| Calc. Temp. 900-Res. Time 90 | 36.8 | 0.0350 | 45.9 | 0.179 | 15.57 | 3.12 | - | - | - | - |
| Calc. Temp. 1000-Res. Time 30 | 30.4 | 0.0208 | 49.8 | 0.235 | 18.84 | 3.13 | - | - | - | - |
| Calc. Temp. 1000-Res. Time 60 | 43.2 | 0.0149 | 39.8 | 0.235 | 23.61 | 3.26 | - | - | - | - |
| Calc. Temp. 1000-Res. Time 90 | 66.3 | 0.0092 | 18.9 | 0.103 | 25.12 | 3.32 | - | - | - | - |
| Calc. Temp. 1100-Res. Time 60 | >100 | - | 4.2 | 0.024 | 22.46 | 3.43 | 4.5 | 9.9 | 20.9 | 82.6 |
| Calc. Temp. 1300-Res. Time 60 | - | - | - | - | - | - | - | 1.0 | 1.9 | 7.9 |
| Test | Reference | Mix | Binder (%) | w/b | MgO | Age (days) | Results | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OPC | FA | S | MS | MK | (%) | Properties | ||||||
| Compressive strength | Liu et al. [39] | P | 100 | - | - | - | - | 0.265 | 1–5 | - | 3–28 | The strength of pastes with MgO reduced up to 15% with the increase of age and of MgO content, compared to the reference pastes (ordinary Portland cement). |
| Jin et al. [49] | P | - | - | 100 | - | - | 0.31 | 2.5–7.5 | Reactivity 10–220 s | 1–90 | The strength of alkali-activated slag cement increased with increasing the amount of high reactivity MgO. Unclear trend was observed with increasing the amount of medium reactivity MgO, at different ages. However, among different amounts of medium reactivity MgO, mixes containing 2.5% obtained maximum increases at 7–90 days, when compared to the strength of control mix. | |
| - | Abdalqader and Al-Tabbaa [40] | P | 25–75 | 15–25 | 45–60 | - | - | 0.31 | 5–10 | Reactivity 170 s | 3–56 | The average strength of mixes containing only FA was higher than those containing FA, slag, and MgO, at all ages. Thus, maximum strength was registered in mixes with 25% FA and 75% OPC, followed by those containing 30% OPC, 60% slag, and 10% MgO. |
| - | Mo et al. [41] | P | 10–60 | 20–40 | - | - | - | 0.4 | 20–60 | Calcination 800 °C | 28 | Cement pastes were exposed to different CO2 periods and CO2 pressures (0.55 MPa for 3 and 15 h, and 0.10 MPa for 1 and 14 days). In mixes without CO2 exposure, the strength decreased with increasing the amount of FA and MgO. Exposing cement pastes to 0.55 MPa CO2 for 3 h led to 7–72% strength increase. In that case, higher replacement of cement caused higher strength increments with CO2 exposure. The strength of mixes exposed to 0.55 MPa CO2 for 3 h was higher than that of mixes under 0.10 MPa for 1 day. In general, the strength of cement pastes increased with increasing CO2 pressure or elongating the exposure time. |
| - | Mo et al. [42] | M | 100 | - | - | - | - | 0.5 | 5–8 | Reactivity 50 s | 3–90 | Reduction of strength occurred with increasing the amount of MgO at all ages, when compared to that of reference mortars. This was attributed to the lower C-S-H formation due to cement weight reduction and replacing it by MgO. |
| - | - | - | 25–100 | 20–35 | 20–40 | - | - | 0.5 | 5–8 | Reactivity 50 s | 3–90 | At 3 days, the incorporation of slag and FA significantly decreased the strength of reference and MgO mixes. This difference decreased at 28 and 90 days. Comparing the strength of the same mixes at 90 days to 3 days, the strength development of 8% MgO mixes with FA and slag (157–313%) were higher than that of 8% MgO-mortars without FA and slag (47%). This was attributed to pozzolanic or hydraulic reaction of FA and slag. Higher FA amount decreased the strength while higher slag increased it. |
| - | Mo et al. [43] | M | 100 | - | - | - | - | 0.5 | 8 | Reactivity 50–400 s | 3–90 | The incorporation of four MgO types (reactivity values: 50, 100, 200, 400) caused slight strength reduction of cement mortar, particularly at late age, when compared to that of conventional reference mortar. The reactivity of MgO showed insignificant influence on the strength of cement mortars. |
| - | - | - | 40–100 | 20 | 40 | - | - | 0.5 | 8 | Reactivity 50–400 s | 3–90 | The strength of reference and MgO-mortars significantly decreased with the addition of FA and slag at 3 days. This difference highly decreased at 28 days. At 90 days, the strength of MgO-mortars with FA and slag was slightly higher than that of reference and MgO-mortars without FA and slag. |
| - | Moradpour et al. [47] | M | 100 | - | - | - | - | 0.5 | 1–5 | - | 7–90 | The strength increased in mortars with nano-MgO, regardless of curing age and MgO content, when compared to that of conventional reference mortars. In fact, MgO mortars had 1.1–2.0 times higher strength. This trend was more obvious with lower MgO content. |
| - | Wei et al. [50] | M | - | - | - | 10–40 | - | 0.5 | 60–90 | - | 3–28 | Mortars were produced without OPC and with different amounts of microsilica and MgO. Maximum strength was obtained in mixes containing 30% microsilica and 70% MgO, at 28 days. This confirmed that full replacement of cement by microsilica and MgO can lead to production of mixes with the same strength as conventional mortars. |
| - | Dung and Unluer [51] | C | 100 | - | - | - | - | 0.5–0.6 | - | - | 3–28 | The incorporation of HCI (hydration agent-HA) and NaHMP (dispersion agent, DA) agents in concrete mixes containing MgO-based cement increased compressive strength up to 50% when compared to that of control mix. This trend was more pronounced in mixes containing only DA, followed by those with HA and DA, and only HA. |
| - | Dung and Unluer [52] | C | 100 | 0.6 | - | - | 3–28 | The incorporation of different HA types (magnesium acetate, magnesium chloride, hydrochloric acid) and HA concentrations (0.05–0.1 M) into MgO concrete enabled strength development increments up to 107% and 53%, when compared to that of the corresponding MgO-concrete mixes (without HA) and conventional concrete mixes, respectively. This trend was more obvious under accelerated curing conditions than under ambient conditions. | ||||
| - | Pu and Unluer [48] | C | 100 | - | - | - | - | 0.38–0.80 | 5–10 | - | 1–28 | The strength of concrete blocks made with 90–95% aggregates and 5–10% MgO or OPC was investigated under carbonation. Maximum strength was obtained in concrete with 10% MgO, followed by those with both MgO (5%) and OPC (5%). The strength of concrete containing 10% MgO was 83% higher than that of concrete with 10% OPC after 7 days. |
| - | Choi et al. [44] | C | 80 | 20 | - | - | - | 0.48–0.65 | 5 | Calcination 1000 ºC | 7–540 | The addition of 5% MgO into concrete mixes produced with 20% FA and different w/b (0.48, 0.65) led to strength decrease (4–13%) at 7 and 28 days. This was attributed to the slow MgO hydration up to 28 days tested in MgO-mortars with X-ray analysis. At 56–540 days, the strength of MgO-concrete mixes became similar to that of the reference mixes. |
| - | Mavroulidou et al. [45] | C | 40–80 | 20–25 | - | - | 15-25 | 0.55 | 5–10 | Reactivity 976 s | 28 | Strength of concrete mixes produced with different amounts of FA and metakaolin slightly decreased with increasing the amount of MgO. This was attributed to MgO’s contribution to porosity increase and production of low strength magnesium silicate hydrates, and lack of reaction between FA and brucite to formulate M-S-H gel, and between cement and MgO to formulate more products. |
| - | Unluer and Al-Tabbaa [53] | C | - | 5 | - | - | - | 0.67–1.44 | 10 | - | 7 | Cement was produced with 5–10% of MgO and 0–5% of heavy or light hydrated magnesium carbonates. Concrete blocks were produced with 10% cement and 5% FA and cured under ambient and accelerated carbonation. For mixes under accelerated carbonation, only concrete containing 8% MgO and 2% heavy hydrated magnesium carbonates obtained higher strength that that of the reference mix, containing 10% MgO. This was attributed to the lower water-demand and denser microstructure of heavy hydrated magnesium carbonates. Under natural curing conditions, reference concrete containing only 10% MgO obtained the maximum strength. The strength decreased with incorporation of hydrated magnesium carbonates, due to absence of sufficient CO2. |
| - | Unluer and Al-Tabbaa [54] | C | - | 5 | - | - | - | 0.6–0.9 | 10 | - | 1–7 | Under accelerated carbonation (10% CO2), MgO-concrete blocks with FA obtained strength values about 2 times higher than those of MgO mixes without FA. This was attributed to the higher porosity in FA mixes, leaving more space for hydrated magnesium carbonate formulation and strength development. Mixes with w/b 0.6 and 0.9 presented poor strengths due to low compaction and presence of saturated pores (preventing CO2 transportation and hydrated magnesium carbonate formulation), respectively. Maximum strength was obtained in MgO mixes with w/b 0.7, with and without FA. The strength of mixes gradually increased with increasing CO2 concentration from 0% to 20%. The strength development from 1 day to 7 days decreased with increasing CO2 concentration. |
| - | Gao et al. [46] | C | 100 | - | - | - | - | 0.48 | 4–12 | Calcination 1150 °C | 3 | Strength of concrete increased with increasing the autoclave time and decreased with increasing the autoclave temperature. Maximum strength was obtained in mixes with 4% MgO, followed by 8% and 12%. |
| - | - | - | 70–100 | 30–50 | - | - | - | 0.48 | 4–12 | Calcination 1150 °C | 3 | Strength of FA-concrete mixes decreased with increasing MgO content and increased with increasing autoclave time and temperature. |
| - | Gonçalves et al. [55] | M | 100 | - | - | - | - | 0.50 | 0–20 | Calcination 800 °C | 28 | Replacement of cement with 20% of MgO led to a decrease of the compressive strength (28%). |
| Flexural strength | Mo et al. [42] | M | 100 | - | - | - | - | 0.5 | 5–8 | Reactivity 50 s | 3–90 | Slight reduction of flexural strength with increasing the amount of MgO at all ages, when compared to that of reference mortars. |
| - | - | 25–100 | 20–35 | 20–40 | - | - | 0.5 | 5–8 | Reactivity 50 s | 3–90 | At 3 days, the incorporation of slag and FA significantly decreased the strength of reference and MgO mixes. At 28 and 90 days, the strength of MgO-mortars with FA and slag became similar or even higher than that of reference or MgO mix without FA and slag. | |
| - | Mo et al. [43] | M | 100 | - | - | - | - | 0.5 | 8 | Reactivity 50–400 s | 3–90 | The incorporation of four MgO types (reactivity values: 50, 100, 200, 400) had insignificant influence on the strength of mortars, regardless of MgO reactivity level and mortar curing age. |
| - | - | - | 40–100 | 20 | 40 | - | - | 0.5 | 8 | Reactivity 50–400 s | 3–90 | The strength of reference and MgO-mortars significantly decreased with the addition of FA and slag at 3 days. This difference highly decreased at 28 days. At 90 days, the strength of MgO-mortars with FA and slag was slightly higher than that of reference and MgO-mortars without FA and slag |
| - | Moradpour et al. [47] | M | 100 | - | - | - | - | 0.5 | 1–5 | - | 7–90 | The strength increased in mortars with nano-MgO, regardless of curing age and MgO content, when compared to that of conventional reference mortars. This trend was more obvious with lower MgO content. |
| - | Wei et al. [50] | M | - | - | - | 10–40 | - | 0.5 | 60–90 | - | 3–28 | Mortars were produced without OPC and with different amounts of microsilica and MgO. Maximum strength was obtained in mixes containing 30% microsilica and 70% MgO, at 3 and 28 days. This confirmed that full replacement of cement by microsilica and MgO can lead to production of mixes with the same strength as conventional mortars. |
| - | Mavroulidou et al. [45] | C | 50–80 | 20–25 | - | - | 15 | 0.55 | 5–10 | - | 28 | Strength of concrete mixes produced with metakaolin and different amounts of FA decreased or remained similar with increasing the amount of MgO. |
| - | Gao et al. [46] | C | 100 | - | - | - | - | 0.48 | 4–12 | Calcination 1150 °C | 3 | Strength of concrete increased with increasing the autoclave time and decreased with increasing the autoclave temperature. Maximum strength was obtained in mixes with 4% MgO, followed by 8% and 12%. |
| - | - | - | 70–100 | 30–50 | - | - | - | 0.48 | 4–12 | Calcination 1150 °C | 3 | Strength of FA-concrete mixes decreased with increasing MgO content and increased with increasing autoclave time and temperature. |
| - | Gonçalves et al. [55] | M | 100 | - | - | - | - | 0.50 | 0–20 | Calcination 800 °C | 28 | Flexural strength decreases (between 27% and 30%) can be observed with increasing replacement of cement with MgO, up to 20%. |
| Tensile strength | Mavroulidou et al. [45] | C | 40–80 | 20–50 | - | - | 10-30 | 0.55 | 5-10 | - | 28 | Strength of concrete mixes produced with different amounts of FA and metakaolin slightly decreased with increasing the amount of MgO. |
| Elastic modules | Choi et al. [44] | C | 80 | 20 | - | - | - | 0.48–0.65 | 5 | Calcination 1000 °C | 28–360 | Concrete mixes were produced with 20% FA, and different w/b (0.48, 0.65), and water cured for 28 and 360 days. The addition of MgO had almost no influence on the elastic modules of concrete mixes after 100, 200, and 300 cycles of freeze-thaw. |
| - | Gonçalves et al. [55] | M | 100 | - | - | - | - | 0.50 | 0–20 | Calcination 800 °C | 28 | There was a slight decrease (between 9% and 15%) in the mortars’ modulus of elasticity with 20% of MgO. This decrease is most likely due to the need to add more water to obtain equivalent workability levels. |
| Micro hardness | Mo and Panesar [56] | P | 100 | - | - | - | - | 0.5 | 10–40 | Calcination 800 °C | 7–56 | The microhardness of MgO-cement pastes was 25–52% higher than that of reference mixes, under carbonated condition. Exception occurred with cement paste having 40% MgO, tested at 7 days, obtaining 16% lower microhardness. Under non-carbonated condition, all MgO-cement pastes had similar or lower (6–36%) microhardness than that of reference mixes. |
| Test | Reference | Mix | Binder (%) | w/b | MgO | Age (days) | Results | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OPC | FA | S | MK | (%) | Properties | ||||||
| Water permeability | Moradpour et al. [47] | M | 100 | - | - | - | 0.5 | 1–5 | - | 28 | The permeability decreased by 7–33% in mortars with nano-MgO, when compared to that of conventional reference mortars. This trend was more obvious in mortars with small MgO contents. |
| - | Dung and Unluer [51] | C | 100 | - | - | - | 0.5–0.6 | - | - | 14–28 | The incorporation of HCI (hydration agent-HA) and NaHMP (dispersion agent-DA) agents in concrete mixes containing MgO-based cement decreased water permeability by 2–42% when compared to that of the control mix. This trend was more pronounced in mixes containing HA and DA under ambient curing and in mixes with only DA under accelerated curing conditions. |
| - | Dung and Unluer [52] | C | 100 | - | - | - | 0.6 | - | - | 14–28 | The incorporation of different HA types (Magnesium acetate, magnesium chloride, hydrochloric acid) and HA concentrations (0.05–0.1 M) into MgO–concrete reduced the permeability by 2–74% when compared to that of conventional concrete mixes. This trend was more obvious under accelerated curing conditions than under ambient conditions. |
| Water absorption | Mavroulidou et al. [45] | C | 45–80 | 20–25 | - | 15–25 | 0.55 | 5–10 | Reactivity 976 s | 28 | Absorption of concrete mixes produced with different amounts of FA and metakaolin decreased with the addition of 5% MgO. The water absorption of 10% MgO–concrete mixes was higher than that of those with 5% MgO, but still lower than reference FA–metakaolin–concrete mixes. This was attributed to the lower compaction of mixes with higher MgO and metakaolin contents, caused by higher water demand. |
| Porosity | Mo and Panesar [56] | P | 100 | - | - | - | 0.5 | 10–40 | Calcination 800 °C | 7–56 | The total pore volume of cement pastes made with MgO was up to 32% lower and 10% higher than that of the reference paste, under carbonation and non-carbonation conditions, respectively. |
| - | Mo et al. [43] | P | 10–60 | 20–40 | - | - | 0.4 | 20–60 | Calcination 800 °C | 28 | Cement pastes were exposed to different CO2 periods and CO2 pressures (0.55 MPa for 3 and 15 h, and 0.10 MPa for 1 and 14 days). In mixes without CO2 exposure, the coarse pores and the total pore volume increased with increasing FA and MgO. Longer CO2 exposure and higher pressure decreased the porosity of all mixes. The CO2 exposure decreased fine pores (<0.2 μm) in mixes with higher FA and MgO content and coarser pores (0.2–2.0 μm) in mixes with higher cement content. |
| - | Liu et al. [39] | M | 100 | - | - | - | 0.5 | 1–5 | - | 28 | The total porosity of mortars with MgO was up to 19% higher than that of reference mix. Higher MgO content led to higher porosity in both 2-dimensionally restrained and unrestrained samples. |
| - | Mo et al. [42] | M | 100 | - | - | - | 0.5 | 8 | Reactivity 50 s | 3–90 | MgO-mortar had lower derivative porosity (size range 0.05–0.2 μm) and cumulative porosity than those of reference mix. |
| - | - | - | 25–100 | 20–35 | 20–40 | - | 0.5 | 8 | Reactivity 50 s | 3–90 | At 28 days, maximum porosity was obtained in reference mortar and mix with only MgO, at pore range of 0.02–0.2 μm. Taking into account pores smaller than 0.02 μm, MgO-mortars with 20–40% slag and 20–35% FA had higher porosity than that of reference mortar and mix with only MgO. |
| - | Pu and Unluer [48] | C | 100 | - | - | - | 0.42–0.80 | 5–10 | - | 1–14 | The porosity of concrete blocks made with 90–95% aggregates and 5–10% MgO or OPC was investigated under carbonation. At 1 day of age, maximum porosity was obtained in concrete with 10% MgO, followed by those with 5% MgO, 10% OPC, and both MgO (5%) and OPC (5%). At 14 days, maximum porosity decrements were obtained in concrete mixes containing 10% MgO or 10% OPC, when compared to those of the same mixes at 7 days. |
| - | Choi et al. [44] | C | 80 | 20 | - | - | 0.48–0.65 | 5 | Calcination 1000 °C | 28–360 | The addition of 5% MgO into concrete mixes produced with 20% FA and w/b of 0.65 led to higher total porosity at 28 days and lower at 360 days. This was attributed to the filler effect of MgO hydration products at longer ages. The addition of MgO decreased pores with 0.03–0.3 μm and 0.6–2.0 μm, and increased those with 0.01–0.03 μm and 0.3–0.6 μm, when compared to FA-concrete without MgO. MgO had similar influence in mixes with w/b of 0.48 and 0.65. |
| - | Unluer and Al-Tabbaa [53] | C | - | 5 | - | - | 0.67–1.44 | 10 | - | 7 | Cement was produced with 5–10% of MgO and 0–5% of heavy or light-hydrated magnesium carbonates. Concrete blocks were produced with 10% cement and 5% FA and cured under ambient and accelerated carbonation. Maximum porosity decreased after the carbonation exposure was obtained in concrete containing 8% MgO and 2% heavy hydrated magnesium carbonates, when compared to mixes with 5% MgO and 5% hydrated magnesium carbonates. |
| Carbonation | Mo et al. [41] | P | 10–60 | 20–40 | - | - | 0.4 | 20–60 | Calcination 800 °C | 28 | Cement pastes were exposed to different CO2 periods and CO2 pressures (0.55 MPa for 3 and 15 h, and 0.10 MPa for 1 and 14 days). Under 0.10 MPa CO2, cement pastes were partially or completely carbonated. Higher CO2 pressure increased the speed of carbonation. In addition, cement pastes with lower cement content (higher FA and MgO) showed higher carbonation. |
| - | Choi et al. [44] | C | 80 | 20 | - | - | 0.48–0.65 | 5 | Calcination 1000 °C | 28–180 | Concrete mixes were produced with 20% FA, and different w/b (0.48, 0.65), water cured for 28 and 360 days, and then carbonated for 28–180 days. Carbonation of concrete mixes water-cured for 28 days was not influenced by the MgO addition up to 180 carbonation days. The influence of MgO addition was more beneficial in mixes with 360 water curing days, at all carbonation ages. This was attributed to the MgO filler effect to decrease concrete porosity at longer ages. |
| - | Pu and Unluer [48] | C | 100 | - | - | - | 0.42–0.80 | 10 | - | 1–14 | The carbonation of concrete blocks made with 90–95% aggregates and 5–10% MgO or OPC was investigated at 1 and 14 days. For this, concrete samples were cut at three different levels (0, 20, 35 mm). In all cases, the carbonation was higher at longer ages (14 days) and on the outer side of the specimens. The carbonation degree of MgO–concrete samples (45%) was twice as high as the reference mix (22%) at 14 days. |
| - | Gonçalves et al. [55] | M | 100 | - | - | - | 0.50 | 0–20 | Calcination 800 °C | 1–91 | The carbonation depth increased with increasing content of MgO. The incorporation of 20% of MgO increases the carbonation depth between 139% and 483%, in the accelerated carbonation test for 28 days. |
| Chloride resistance | Choi et al. [44] | C | 80 | 20 | - | - | 0.48–0.65 | 5 | Calcination 1000 °C | 28–360 | Concrete mixes were produced with 20% FA, and different w/b (0.48, 0.65), and water cured for 28 and 360 days. The chloride ion migration coefficient increased with incorporating MgO in mixes water-cured for 28 days and decreased in those water-cured for 360 days. This was confirmed by porosity test, showing reduced total porosity and pores with 0.03–0.30 μm with the addition of MgO in mixes cured for 360 days. |
| Freezing and thawing | Choi et al. [44] | C | 80 | 20 | - | - | 0.48–0.65 | 5 | Calcination 1000 °C | 28–360 | Concrete mixes were produced with 20% FA, and different w/b (0.48, 0.65), and water cured for 28 and 360 days. The MgO might have had little effect on the freeze/thaw resistance of concrete. The authors justify that with the Mercury Intrusion Porosimetry (MIP) analysis, which indicates an absence of alteration of pores larger than 10 µm within MgO concrete. |
| Expansion | Liu et al. [39] | P | 100 | - | - | - | 0.265 | 1–6 | - | 1–210 | Cement pastes obtained higher expansion values with increasing the MgO content, when compared to that of reference mixes, at all curing temperatures (20 °C and 50 °C). |
| - | Mo et al. [37] | P | 100 | - | - | - | 0.3 | 8 | Calcination 900–1300 °C | 1–300 | Cement pastes were produced with five types of MgO, having different neutralization times (46–1966 s), calcination temperatures (900–1300 °C), and residence times (30–90 min), and cured at 20 °C and 40 °C in water. At 20 °C and age of 300 days, the expansion values obtained in mixes containing MgO of lower neutralization time (46–325 s) were significantly higher than those of reference paste and mixes with higher neutralization time MgO (1266–1966 s). At 40 °C, higher values were obtained in mixes containing MgO with higher neutralization times, when compared to that of reference mix. |
| - | Mo et al. [57] | P | 100 | - | - | - | 0.28 | 2.75–7.00 | Calcination 1000 °C | 1–91 | Cement pastes were produced with three blended expansive agents of dolomite and magnesite (1:0, 9:11, 3:7), and cured at 20 °C and 40 °C in water. At 20 °C, higher expansion values were obtained in mixes containing higher blended expansive agent amounts and magnesite ratios, when compared to those of reference pastes. Similar trend was observed at 40 °C. The expansion of cement pastes containing blended expansive agent at 40 °C were greater and faster than those of the same samples tested at 40 °C. |
| - | Mo et al. [43] | P | 100 | - | - | - | 0.38 | 8 | Reactivity 50–400 s | 1–240 | Cement pastes were produced with the incorporation of four MgO types (reactivity values: 50, 100, 200, 400 s), and cured at 20 °C and 38 °C in water. At 20 °C, MgO with higher reactivity led to higher expansion, when compared to that of the reference mix. By contrast, at 38 °C, mixes with lower reactivity MgO presented higher expansion. |
| - | - | - | 40-100 | 20 | 40 | - | 0.5 | 8 | Reactivity 50–400 s | 1–240 | For samples cured in both 20 °C and 38 °C water, the incorporation of FA and slag in MgO-pastes led to lower expansion values, due to the reduced MgO amount that was added by cement weight. Minimum expansion value was obtained by reference cement paste, followed by the one with FA, slag and the most reactive MgO (50 s). |
| - | Mo et al. [42] | P | 100 | - | - | - | 0.38 | 5–8 | Reactivity 50 s | 1–210 | Compared to reference mix, increasing the amount of MgO significantly increased the cement paste expansion, regardless of testing age. |
| - | - | - | 25–100 | 20–35 | 20–40 | - | 0.38 | 5–8 | Reactivity 50 s | 1–210 | Maximum expansion was observed in cement paste with 8% MgO, followed by the one containing 5% MgO, compared to that of the reference mix. Increasing the amount of slag and FA decreased the cement paste expansion. This was attributed to the MgO content reduction (which was added by weight of cement) with reduction of cement weight in FA and slag mixes. |
| - | Moradpour et al. [47] | P | 100 | - | - | - | 0.25 | 1–5 | - | 1 | The autoclave expansion of cement pastes produced with 1–5% nano-MgO was 0–33% higher than that of reference mortar, after being under autoclaved pressure for 3 h. Higher MgO amounts led to higher expansion. |
| - | Gao et al. [58] | C | 100 | - | - | - | 0.48 | 4–12 | - | 2–180 | Under the same curing conditions (90% RH, 20 °C), concrete mixes produced with higher MgO content registered significantly greater expansion, when compared to that of reference concrete. |
| - | Gao et al. [46] | C | 100 | - | - | - | 0.48 | 4–12 | Calcination 1150 °C | 3 | Expansion of concrete increased with increasing the amounts of MgO and temperature and time of autoclave. |
| - | - | - | 70–100 | 30–50 | - | - | 0.48 | 4–12 | Calcination 1150 °C | 3 | Under autoclave curing, expansion decreased with increasing FA content, regardless of MgO incorporation. |
| - | Gao et al. [58] | C | 100 | - | - | - | 0.49–0.67 | 6–10 | - | 1–360 | Concrete mixes were produced with purified MgO (conventional mix w/c 0.67) and calcined MgO (hydraulic mix w/c 0.49). Reference concrete in both conventional and hydraulic mixes shrunk up to 360 days. Expansion of conventional mixes was higher than that of hydraulic mixes, regardless of MgO content. Expansion increased with increasing MgO content, at all ages. |
| - | - | - | 50–100 | 30–50 | - | - | 0.49–0.67 | 6–10 | - | 1–360 | Expansion of conventional concrete mixes with 10% purified MgO and hydraulic mixes with 6% calcined MgO decreased with increasing FA content. This behaviour was attributed to FA reaction with Ca(OH)2 and Mg(OH)2 and reduced the expansion stress formulated by MgO. |
| Shrinkage | Mo et al. [43] | P | 100 | - | - | - | 0.38 | 8 | Reactivity 50–400 s | 1–5 | Cement pastes were produced with the incorporation of four MgO types (reactivity values: 50, 100, 200, 400 s). The autogenous shrinkage of cement pastes significantly reduced with decreasing the reactivity of MgO, when compared to that of reference mix. This was attributed to MgO hydration compensating the shrinkage. |
| - | - | - | 40-100 | 20 | 40 | - | 0.5 | 8 | Reactivity 50–400 s | 1–5 | The incorporation of slag and FA decreased the shrinkage of cement pastes. Mixes with FA, slag and MgO with reactivity value of 50 s slightly expanded at the end of the test, due to MgO hydration. The deformation curves in FA-slag-mortars with 50, 100, 200 and 400 MgO reactivity values needed 9, 22, 34, and 34 h to rise up after their steep fall. |
| - | Mo et al. [42] | P | 100 | - | - | - | 0.38 | 5–8 | Reactivity 50 s | 1–5 | Maximum autogenous shrinkage was obtained in the reference cement paste, followed by mixes containing 5% MgO, and 8% MgO. This was attributed to the compensation of the shrinkage by MgO hydration. |
| - | - | - | 25–100 | 20–35 | 20-40 | - | 0.38 | 5–8 | Reactivity 50 s | 1–5 | The autogenous shrinkage of mixes containing slag and FA were lower than that of the reference mix, regardless of the MgO addition. Mixes with 8% MgO and different amounts of FA and slag obtained no shrinkage by the end of the test. In fact, 8% MgO mixes with 20–40% slag and 20% FA exhibited small expansions after 24 h. |
| - | Jin et al. [49] | P | - | - | 100 | - | 0.31 | 2.5–7.5 | Reactivity 10–100 s | 1–90 | Compared to the shrinkage of alkali-activated slag cement at 90 days, the maximum decreases (26%) was obtained in mixes containing 7.5% high reactivity MgO, followed by those with 7.5% medium reactivity MgO, and 5.0% high reactivity MgO. |
| - | Gonçalves et al. [55] | M | 100 | - | - | - | 0.50 | 0–20 | Calcination 800 °C | 1–91 | The use of 15% of MgO as cement replacement led to notable decreases in shrinkage, up to less 400 μm/m in 91-day shrinkage measurements when compared to those of OPC concrete (439 μm/m at 91 days). |
| - | Kabir and Hooton [59] | C | 100 | - | - | - | 0.50 | 0–15 | Reactivity 55–210 s | 1–180 | 7-day water curing of concrete prisms having different levels of MgO with reactivity of 55 s admixtures led to reductions in drying shrinkage: a reduction of more than 50% when 15% of MgO was used was observed. However, 210 s MgO did not mitigate shrinkage even at a 15% replacement level. Prisms cured in wet conditions for a month and having 15% of 55 s MgO ended up with a positive permanent expansion after 6 months of exposure to drying. |
| Reference | Mix | Age (day) | Results |
|---|---|---|---|
| Mo et al. [37] | P | 270 | Cement pastes were produced with three types of MgO, having different neutralization times (46–1966 s). Hydration products in Mg(OH)2 were smaller and more irregular than those hydrated in water. Cracks were observed at the MgO particle interface. |
| Mo et al. [43] | P | 90 | Cement pastes were produced with the incorporation of two MgO types (reactivity values: 50, 400), FA and slag, and cured in 38 °C water. In mixes without FA and slag, MgO particles were surrounded by hydration products of cement. Sheets with similar features to brucite were observed, having some empty inner pores. In mixes with FA and slag, rims of pozzolanic reaction were mainly formulated around coarser slag particles. The finer slag particles appeared to be fully hydrated. |
| Mo et al. [41] | P | 28 | Cement pastes were produced with 20–40% FA and 20–60% MgO, and exposed to different CO2 periods and CO2 pressures (0.55 MPa for 3 h, and 0.10 MPa for 14 days). Exposing cement pastes to CO2 formulated interconnected rounded-shape products (CaxMg1-xCO3), bounding FA particles. Increasing CO2 pressure further densified the microstructure of cement pastes. Mixes with low cement content (10%) presented loose microstructure, which were enhanced with the CO2 exposure by interconnecting hydration products. |
| Abdalqader and Al-Tabbaa [40] | P | 28 | The microscopic analysis of mixes produced with OPC (25%, 75%), FA (15–25%), slag (45–60%), and MgO (5%, 10%) appeared to be similar. The formation of brucite was not detected, which may be consumed by slag. The characteristic platelets of hydrotalcite were also not defined due to its tiny size. |
| Jin et al. [49] | P | 1–28 | At 1 day, in the microstructure of alkali-activated slag cement, and those containing medium reactivity MgO and 2.5% high reactivity MgO, slag particles covered with reticulated C–S–H was observed. Dense C–S–H gels were found in mixes with 5.0% and 7.5% of high reactivity MgO, which means denser matrix microstructure, accelerated slag hydration, and higher strength. At 14 days, all mixes presented similar microstructures. An exception was detected in mixes with 2.5% high and low reactivity MgO, by showing fibrous Ht and hydrogarnet, respectively. |
| Moradpour et al. [47] | M | 28 | Mortars produced with 1%, 3% and 5% nano-MgO had denser microstructure than that of reference mix. This was attributed to the expansion and filler effects of MgO. No obvious differences were detected between mixes containing different MgO amounts. The backscattered electron mode of scanning electron microscopy (SEM) presented modified C–S–H in mixes with MgO. |
| Mo et al. [42] | M | 28–90 | The microscopy of a mortar produced with 5% MgO, 40% slag, and 20% FA showed that FA particles and their surroundings were covered with hydration products. The densified structure may have been useful for strength increase. Pozzolanic or hydraulic products were grown from outside to the unhydrated inside of slag particles. The densified slag–FA interface contributed to strength increase. Unlike finer slag particles, the larger ones were not fully hydrated. |
| Dung and Unluer [51] | C | 14 | MgO–concrete mixes were produced with and without HCI (hydration agent-HA) and NaHMP (dispersion agent-DA), and tested under ambient and accelerated carbonation condition. For ambient conditions, hydrated magnesium carbonate formation was 5–10 times higher in mixes containing HA and/or DA, when compared to that of concrete without additional agents, which eventually led to lower water absorption and higher compressive strength. Under accelerated carbonation, mixes containing HA presented a large amount of nesquehonite formation, as opposed to the domination of non-carbonated brucite under ambient condition. |
| Unluer and Al-Tabbaa [53] | C | 7 | Cement was produced with 5–10% of MgO and 0–5% of heavy or light hydrated magnesium carbonates. Concrete blocks were produced with 10% cement and 5% FA and cured under natural and accelerated carbonation. Under accelerated carbonation, reference mix produced with 10% MgO presented dypingite/hydromagnesite abundance, while other containing MgO and hydrated magnesium carbonates also showed nesquehonite. In mixes with 5% MgO and 2% heavy hydrated magnesium carbonates, dypingite/hydromagnesite and nesquehonite were observed to have increased, respectively, which eventually increased the mechanical strength of mixes containing 2%. In some mixes with 5% MgO and 5% light hydrated magnesium carbonates, uncarbonated brucite was defined, leading to lower concrete strength. Under natural curing, small quantities of brucite and uncarbonated MgO were detected, explaining the low strength of concrete mixes without carbonation acceleration. |
| Gao et al. [58] | C | 28–360 | Microscopic analysis of concrete mixes produced with 6% purified MgO showed that samples with 360 days obtained denser microstructures when compared to those of 28-day samples. This was attributed to MgO hydration products filling the pores at later ages. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nobre, J.; Ahmed, H.; Bravo, M.; Evangelista, L.; de Brito, J. Magnesia (MgO) Production and Characterization, and Its Influence on the Performance of Cementitious Materials: A Review. Materials 2020, 13, 4752. https://doi.org/10.3390/ma13214752
Nobre J, Ahmed H, Bravo M, Evangelista L, de Brito J. Magnesia (MgO) Production and Characterization, and Its Influence on the Performance of Cementitious Materials: A Review. Materials. 2020; 13(21):4752. https://doi.org/10.3390/ma13214752
Chicago/Turabian StyleNobre, José, Hawreen Ahmed, Miguel Bravo, Luís Evangelista, and Jorge de Brito. 2020. "Magnesia (MgO) Production and Characterization, and Its Influence on the Performance of Cementitious Materials: A Review" Materials 13, no. 21: 4752. https://doi.org/10.3390/ma13214752
APA StyleNobre, J., Ahmed, H., Bravo, M., Evangelista, L., & de Brito, J. (2020). Magnesia (MgO) Production and Characterization, and Its Influence on the Performance of Cementitious Materials: A Review. Materials, 13(21), 4752. https://doi.org/10.3390/ma13214752









