Abstract
This paper presents a literature review concerning the characteristics of MgO (magnesium oxide or magnesia) and its application in cementitious materials. It starts with the characterization of MgO in terms of production processes, calcination temperatures, reactivity, and physical properties. Relationships between different MgO characteristics are established. Then, the influence of MgO incorporation on the properties of cementitious materials is investigated. The mechanical strength and durability behaviour of cement pastes, mortars and concrete mixes made with MgO are discussed. The studied properties of MgO–cement mixes include compressive strength, flexural strength, tensile strength, modulus of elasticity, water absorption, porosity, carbonation, chloride ion penetration, shrinkage, expansion, and hydration degree. In addition, microscopic analyses of MgO-cement mixes are also assessed. Summarizing the results of different studies, it is concluded that MgO incorporation in cementitious materials generally decreases the mechanical strength and shrinkage, and increases the porosity, expansion, carbonation and chloride ion migration. However, it should be emphasized that the properties of the specific MgO used (mainly the calcination temperature, the reactivity and the surface area) have a significant influence on the characteristics of the cementitious materials produced.
1. Introduction
The demand for cement and natural aggregates has been exponentially increasing due to rapid construction development. In fact, in 2014, about 40 billion tonnes of aggregates and 4 billion tonnes of cement were required in the construction sector worldwide [1,2]. Consequently, a significant amount of carbon dioxide (CO2) is released to the air during the production stage of these materials. For example, to produce 1 tonne of cement it is necessary to use 125 kW of electricity and to emit about 0.89 tonnes of CO2 emissions to the atmosphere [3,4]. One way to overcome this issue is by incorporating sustainable materials, such as fly ash (FA), silica fume, slag, metakaolin, and recycled aggregates in concrete [5,6,7,8,9,10].
Another alternative approach towards sustainable concrete is through MgO incorporation. Cements with high MgO content gained significant popularity in the last decade, mainly due to the growing concern about climate changes, that is, with the intention and the need to mitigate the CO2 emissions associated with the production of conventional Portland cements. Some authors believe that it is possible to produce such cements, with a high MgO content, with reduced CO2 emissions [11]. Other authors even believe that it is possible to produce cement that has a positive CO2 balance, by capturing atmospheric CO2 to form magnesium minerals (carbonates and hydroxycarbonates). The carbonation of MgO can be described, in general, as the formation of magnesite from MgO, through the absorption of carbon dioxide [11].
The utilization of MgO can, in some conditions of calcination and reactivity, decrease the thermal shrinkage [12,13], reduce the cost of concrete by decreasing costly cooling measures, and accelerate construction process speed by continuously casting concrete without needing as many cold joints [14]. However, the main motivation for the development and upscaling of MgO-based cements was that of an environmental nature. The lower temperatures required for the production of MgO compared to those required for the conversion of CaCO3 into ordinary Portland cement (OPC) and the energy savings associated with that reduced temperature led many to envision MgO-based cements as central to the future of environmentally friendly cement production. Likewise, MgO’s ability to absorb CO2 from the atmosphere to form a variety of carbonates and hydroxycarbonates fits well into the discussion of “carbon neutral” cements, which could absorb almost as much CO2 during its lifetime as that emitted during their manufacture. These two interconnected aspects have led to a recent rise in interest, both academic and commercial, in the area of MgO-based cements.
Currently, research has been focusing on the mechanical and durability-related properties of cementitious materials with MgO. However, an extensive and critical revision based on MgO characterization and properties of MgO-cementitious materials has not been conducted yet. Therefore, the scope of this review is to present MgO’s characterization in terms of production processes, calcination temperatures, reactivity, chemical and physical properties and microscopic features, and highlight the influence of MgO incorporation in cement pastes, mortars and concrete in terms of mechanical properties (compressive strength, flexural strength, tensile strength, modulus of elasticity), durability behaviour (water absorption, porosity, carbonation, chloride ion penetration, shrinkage), hydration degree, and microstructural analysis. The authors consider that the existence of a review paper that presents the available results up to date in a structured way will be an important support tool to understand whether or not the use of cementitious materials with MgO as replacement of Portland cement is viable in a given application. On the other hand, through the joint analysis of the existing investigations, it is also intended to understand the extension (percentage) of replacement that can be used and which MgO is best suited to the applications evaluated herein.
A very particular strategy was followed in the development of the literature review presented in this paper. First, an initial list of publications was collected, based on several factors: relevance of the title in relation to the topic; cementitious materials type; MgO type; and available data for statistical analysis. For each work collected, an expedient analysis was made in order to establish the relevance of its contents to the research, as well as the tests performed, main outcomes and conclusions. This information was then properly identified and transcribed into a spreadsheet, containing various topics of interest for all publications. As each work was individually evaluated, the relevant data regarding the production of MgO-cementitious material (i.e., cementitious materials type, mix design, curing conditions, etc.) were also collected. Then, an initial table of contents was proposed to serve as a guide for the subsequent investigation. This allowed a comprehensive exploration of the existing information on various factors relating to the use of MgO in the several properties of cementitious materials. As a result, the key points were revealed from the analysis and evaluation data. This allowed drawing several conclusions on the effects of using distinct types of MgO on cementitious materials, and thus enable its use in construction applications.
2. Magnesia Production and Its Use in Cementitious Materials
Magnesium is the eighth most abundant element in the Earth’s crust, at ~2.3% by weight, present in a range of rock formations such as dolomite, magnesite and silicate. Magnesium is also the third most abundant element in solution in seawater, with concentrations of ~1300 ppm. The current global production of MgO is 14 million tonnes annually (USGS, 2012), compared with that of OPC of over 2.6 billion tonnes, with current costs of around ~£200/tonne for reactive MgO (calcined), compared to ~£70/tonne for OPC. The cement production process implemented in most industries is known as the dry process and consists mainly of the following steps: grinding and homogenization of raw materials (obtaining raw flour); clinkerization of the raw flour in rotary kilns (clinker production); subsequent clinker cooling; grinding of clinker and addition of gypsum to obtain cement; bagging and shipping of the final product. This process requires high energy consumption and, since it requires temperatures of up to 1400 °C, it emits a large amount of polluting gases. In turn, magnesia (magnesium oxide, MgO) is mainly produced from the calcination of magnesite in a process similar to the production of lime from limestone. A smaller proportion of the world’s MgO production comes from seawater and brine sources, or other sources [15].
2.1. Calcination of Magnesite
The most common method used for MgO production is the calcination of magnesite () because of the higher energy requirements for production through the wet route. To produce 1 tonne of MgO from fully decomposed pure magnesite, about 1.08 tonnes of CO2 can be generated, while OPC production results in 0.85 tonnes of CO2 [16,17]. However, the amounts of CO2 released and MgO produced are highly dependent on the temperature and CO2 pressure used. A kiln with variable temperature is used for magnesite calcination, depending on the required MgO reactivity. In general, four types of MgO are produced [15,16]: light-burned or caustic-calcined MgO (calcined at 700–1000 °C), with the highest reactivity and greatest specific surface area; hard-burned MgO (calcined at 1000–1500 °C), with lower reactivity and specific surface area than those of light-burned MgO; dead-burned MgO or periclase (calcined at 1400–2000 °C), with the lowest specific surface area, making them almost unreactive; fused MgO (calcined at 2800 °C) with the lowest reactivity.
2.2. Calcination of Magnesium Hydroxide
Calcining magnesium hydroxide includes heating a filter cake containing 50–72% magnesium hydroxide solids (). This procedure is similar to that of MgO production in either a brine or seawater process (later discussed in Section 2.3). After placing the filter cake in a kiln, the decomposition reaction starts to take place at 350 °C and it quickly increases above this temperature. During this calcination, several inconsequential processes occur, including filter cake dehydration, dry magnesium hydroxide decomposition, and MgO sintering. The removal of chemically bound water from magnesium hydroxide is a difficult process without raising the temperature above 1000 °C [18].
2.3. Seawater and Brine
MgO can be produced from alkaline precipitation of brucite (Mg(OH)2) from seawater or Mg rich brine. In the former method, Mg concentration is about 1.4 g/L [19]. The seawater is pre-treated with sulphuric acid to reduce the pH to 4 to remove the carbonates (). Then, an alkali (lime or sodium hydroxide) is added to raise the pH above the brucite precipitation point (pH 10.5). Sodium hydroxide is used to obtain MgO with low Ca content (). Lime from dolomitic limestone is used to reduce the required additive quantity (). After brucite slurry filtration, the filter cake obtained is decomposed at temperatures above 350 °C, requiring higher energy than that of the magnesite calcination method (Section 2.1).
Another MgO production method is by carbonation, involving CO2 sequestration through carbonating Mg rich solutions [19,20,21]. For this purpose, natural (seawater or brine) or waste-based solutions (water from oil extraction, rejected brines from a desalinisation process) are used. The reaction between Mg2+ and CO2 sparged in the solution leads to the precipitation of Mg carbonate. The Mg carbonate type produced depends on the CO2 pressure and temperature [20]. For example, the formation of hydrated magnesium carbonates (nesquehonite and hydromagnesite), and magnesite occurs at temperatures of 25 °C, 120 °C and 120 °C, and CO2 pressures of 1 bar, 3 bar, and 100 bar, respectively. After that, the obtained Mg carbonate is calcined to formulate MgO.
2.4. Extraction of Magnesia from Mg-Bearing Minerals
This method involves geological CO2 sequestration, where rocks containing chemical groups capable of carbonation are decomposed to suitable precursors to react with CO2 [22,23,24]. The magnesium silicates decomposition can be facilitated by various methods. The first one is through Mg silicate acid digestion (), followed by brucite precipitation (), and its calcination () [22]. The second method is through Mg silicate carbonation with subsequent Mg carbonate calcination () [24]. The Mg carbonate type from this process depends on the CO2 pressure and temperature. For example, formulation of magnesite occurs at 155 °C and 126 bar, and hydrated magnesium carbonates at lower temperature and pressure [24]. After that, the carbonates would be calcined to produce MgO, CO2 and possibly H2O.
2.5. MgO in Cementitious Materials
In general, two main methods are used to add MgO in cementitious materials. One is by increasing the periclase (magnesium oxide mineral) content in cement clinker to produce high magnesia cement. This method has been used in dam concrete for about 40 years in China [25]. The second method is by preparing MgO from magnesite (MgCO3) calcination and then incorporating the material in concrete as an expansive additive [25]. When using the second method, it is important to homogenously disperse MgO in concrete by using an adequate mixing process to avoid heterogeneous expansion that could lead to concrete destruction.
The addition of MgO to conventional Portland cements results in the formation of Mg(OH)2 ( and its subsequent carbonation (, giving rise to hydrated magnesium carbonates. This type of cement was designed to replace Portland cement in large quantities, thereby deriving environmental benefits with respect to CO2 emissions. However, due to the long-term dimensional instability seen in concrete with cements with high MgO content, existing standards strictly limit the MgO content that can be used in Portland cements [11].
3. Magnesia Characterization
3.1. Physical Properties
MgO is commonly used as a raw material for Portland cement. This MgO is obtained by calcination at 1400–2000 °C of MgCO3, when clinker is produced. These high temperatures allow a large crystalline structure to be formed, with reduced reactivity and hydration rate. This behaviour causes a slow expansion, leading to extensive cracking of the cement paste [26]. Due to the referred behaviour, the standard EN 197 indicates that the MgO content in the cement should not exceed 5% (of cement mass). However, by varying the calcination temperature, MgO with completely different physical-chemical properties can be obtained.
The main physical properties of MgO are summarized in Table 1. MgO thermal conductivity has been determined by different studies, covering polycrystalline sintered MgO at lower temperatures [27] and sintered MgO at higher temperatures [28]. Table 1 shows values between 0.03 and 0.10 cal s−1 cm−2 °C−1 cm.

Table 1.
Physical properties of magnesia.
It was also found that MgO’s electrical resistance is very high, which makes it an excellent high-temperature electrical insulator. The relationship between specific resistance (ρ) of magnesia and temperature (T) can be expressed by , where A and B are specific constants. Table 1 displays the specific resistivity for sintered and high-purity MgO.
The thermal expansion of periclase is the greatest of all pure refractory oxides and approaches the expansion of metals. Expansion measurements have been carried out on single periclase crystals and high-purity sintered MgO (Table 1). Values for the specific heat capacity from ambient to higher temperatures are also shown in Table 1.
Regarding the structural properties, MgO has compressive strength of 0.83–1.44 GPa, tensile strength of 96 MPa, elastic modulus of 210–317 GPa, and flexural strength of 90 MPa, according to general references, as reported by Shand [16].
3.2. Reactivity
MgO reacts with water and diluted acids, and its reactivity (rate and degree of reaction) depends considerably on the physical properties and purity of the material [33]. MgO reactivity increases by reducing its particle size and, consequently, increasing its specific surface area [33]. MgO surface area and particle size are both controlled by the production conditions (raw material type and purity, calcination temperature, and residence time during calcination). Similar to other types of metal oxide, MgO has various surface defects, significantly influencing their reactivity [34]. The usual one-dimensional surface defects are in the step-shape and may have defect points [16]. In general, two types of MgO defects have been investigated: oxygen vacancy and Mg vacancy [16]. MgO reactivity is also influenced by the chemical nature of the precursor from which MgO is produced [35]. The arbitrary industrial classification of MgO reactivity serves for the appropriate grade selection depending on the application from hard-burned (slow hydration) to reactive (fast hydration) [16].
MgO reactivity is assessed according to the neutralisation rate of weak acid solutions, including citric acid [36] and acetic acid [16]. In the former testing method, citric acid is dissolved in distilled water, followed by adding Bromothymol blue (pH indicator) and MgO. The time taken for the solution to change colour is measured. For the latter testing method, MgO is added into distilled water, followed by adding phenolphthalein indicator and acetic acid with continuous mixing. The time is measured until development of the red phenolphthalein colour.
For MgO production, MgCO3 undergoes calcination in a furnace at a given temperature (calcination temperature), and a residence time (calcination time). The activity of the resulting MgO is evaluated by measuring the time needed to fully neutralize an acidic solution (neutralization time). MgO with higher reactivity tends to need shorter neutralization time [37]. The surface area and neutralization time of MgO are influenced by the time and temperature of calcination, as shown by Mo et al. [37]. In their study, magnesite was crushed into fine particles (<80 μm) and calcined in a furnace at a given temperature and calcination time. Figure 1a shows that the specific surface area of MgO decreases by increasing the temperature and residence time (calcination time). This was attributed to the particle growth of MgO due to the continued sintering, leading to total pore volume reduction and pore size enlargement. Figure 1a also shows that the neutralization time of MgO increases by increasing the calcination temperature and the residence time. The residence time has higher effect on the neutralization time, at higher temperature. Figure 1b shows the inverse relationship between MgO’s neutralization time and surface area. The neutralization time decreased considerably when the surface area was smaller than 1.3 m2/g. This decrement became less apparent for specific surface areas bigger than 20 m2/g. This was attributed to the influence of the surface area and hydration activity of MgO particles (related to its crystal size) on the MgO activity. In the study of Wogelius et al. [38], it was reported that MgO hydration activity was related to its surface structure, meaning that low-defect surface MgO had lower activity. At an early stage of sintering, more defects exist in MgO, leading to higher grain activity. MgO crystal lattice’s atoms array gradually become regular, contributing to fewer defects and lower hydration activity.
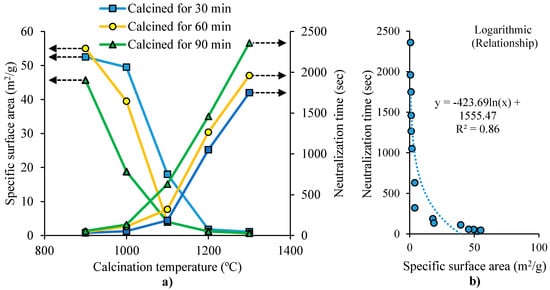
Figure 1.
(a) Effect of calcination temperature and residence time on the surface area and neutralization time of MgO. (b) Relationship between the surface area and the neutralization time of MgO (adapted from Mo et al. [37]).
Mo et al. [37] reported that the density of MgO and MgO grain size increased with the increase of calcination temperature and residence time, while MgO lattice distortion decreased (Table 2). The study also investigated the hydration degree of MgO for samples with 60 min of residence time, at 2, 7, 15 and 30 days, using microscopic analysis (Table 2). The results showed that the most rapid hydration occurred at 900 °C, where about 97% and 100% of MgO has been hydrated at 2 and 7 days, respectively. For the sample at 1100 °C, MgO hydration degree was 4.5% and 9.9% at 2 days and 7 days, respectively. The sample at 1300 °C had the slowest hydration. In conclusion, MgO grain hydration activity has higher influence than its surface area on MgO hydration activity.

Table 2.
Influence of calcination temperature (calc. temp.) and residence time (res. time) on microstructure and hydration degree of MgO [37].
4. Characterization of Cementitious Materials with Additional MgO
Several experimental campaigns conducted over the years have proven that the performance of concrete produced with cements with reactive MgO can be quite interesting. So, in the next sub-chapters, the results obtained by several investigations on the properties of such concrete mixes are analysed.
4.1. Mechanical Properties
The mechanical properties of cement pastes, mortars and concrete mixes produced with different types and amounts of MgO, studied by different researchers, are summarized in Table 3. In general, incorporating MgO leads to strength decrease of cement pastes, mortars and concrete. In fact, this behaviour was observed in compressive strength [39,40,41,42,43,44,45,46], flexural strength [42,43,46,47], and tensile strength [48]. The incorporation of higher amounts of MgO caused further strength decrements [39,43,46,47,49].

Table 3.
Mechanical properties of cementitious materials with MgO.
Liu et al. [39] studied the influence of calcined MgO addition in cement pastes. Mixes were produced with 0%, 2% and 3% of MgO and water to binder ratio (w/b) of 0.265. The compressive strength of the cement pastes was tested at 3, 7 and 28 days. There was a compressive strength reduction with increased MgO content and age of samples, relative to a reference paste without additional MgO (ordinary Portland cement mix).
Abdalqader and Al-Tabbaa [40] studied the compressive strength of cement pastes made with OPC (30%, 75%), FA (15–25%), slag (45–60%), and MgO (5%, 10%) with 170 s reactivity according to the acetic acid test, at 3, 7, 28 and 56 days. The average compressive strength of mixes containing OPC (75%) and FA (25%) was higher than those containing FA, slag, and MgO, at all ages. This was attributed to the smaller amount of OPC in the latter binders to activate FA and slag. It was also found that the use of 10% MgO, 60% slag and 30% Portland cement gave rise to cement pastes with strengths similar to those with 75% Portland cement and 25% fly ash. Therefore, it can be concluded that the combined use of MgO and slag causes the occurrence of chemical reactions that favour the strength of these cementitious materials.
Mo et al. [42] studied the effect of MgO with 50 s reactivity according to the citric acid test on the flexural and compressive strength of mortars. Mixes were produced with 5% and 8% of MgO as a replacement for cement clinker during the inter-grinding process. The addition of MgO induced reductions of the flexural and compressive strength at all ages. At 90 days, the compressive strength in mortars with 5% and 8% of MgO decreased by 13.7% and 19.7%, respectively, in comparison with that of conventional mortars. The authors attributed the reduction to the less frequent C–S–H formation due to OPC reduction in MgO mixes.
In summary, the results available to date in the literature on the mechanical behaviour of cementitious materials with reactive MgO allow the following conclusions:
—Compressive strength generally decreases with the introduction of MgO regardless of the replacement rate and reactivity of MgO. This reduction is of about 10% and 30%, when using 5% and 20% of MgO, respectively;
—Flexural strength also decreases with the use of reactive MgO in detriment of Portland cement. This reduction is similar to that found for compressive strength;
—The modulus of elasticity slightly decreases with the use of reactive MgO instead of Portland cement. This reduction equals 10% when 20% of MgO is used. This decrease is most likely due to the higher quantity of water required to obtain similar workability levels. Some investigations even point to the maintenance of this property with the use of MgO;
—The generalized decrease in mechanical properties is due to less frequent C–S–H formation due to OPC reduction in MgO mixes;
—Even though this decrease is generally observed at any age, it appears that the use of MgO in cementitious materials makes them approach their final strength earlier, especially when using highly reactive MgO;
—The joint introduction of MgO and slag causes the occurrence of chemical reactions that favour the strength of these cementitious materials;
—The carbonated curing conditions allow cementitious materials with MgO to reach a higher increase of their mechanical strength than cementitious materials without MgO. This was attributed to the higher porosity in the first mixes, leaving more space for hydrated magnesium carbonate formulation and strength development;
—The incorporation of different HA types (magnesium acetate, magnesium chloride, hydrochloric acid) in MgO concrete enabled higher strength development increments, when compared with the same incorporation in conventional concrete mixes.
4.2. Durability Behaviour
The durability behaviour of cementitious materials with MgO, studied by different researchers, is summarized in Table 4. In general, the incorporation of MgO leads to lower water absorption [45,47,52], higher carbonation [41,48], higher chloride ion migration coefficient [41], higher initial expansion [37,39,42,43,46,47,57,58], and lower shrinkage [42,43,53].

Table 4.
Durability of cementitious materials with MgO.
Moradpour et al. [47] studied the water permeability of mortars produced with nano-MgO, at 28 days. The results showed that the permeability decreased in mortars containing nano-MgO particles. The researchers defined 1% of MgO as an optimal amount to obtain maximum improvements of the mechanical strength and water absorption of mortars. Dung and Unluer [51,52] also studied the water absorption of concrete mixes produced with MgO and found a decrement when compared to that of conventional mixes.
Mo and Panesar [56] studied the porosity of cement pastes produced with 10%, 20% and 40% reactive MgO (calcined at 800 °C), under carbonation and non-carbonation conditions. The researchers found that incorporating 20% of MgO could lead to 32% pore volume decrease at 28 days, when compared to that of reference cement paste, under accelerated carbonation. By contrast, a 6–10% pore volume increase was found in pastes with 10–40% MgO, under non-carbonation condition.
Liu et al. [39] investigated the expansion behaviour of cement pastes produced with 2%, 3%, 4%, 5% and 6% of MgO. The length variation was measured in small prisms (10 × 10 × 40 mm) up to 210 days, at two different temperatures (20 °C and 50 °C) in water. The expansion increment rapidly took place at early ages (1–30 days), it slowed down between 30 to 90 days and became even after 90 days. Cement pastes presented higher expansion values with the increment of MgO content and curing temperature.
The results available to date in the literature on the durability-related behaviour of cementitious materials with reactive MgO, prompt the following conclusions:
—Porosity generally increases with the incorporation of MgO regardless of the replacement ratio and of its reactivity. This increase will be higher than 10% when using more than 5% MgO. However, the results in the literature are quite variable, with some studies showing improvements in this property with the use of MgO at ratios lower than 5%;
—Carbonated curing conditions allow cementitious materials with MgO to decrease their porosity to a higher extent than that found for cementitious materials without MgO. This was attributed to the higher initial porosity in the first mixes, leaving more space for hydrated magnesium carbonate formulation;
—Carbonation depth increases with the use of reactive MgO to the detriment of Portland cement. This increase can reach up to 400% for ratios of 20% MgO;
—The MgO may have little effect on the freeze/thaw resistance of concrete. The authors justify this through MIP analysis that indicate that pores larger than 10 µm within the MgO concrete do not present any changes;
—The expansion of cementitious materials is widely controlled by the MgO content, neutralization time, curing temperature, and MgO reactivity level. Overall, higher MgO content leads to higher initial values of expansion. MgO with higher reactivity led to higher expansion;
—As for shrinkage, the MgO incorporation effect in cementitious materials is dependent on the MgO reactivity [38,41] and MgO content [38,40]. However, the shrinkage is always lower with the incorporation of MgO. The use of 15% of MgO as cement replacement led to remarkable decreases in shrinkage, which may be of only about 10% of the 91-day shrinkage of OPC concrete.
4.3. Hydration Degree
The hydration of cementitious materials produced with additional MgO was studied by Dung and Unluer [52] and Mo et al. [42] through isothermal calorimetry and thermogravimetric analysis. In the study of Dung and Unluer [51], MgO-cement pastes were produced with and without HCI (hydration agent, HA) and NaHMP (dispersion agent, DA). The heat flow (Figure 2a) and cumulative heat (Figure 2b) of cement pastes were analysed through isothermal calorimetry at 30 °C for 72 h. Thermogravimetric analysis was assessed to evaluate MgO hydration degree with and without DA and HA, under up to 600 °C at 1, 3 and 14 days (Figure 2c).
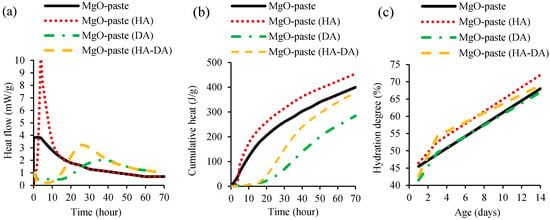
Figure 2.
Isothermal calorimetry of MgO-cement pastes showing (a) heat flow and (b) cumulative heat. (c) Thermogravimetric analysis (hydration degree) of MgO-cement pastes (adopted from [51]).
The isothermal calorimetry results showed that MgO dissolution and brucite formation occurred after the first few hours of mixing MgO-cement pastes with and without HA. This first peak of MgO-cement paste with HA was significantly higher than that of the mix without HA. The addition of DA in mixes with and without HA slowed down this action to 10 to 15 h, followed by a lower peak of hydration and MgO dissolution. After 24 h and 38 h, a wide brucite formation peak was observed in mixes containing both DA and HA and only DA, respectively. In conclusion, the incorporation of HA increased the MgO hydration, while the opposite effect was observed with the addition of DA, associated with the deflocculating effect of the latter. The thermogravimetric analysis (Figure 2c) also showed hydration increase with the addition of HA in mixes with or without DA, at 3 and 14 days, when compared to that of reference mix without HA and DA. The same authors [52] also investigated the influence of using 0.05% and 1% of different HA types, namely magnesium chloride (MgCl2), magnesium acetate ((CH3COO)2Mg), and hydrochloric acid (HCl). MgO-cement pastes containing HA obtained higher values in terms of heat flow, cumulative heat and hydration degree, when compared to mixes without HA. The incorporation of HAs enhanced the dissolution of reactive MgO and the precipitation of brucite, which, in turn, increased the rate and degree of reactive MgO hydration. Increasing the amount of HA led to increase of hydration. The isothermal calorimetry results showed that the maximum increases was achieved with the addition of MgCl2, followed by (CH3COO)2Mg, and HCl. In the thermogravimetric analysis, the sequence was (CH3COO)2Mg, MgCl2, and HCl.
Mo et al. [42] made an isothermal calorimetry analysis of cement pastes made with 8% MgO, with or without 20–0% slag and 20–35% FA, during the first 72 h after mixing. In the first 7 h, no obvious differences were observed between the heat flow of the reference mix without MgO and MgO mixes. This was attributed to the accelerated formation of C-S-H. However, the addition of MgO led to decrease of maximum heat flow peak between 7–12 h and increase of heat flow between 12–48 h. Compared to the reference cement paste and that containing only MgO, mixes with FA, slag, and MgO presented longer periods of acceleration and induction. In fact, the MgO–cement pastes with 40% slag and 20% FA and the one with 20% slag and 35% FA reached their maximum heat flow 2 h after reference mix. This was attributed to small cement hydration retardation caused by slag and FA.
4.4. Microscopic Analysis
The analysed observations based on scanning electron microscopy (SEM) of cementitious materials made with additional MgO, collected from different studies, are summarized in Table 5. Each study presented different aspects and phenomena, including the ability of MgO to create cracks in cementitious materials [37] and densify its microstructure [47,49].

Table 5.
Microscopic analysis results of cementitious materials with MgO.
Mo et al. [37] assessed the morphology of cement pastes produced with MgO having different neutralization times (46, 325, and 1966 s), water-cured for 270 days at 40 °C. Due to the influence of alkali on MgO hydration, the hydration products in Mg(OH)2 were more irregular and smaller, when compared to reference paste without additional MgO. On the other hand, cracks were observed at the sintered MgO particle interface, associated with its expansion at the particle boundary. The same researchers [43] produced cement pastes with incorporation of two MgO types (reactivity values: 50 and 400 s), FA and slag, and cured them in 38 °C water. In mixes without FA and slag, the MgO particles were surrounded by hydration products of cement. Sheets with similar features of brucite were observed, with some empty inner pores. In mixes with FA and slag, rims of pozzolanic reaction were mainly formulated around coarser slag particles. The finer slag particles appeared to be fully hydrated.
Moradpour et al. [47] observed that mortars produced with 1%, 3% and 5% nano-MgO had denser microstructure than that of the reference mix. The authors attributed this fact to the expansion and filler effects of MgO. The backscattered electron mode of SEM showed modified C–S–H in mixes with MgO.
5. Conclusions
From the review of published literature focused on the mechanical strength and durability behaviour of cement pastes, mortars and concrete mixes produced with additional MgO, the following main conclusions were drawn:
- The compressive strength, flexural strength and tensile strength of cementitious materials decreased with the incorporation and increase in the MgO content, regardless of the material being added directly to the mix or to the cement clinker. This was mainly attributed to the porosity increment and lower hydration of the MgO mixes, when compared to conventional mixes without MgO. The reactivity of MgO showed insignificant influence on the strength of cementitious materials;
- The incorporation of MgO could lead to porosity decrease, when compared to that of conventional reference mixes, under accelerated carbonation. By contrast, porosity increased with the addition of MgO under ambient carbonation condition;
- The carbonation of concrete mixes produced with MgO tends to be higher than that of conventional concrete mixes. This trend becomes more evident with higher MgO incorporation levels;
- The chloride ion migration coefficient increased by incorporating MgO in mixes water-cured for 28 days and decreased in those water-cured for 360 days. This decrease was attributed to the reduced porosity with the addition of MgO in mixes cured for 360 days;
- The initial expansion of concrete mixes increased by increasing the MgO content;
- The shrinkage of cementitious materials decreased with the incorporation of MgO due to the compensation of the shrinkage by MgO hydration, during 1–5 days. The shrinkage of cementitious materials fell significantly by increasing the reactivity of MgO;
- The hydration degree of cementitious material mixes was not changed by the addition of MgO, during the first 7 h of mix production. This was attributed to the accelerated formation of C–S–H. However, the addition of MgO led to a decrease of maximum heat flow peak between 7–12 h and increase of heat flow between 12–48 h. The incorporation of hydration agent increased the MgO hydration, while the opposite effect was observed with the addition of dispersion agent, associated with the deflocculating effect of the latter;
- Microscopic analysis showed that cementitious materials produced with MgO may have had denser microstructure when compared to that of conventional reference mixes. This was attributed to MgO hydration products filling the pores and to the expansion effect of MgO.
In the future, it would be interesting to prepare a systematic literature review by subjecting the existing data to a statistical analysis. Based on the type and content of the reactive MgO, the existing results allow the development of models for predicting different properties.
Author Contributions
Conceptualization, M.B., L.E. and J.d.B.; methodology, J.N., M.B., L.E. and J.d.B.; validation, M.B., L.E. and J.d.B.; data curation, J.N., H.A. and M.B.; writing—original draft preparation, J.N. and H.A.; writing—review and editing, J.N., H.A. and M.B.; supervision, L.E. and J.d.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors wish to thank CERIS (Civil Engineering Research and Innovation for Sustainability) research centre and FCT (Foundation for Science and Technology).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Freedonia. World Construction Aggregates-Demand and Sales Forecasts, Market. Share, Market. Size, Market. Leaders; Industry study No. 3389 The Freedonia Group: Cleveland, OH, USA, 2016; p. 390. [Google Scholar]
- USGS. Commodity Statistics and Information Mineral. Yearbooks; USA Geological Survey: Washington, DC, USA, 2015.
- Marinković, S.; Radonjanin, V.; Malesev, M.; Ignjatovic, I. Comparative environmental assessment of natural and recycled aggregate concrete. Waste Manag. 2010, 30, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- de Schepper, M.; Heede, P.; Driessche, I.; de Belie, N. Life cycle assessment of completely recyclable concrete. Materials 2014, 7, 6010–6027. [Google Scholar] [CrossRef] [PubMed]
- Kurda, R.; de Brito, J.; Silvestre, J.D. Combined economic and mechanical performance optimization of recycled aggregate concrete with high volume of fly ash. Appl. Sci. 2018, 8, 1189. [Google Scholar] [CrossRef]
- Kurda, R.; de Brito, J.; Silvestre, J.D. Water absorption and electrical resistivity of concrete with recycled concrete aggregates and fly ash. Cem. Concr. Compos. 2019, 95, 169–182. [Google Scholar] [CrossRef]
- Berndt, M.L. Properties of sustainable concrete containing fly ash, slag and recycled concrete aggregate. Constr. Build. Mater. 2009, 23, 2606–2613. [Google Scholar] [CrossRef]
- Kou, S.C.; Poon, C.S.; Agrela, F. Comparisons of natural and recycled aggregate concretes prepared with the addition of different mineral admixtures. Cem. Concr. Compos. 2011, 33, 788–795. [Google Scholar] [CrossRef]
- Ferdous, W.; Manalo, A.; Wong, H.; Abousnina, R.; Ajarmeh, O.; Zhuge, Y.; Schubel, P. Optimal design for epoxy polymer concrete based on mechanical properties and durability aspects. Constr. Build. Mater. 2020, 232, 117–229. [Google Scholar] [CrossRef]
- Abousnina, R.; Manalo, A.; Ferdous, W.; Lokuge, W.; Benabed, B.; Al-Jabri, K. Characteristics, strength development and microstructure of cement mortar containing oil-contaminated sand. Constr. Build. Mater. 2020, 252, 119155. [Google Scholar] [CrossRef]
- Walling, S.; Provis, J. Magnesia-based cements: A journey of 150 years, and cements for the future? Chem. Rev. 2016, 116, 4170–4204. [Google Scholar] [CrossRef]
- Yuan, M.; Tang, M. Study on the mechanism of autogenous expansion of concrete used in Baishan Dam. J. Nanjing Inst. Chem. Technol. 1984, 2, 15–18. (In Chinese) [Google Scholar]
- Mehta, P. History and status of performance tests for evaluation of soundness of cements. In Cement Standards-Evolution and Trends, ASTM STP663-EB; American Society for Testing and Materials: Philadelphia, PA, USA, 1977; pp. 35–60. [Google Scholar]
- Du, C. A review of magnesium oxide in concrete. Concr. Int. 2005, 27, 45–50. [Google Scholar]
- Al-Tabbaa, A. Chapter 19: Reactive magnesia cement. In Eco-Efficient Concrete; Pacheco-Torgal, F., Jalali, S., Labrincha, J., John, V., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 523–543. [Google Scholar]
- Shand, M.A. The Chemistry Technology of Magnesia; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Taylor, F.H.W. Cement Chemistry; Academic Press: New York, NY, USA, 1990. [Google Scholar]
- Gregg, S.J.; Packer, R.K. The production of active solids by thermal decomposition. Part VI. The calcination of magnesium hydroxide. J. Chem. Soc. 1955, 51–55. [Google Scholar] [CrossRef]
- Ferrini, V.; De-Vito, C.; Mignardi, S. Synthesis of nesquehonite by reaction of gaseous CO2 with Mg chloride solution: Its potential role in the sequestration of carbon dioxide. J. Hazard. Mater. 2009, 168, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Hänchen, M.; Prigiobbe, V.B.R.; Mazzotti, M. Precipitation in the Mg-carbonate system-effects of temperature and CO2 pressure. Chem. Eng. Sci. 2008, 63, 1012–1028. [Google Scholar] [CrossRef]
- Back, M.; Bauer, M.; Stanjek, H.; Peiffer, S. Sequestration of CO2 after reaction with alkaline earth metal oxides CaO and MgO. Appl. Geochem. 2011, 26, 1097–1107. [Google Scholar] [CrossRef]
- Teir, S.; Eloneva, S.; Fogelholm, C.; Zevenhoven, R. Fixation of carbon dioxide by producing hydromagnesite from serpentinite. Appl. Energy 2009, 86, 214–218. [Google Scholar] [CrossRef]
- Zevenhoven, R.; Teir, S. Long term storage of CO2 as magnesium carbonate in Finland. In Proceedings of the 3rd Annual Conference on Carbon Capture and Sequestration, Alexandria, Egypt, 3–6 May 2004; pp. 1–11. [Google Scholar]
- Maroto-Valer, M.; Fauth, D.; Kuchta, M.; Zhang, Y.; Adresen, J. Activation of magnesium rich minerals as carbonation feedstock materials for CO2 sequestration. Fuel Process. Technol. 2005, 86, 1627–1645. [Google Scholar] [CrossRef]
- Mo, L.; Deng, M.; Tang, M.; Al-Tabbaa, A. MgO expansive cement and concrete in China: Past, present and future. Cem. Concr. Res. 2014, 57, 1–12. [Google Scholar] [CrossRef]
- Jin, F.; Al-Tabbaa, A. Characterisation of different commercial reactive magnesia. Adv. Cem. Res. 2013, 26, 101–113. [Google Scholar] [CrossRef]
- Francl, J.; Kingery, W.D. Thermal conductivity: IV, apparatus for determining thermal conductivity by a comparative method: Data for Pb, Al2O3, BeO, and MgO. J. Am. Ceram. Soc. 1954, 37, 80–84. [Google Scholar] [CrossRef]
- McQuarrie, M. Thermal conductivity: High-temperature method and results for alumina, magnesia, and beryllia from 1000 °C to 1800 °C. J. Am. Ceram. Soc. 1954, 37, 84–88. [Google Scholar] [CrossRef]
- Austin, J.B. The thermal expansion of some refractory oxides. J. Am. Ceram. Soc. 1931, 14, 795–810. [Google Scholar] [CrossRef]
- Ebert, H.; Tingwaldt, C. Expansion Measurements at temperatures up to 2000 °C. Physics 1936, 37, 471–474. [Google Scholar]
- Diepschlag, E.; Wulfesting, F. Electric conductivity of magnesia and some other refractory materials. Iron Steel Ind. 1929, 24, 32. [Google Scholar]
- Foëx, M. Electrical conductivity of beyllia and of magnesia at high temperatures. Comptes Rendus 1942, 214, 665–666. [Google Scholar]
- Liska, M.; Wilson, A.; Bensted, J. 13.4-MgO cements. In Lea’s Chemistry of Cement and Concrete, 5th ed.; Hewlett, P., Liska, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 609–620. [Google Scholar]
- Wenhua, S.; Chong, C.; Honghua, Z.; Kehao, C. Relationship between crystalline size and lattice distortion of MgO and its activity. J. Wuhan Univ. Technol. 1991, 13, 21–24. (In Chinese) [Google Scholar]
- Smithson, G.; Bakhshi, N. The kinetics and mechanism of the hydration of magnesium oxide in a batch reactor. Can. J. Chem. Eng. 1969, 47, 508–513. [Google Scholar] [CrossRef]
- Strydom, C.; Merwe, E.; Aphane, E. The effect of calcining conditions on the rehydration of dead burnt magnesium oxide using magnesium acetate as a hydrating agent. J. Therm. Anal. Calorim. 2005, 80, 659–662. [Google Scholar] [CrossRef]
- Mo, L.; Deng, M.; Tang, M. Effects of calcination condition on expansion property of MgO-type expansive agent used in cement-based materials. Cem. Concr. Res. 2010, 40, 437–446. [Google Scholar] [CrossRef]
- Wogelius, R.; Refson, K.; Fraser, D.; Grime, G.; Goff, J. Periclase surface. Geochim. Cosmochim. Acta 1995, 59, 1875–1881. [Google Scholar] [CrossRef]
- Liu, Z.; Cui, X.; Tang, M. MgO-type delayed expansive cement. Cem. Concr. Res. 1991, 21, 1049–1057. [Google Scholar]
- Abdalqader, A.F.; Al-Tabbaa, A. Mechanical and microstructural characterisation of multicomponent blended cements incorporating reactive magnesia. In Proceedings of the 1st Concrete Innovative Conference (CIC), Oslo, Norway, 11–13 June 2014; The Norwegian Concrete Association-Norsk Betongforening: Oslo, Norway, 2014; p. 93. Available online: https://www.researchgate.net/profile/Ahmed_Abdalqader/publication/269400461_MECHANICAL_AND_MICROSTRUCTURAL_CHARACTERISATION_OF_MULTICOMPONENT_BLENDED_CEMENTS_INCORPORATING_REACTIVE_MAGNESIA/links/558429dd08ae71f6ba8c3521/MECHANICAL-AND-MICROSTRUCTURAL-CHARACTERISATION-OF-MULTICOMPONENT-BLENDED-CEMENTS-INCORPORATING-REACTIVE-MAGNESIA.pdf (accessed on 21 January 2020).
- Mo, L.; Zhang, F.; Deng, M. Effects of carbonation treatment on the properties of hydrated fly ash-MgO-Portland cement blends. Constr. Build. Mater. 2015, 96, 147–154. [Google Scholar] [CrossRef]
- Mo, L.; Liu, M.; Al-Tabbaa, A.; Deng, M.; Lau, W.Y. Deformation and mechanical properties of quaternary blended cements containing ground granulated blast furnace slag, fly ash and magnesia. Cem. Concr. Res. 2015, 71, 7–13. [Google Scholar] [CrossRef]
- Mo, L.; Liu, M.; Al-Tabbaa, A.; Deng, M. Deformation and mechanical properties of the expansive cements produced by inter-grinding cement clinker and MgOs with various reactivities. Constr. Build. Mater. 2015, 80, 1–8. [Google Scholar] [CrossRef]
- Choi, S.; Jang, B.; Kim, J.; Lee, K. Durability characteristics of fly ash concrete containing lightly-burnt MgO. Constr. Build. Mater. 2014, 58, 77–84. [Google Scholar] [CrossRef]
- Mavroulidou, M.; Morrison, T.; Unsworth, C.; Gunn, M. Properties of concrete made of multicomponent mixes of low-energy demanding binders. Constr. Build. Mater. 2015, 101, 1122–1141. [Google Scholar] [CrossRef]
- Gao, P.-W.; Wu, S.-X.; Lu, X.-L.; Deng, M.; Lin, P.-H.; Wu, Z.-R.; Tang, M.-S. Soundness evaluation of concrete with MgO. Constr. Build. Mater. 2007, 21, 132–138. [Google Scholar] [CrossRef]
- Moradpour, R.; Taheri-Nassaj, E.; Parhizkar, T.; Ghodsian, M. The effects of nanoscale expansive agents on the mechanical properties of non-shrink cement-based composites: The influence of nano-MgO addition. Compos. Part. B 2013, 5, 193–202. [Google Scholar] [CrossRef]
- Pu, L.; Unluer, C. Investigation of carbonation depth and its influence on the performance and microstructure of MgO cement and PC mixes. Constr. Build. Mater. 2016, 120, 349–363. [Google Scholar] [CrossRef]
- Jin, F.; Gu, K.; Al-Tabbaa, A. Strength and drying shrinkage of reactive MgO modified alkali-activated slag paste. Constr. Build. Mater. 2014, 51, 395–404. [Google Scholar] [CrossRef]
- Wei, J.; Chen, Y.; Li, Y. The reaction mechanism between MgO and microsilica at room temperature. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2006, 21, 88–91. [Google Scholar]
- Dung, N.; Unluer, C. Improving the performance of reactive MgO cement-based concrete. Constr. Build. Mater. 2016, 126, 747–758. [Google Scholar] [CrossRef]
- Dung, N.; Unluer, C. Sequestration of CO2 in reactive MgO cement-based mixes with enhanced hydration mechanisms. Constr. Build. Mater. 2017, 143, 71–82. [Google Scholar] [CrossRef]
- Unluer, C.; Al-Tabbaa, A. Impact of hydrated magnesium carbonate additives on the carbonation of reactive MgO cements. Cem. Concr. Res. 2013, 54, 87–97. [Google Scholar] [CrossRef]
- Unluer, C.; Al-Tabbaa, A. Enhancing the carbonation of MgO cement porous blocks through improved curing conditions. Cem. Concr. Res. 2014, 59, 55–65. [Google Scholar] [CrossRef]
- Gonçalves, T.; Silva, R.V.; de Brito, J.; Fernández, J.; Esquinas, A. Mechanical and durability performance of mortars with fine recycled concrete aggregates and reactive magnesium oxide as partial cement replacement. Cem. Concr. Compos. 2020, 105, 103420. [Google Scholar] [CrossRef]
- Mo, L.; Panesar, D.K. Effects of accelerated carbonation on the microstructure of Portland cement pastes containing reactive MgO. Cem. Concr. Res. 2012, 42, 769–777. [Google Scholar] [CrossRef]
- Mo, L.; Deng, Y.; Lu, A.; Deng, M. Preparation of MgO- and CaO-bearing expansive agent used for cement-based materials. Key Eng. Mater. 2013, 539, 211–214. [Google Scholar] [CrossRef]
- Gao, P.; Xu, S.; Chen, X.; Li, J.; Lu, X. Research on autogenous volume deformation of concrete with MgO. Constr. Build. Mater. 2013, 40, 998–1001. [Google Scholar] [CrossRef]
- Kabir, H.; Hooton, R. Evaluating soundness of concrete containing shrinkage-compensating MgO admixtures. Constr. Build. Mater. 2020, 253, 119141. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).